Summary
Genetic deficiency of dystrophin leads to disability and premature death in Duchenne muscular dystrophy (DMD), affecting the heart as well as skeletal muscle. Here, we report that clinical-stage cardiac progenitor cells, known as cardiosphere-derived cells (CDCs), improve cardiac and skeletal myopathy in the mdx mouse model of DMD. Injection of CDCs into the hearts of mdx mice augments cardiac function, ambulatory capacity, and survival. Exosomes secreted by human CDCs reproduce the benefits of CDCs in mdx mice and in human induced pluripotent stem cell-derived Duchenne cardiomyocytes. Surprisingly, CDCs and their exosomes also transiently restored partial expression of full-length dystrophin in mdx mice. The findings further motivate the testing of CDCs in Duchenne patients, while identifying exosomes as next-generation therapeutic candidates.
Keywords: muscular dystrophy, exosomes, dystrophin, cardiosphere-derived cells, microRNA, cardiomyopathy
Graphical Abstract

Highlights
-
•
CDCs improve cardiac and skeletal myopathy in the mdx mouse model of DMD
-
•
CDC exosomes reproduce the benefits of CDCs
-
•
CDCs and their exosomes transiently restored partial expression of dystrophin
In this article, Marbán and colleagues show that exosomes mediate benefits of cell therapy in mouse and human models of Duchenne muscular dystrophy.
Introduction
Absence of dystrophin in Duchenne muscular dystrophy (DMD) leads to membrane fragility and secondary damage to muscle (both skeletal and cardiac) (Shirokova and Niggli, 2013). Early disability is due predominantly to the skeletal myopathy, but heart failure is the most common cause of death (Verhaert et al., 2011). Cardiosphere-derived cells (CDCs) may represent a viable therapeutic option. CDCs are progenitor cells intrinsic to the heart; in clinical trials after myocardial infarction, CDCs promote cardiomyogenesis and reverse established scar (Makkar et al., 2012, Malliaras et al., 2014). Multiple lines of evidence now indicate that most of the beneficial effects of CDCs are indirect. In the extreme, allogeneic CDCs are cleared completely within several weeks, but their functional and structural benefits persist for at least 6 months (Malliaras et al., 2012). CDCs secrete diffusible factors that promote angiogenesis, recruit endogenous progenitor cells, and coax surviving heart cells to proliferate (Chimenti et al., 2010, Li et al., 2010); transplanted CDCs suppress maladaptive remodeling (Lee et al., 2011), apoptosis (Cheng et al., 2012, Li et al., 2010), fibrosis (Tseliou et al., 2013), and inflammation after myocardial infarction (Tseliou et al., 2013) and in non-ischemic cardiomyopathy (Aminzadeh et al., 2015b). These diverse mechanisms appear to be mediated via the secretion of exosomes laden with noncoding RNA, including microRNAs (miRNAs) (Ibrahim et al., 2014), consistent with the notion that exosomes contain a plethora of bioactive molecules that target multiple signaling pathways synergistically (Vyas and Dhawan, 2016). In a murine model of myocardial infarction, CDC-secreted exosomes (CDC exosomes) mimic the functional and structural benefits of CDCs, while blockade of exosome biosynthesis renders CDCs ineffective (Ibrahim et al., 2014). Given the clinical data with CDCs, and the complementarity between their therapeutic actions and the pathophysiological processes underlying Duchenne cardiomyopathy (oxidative stress [Menazza et al., 2010, Williams and Allen, 2007], inflammation [Wehling-Henricks et al., 2010], fibrosis [Tandon et al., 2015], and mitochondrial dysfunction [Burelle et al., 2010]), we reasoned that CDCs and their exosomes might be useful in treating Duchenne cardiomyopathy. Our early work reported in abstract form (Aminzadeh et al., 2014, Aminzadeh et al., 2015a) revealed striking phenotypic correction by CDCs in mdx dystrophic mice, motivating the HOPE-Duchenne clinical trial (Ascheim and Jefferies, 2016) of CDCs in DMD patients. Initially, we had not aspired to restore skeletal muscle function, but merely to offset the pathophysiological consequences of dystrophin deletion in the heart. We now report that CDCs and their secreted exosomes potently improve not only cardiac but also skeletal muscle structure and function, contributing to major systemic benefits after injection of CDCs into the heart. An unanticipated, minor restoration of dystrophin expression was also observed, but this cannot explain all of the observed benefits.
Results
CDC Transplantation in mdx Hearts
Intramyocardial injection of first and second (lower) doses of CDCs into the hearts of mdx mice improved left ventricular function (as manifested by ejection fraction [EF]) and volumes, relative to placebo, for at least 6 months (Figures 1A and S1A). The CDC-induced improvement in EF persisted beyond the point at which no surviving CDCs were detectable in mdx hearts (3 weeks after CDC delivery; Figure S1B). In addition to improving EF, CDC injection enhanced ambulatory function (Figure 1B). Ten-month-old wild-type mice (WT) and mdx mice (distinct from the mdx mice studied in Figure 1A) were subjected to weekly high-intensity treadmill exercise, starting 3 weeks after single-dose CDC or vehicle administration. CDC-treated mdx mice showed a substantial increase in maximal exercise capacity, relative to vehicle-treated mdx mice, over the 3 months that exercise capacity was measured; survival also differed in the two groups (Figure 1C). By ∼23 months of age, all vehicle-treated mdx mice had died, whereas >50% of CDC-treated mdx mice remained alive (Figure 1C). In investigating the mechanism, we studied known (anti-oxidative, anti-inflammatory, anti-fibrotic, and cardiomyogenic) effects of CDCs (Aminzadeh et al., 2015b, Cheng et al., 2012, Chimenti et al., 2010, Davis et al., 2009, Ibrahim et al., 2014, Lee et al., 2011, Li et al., 2010, Makkar et al., 2012, Makkar et al., 2014, Malliaras et al., 2012, Smith et al., 2007, Tseliou et al., 2013, White et al., 2013). Injection of CDCs led to major changes in the expression of genes related to oxidative stress, inflammation, and mitochondrial integrity (Figures S1C–S1G). The NRF2 antioxidant pathway was activated in CDC-treated mdx heart (Figure 1D). NRF2 is normally repressed by KEAP1, but oxidative stress (as well as NRF2 phosphorylation by protein kinases such as AKT) causes dissociation of the NRF2-KEAP1 complex, culminating in nuclear translocation of NRF2 and transcriptional activation of antioxidant enzymes (Martin et al., 2004). In mdx hearts, levels of phosphorylated AKT (Figure 1E), total NRF2 (Figure 1F), and nuclear NRF2 (Figure 1G) were high (as expected in response to oxidative stress); CDC treatment further increased their protein levels (Figures 1D–1G) and those of downstream gene products (hemeoxygenase-1 [HO-1], catalase, superoxide dismutase-2 [SOD-2], and the catalytic subunit of glutamate-cysteine ligase [GCLC]; Figures 1H and S1G). Concomitantly, oxidative stress was attenuated, as demonstrated by a profound reduction of malondialdehyde adducts (Figure 1I). Histologic analysis revealed extensive fibrosis in vehicle-treated mdx hearts, but much less in CDC-treated mdx hearts (comparable with an age-matched WT control; Figure S2A). Likewise, CDC treatment largely reversed the accumulation of collagens I and III in mdx heart tissue 3 weeks after treatment (Figure S2B). CDCs inhibited the inflammation (Figures 1J and 1K) and mitochondrial dysfunction (Figures 1L–1N) characteristic of mdx cardiomyopathy. Nuclear factor κB (NF-κB), the master regulator of pro-inflammatory cytokines and chemokines (Carlson et al., 2005), was activated in vehicle mdx hearts (Figure 1K, top panel). Increases in phosphorylated IκB and nuclear p65 were accompanied by upregulation of MCP1 (monocyte chemoattractant protein1) and accumulation of CD68+ macrophages and CD3+ T cells (Figure 1K, bottom panel). CDC treatment reversed activation of NF-κB and decreased the number of inflammatory cells in mdx hearts 3 weeks after CDC injection (Figures 1J, 1K, and S2C). Mitochondrial structure and function are abnormal in muscular dystrophy-associated heart failure (Burelle et al., 2010). Whole-transcriptome analysis revealed major changes in the expression of genes related to mitochondrial integrity in mdx hearts (Figure S1D). Consistent with this finding, CDCs restored mitochondrial ultrastructure (Figure 1L), increased mtDNA copy numbers (but not mitochondrial number; Figure S3A), augmented levels of respiratory chain subunits (Figure 1M), and normalized the deficient respiratory capacity of isolated mdx mitochondria (Figure 1N). Of note, the salutary mitochondrial changes were associated with upregulation of antioxidant enzymes and reductions of oxidative stress and inflammation (Figures 1D–1K, S1E–S1G, and S5). We also probed the effects of CDCs on cardiomyogenesis. Vehicle-treated mdx hearts exhibited a modest increase in the numbers of cycling (Ki67+) and proliferating (aurora B+) cardiomyocytes (Figures S3B and S3C), presumably as a compensation for ongoing cardiomyocyte loss. CDCs are known to increase endogenous cardiomyogenesis in ischemic (Cheng et al., 2012, Lee et al., 2011, Li et al., 2012, Makkar et al., 2012, Malliaras et al., 2014) and non-ischemic models (Aminzadeh et al., 2015b). Similar effects were seen in the mdx heart: CDC treatment promoted cardiomyocyte cycling and proliferation, as demonstrated by a marked increase in Ki67+ and aurora B+ cardiomyocytes (Figures S3B and S3C).
