Summary
Investigation of human muscle regeneration requires robust methods to purify and transplant muscle stem and progenitor cells that collectively constitute the human satellite cell (HuSC) pool. Existing approaches have yet to make HuSCs widely accessible for researchers, and as a result human muscle stem cell research has advanced slowly. Here, we describe a robust and predictable HuSC purification process that is effective for each human skeletal muscle tested and the development of storage protocols and transplantation models in dystrophin-deficient and wild-type recipients. Enzymatic digestion, magnetic column depletion, and 6-marker flow-cytometric purification enable separation of 104 highly enriched HuSCs per gram of muscle. Cryostorage of HuSCs preserves viability, phenotype, and transplantation potential. Development of enhanced and species-specific transplantation protocols enabled serial HuSC xenotransplantation and recovery. These protocols and models provide an accessible system for basic and translational investigation and clinical development of HuSCs.
Keywords: human satellite cell purification, serial transplantation, satellite cell cryopreservation
Highlights
-
•
High-efficiency purification permits serial transplantation of human satellite stem cells
-
•
Cryopreservation preserves satellite cell function and phenotype
-
•
1 gram of adult skeletal muscle yields 104 highly purified satellite cells
-
•
Purified uncultured endogenous human satellite cells can be stored and shared
Garcia and colleagues report methods for efficient purification of satellite cells from human skeletal muscle. They use their approaches to demonstrate stem cell functions of endogenous satellite cells and to make human satellite cells accessible for sharing among researchers.
Introduction
Muscle regeneration in most species is mediated by satellite cells, anatomically defined based on their position between the skeletal muscle fiber plasma membrane and the basal lamina (Mauro, 1961). Satellite cells are a heterogeneous pool of muscle progenitors, a subset of which fulfill criteria of adult stem cells in that they engraft, proliferate, respond to injury by regenerating mature muscle, reoccupy the muscle satellite cell niche, and self-renew (Collins et al., 2005, Kuang et al., 2007, Montarras et al., 2005, Sacco et al., 2008, Sherwood et al., 2004). Endogenous characteristics of satellite cell populations, including activation state and stemness, are rapidly altered in culture (Brimah et al., 2004, Charville et al., 2015, Cooper et al., 2001, Montarras et al., 2005), limiting investigation of in vivo properties, natural heterogeneity, and regenerative capacity. For human satellite cells (HuSCs), experimental tractability is further complicated by source scarcity and less predictable yield that may in turn be related to variable source properties such as muscle type, age, and delay in preparation after tissue procurement. This limits preclinical investigation and slows clinical translation.
Several standard and classical experimental paradigms used to study tissue stem cells, such as serial transplantation, have been unavailable for use in human muscle stem cell biology because of difficulty obtaining adequate tissue, limited ability to isolate pure populations of satellite cells, and challenges with xenotransplantation. Whereas the mouse has proved extremely valuable for understanding muscle satellite cell biology that is highly relevant to humans, there are limitations in terms of how closely human muscle biology will mimic that of laboratory rodents. Therefore it is imperative to study naturally occurring HuSCs in order to address clinical muscle disorders. Although endogenous human muscle progenitors and satellite cells have recently been characterized and transplanted (Alexander et al., 2016, Castiglioni et al., 2014, Charville et al., 2015, Uezumi et al., 2016, Xu et al., 2015), the lack of readily available sources of preserved HuSCs has sequestered HuSC investigation from most muscle researchers. Overcoming current limitations in human muscle stem cell research will advance muscle regeneration research and should lead to more precise clarification of muscle stem cell targets relevant for durable and impactful therapeutic interventions.
Here, we report and provide methods for high-grade purification of satellite cells from adult human skeletal muscle and methods for predictable isolation, yield, and storage, that together enable more sophisticated and better controlled experimentation than was previously feasible. Cryopreservation retains satellite cell properties and permits direct comparisons of same-source satellite cells after different treatments. Improved engraftment techniques and methods to separate HuSCs from mouse tissue have enabled serial transplantation of human satellite stem cells. The approaches developed in this study resolve technical hindrances impeding HuSC and human muscle stem cell investigation.
Results
Efficient High-Yield Purification of Satellite Cells from Human Skeletal Muscles
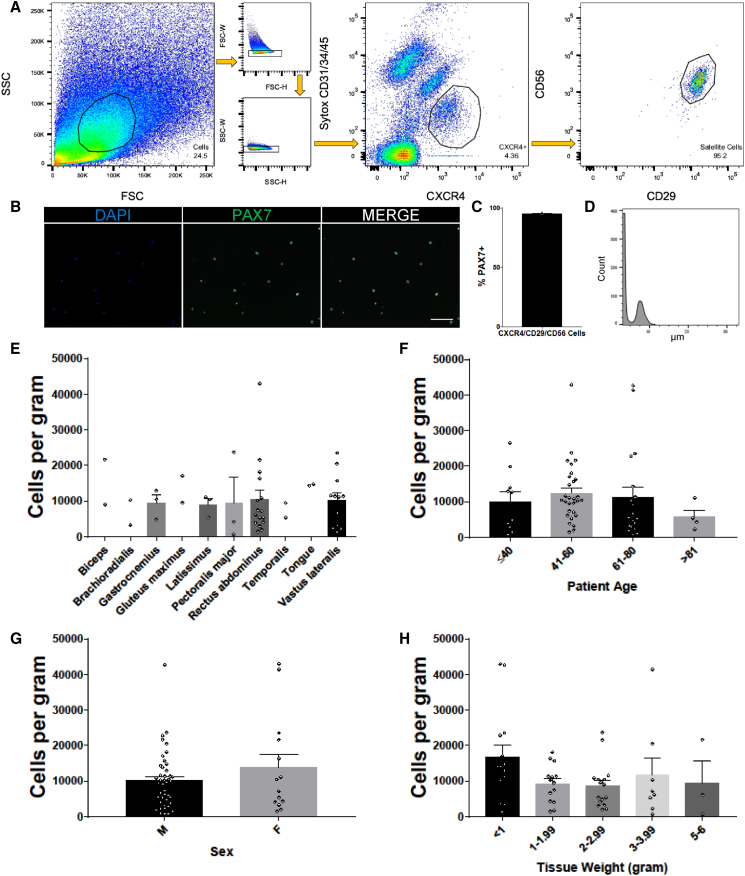