Figure 1.
CDC Transplantation into mdx Hearts
Function, survival, antioxidant pathways, inflammation, and mitochondrial dysfunction improved by CDC transplantation into mdx mice.
(A) Ejection fraction (EF) in CDC-injected mdx mice (Mdx + CDC) and vehicle-injected mdx mice (Mdx + vehicle) in response to injections at baseline (10 months of age) and 3 months later (WT, n = 7; Mdx + vehicle and Mdx + CDC, n = 12 each).
(B) Exercise capacity in mice subjected to weekly high-intensity treadmill exercise, starting 3 weeks after single-dose CDC or vehicle administration (WT, n = 7; Mdx + vehicle and Mdx + CDC, n = 11 each). Cardiac (A) and treadmill (B) experiments were performed separately on different groups of experimental mice.
(C) Kaplan-Meier analysis of survival in the same animals as (B) shows lower survival in vehicle-treated mdx mice than in CDC-treated mdx mice or WT controls (p < 0.001, log rank test); the latter two groups, however, were statistically comparable.
(D) Immunohistochemical images of NRF2 in mdx mouse hearts 3 weeks after administration of vehicle or CDCs. Age-matched WT mice served as control.
(E–I) Western blots and pooled data for protein abundance of phospho-AKT (AKT-pT308, AKT-pS473; E), cytoplasmic phospho-NRF2 (NRF2- pS40; F), nuclear NRF2 (G), NRF2 downstream gene product, hemeoxygenase-1 (HO-1; H), and malondialdehyde protein adducts (I) in mdx mouse hearts 3 weeks after administration of vehicle or CDCs (WT, n = 4; Mdx + vehicle and Mdx + CDC, n = 6 each).
(J) Immunohistochemical images of hearts stained for inflammatory cell markers CD68, CD20, and CD3. Black arrows point to CD68+ (upper row), CD20+ (middle row), and CD3+ (lower row) cells.
(K) Western blots, pooled data, and bar graph (lower right) representing protein abundance of nuclear p65, p-IκB (NF-κB pathway), and MCP1 (monocyte chemoattractant protein1) and average number of indicated inflammatory cells and in mdx mouse hearts.
(L) Transmission electron microscopy (TEM) images from mdx mouse hearts 3 weeks after administration of vehicle (Mdx + vehicle) or CDCs (Mdx + CDC). Age-matched WT mice served as control.
(M and N) Representative western blots and pooled data for mitochondrial respiratory chain subunits in WT and vehicle/CDC mdx heart tissues (M) and oxygen consumption rate (OCR) of mitochondria isolated from the hearts of WT and CDC- or vehicle-treated mdx mice (N) 3 weeks after treatment (WT, n = 3; Mdx + vehicle and Mdx + CDC, n = 8 each). Substrates (pyruvate, malate, and ADP), a selective uncoupler (FCCP) and blockers (oligomycin [Olig.]; antimycin and rotenone [Anti. & Rot.]) of oxidative phosphorylation were applied when indicated. Pooled data are means ± SEM, CM: cardiomyocyte.
∗p < 0.05 versus Mdx + CDC; #p < 0.005 versus Mdx + CDC; †p < 0.05 versus Mdx + Vehicle and WT; ‡p < 0.002 versus Mdx + CDC and WT. Scale bars: 10 μm (D and J); 5 μm (L).
CDC Exosome Transplantation in mdx Hearts
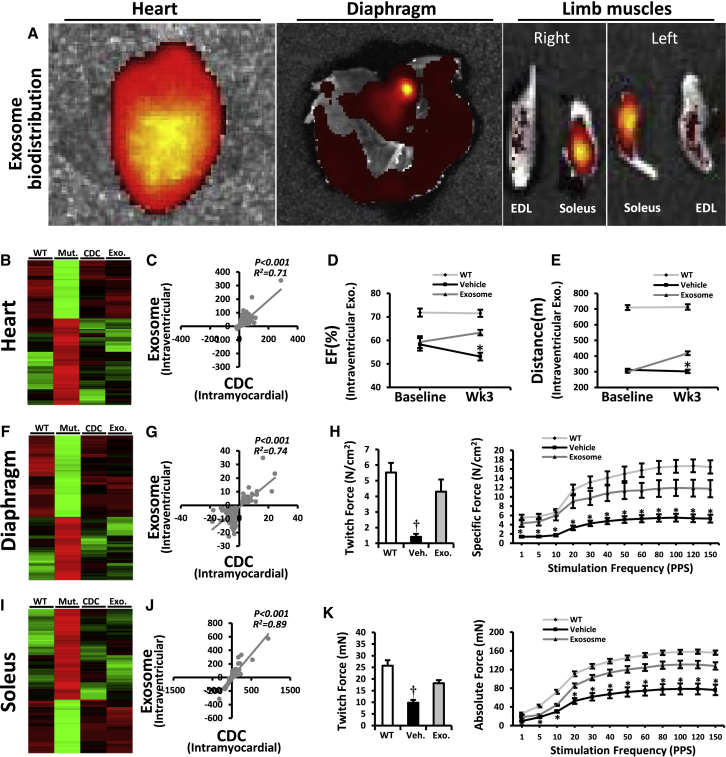
CDC exosomes mimic the functional and structural benefits of CDCs in a murine model of myocardial infarction (Ibrahim et al., 2014). In mdx mice, likewise, exosomes, isolated from media conditioned by hypoxic CDCs, reproduced the benefits of CDCs (Figures 2A–2C and S4A–S4D). Two repeat doses of human CDC exosomes (separated by 3 months) led to sustained improvement in EF, relative to vehicle injection (Figures 2A and S4B), with a minimal but detectable humoral response in the non-immunosuppressed mdx mice (Figure S4C). Collagen I and III levels decreased (Figure 2B) while cycling (Ki67+, Figure 2C upper row) and proliferating (aurora B+, Figure 2C lower row) cardiomyocytes increased in CDC exosome-injected mdx hearts.
Figure 2.
CDC Exosome Injection into mdx Hearts Reproduces the Benefits of CDCs
(A) Sustained functional benefit for at least 3 months with each of two sequential CDC exosome injections in mdx mice (n = 11).
(B) Western blots and pooled data for cardiac collagen IA and IIIA.
(C) Immunohistochemical images and pooled data (WT, n = 4; vehicle and CDC exosome-treated [Mdx (XO)], n = 6 each) from mdx mouse hearts stained for Ki67 [upper row] and Aurora B [lower row]. Arrows point to Ki67+ (upper row) and Aurora B+ (lower row) cardiomyocytes.
Data are means ±SEM; ∗p < 0.05 versus Mdx + exosome; ‡p < 0.01 versus Mdx + exosome and WT; †p < 0.02 versus Mdx + vehicle and WT mice. Scale bar: 10 μm.
Remote Effects of CDC Transplantation in mdx Heart
Intramyocardial injection of CDCs and their exosomes improved Duchenne cardiomyopathy, reversing key pathophysiological processes in the mdx mouse heart (Figures 1 and 2). These changes were associated with a substantial increase in exercise capacity, which was disproportionate to the improvement in cardiac function: EF increased by <10% (Figure 1A), while ambulatory capacity doubled (Figure 1B). To further evaluate the mechanism of enhanced exercise capacity in CDC-treated mdx mice, we isolated and examined three distinct skeletal muscles: the diaphragm (DIA, a key respiratory muscle), and two limb muscles (soleus and extensor digitorum longus [EDL], representative of slow and fast twitch muscles, respectively) 3 weeks after intramyocardial injection of CDCs or vehicle. Whole-transcriptome analysis in DIA revealed downregulation of pathways related to intracellular [Ca2+] excess, oxidative stress, and inflammation after intramyocardial CDC injection (Figures 3A and S5A). Decreases in malondialdehyde protein adducts (Figure 3B), repressed NF-κB, reduced infiltration of inflammatory cells (Figure 3C), and diminished fibrosis (Figure 3D) paralleled a marked improvement in the contractile function of DIA (Figures 3E–3G). Similarly, soleus (Figures 3H–3K) and EDL (Figures S5B–S5D) showed notable improvements at transcriptomic, histologic, and functional levels; soleus fibrosis (Figure S5D) was attenuated and contractile force (Figures 3I–3K) was augmented. Changes in gene expression in DIA and soleus were significantly correlated (Figure 3L).
Figure 3.
CDC Transplantation in mdx Hearts Conferred Beneficial Effects on Diaphragm and Soleus Muscles
(A) Two-dimensional hierarchical clustering using genes with at least two times fold change difference between vehicle/CDC mdx diaphragms.
(B and C) Western blots and pooled data for protein abundance of malondialdehyde protein adducts (B), cytoplasmic p-p65 and p-IκB (C; NF-κB pathway; WT, n = 4; vehicle and CDC, n = 6 each) and immunohistochemical images of diaphragm stained for inflammatory cell markers CD20 and CD3; bar graph represents the average number of indicated inflammatory cells 3 weeks after administration of vehicle or CDCs into mdx hearts.
(D) Representative Masson trichrome images and morphometric analysis in diaphragms 3 weeks after administration of vehicle or CDCs into the hearts of mdx mice.
(E–G) In vitro measurement of isometric diaphragm contractile properties: twitch force (E), maximum tetanic force (F), and force/frequency relationships (G) 3 weeks after CDC/vehicle mdx heart treatments.
(H) Two-dimensional hierarchical clustering using genes with at least two times fold change difference between vehicle/CDC mdx soleus.
(I–K) In vitro measurement of isometric soleus contractile properties: twitch force (I), maximum tetanic force (J), and force/frequency relationships (K) 3 weeks after CDC/vehicle treatment of mdx hearts.
(L) Correlation of fold changes in expression of same genes in diaphragm and soleus 3 weeks after intramyocardial CDC injection in mdx mice.
(M) Three-dimensional plot depicting principal components analysis (PCA) of RNA-seq expression data from exosomes isolated from hypoxic conditioned media and effluents of CDC- or vehicle-treated mdx hearts. The effluent of isolated mdx hearts undergoing Langendorff perfusion was collected for exosome isolation and subsequent RNA-seq 3 days after intramyocardial CDC/vehicle injection. PCA analysis showed clustering of CDC exosomes (red) with exosomes isolated from effluent of CDC mdx hearts (blue), but not vehicle-injected mdx hearts (stippled), indicating that CDC exosomes were shed from mdx hearts at least 3 days after intramyocardial CDC injection. Effluents of mdx hearts from the same group were pooled (n = 3 for each group).
Data are means ±SEM; ∗p < 0.05 versus Mdx + CDC; †p < 0.05 versus Mdx + CDC and WT mice. Scale bars: 10 µm (C); 100 µm (D).
As a basis for the remote effects of intramyocardial injection of CDCs on skeletal muscle, we considered the possibility that exosomes secreted by CDCs lodged in the heart might exit in the venous effluent and exert remote signaling. Principal components analysis revealed that CDC exosomes were very similar in their RNA content to the exosomes isolated from effluents of isolated CDC-treated mdx hearts, but quite distinct from exosomes in the effluents of vehicle-treated mdx hearts 3 days after intramyocardial CDC injection (Figure 3M), pinpointing exosomes as likely mediators of the secondary systemic effects. Such secondary effects are extensive: whole-transcriptome analysis of liver (Figure S6A) 3 weeks after intramyocardial CDC injection revealed downregulation of inflammatory pathways in liver analogous to what we found in heart and skeletal muscle. Thus, CDCs' secondary effects are not restricted to muscle.
Systemic CDC Exosome Injection
To further evaluate the potential of exosomes to mediate systemic benefits, we injected CDC exosomes into the left ventricular cavity of mdx hearts. Six hours post injection, fluorescently labeled CDC exosomes were evident not only in the heart and skeletal muscle (Figure 4A) but also in brain, liver, lung, spleen, gut, and kidneys (Figure S6B). Changes in mdx heart (Figures 4B–4E), diaphragm (DIA; Figures 4F–4H), and soleus (Figures 4I–4K) 3 weeks after intraventricular CDC exosome injection mimicked the modifications seen in these organs after intramyocardial CDC injection (Figure 3). Taken together, the results in Figures 1, 2, 3, and 4 implicate CDC exosomes as mediators of the local and remote effects of intramyocardial CDC injection.
Figure 4.
Systemic CDC Exosome Injection Mimicked the Cardiac and the Remote Effects of Intramyocardial CDC Injection in mdx Mice
(A) Systemic biodistribution of CDC exosomes after intraventricular injection in mdx mice. CDC exosomes were stained with fluorescent lipid dye and tracked 6 hr later using bioluminescence imaging.
(B) Two-dimensional hierarchical clustering using genes from hearts of non-treated mdx mice and of mdx mice treated intramyocardially with CDCs or intraventricularly with CDC exosomes. Genes with at least 2-fold differences with corresponding transcripts in non-treated mdx mice were included.
(C) Correlation of fold changes in expression of same genes 3 weeks after intramyocardial CDC injection or intraventricular CDC exosome injection in mdx hearts.
(D and E) EF and exercise capacity in mdx mice 3 weeks after intraventricular injection of vehicle/CDC exosome (WT, n = 5; Mdx + vehicle and Mdx + CDC exosome, n = 9 each).
(F) Two-dimensional hierarchical clustering using genes from diaphragm of non-treated mdx mice and of mdx mice treated intramyocardially with CDCs or intraventricularly with CDC exosomes. Genes with at least 2-fold differences with corresponding genes in non-treated mdx mice were included.
(G) Correlation of fold changes in expression of the same genes in diaphragm 3 weeks after intramyocardial CDC injection or intraventricular CDC exosomes injection.
(H) Diaphragm isometric twitch force and force/frequency relationships 3 weeks after intraventricular CDC exosome injection.
(I–K) Two-dimensional hierarchical clustering (I), correlation analysis (J), and isometric twitch force and force/frequency relationships (K) from soleus muscle 3 weeks after intraventricular CDC exosome injection.
Data are means ± SEM; ∗p < 0.05 versus Mdx + CDC exosome; †p < 0.05 versus Mdx + CDC exosome and WT mice.