Building on our previously published strategy for isolation of HuSCs (Garcia et al., 2017, Xu et al., 2015) we developed an enhanced protocol that standardizes yield and greatly improves isolation efficiency (see Experimental Procedures and Supplemental Experimental Procedures for details of the protocol). In agreement with prior reports by others (Bareja et al., 2014, Castiglioni et al., 2014) we found that CXCR4 marks HuSCs. Isolation strategies that solely use either CXCR4 or CD29/CD56-positive markers require more conservative gating of incompletely separated populations to avoid capturing potential non-satellite cells, therefore also potentially excluding satellite cells that are not well separated (Figure S1). For example, the rightmost panels (top three rows) and second from right panels (bottom three rows) of Figure S1A show different experiments that have variable overlap of the populations. We also determined that satellite cells in adult human muscle are negative for CD34 surface expression (Figure S2), in contrast to mouse satellite cells (Beauchamp et al., 2000, Fukada et al., 2004, Montarras et al., 2005, Sherwood et al., 2004) and in agreement with prior reports identifying CD34-negative unipotent human myogenic cells (Pisani et al., 2010) and CD34-low or -negative fetal and adult HuSCs (Castiglioni et al., 2014). Negative selection with CD34 was therefore introduced into the purification strategy. To enhance separation, we developed a combinatorial strategy using negative and stepwise positive selection. We also investigated various tissue dissociation procedures and found that enzymatic sample digestion with the use of collagenase and trypsin was superior to pronase and collagenase that we had used previously. Optimized tissue dissociation preserved epitopes of the surface proteins assessed in this study, as demonstrated by similar fluorescence intensity with or without trypsin, and resulted in improved separation of satellite cells from non-satellite cells (Figure S2). Possibly, other satellite cell surface proteins that were not evaluated here could be affected by the enzymatic digestion protocol. Using these advances, an optimized isolation protocol was developed: Muscle digestion is followed with depletion of endothelial and hematopoietic cells using magnetic column separation with CD31 and CD45 metal beads. Remaining cells are then sorted via flow cytometry for viable singlets with the following markers: Sytox−/CD31−/CD34−/CD45−/CXCR4+/CD29+/CD56+ (Figure 1A). Primary gating with simultaneous negative depletion and positive selection with CXCR4 initially plots satellite cells as distinct from the majority of non-satellite cells, and subsequent gating on CD56/CD29 further distinguished highly separable and pure HuSCs. CXCR4 also separates the satellite cell population from small autofluorescent fiber fragments, significantly reducing the sorting abort rate. We have found that this strategy greatly improves efficiency during sorting. In particular, the satellite cell population can be rapidly identified during the initial part of the flow run, and the absolute time required to sort a given sample is reduced, partly because addition of trypsin reduces the difficult to digest muscle fiber pellet. Examples of various satellite cell isolations are shown in Figure S1, highlighting the above improvements, which together contribute to high and reproducible HuSC yields. Reliability of successful isolation is ensured by the use of the sequential positive selection, which enables separation of HuSCs in the occasional instance of technically substandard separation in one step, as shown in row 4 of Figure S1. Isolated cells were confirmed to be highly purified by detectable PAX7 immunostaining in nearly all cells (95% ± 0.58%) (Figures 1B and 1C). Average satellite cell size, determined by Moxi Flow as per the manufacturer’s instructions, was 8.2 μm in diameter with a range of 6–12.5 μm (Figure 1D). In ten different skeletal muscle types from adults >18 years of age, including two cranial skeletal muscles (tongue and temporalis), we isolated roughly similar numbers of cells from each muscle on the day after biopsy. The most frequent samples yielded 10,000 or more HuSCs per gram of tissue (Figure 1E). Yield was slightly decreased in samples from elderly (>81 years) individuals (Figure 1F). The yield of satellite cells isolated from tissues of females tended to increase when controlled for age and muscle type, but limited sample size likely precluded definitive determination of variations based on sex (Figure 1G). Satellite cell isolation was efficient over a wide range of starting tissue weights from less than 1 g to 6 g. There was increased variability of yield from larger tissue samples, likely related to technical aspects of upscaling the processing (Figure 1H). Together, these technical advances provide a refined protocol for reproducible, stringent, and efficient HuSC isolation.
Figure 1.
Optimized Isolation of Human Satellite Cells from Postnatal Muscle Tissue
(A) Representative flow-cytometry profiles of HuSCs gated for live singlets expressing the surface marker profile CD31−/CD34−/CD45−/CXCR4+/CD29+/CD56+. Cells gated are outlined in black within each plot. The percentage of events in each gating step is shown in each plot (n = 57).
(B) HuSCs were collected stained for PAX7 expression (n = 3). Scale bar 100 μm.
(C) Bar graph representation of PAX7 immunoreactivity in seeded HuSCs (n = 3).
(D) Representative histogram of HuSC diameters after a satellite cell isolation from a single muscle sample (n = 3). Left peak consists of small debris.
(E) Bar plot showing the average number of HuSCs isolated per gram, stratified by muscle type. There were no statistically significant differences (n = at least two samples per muscle type).
(F) Bar plot depicting the average number of cells isolated per gram stratified by donor age. There were no statistical differences among any of the age groups when grouped by age (p = 0.610) or with linear regression analysis (p = 0.474).
(G) Bar plot depicting the average number of cells isolated per gram separated by donor gender shows no statistically significant difference (p = 0.343).
(H) Bar plot depicting the average number of cells isolated per gram arranged by tissue weight shows no statistically significant differences among any of the weight groups.
Data presented as mean ± SEM. See Table S1 for complete sample details and individual n values, which denote individual donors and experiments, and Table S2 for all statistics and p values. See also Figures S1 and S2.