CDC Exosome Injection into mdx Skeletal Muscle
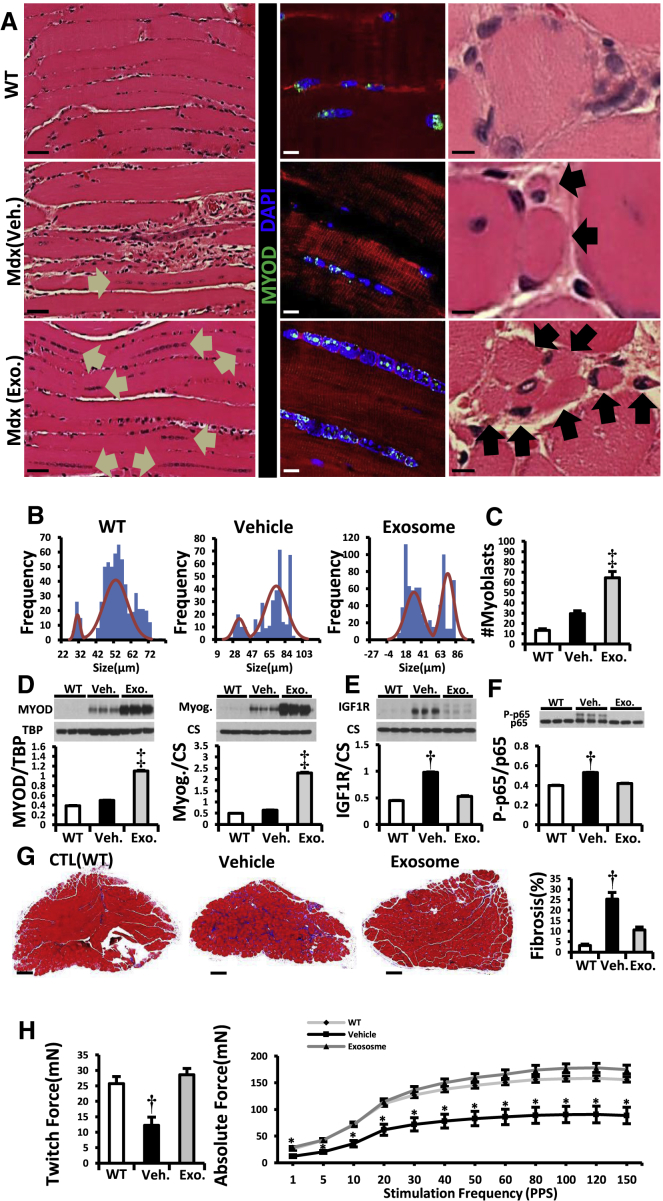
To investigate primary effects on skeletal muscle, we injected CDC exosomes directly into the soleus in mdx mice. Histologic analysis revealed a paucity of surviving myofibers in vehicle-injected mdx soleus relative to WT controls, and those that remained were hypertrophic (Figure 5A). CDC exosomes markedly increased the total number of myofibers and shifted the size distribution to smaller diameters, indicative of myofiber proliferation 3 weeks after injection (Figure 5B). Consistent with this interpretation, the number of MYOD+ cells was augmented after CDC exosome injection (Figures 5A and 5C), with increased tissue levels of MYOD and myogenin, the major transcription factors orchestrating myoblast determination and differentiation, respectively (Bentzinger et al., 2012) (Figure 5D). In physiologic muscle growth, insulin growth factor (IGF)-1 is commonly implicated as an upstream signal (Schiaffino and Mammucari, 2011), but the effects of CDC exosomes on mdx soleus muscle were independent of IGF-1 receptors (Figure 5E). Along with enhanced muscle regeneration, intrasoleus CDC exosome injection decreased inflammation (Figure 5F) and fibrosis (Figure 5G). The net effect was complete restoration of contractile force in soleus muscles injected with CDC exosomes (Figure 5H).
Figure 5.
Intramuscular Injection of CDC Exosomes Resulted in Muscle Growth and Reversal of Pathophysiologic Abnormalities
(A) H&E and immunohistochemical images of soleus muscle stained for MYOD (WT, vehicle, and CDC exosome-treated [Mdx (exosome)] mdx mouse soleus). Arrows in H&E images point to the lined-up nuclei (left column) and myofibers (right column). In the immunohistochemistry, linearly arranged nuclei were positive for MYOD (middle column).
(B and C) Frequency distribution of myofiber sizes and number of myoblasts (MYOD+) 3 weeks after vehicle/CDC exosome injection in mdx soleus muscles (WT, n = 5; vehicle and exosome, n = 9 each).
(D–F) Western blots and pooled data for protein abundance of MYOD, myogenin (D), IGF1 receptor (IGF1R; E) and cytoplasmic p-p65 (F) in mdx soleus muscles 3 weeks after intrasoleus vehicle/CDC exosome injection (WT, n = 4; vehicle and exosome, n = 6 each).
(G) Representative Masson trichrome images and morphometric analysis in mdx soleus muscles 3 weeks after administration of vehicle or CDC exosomes into mdx soleus (WT, n = 5; vehicle and exosome. n = 9 each).
(H) In vitro measurement of soleus isometric twitch force and force/frequency relationships 3 weeks after vehicle/CDC exosome injection into mdx soleus muscles.
Pooled data are means ± SEM. ∗p < 0.05 versus Mdx + CDC exosome; †p < 0.05 versus Mdx + CDC exosome and WT mice; ‡p < 0.002 versus Mdx + vehicle and WT mice. Scale bars: 5 μm (A, right column), 10 μm (A, middle column), 50 μm (A, left column), and 200 μm (G).
CDC Exosomes in Human Duchenne Cardiomyocytes Derived from iPSCs
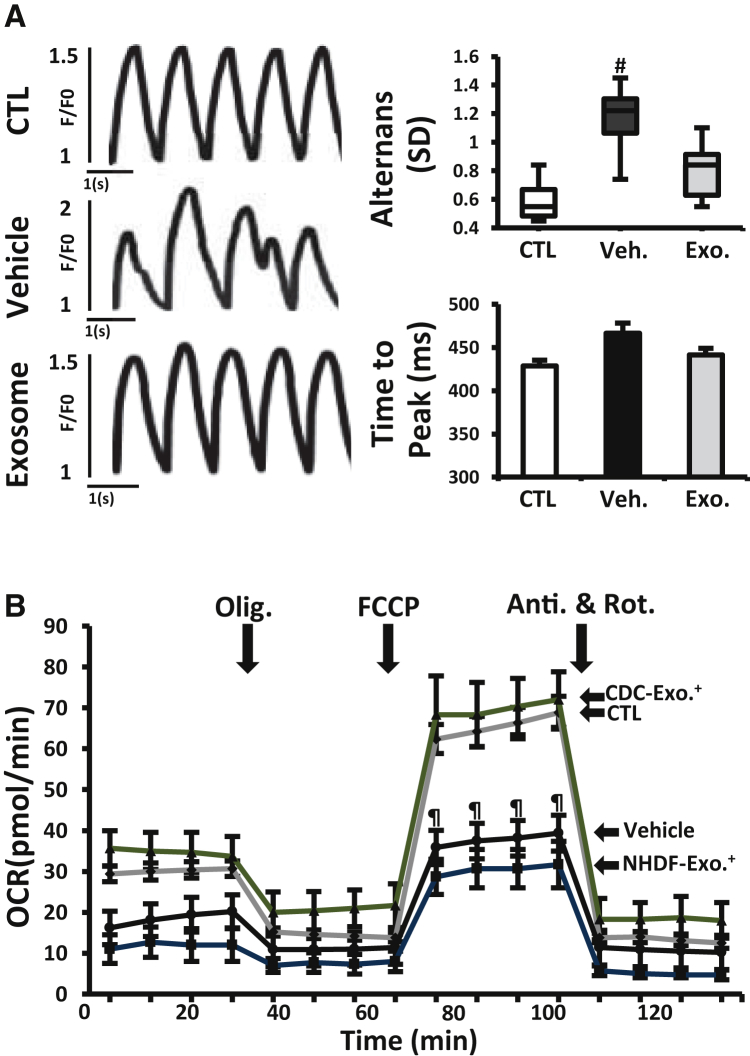
Demonstration of efficacy in multiple models of DMD would bolster the notion that CDC exosomes may be viable therapeutic candidates. Duchenne human induced pluripotent stem cell (iPSC)-derived cardiomyocytes (DMD CMs) exhibit a number of phenotypic deficits characteristic of DMD, including decreased oxygen consumption rate (OCR) reminiscent of that observed in mdx heart mitochondria (Figure 1N), and abnormal calcium cycling (Guan et al., 2014). Priming DMD CMs with CDC exosomes 1 week earlier suppressed beat-to-beat calcium transient alternans during 1 Hz burst pacing (a measure of arrhythmogenicity; Clusin, 2008) (Figure 6A) and normalized OCR (Figure 6B). The congruence of experimental findings in the two DMD models is noteworthy: the mdx mouse has a nonsense mutation in exon 23 of the murine dystrophin gene leading to a premature termination codon (PTC), while the DMD patient whose iPSCs were studied here has a fundamentally different genetic lesion in the human dystrophin gene (exon 50 deletion with frameshift; Guan et al., 2014). Thus, CDC exosomes exert salutary effects in at least two classes of DMD mutations.
Figure 6.
CDC Exosomes in Human Duchenne Cardiomyocytes Derived from iPSCs
(A) Calcium transients from normal and DMD CM measured during 1 Hz burst pacing. Duchenne cardiomyocytes were primed with vehicle or CDC exosomes (exosomes) 1 week before assessment. Bar graphs of calcium transient alternans (variation in beat-to beat calcium transient amplitude) and time to peak (n = 10 cells in each group).
(B) Oxygen consumption rate (OCR) in DMD CMs primed with CDC exosomes or exosomes from normal human dermal fibroblasts (NHDF, as control; NHDF exosome) 1 week before OCR measurement. Normal (CTL) and non-treated DMD CM (vehicle) were studied in parallel. Results from four independent experiments performed in three replicates are shown. See Figure 1 legend for abbreviations. All data are means ± SEM except for the boxplot (means ± SD).
#p < 0.03 versus CDC exosome and CTL (normal cardiomyocyte); ¶p < 0.02 versus CDC exosome.
Dystrophin Expression after Injection of CDCs or Their Exosomes in mdx Mice
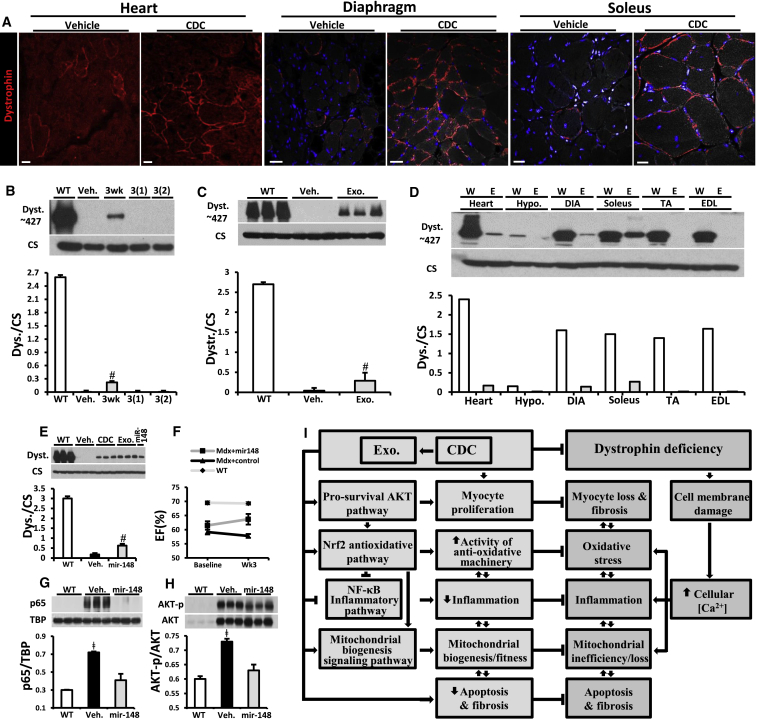
In assessing the seemingly unlikely possibility that CDCs and/or CDC exosomes might restore expression of dystrophin, we were surprised to contradict our preconception. Figure 7 shows immunohistochemical images (Figure 7A) and immunoblots (Figures 7B–7D) demonstrating partial, transient dystrophin expression after injection of CDCs (Figures 7A and 7B) or exosomes (Figures 7C and 7D). Three weeks after intramyocardial injection of CDCs, immunohistochemistry of heart (Figure 7A, left), diaphragm (center) and soleus (right) revealed apparent restoration of dystrophin expression, with appropriate membrane localization, to all these muscles. While immunohistochemical images can be difficult to interpret quantitatively (van Putten et al., 2013), western blots of CDC-injected hearts (Figure 7B) reveal the re-expression of full-length dystrophin (427 kDa) to be relatively low (≤10% of WT levels 3 weeks post injection), and transient (no detectable bands 3 months post injection). Interestingly, partial restoration of dystrophin was also evident after intramyocardial injection of CDC exosomes (Figure 7C). Consistent with the transient nature of dystrophin re-expression after CDC injection (Figure 7B), CDC exosomes led to visible dystrophin bands in the heart 1 and 3 weeks, but not 3 months, after systemic injection (Figure S6C). Among various tissues collected and analyzed 1 week after systemic exosome delivery (Figure 7D), detectable levels of dystrophin were evident in heart, diaphragm, and soleus but not in tibialis anterior and EDL muscles or in the hypothalamus. Thus, CDCs and their exosomes induce measurable dystrophin re-expression in heart and certain types of skeletal muscle, but the effect is transient.
Figure 7.
Exosomes Mediate Reversal of Key Pathophysiological Features of Duchenne Muscular Dystrophy
(A) Immunohistochemical images of dystrophin in mdx mouse heart, diaphragm, and soleus treated with and without CDC at 10 months of age.
(B) Western blot and pooled data for dystrophin protein in WT control mouse heart and mdx mouse hearts 3 weeks and 3 months after first intramyocardial CDC injection, 3(1), and 3 months after second (repeat) CDC injection into myocardium, 3(2), versus Mdx + vehicle (Veh.); (Mdx + vehicle and Mdx + CDC, n = 6 each).
(C) Western blot and pooled data for dystrophin protein in WT control mouse heart and mdx mouse hearts 3 weeks after CDC exosome injection into myocardium (Mdx + vehicle and Mdx + exo, n = 6 each).
(D) Western blot showing protein content of dystrophin in WT control and in mdx mouse (Exo) heart, hypothalamus (hypo.), diaphragm (DIA), soleus, tibialis anterior (TA), and extensor digitorum longus (EDL) 1 week after systemic CDC exosome delivery by intraventricular injection (n = 2).
(E) Western blots and pooled data for protein abundance of dystrophin isoform: dp427 in mdx mouse hearts 3 weeks after intramyocardial injection of vehicle, CDC, CDC exosomes, or mimics of Mir-148a.
(F) Ejection fraction (EF) at baseline and 3 weeks after intramyocardial injection of Mir-148a or miRNA control in mdx mice. WT EF values are also shown for reference, n = 5 per group.
(G and H) Western blots and pooled data for nuclear p65 (G) and phosphorylated AKT (H) in mdx mouse hearts 3 weeks after Mir-148a treatment (vehicle and Mir-148a, n = 6 each).
(I) Schematic of pathophysiological mechanisms operative in Duchenne cardiomyopathy and the cellular mechanisms recruited by CDCs and their exosomes. All organs were from mice 10 months old at baseline.
All data are means ± SEM. #p < 0.05 versus Mdx + vehicle and WT; ǂ p < 0.05 versus Mir-148a and WT. Scale bars: 25 μm (A, heart); 25 μm (A, diaphragm); 20 μm (A, soleus).