Increased Engraftment of Xenotransplanted Human Satellite Cells by Broad Transplant Distribution
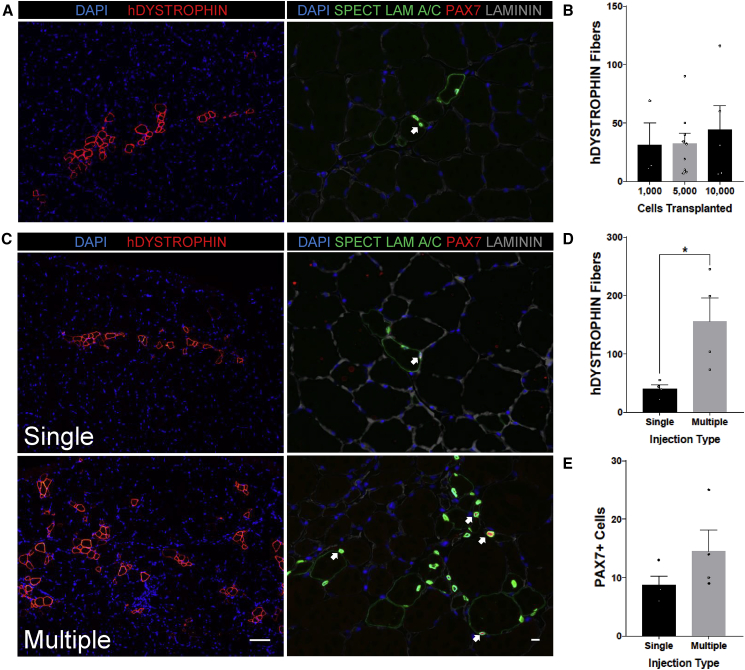
To evaluate whether the extent of HuSC engraftment into minimally damaged muscle could be enhanced, we transplanted 10-fold variable numbers of HuSCs. Nod SCID Gamma (NSG) mice underwent hindlimb irradiation (see Experimental Procedures) and were transplanted with either 1,000, 5,000, or 10,000 HuSCs using a single injection into the tibialis anterior (TA) muscle (Figure 2A). Engraftment was evaluated using immunostaining for human DYSTROPHIN and PAX7 with human SPECTRIN/LAMIN A/C. While engraftment was efficient over the range of transplantation cell dose, the number of engrafted human fibers did not differ among mice transplanted with 1,000, 5,000, or 10,000 satellite cells (Figure 2B). This finding suggested that locally transplanted cells may saturate receptive niches or that engraftment may be otherwise limited in the acute period of this model, and since neither murine satellite cells (Sacco et al., 2008), human myoblasts (Skuk et al., 2010), or HuSCs (Xu et al., 2015) migrate significantly far from the immediate site of transplantation, we adopted a transplantation strategy utilizing multiple injection sites. We used nine injections of approximately 5.5 μL, each containing approximately 220 HuSCs suspended in 0.5% bupivacaine, totaling 50 μL per NSG TA. We found that when compared with mice transplanted with a single injection, the same dose of HuSC distributed in multiple injections resulted in increased engraftment area of human fibers (Figure 2C). We observed that the proportion of engrafted PAX7-positive HuSCs increases as well when the multiple-injection protocol is utilized, as evidenced by staining for human-specific SPECTRIN combined with the costaining of PAX7 and LAMIN A/C (Figures 2C and S3). The average human fiber formation in mice transplanted with 2,000 HuSCs utilizing multiple injections was increased to an average of 155 fibers, over a 2.5-fold increase in comparison with mice transplanted with the same number of cells under the single injection protocol with an average of 40 fibers (Figure 2D). The average number of human-derived PAX7 cells appeared to increase in multiple-injection recipient muscle, but limited sample size precluded definitive quantification (Figure 2E). This level of engraftment corresponds to a conservatively estimated engraftment efficiency of 1 human-derived fiber per 20 HuSCs transplanted. The engraftment data reported here represent a conservative analysis, and it is possible that actual engraftment was higher if some human-derived fibers within the muscle did not reach the analyzed section. This is possible since injections were done throughout the recipient muscle. However, we have previously shown that individual human-derived fibers generally extend along most of the length of the recipient muscle (Xu et al., 2015), mitigating this issue and supporting the analysis method used here. These findings indicate a high capacity for engraftment by individual HuSCs, and show that distribution over a wider area, possibly accessing a higher number of receptive niches in the acute setting, is more important than the absolute number of cells transplanted.
Figure 2.
Enhanced Engraftment of HuSCs by Multiple-Site Injection
(A) Representative images of a conventional transplant with a single injection with 10,000 cells per TA (n = 5, biological replicates). TA cross-sections were stained with human DYSTROPHIN (left) for human fibers or with human-specific SPECTRIN, LAMIN A/C, Laminin, and PAX7 for HuSCs (right). Satellite cells are marked with an arrow.
(B) Bar graph representation of human fiber engraftment with varying dosage of transplanted cells. Isolated satellite cells were transplanted in a single injection of either 1,000 (n = 3), 5,000 (n = 9), or 10,000 (n = 5) cells. n Values denote biological replicates. Human DYSTROPHIN-positive fibers were counted in TA cross-sections, and the y axis value indicates the number of fibers within the cross-section containing the maximum number of human-derived fibers. The number of engrafted fibers was not significantly different among the three groups.
(C) Satellite cells were transplanted in either a single injection (top panels) or with multiple injection sites (bottom panels) with a dose of 2,000 cells per TA. TA cross-sections were stained with human DYSTROPHIN (left) for human fibers or with human-specific SPECTRIN, LAMIN A/C, Laminin, and PAX7 for HuSCs (right) (n = 4 biological replicates). Satellite cells are marked with arrows. Scale bars, 100 μm (left panels; also applies to A) and 10 μm (right panels; also applies to A).
(D) Bar graph showing the engraftment of human myofibers after transplantation as assessed by human DYSTROPHIN staining (n = 4 per group, individual mice).
(E) Bar graph showing the average engraftment of PAX7-positive HuSCs per cross-section after transplantation (n = 4 per group, individual mice).
Data presented as mean ± SEM. ∗p < 0.05. All samples were processed the morning after tissue collection, within 12 hr after muscle biopsy. All mice were analyzed 5 weeks after transplantation. See also Figure S3.