Many, if not most, of the effects of exosomes are attributable to their RNA and protein payloads (Vyas and Dhawan, 2016). In CDC exosomes, dystrophin protein was undetectable (Figure S6D), and dystrophin transcripts were absent by RNA sequencing (RNA-seq) and undetectable by qPCR (Figure S6D), so dystrophin restoration is not due to exosomally mediated transfer of its protein or mRNA. Nevertheless, regulatory RNA may act directly or indirectly to increase dystrophin expression, e.g., by readthrough of PTCs (Advani and Dinman, 2016). RNA-seq of CDC exosomes grown under our conditions revealed 144-fold augmentation of miRNA 148a (Mir-148a; Figure S6E), which we tested as a candidate effector. Intramyocardial injection of Mir-148a restored expression of dystrophin in mdx hearts 3 weeks after administration (Figure 7E). Like CDC exosomes, Mir-148a (but not a scrambled miRNA) increased EF in mdx mice (Figure 7F). The unexpected bioactivity of Mir-148a on dystrophin occurred in parallel to suppression of known Mir-148a targets (NF-κB p65 and phospho-AKT; Bao and Lin, 2014) (Figures 7G and 7H). Analysis of exon-intron junctions for dystrophin transcripts in mdx hearts exposed to CDCs, CDC exosomes, or Mir-148a, shows no treatment-related exon skipping or alternative splicing (Figure S7A). Thus, we have excluded exosomal dystrophin mRNA or protein transfer, as well as RNA splicing, while implicating Mir-148a as a potential mediator of enhanced full-length dystrophin protein synthesis.
Discussion
We propose that CDCs act by secreting exosomes, which are taken up by surrounding myocardium and by distant skeletal muscle, antagonizing multiple pathophysiological pathways active in DMD. The congruent effects in mdx mice (Figures 1, 2, 3, 4, and 5) and in exon 50-deleted DMD hCM (Figure 6) highlight the ability of CDC exosomes to benefit multiple disease-causing dystrophin mutations. We found, unexpectedly, that CDCs and their exosomes increase dystrophin expression in the mdx mouse model. Various lines of evidence exclude alternative splicing, mRNA, or protein transfer, supporting the idea that translational readthrough enhances dystrophin expression in the exon 23 PTC mdx mutant, perhaps by exosomally mediated transfer of Mir-148a. Additional work will be required to pinpoint the precise mechanism of enhanced dystrophin expression and to determine if the effect is generalizable to other types of DMD mutations. Nevertheless, the dystrophin-independent actions of CDCs and their exosomal contents should be generalizable. After all, CDCs and their exosomes work quite well in cardiomyopathies not associated with dystrophin deficiency (Aminzadeh et al., 2015b, Gallet et al., 2017, Ibrahim et al., 2014). The protean actions of CDCs and their exosomes include improved mitochondrial function, enhanced myocyte proliferation, and suppression of oxidative stress, inflammation, and fibrosis (Figure 7). Observed effects, which must be independent of dystrophin restoration, include the suppression of inflammation in mdx liver (an organ with no dystrophin expression; Love et al., 1991) as well as the persistent benefits in heart 3 months after injection of CDCs or CDC exosomes (at which time dystrophin is no longer detectable). Notably, CDCs and their exosomes not only reverse the dystrophic phenotype but also forestall disease progression: the functional benefits of cardiac injections of CDCs or their exosomes persist for at least 3 months, and single doses of CDCs decrease mortality more than 1 year later in mdx mice. These findings beg the investigation of exosomally triggered epigenomic modifications as a potential basis for the durable benefits (Lee et al., 2012), but such experiments are beyond the scope of this report.
The HOPE-Duchenne trial was designed to assess single-dose delivery of CDCs to the heart (Ascheim and Jefferies, 2016). Exploratory efficacy data reported recently from this 25-patient randomized trial give credence to the idea that CDCs may benefit not only the cardiomyopathy but also the skeletal myopathy of DMD (Jefferies et al., 2017). A follow-on placebo-controlled trial of CDCs in DMD patients, HOPE-2, is currently being planned (Kegel, 2017). Given the preclinical insights reported here and elsewhere (Rogers et al., 2017), CDCs will be administered systemically in HOPE-2, thereby avoiding the need for cardiac catheterization.
In addition to CDCs themselves, exosomes derived from CDCs are promising next-generation therapeutic candidates (TCs). Although CDCs and their exosomes exert multiple, synergistic benefits (Figure 7I), focused dystrophin augmentation, even if at much lower levels than normal (van Putten et al., 2013), may be no less valuable a concept to pursue. CDC-exosomes carry all the instructions required to induce dystrophin re-expression, and clinical-grade manufacturing is feasible (Marbán, 2018). Nevertheless, a defined agent would have considerable reductionist appeal. Emergent insights pinpoint Mir-148a as a defined agent that increases dystrophin expression, making it a promising third-generation TC. The dystrophin-enhancing effects of CDC exosomes, and presumably of Mir-148a, wear off between 3 weeks and 3 months, but repeated delivery of either agent (∼monthly) may lead to sustained dystrophin enhancement; however, we have not yet tested this conjecture. All three TCs (CDCs, CDC exosomes, and Mir-148a) have mechanisms of action complementary to, and potentially synergistic with, those of the approved exon-skipping agent etiplirsen, or of other treatment approaches currently in active development for DMD (e.g., myoediting).
Experimental Procedures
Please see Supplemental Information for expanded methods.
Animal Study
We studied the mdx mouse model of DMD (C57BL/10ScSn-Dmdmdx/J) and WT strain-matched mice (C57BL/10ScSnJ WT mouse heart) (Jackson Laboratory, USA) from 10 months of age. All mice studied were female. To optimize the process of CDC transplantation, preliminary dose-response experiments were performed, which identified 1 × 105 cells in the first injection and 1 × 104 cells in the second injection (3 months after the first injection) as effective doses, consistent with prior dose-ranging experiments in ischemic and non-ischemic mouse models (Aminzadeh et al., 2015b, Shen et al., 2012). A total of 1 × 105 cells/40 μL of PBS (first injection) or 1 × 104 cells/40 μL of PBS (second injection) or PBS alone were injected into left ventricular (LV) myocardium divided equally among four sites as described (Aminzadeh et al., 2015b, Nagaya et al., 2005). The LV was visually divided into three zones: basal, middle, and apical, with one injection in the basal, two in the middle, and one in the apical zone. Ten-month-old CDC/mdx and vehicle/mdx mice were injected with CDCs (Mdx + CDC, n = 12) or vehicle (placebo: Mdx + vehicle [PBS], n = 12) twice (3 month interval), respectively. Injections were during open-chest thoracotomy via a 28.5 gauge needle. All surgical procedures were carried out while the animals were under general anesthesia (dexmedetomidine 0.5 mg/kg/ketamine 75 mg/kg; intraperitoneally; once before surgery). Similar protocols were used for injection of CDC exosomes into myocardium. Intraventricular single injection of CDC exosomes, (10.32 ± 3.28) × 109/150 μL of PBS, or PBS alone into the LV cavity were performed during open-chest thoracotomy via a 28.5 gauge needle. Intramuscular injection of exosomes into soleus (SOL) muscles were performed at a single site at the lower 1/3 of the muscle using a 25 μL Hamilton syringe (with 0.5 μL marks) with a 31 gauge needle. The needle was advanced up to the upper 1/3 of the muscle and then slowly retracted through the belly as exosomes, (20.64 ± 2.12) × 107/3 μL, were injected. Among all mice studied, there was only one peri-operative death. To ensure ethical and humane treatment of animals, we adhered to the principles recommended in the Guide for Care and Use of Laboratory Animals with oversight and approval by the Cedars-Sinai Health System Institutional Animal Care and Use Committee and the Department of Comparative Medicine (IACUC#3809).
CDCs, CDC Exosomes, NHDF Exosomes
CDCs
Mouse CDCs were expanded from female WT strain-matched mouse hearts (C57BL/10ScSnJ) as described (Smith et al., 2007). Briefly, ventricular tissues were minced into ∼1 mm explants, partially digested enzymatically and plated on adherent (fibronectin-coated) culture dishes. These explants spontaneously yield outgrowth cells (explant-derived cells), which were harvested once confluent and plated in suspension culture (105 cells/mL on poly-D-lysine-coated dishes) to enable self-assembly of three-dimensional cardiospheres. Subsequent replating of cardiospheres on adherent culture dishes yielded CDCs, which were used in all experiments at passage one.
CDC Exosomes
Exosomes were isolated from serum-free media conditioned overnight (24 hr) by cultured human male CDCs from two different cell lines (Gallet et al., 2017, Ibrahim et al., 2014) (CDC exosome) (or normal human dermal fibroblasts [NHDF] as a control) in hypoxia (2% O2; default condition) or normoxia (20% O2, solely for studies comparing RNA content of exosomes). Exosomes from both cell lines, either frozen and thawed or prepared fresh, yielded similar results. Ultracentrifugation (100,000 × g for 1 hr) was used to isolate exosomes from conditioned media after sequential centrifugations at 300 × g (10 min) and 10,000 × g (30 min) and filtration with 0.22 μm filters (Lässer et al., 2012). Isolated exosomes were re-suspended in PBS (for in vivo and in vitro experiments) and the ratio of exosome to protein was measured using a Nanosight particle counter (Webber and Clayton, 2013) and Micro BCA Protein Assay Kit (Life Technologies, Grand Island, NY), respectively. Preliminary dose-response studies identified (2.24 ± 1.34) × 107 and (6.19 ± 3.68) × 108 exosomes from hypoxic CDCs as effective doses for in vitro and in vivo (intramyocardial CDC exosome injection) experiments, respectively. Exosomes were characterized by the most rigorous of criteria (Lötvall et al., 2014): linear iodixanol density gradient, transmission electron microscopy (TEM), key membrane proteins, and the biological effect. TEM images of sequentially centrifuged exosomes with and without purification with a linear iodixanol density gradient are shown (Figure S7B). The vesicles are variable in size and morphology, consistent with previous work (Zabeo et al., 2016). Western blots on lysed exosomes showed key proteins characteristic of exosomes: CD63, CD81, and TSG (Figure S7C). Biological activity of sequentially centrifuged exosomes with (Exo1) and without (Exo2) purification with linear iodixanol density were compared by injection into mdx soleus muscles and evaluation of mdx soleus transcriptome 3 weeks after injection (Figure S7D). Correlation of fold changes in expression of the same genes 3 weeks after Exo1 and Exo2 injection in mdx soleus muscles (Figure S7E) demonstrated similar effects of Exo1 and Exo2 and supported the notion that the bioactivity of the vesicles isolated by our default protocol is genuinely due to exosomes and not to another type of vesicles that might have been co-purified by ultracentrifugation.
iPSC-Derived Cardiomyocytes
Urine-derived cells were collected and used as source material for iPSCs, from a male subject with genetically confirmed DMD (exon 50 deletion), and an unaffected control subject, as described (Guan et al., 2014). iPSCs were differentiated to cardiomyocytes following an established protocol with modifications. Briefly, iPSC colonies were detached by 10 min incubation with Versene (Life Technologies, Carlsbad, CA), triturated to a single-cell suspension, and seeded onto Matrigel-coated plastic dishes at a density of 250,000 cells/cm2 in mTeSR1 medium and cultured for 4 more days. Differentiation was then initiated by switching the medium to RPMI 1640 medium supplemented with 2% insulin-reduced B27 (Life Technologies) and fresh L-glutamine.
Author Contributions
M.A.A. designed and performed in vivo experiments and data analysis; manufactured exosomes; performed western blots; and wrote first draft of the paper. R.G.R. performed in vivo experiments and data analysis; manufactured exosomes; and performed western blots. M.F. performed and analyzed isolated skeletal muscle experiments. R.E.T. assisted with experiments and data analysis. X.G. and M.K.C. prepared and provided cardiomyocytes differentiated from human induced pluripotent cells and performed respirometry experiments on cardiomyocytes differentiated from human induced pluripotent cells. A.M.A., D.J.T., and R.A.G. performed and analyzed respirometry on isolated mitochondria. A.I., R.A.V., and M.L. assisted with experimental design, data analysis, and manuscript drafting. X.D. performed RNA sequencing and analyzed data. A.T. and J.M.G. measured calcium transients in cardiomyocytes differentiated from human induced pluripotent cells and analyzed data. E.M. was responsible for experimental design, data analysis, and preparation of the final manuscript.
Acknowledgments
This work was supported by grants from Coalition Duchenne and the NIH (R01 HL124074). We thank Liang Li for skilled technical assistance. E.M. is a founder of Capricor and a member of its scientific advisory board. A.I. consults for Capricor.
Published: February 22, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.01.023.
Supplemental Information
References
- Advani V.M., Dinman J.D. Reprogramming the genetic code: the emerging role of ribosomal frameshifting in regulating cellular gene expression. Bioessays. 2016;38:21–26. doi: 10.1002/bies.201500131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminzadeh M.A., Tobin R., Smith R., Marban L., Marban E. Heart-derived cell therapy for Duchenne cardiomyopathy: cardiosphere-derived cells and their exosomes improve function, restore mitochondrial integrity and reverse degenerative changes in the hearts of Mdx mice. Circ. Res. 2014;115:e90. [Google Scholar]
- Aminzadeh M.A., Durvasula P., Tobin R., Guan X., Andres A., Taylor D., Ibrahim A., Sun B., Torrente A., Goldhaber J. Exosome-mediated reversal of Duchenne cardiomyopathy. Circulation. 2015;132:A16015. [Google Scholar]
- Aminzadeh M.A., Tseliou E., Sun B., Cheng K., Malliaras K., Makkar R.R., Marbán E. Therapeutic efficacy of cardiosphere-derived cells in a transgenic mouse model of non-ischaemic dilated cardiomyopathy. Eur. Heart J. 2015;36:751–762. doi: 10.1093/eurheartj/ehu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascheim D., Jefferies J.L. 2016. A randomized, open-label study of the safety and efficacy of multi-vessel intracoronary delivery of allogeneic cardiosphere-derived cells in patients with cardiomyopathy secondary to Duchenne muscular dystrophy.https://clinicaltrials.gov/ct2/show/NCT02485938 [Google Scholar]
- Bao J.L., Lin L. MiR-155 and Mir-148a reduce cardiac injury by inhibiting NF-kappaB pathway during acute viral myocarditis. Eur. Rev. Med. Pharmacol. Sci. 2014;18:2349–2356. [PubMed] [Google Scholar]
- Bentzinger C.F., Wang Y.X., Rudnicki M.A. Building muscle: molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008342. doi: 10.1101/cshperspect.a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burelle Y., Khairallah M., Ascah A., Allen B.G., Deschepper C.F., Petrof B.J., Des Rosiers C. Alterations in mitochondrial function as a harbinger of cardiomyopathy: lessons from the dystrophic heart. J. Mol. Cell. Cardiol. 2010;48:310–321. doi: 10.1016/j.yjmcc.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C.G., Samadi A., Siegel A. Chronic treatment with agents that stabilize cytosolic IκB-α enhances survival and improves resting membrane potential in MDX muscle fibers subjected to chronic passive stretch. Neurobiol. Dis. 2005;20:719–730. doi: 10.1016/j.nbd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Cheng K., Malliaras K., Li T.-S., Sun B., Houde C., Galang G., Smith J., Matsushita N., Marbán E. Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplant. 2012;21:1121–1135. doi: 10.3727/096368911X627381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimenti I., Smith R.R., Li T.S., Gerstenblith G., Messina E., Giacomello A., Marbán E. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W.T. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1–H10. doi: 10.1152/ajpheart.00802.2007. [DOI] [PubMed] [Google Scholar]