Human Satellite Cell Viability and Engraftment Capacity Are Maintained in Stored Muscle before Processing
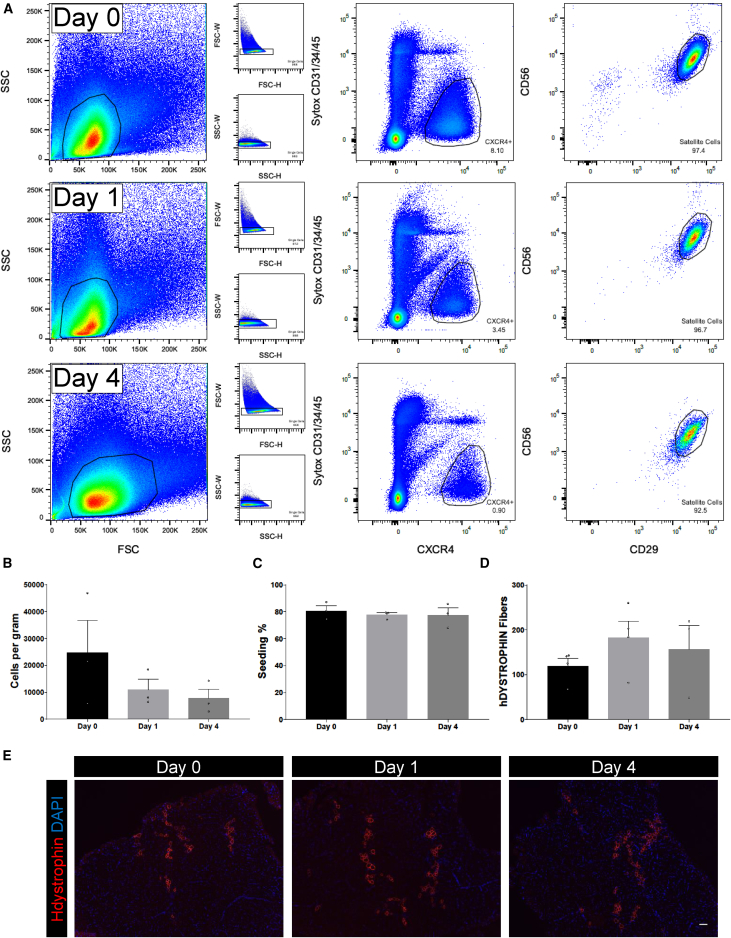
The need to process muscle samples immediately after acquisition remains an open question, and the possibility of longer-term storage would facilitate experimentation and sharing of valuable samples among researchers. We therefore determined whether functioning HuSCs can be extracted from tissue several days after biopsy. Prior work indicated the capacity of skeletal muscle cells from human adult muscle to survive for several days post mortem (Latil et al., 2012). To determine whether HuSCs can be isolated from stored muscle tissue, we isolated satellite cells from three different post-biopsy time points: immediately (day 0), 1 day post biopsy (day 1), and 4 days post biopsy (day 4). Three separate muscle samples from different donors were harvested and divided into 2-g pieces at the time of biopsy. All muscle samples were stored in 30% fetal bovine serum (FBS) at 4°C until use. Each 2-g sample was processed as described in Figure 1A at each post-biopsy time point. Flow-cytometry profiles were similar across the three time points and HuSCs were isolated from each time point in similar proportions (Figure 3A). Satellite cell yield tended to decrease from day 0 to day 4, but reproducible yield was maintained at 4 days (Figure 3B). To assess cell viability, we performed a test of cell-seeding aptitude. For each experimental time point, cells were sorted into Terasaki plate wells at a density of 25 cells per well (Figure 3C), with 20 total wells per replicate. The cells were cultured for 24 hr, at which time the wells were fixed and cell number was quantified. Seeding of cells isolated was greater than 70% in each time point and experimental replicate. There was no significant difference among seeded cells isolated on day 0, day 1, or day 4. To confirm satellite cell identity, we cultured sorted cells from each time point for 3 hr and stained them for PAX7 (Figure S4). PAX7 expression was not significantly different in cells isolated from the experimental groups compared with what is typically seen, as in Figure 1C. Next, we tested satellite cell engraftment function by xenotransplantation as described above. We transplanted 2,000 HuSCs into irradiated TA muscles and analyzed mice 5 weeks post transplantation for the presence of human muscle fibers via human-specific DYSTROPHIN staining. The engraftment of human fibers was similar at the three experimental time points with an average of greater than 100 human fibers at each time point, demonstrating efficient engraftment of human fibers from cells isolated from muscle up to 4 days after removal from the body (Figures 3D and 3E). These findings establish the feasibility of sample sharing and more flexible experimental planning and design.
Figure 3.
HuSC Isolation from Stored Muscle
(A) HuSCs were isolated as previously described from resected adult muscle either immediately after resection or after a storage period of 1 or 4 days in 30% FBS at 4°C. Representative flow-cytometry profiles of HuSC isolation after each condition are shown (n = 3 biological replicates). Cells gated are outlined in black within each plot. The percentage of events in each gating step is shown in each plot.
(B) Bar graph depicting the average number of HuSCs isolated per gram of muscle on each day processed. No statistically significant difference among the three groups (n = 3 biological replicates).
(C) Bar graphs demonstrating the average percentage of HuSCs adhering onto Terasaki wells after isolation and seeding. There was no statistically significant difference among the three groups (n = 3 biological replicates).
(D) Bar graph showing the number of human myofibers engrafted in each mouse TA after xenotransplantation with 2,000 HuSCs into NSG TA muscles with cells isolated on day 0 (n = 4), day 1 (n = 4), or day 4 (n = 3) after biopsy (n values denote individual mice). There was no significant difference in the average engraftment of each condition.
(E) Representative images of human myofiber engraftment after xenotransplantation (n = 3 biological replicates). Scale bar, 100 μm.
Data presented as mean ± SEM. See also Figure S4.
Human Satellite Cells Engraft and Produce Dystrophin in Immunodeficient MDX Mice
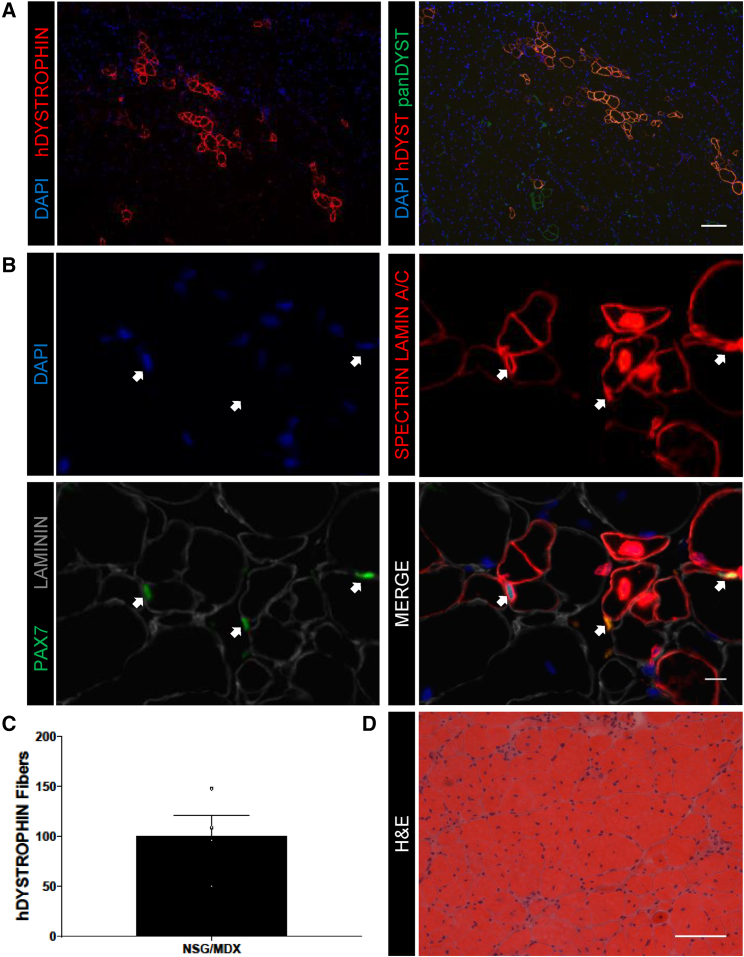
To develop a model system for investigating HuSC transplantation into dystrophic muscle, we adapted HuSC xenotransplantation to dystrophin-deficient hosts. The MDX mouse is the most commonly used mouse model of Duchenne muscular dystrophy (Bulfield et al., 1984, DiMario et al., 1991, Dumont et al., 2015, Hoffman et al., 1987, Straub et al., 1997). Although derived human muscle cells and cultured muscle progenitors have been transplanted into different immunodeficient MDX mouse strains (Chirieleison et al., 2012, Darabi et al., 2012, Goudenege et al., 2012, Meng et al., 2014), HuSCs have not. The lack of a standard benchmark of endogenous HuSCs limits interpretation and comparison of efficacy and stem cell properties of transplanted cells from various sources. To test the capacity of HuSCs to engraft human muscle in the MDX mouse, we crossed MDX and NSG mice to generate an NSG/MDX compound mutant line (see Experimental Procedures). We transplanted HuSCs into 8-week-old fifth-generation NSG/MDX mice, which were then euthanized at 13 weeks, at which time the TA muscles were processed and stained for analysis of human fiber engraftment. The engraftment of human muscle and expression of DYSTROPHIN was confirmed with human-specific DYSTROPHIN staining (Figure 4A). Costaining with human-specific DYSTROPHIN and pan-sensitive Dystrophin demonstrated several small foci of revertant mouse fibers expressing Dystrophin as expected (Hoffman et al., 1990). The presence of sublaminar, PAX7-positive HuSCs was confirmed with SPECTRIN, PAX7, LAMIN A/C, and Laminin staining, indicating repopulation of HuSCs in the satellite cell niche (Figure 4B). To determine the average engraftment of NSG/MDX transplanted with HuSCs, we transplanted NSG/MDX TAs with 7,000 cells each (Figure 4C). Engraftment was similar to that observed in transplanted NSG mice and averaged 101 human fibers identified by human-specific DYSTROPHIN with a range of 50–148. This similarity did not merit formal direct comparison of engraftment into NSG versus NSG/MDX backgrounds. H&E evaluation of transplanted NSG/MDX TAs demonstrated typical hallmarks of muscle degeneration and regeneration (Bulfield et al., 1984) including frequent centralized nuclei and a broad range of myofiber diameters (Figure 4D). Engraftment of HuSCs in NSG/MDX and in NSG muscle was similar in terms of efficiency of human-derived fiber formation.
Figure 4.
Xenotransplantation of HuSCs into NSG/MDX Compound Mutant Mice
(A) Representative images of engrafted human fibers in NSG/MDX TA muscle after transplantation with HuSCs (n = 4 biological replicates). Left: human-specific DYSTROPHIN. Right: costaining for human-specific DYSTROPHIN and pan-sensitive Dystrophin. Orange fibers represent costaining and green fibers represent revertant fibers. Scale bar, 100 μm.
(B) Representative images of HuSC engraftment after transplantation into NSG/MDX TA (n = 4 biological replicates). HuSCs are denoted by sublaminar location and expression of human-specific LAMIN A/C and PAX7, marked by arrows. Scale bar, 10 μm.
(C) Bar graph showing quantification of human fiber engraftment in the NSG/MDX TAs identified by human-specific DYSTROPHIN staining (n = 4 individual mice). Data presented as mean ± SEM.
(D) Representative H&E of an NSG/MDX TA cross-section after transplantation with HuSCs (n = 4 biological replicates). Scale bar, 100 μm.