- Davis D.R., Zhang Y., Smith R.R., Cheng K., Terrovitis J., Malliaras K., Li T.S., White A., Makkar R., Marbán E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet R., Dawkins J., Valle J., Simsolo E., de Couto G., Middleton R., Tseliou E., Luthringer D., Kreke M., Smith R.R. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 2017;38:201–211. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X., Mack D.L., Moreno C.M., Strande J.L., Mathieu J., Shi Y., Markert C.D., Wang Z., Liu G., Lawlor M.W. Dystrophin-deficient cardiomyocytes derived from human urine: new biologic reagents for drug discovery. Stem Cell Res. 2014;12:467–480. doi: 10.1016/j.scr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A.G.-E., Cheng K., Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies J., Byrne B., Taylor M., Lima J., Smith R.R., Maliaris K., Fedor B., Rudy J., Pogoda J., Marban L. Cardiosphere-derived cells for the treatment of Duchenne cardiomyopathy: results of the Halt cardiOmyopathy ProgrEssion [HOPE]-Duchenne Trial. Circulation. 2017;136:e448. [Google Scholar]
- Kegel M. Capricor set to launch phase 2 trial of cell therapy CAP-1002 in advanced DMD patients. Muscular Dystrophy News Today. 2017 https://musculardystrophynews.com/2017/12/01/fda-clears-capricor-phase-2-trial-dmd-therapy-cap-1002/ [Google Scholar]
- Lässer C., Eldh M., Lötvall J. Isolation and characterization of RNA-containing exosomes. J. Vis. Exp. 2012:e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-T., White A.J., Matsushita S., Malliaras K., Steenbergen C., Zhang Y., Li T.S., Terrovitis J., Yee K., Simsir S. Intramyocardial injection of autologous cardiospheres or cardiosphere-derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post-myocardial infarction. J. Am. Coll. Cardiol. 2011;57:455–465. doi: 10.1016/j.jacc.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Lee Y., Andaloussi S.E., Wood M.J. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum. Mol. Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- Li T.S., Cheng K., Malliaras K., Smith R.R., Zhang Y., Sun B., Matsushita N., Blusztajn A., Terrovitis J., Kusuoka H. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T.S., Cheng K., Lee S.T., Matsushita S., Davis D., Malliaras K., Zhang Y., Matsushita N., Smith R.R., Marbán E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötvall J., Hill A.F., Hochberg F., Buzás E.I., Di Vizio D., Gardiner C., Gho Y.S., Kurochkin I.V., Mathivanan S., Quesenberry P. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014;3:29613. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D., Morris G., Ellis J., Fairbrother U., Marsden R., Bloomfield J., Edwards Y., Slater C., Parry D., Davies K. Tissue distribution of the dystrophin-related gene product and expression in the mdx and dy mouse. Proc. Natl. Acad. Sci. USA. 1991;88:3243–3247. doi: 10.1073/pnas.88.8.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar R., Schatz R., Traverse J., Hamer A., Beattie K., Smith R.R., Kivel F., Marbán L., Marbán E., Henry T.D. ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): the one year Phase I results. Circulation. 2014;130:A20536. [Google Scholar]
- Makkar R.R., Smith R.R., Cheng K., Malliaras K., Thomson L.E., Berman D., Czer L.S., Marbán L., Mendizabal A., Johnston P.V. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K., Li T.S., Luthringer D., Terrovitis J., Cheng K., Chakravarty T., Galang G., Zhang Y., Schoenhoff F., Van Eyk J. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K., Makkar R.R., Smith R.R., Cheng K., Wu E., Bonow R.O., Marbán L., Mendizabal A., Cingolani E., Johnston P.V. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J. Am. Coll. Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbán E. The secret life of exosomes: what bees can teach us about next-generation therapeutics. J. Am. Coll. Cardiol. 2018;71:193–200. doi: 10.1016/j.jacc.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Rojo A.I., Salinas M., Diaz R., Gallardo G., Alam J., de Galarreta C.M.R., Cuadrado A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/AKT pathway and the NRF2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004;279:8919–8929. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- Menazza S., Blaauw B., Tiepolo T., Toniolo L., Braghetta P., Spolaore B., Reggiani C., Di Lisa F., Bonaldo P., Canton M. Oxidative stress by monoamine oxidases is causally involved in myofiber damage in muscular dystrophy. Hum. Mol. Genet. 2010;19:4207–4215. doi: 10.1093/hmg/ddq339. [DOI] [PubMed] [Google Scholar]
- Nagaya N., Kangawa K., Itoh T., Iwase T., Murakami S., Miyahara Y., Fujii T., Uematsu M., Ohgushi H., Yamagishi M. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- Rogers R.G., Fournier M., Aminzadeh M.A., Gouin K., Sanchez L., Marban E. Abstract 16576: intravenous infusion of cardiosphere-derived cells and their exosomes improve dystrophin-deficient cardiomyopathy in mdx mice. Circulation. 2017;136:A16576. [Google Scholar]
- Schiaffino S., Mammucari C. Regulation of skeletal muscle growth by the IGF1-AKT/PKB pathway: insights from genetic models. Skelet. Muscle. 2011;1:1. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D., Cheng K., Marbán E. Dose-dependent functional benefit of human cardiosphere transplantation in mice with acute myocardial infarction. J. Cell. Mol. Med. 2012;16:2112–2116. doi: 10.1111/j.1582-4934.2011.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirokova N., Niggli E. Cardiac phenotype of Duchenne muscular dystrophy: insights from cellular studies. J. Mol. Cell. Cardiol. 2013;58:217–224. doi: 10.1016/j.yjmcc.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.R., Barile L., Cho H.C., Leppo M.K., Hare J.M., Messina E., Giacomello A., Abraham M.R., Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- Tandon A., Villa C.R., Hor K.N., Jefferies J.L., Gao Z., Towbin J.A., Wong B.L., Mazur W., Fleck R.J., Sticka J.J. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in Duchenne muscular dystrophy. J. Am. Heart Assoc. 2015;4:e001338. doi: 10.1161/JAHA.114.001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseliou E., Pollan S., Malliaras K., Terrovitis J., Sun B., Galang G., Marbán L., Luthringer D., Marbán E. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J. Am. Coll. Cardiol. 2013;61:1108–1119. doi: 10.1016/j.jacc.2012.10.052. [DOI] [PubMed] [Google Scholar]
- van Putten M., Hulsker M., Young C., Nadarajah V.D., Heemskerk H., van der Weerd L., t Hoen P.A., van Ommen G.J., Aartsma-Rus A.M. Low dystrophin levels increase survival and improve muscle pathology and function in dystrophin/utrophin double-knockout mice. FASEB J. 2013;27:2484–2495. doi: 10.1096/fj.12-224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaert D., Richards K., Rafael-Fortney J.A., Raman S.V. Cardiac involvement in patients with muscular dystrophies magnetic resonance imaging phenotype and genotypic considerations. Circ. Cardiovasc. Imaging. 2011;4:67–76. doi: 10.1161/CIRCIMAGING.110.960740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N., Dhawan J. Exosomes: mobile platforms for targeted and synergistic signaling across cell boundaries. Cell. Mol. Life Sci. 2016;74:1567–1576. doi: 10.1007/s00018-016-2413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J., Clayton A. How pure are your vesicles? J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.19861. 19861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling-Henricks M., Jordan M.C., Gotoh T., Grody W.W., Roos K.P., Tidball J.G. Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One. 2010;5:e10763. doi: 10.1371/journal.pone.0010763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A.J., Smith R.R., Matsushita S., Chakravarty T., Czer L.S., Burton K., Schwarz E.R., Davis D.R., Wang Q., Reinsmoen N.L. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur. Heart J. 2013;34:68–75. doi: 10.1093/eurheartj/ehr172. [DOI] [PubMed] [Google Scholar]
- Williams I.A., Allen D.G. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- Zabeo D., Cvjetkovic A., Lasser C., Schorb M., Lotvall J., Hoog J.L. Exosomes purified from a single cell type have diverse morphology and composition. bioRxiv. 2016 doi: 10.1080/20013078.2017.1329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.