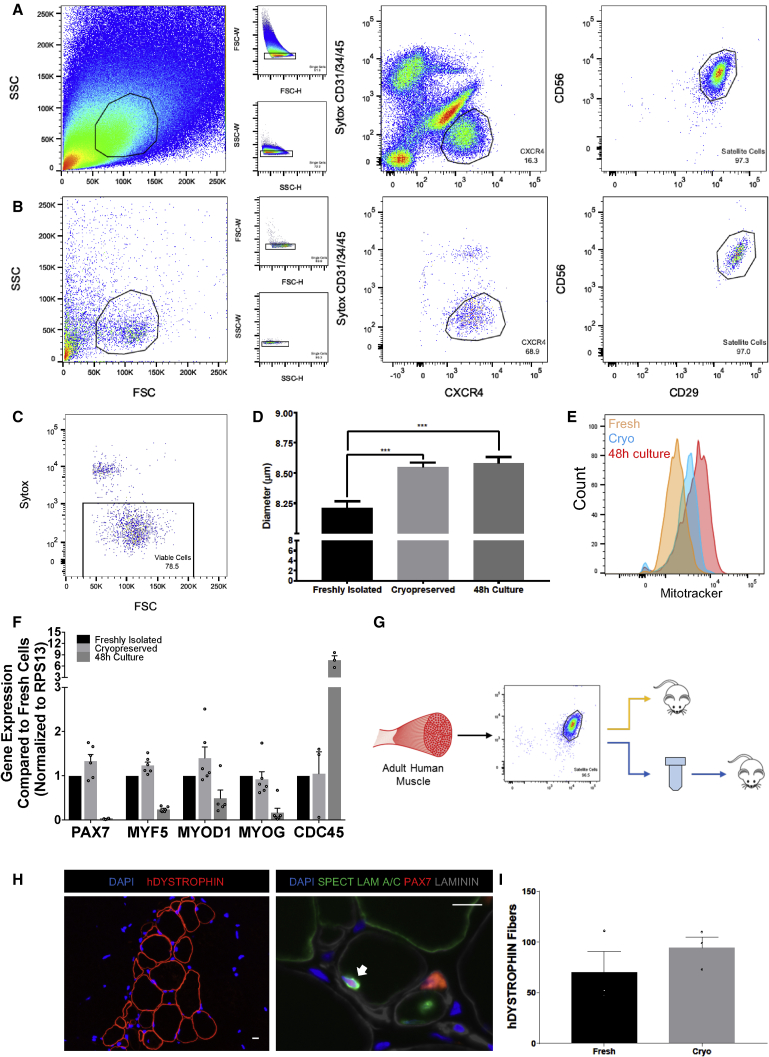
Human Satellite Cells Retain Phenotype and Function after Cryopreservation
The scarcity of muscle tissue from human donors dictates the availability and timing of experimentation with HuSCs and significantly hinders HuSC research. We evaluated whether HuSCs can be preserved with cryopreservation. HuSCs were isolated as described previously (Figure 5A). Sorted cells were then directly frozen in DMEM/F12 with 20% FBS, 1× insulin-transferrin-selenium (ITS), 1× glutamine, 1× gentamicin, and 10% DMSO. After complete preservation in vapor-phase nitrogen the cells were thawed, stained, and reanalyzed with flow cytometry. After thawing, HuSCs retained reactivity to CXCR4, CD29, and CD56 antibodies (Figure 5B) and >75% of the cells were viable based on live dead flow-cytometry assay with Sytox staining (Figure 5C). To test cryopreserved HuSC characteristics, we directly compared cell size and mitochondrial activity with that of cells from the same batch after culture for 48 hr (in DMEM/F12 with 20% FBS, 1× ITS, 1× glutamine, 1× gentamicin) and to freshly isolated HuSCs of the same muscle type in the same experiment. The average size of satellite cells from the freshly isolated, cryopreserved, or 48-hr culture groups were determined by forward scatter on flow cytometry (Figures S5A–S5C) and also by Moxi Flow, which showed average sizes of 8.21 ± 0.05 μm, 8.55 ± 0.04 μm, and 8.58 ± 0.05 μm, respectively (Figure 5D). The maximum diameters of each were 14 μm, 16.5 μm, and 18 μm, respectively, with 1.35% of all cryopreserved cells and 4.19% of all 48-hr culture cells larger than 14 μm. To assess the relative mitochondrial activity of the cryopreserved satellite cells to freshly isolated cells, we performed a mitochondrial activity (MitoTracker) assay. Freshly isolated satellite cells, cryopreserved satellite cells, and 48-hr culture-activated satellite cells were stained with MitoTracker green and analyzed by flow cytometry as per the manufacturer’s instructions. Average mitochondrial activity and relative cell size were increased in the relative order 48-hr culture > cryopreserved > freshly isolated cells (Figure 5E). Next, we compared the level of transcript expression of the myogenic regulatory factors (MRFs) MYF5, MYOD1, and MYOG, as well as PAX7 and the cell cycle gene CDC45, among freshly isolated, cryopreserved, and briefly cultured (48-hr) HuSCs (Figure 5F). The expression of these genes was not different between the freshly isolated and cryopreserved groups. However, the expression of PAX7, MYF5, and MYOG was lower in the 48-hr culture cells compared with both the freshly isolated and cryopreserved cells. MYOD1 expression was lower in 48-hr cells compared with cryopreserved cells. Although at 48 hr of culture HuSCs have not yet proliferated significantly, the expression of CDC45 was higher in the 48-hr culture group compared with both the freshly isolated and cryopreserved groups. To assess retention of function, we next evaluated the ability of the cryopreserved satellite cells to engraft compared with freshly isolated cells from the same muscle tissue (Figure 5G). Frozen satellite cells were thawed and resuspended in bupivacaine as with prior transplantations. The cells were then transplanted into NSG TAs at a dose of 2,000 cells per TA. Human fibers were identified by human DYSTROPHIN staining. Niche-associated, sublaminar, human, and PAX7 positive satellite cells were identified in each preparation using human-specific SPECTRIN, LAMIN A/C, PAX7, and Laminin staining (Figures 5H and S5D). When compared with freshly isolated HuSCs, cryopreserved HuSCs engraft with the same efficiency (p = 0.361) (Figure 5I). Collectively, these results show that cryopreservation of HuSCs results in some alteration of cell size and mitochondrial activity, but can be performed with retention of MRF expression levels and without loss of cell surface marker expression or diminishment of engraftment capacity.
Figure 5.
HuSC Phenotype and Function after Cryopreservation
(A) Flow-cytometry profile of an HuSC isolation from the rectus abdominis muscle of a 43-year-old male.
(B) Isolated HuSCs (A) were cryopreserved and thawed, then restained and sorted. In (A and B), cells gated are outlined in black within each plot. The percentage of events in each gating step is shown in each plot.
(C) Sytox blue flow-cytometry profile for sorted satellite cells in (B) demonstrating >75% viability after cryopreservation.
(D) Representative bar graph showing the average difference in satellite cell size among freshly isolated, cryopreserved, and 48-hr cultured cells (n = 490, 2,037, and 1,942 cells, respectively, from three biological replicates). ∗∗∗p < 0.001.
(E) Representative histograms of freshly isolated satellite cells (orange), cryopreserved cells (blue), and 48-hr cultured cells (red) assessed by MitoTracker flow-cytometry assay (n = 3 biological replicates). “Count” denotes number of cells. Complete profiles are shown in Figure S5.
(F) Bar plots of qRT-PCR data comparing the expression of PAX7, MYF5, MYOD1, MYOG (n = 6), and CDC45 in satellite cells from freshly isolated, cryopreserved, and 48-hr cultured cell groups (n = 3). n Values denote technical replicates from two independent biological samples. Gene expression was normalized to the housekeeping gene RPS13.
(G) Schematic depicting experimental approach to compare the engraftment of cryopreserved and fresh HuSCs from the same muscle tissue.
(H) Representative images after transplantation with 2,000 cryopreserved HuSCs, of human fiber engraftment (left) and of repopulation the niche (right) with HuSCs (arrows) (n = 3 biological replicates). Scale bars, 10 μm.
(I) Bar graph depicting the engraftment of human fibers after transplantation with freshly isolated versus cryopreserved HuSCs quantified by human-specific DYSTROPHIN staining. There was no significant difference in engraftment (n = 3 individual mice).
Data presented as mean ± SEM. See also Figure S5.
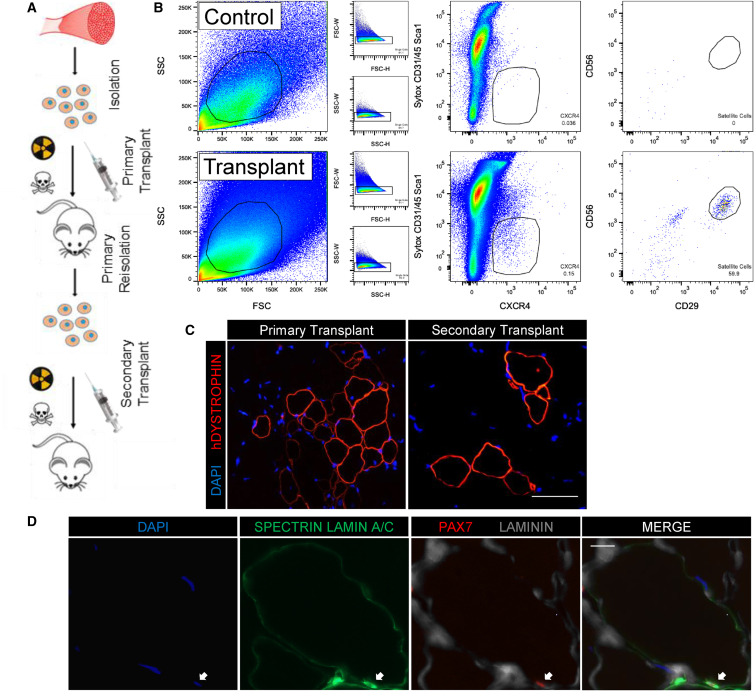
Serial Isolation and Transplantation of Human Satellite Cells
Serial transplantation is a quintessential experimental measure of stem cell function that has not yet been demonstrated for human muscle progenitors, leaving the existence of stem cell identities within the HuSC compartment unproven. The lack of this experimental technique also hinders direct comparison of regenerative potential among different HuSCs. The enhanced HuSC isolation and xenotransplantation methods developed in this research enabled us to test stem cell functions of transplanted HuSCs, utilizing serial isolation and transplantation (Figure 6A). Satellite cells isolated from adult human muscle were transplanted into NSG mice and human cells and muscle were allowed to engraft, return to quiescence, and mature for 10 weeks, as we have previously reported (Xu et al., 2015). After 10 weeks, mice were euthanized and both transplanted TAs and contralateral control TAs were digested and dissociated into single-cell suspensions. Viable singlet cells were sorted by flow cytometry for CD31−/CD45−/Sca1−/CXCR4+/CD29+/CD56+ cells using human-specific antibodies to detect CD56 and CD29 (Figure 6B). CXCR4/CD29/CD56 cells were found and isolated from NSG muscle transplanted with donor cells but not in contralateral control muscle. We isolated 200–1,400 CXCR4/CD29/CD56 HuSCs from each group of four mice that were originally transplanted with 2,500–5,000 donor satellite cells, representing an average recovery rate of approximately 7% with a range of 1%–9%. After reisolation, the satellite cells were transplanted into secondary NSG mice and after 5 weeks the mice were euthanized to assess for engraftment. Human muscle engraftment was confirmed with human-specific DYSTROPHIN in both primary and secondary transplants, indicating preservation of stem cell function through two rounds of transplantation. Since repopulation of the niche and formation of human-derived fibers was observed after two consecutive rounds of isolation and transplantation, donor satellite cells retained stem cell functions and may have undergone self-renewal (Figure 6C). Alternatively, it is possible that a subset of transplanted HuSCs remained quiescent during the first engraftment and was responsible for secondary engraftment, without dividing. Definitive self-renewal assays during HuSC serial transplantation merit further investigation. Although fewer human fibers per section were detected in secondary recipients (83 ± 30 [primary n = 5] versus 9 ± 3 [secondary n = 3]), engraftment efficiency when corrected for the number of transplanted cells did not differ significantly between primary and secondary transplants (p = 0.984). This reflects the smaller number of cells transplanted into secondary recipients, limited by yield of recovery during reisolation. Moreover, we observed an average of 1 PAX7-positive HuSC in the satellite cell niche per 10 fibers per cross-section in both primary and in secondary recipients, confirming both return to quiescence and preservation of engraftment potency of HuSCs that undergo one round of serial transplantation (Figure 6D).
Figure 6.
Serial Isolation and Transplantation of HuSCs
(A) Schematic depicting the experimental design of serial isolation and transplantation of primary HuSCs. Syringe, cell suspension injection; skull and crossbones, bupivacaine; hazard symbol, radiation.
(B) Representative flow-cytometry profiles of a primary reisolation. HuSCs are CXCR4/CD29/CD56-positive located within the outline in the right plot and were only seen in muscle originally transplanted with donor HuSCs (bottom) compared with no HuSCs seen in digests from contralateral control muscle (top) (n = 3). Cells gated are outlined in black within each plot. The percentage of events in each gating step is shown in each plot.
(C) Representative image of human fiber formation in mice transplanted with primary reisolated HuSCs indicating engraftment (n = 3). Scale bar, 100 μm.
(D) Images of HuSC repopulation of the satellite cell niche after secondary transplantation. Immunofluorescence staining for PAX7, SPECTRIN, LAMIN A/C, and Laminin demonstrates human PAX7-positive cells in the sublaminar satellite cell niche in mice transplanted with primary reisolated HuSCs (arrow) (n = 3). Scale bar, 10 μm.
All n values denote biological replicates.
Discussion
The methods developed in this study reproducibly provide robust yields of highly purified adult HuSCs from a wide spectrum of cranial and somitic skeletal muscles. The techniques resulted in first applications of classical experimental stem cell paradigms to this endogenous cell population. These advances, and the demonstrated feasibility of muscle sample and HuSC storage, will make HuSCs widely available to muscle stem cell researchers, enabling use of minimally altered HuSCs for basic and translational research.
Compared with prior studies we have improved HuSC isolation to permit better separation of satellite cells, resulting in highly purified samples with little contamination of non-satellite cells. Although significant variability of yield persists, researchers can expect to obtain approximately 10,000 HuSCs from each gram of skeletal muscle processed from male or female individuals aged 41–60 years (Table S1 and Figure 1F), regardless of muscle type. Such is the case for samples processed immediately or after storage for up to 4 days, findings in agreement with prior work demonstrating survival of muscle progenitors in postmortem skeletal muscle (Latil et al., 2012), and show that although absolute HuSC yield may decrease compared with fresh tissue, robust yield is maintained and transplantability is preserved after storage. This will enable broader use and sharing of human muscle samples. Future work should further identify determinants of and minimize variability of yield. Our experiments with tissue storage did not result in detectable changes in HuSC function, suggesting that altered metabolic state associated with a period of cold storage does not diminish regenerative capacity. In contrast, culture for even short periods without passaging alters progenitor properties such that engraftment capacity per cell is greatly diminished. Whereas much work using human muscle progenitors uses cultured human muscle cells (Brimah et al., 2004, Cooper et al., 2001, Silva-Barbosa et al., 2008, Skuk et al., 2010) and CD133+ cells (Meng et al., 2014), which despite being expanded are uniformly altered from their endogenous states, it is now feasible to perform experiments with bona fide endogenous cells that retain very high fidelity to satellite cells in their natural in vivo states. This can be accomplished with purified HuSCs as demonstrated in this study or with fiber-associated HuSC as previously shown by us and others (Marg et al., 2014, Xu et al., 2015). Minimally altered HuSCs will be useful as a benchmark for other muscle progenitors in efforts to recapitulate natural functions using derived or expanded cells.
This report demonstrates reproducibility of satellite cell preparation from 57 human skeletal muscle samples weighing 6 g and under. Scalability should be readily achievable with larger samples to achieve consistent yields by uniform digestion and downstream processing. For clinical application, the methods presented here can be adapted to meet clinical requirements with compatible reagents. Based on current understanding of limitations of prior clinical trials that used cultured muscle progenitors (Miller et al., 1997, Partridge, 2002, Skuk et al., 2006), readily available unpassaged HuSC preparations should be expected to engraft, survive, and regenerate much more robustly. Furthermore, cryopreservation will greatly facilitate ex vivo manipulation such as genetic modification, as well as repeated administration from the same source. The previously established clinical availability of large expendable human muscles indicates that preparing adequate numbers of satellite cells for therapeutic transplantation into smaller individual human muscles (e.g., face, extraocular, upper extremity, sphincter, and larynx) is feasible without ex vivo expansion.
Availability of highly purified HuSCs readily enables new experimental investigations. Cryopreservation permits direct comparisons of cells that undergo disparate manipulations without confounding factors of source variability such as muscle type, and donor variables such as age, gender, and other unknown factors that could differ among individuals. Using cryopreserved cells will also minimize unwanted experimental variables such as unmatched transplant recipients, reagent batches, and equipment variability that exist when experiments are performed at different times. For example, we could assess how culture affects HuSC mitochondrial activity by using cryopreserved cells from the same sample, treated for different lengths of time and then analyzed simultaneously. Serial transplantation will allow experimental investigation of how in vivo regenerative capacity is affected in aged versus young samples, after pharmacological manipulation and after genetic modification. It will also enable comparative studies of heterogeneous HuSCs separated by distinct surface markers. HuSC transplantation can be further used to study regenerative capacity in vivo using reinjury expansion or by stimulation of regeneration in the NSG/MDX model. Finally, it is feasible to explore the use of in vivo long-term incubation or expansion of HuSCs in non-human animal hosts, a process that could possibly preserve some natural characteristics and functions relative to in vitro systems involving cryopreservation or culture.
The improvements over existing technology were developed by empiric digestion and sorting strategies aimed at achieving complete sample digestion and use of surface marker combinations that allowed more rapid sorting and better separation from contaminating cells. The addition of CXCR4 to the prior CD56/CD29 combination and the negative selection greatly enhanced the efficiency of flow cytometry and effectively removed non-satellite cells. This advance will improve studies that rely on highly pure satellite cell samples such as transcriptional analysis. We also demonstrate that it is not necessarily advantageous to use high numbers of satellite cells for transplantation. Indeed, we show similar levels of human-derived fiber formation after transplantation of hundreds of cells versus tens of thousands, implying that with relatively pure populations of satellite cells, engraftment is limited by niche receptiveness or access in addition to satellite cell survival or other factors affecting their ability to engraft, as suggested by prior reports of high engraftment efficiency from small numbers of HuSCs transplanted within their niches on fibers (Marg et al., 2014, Xu et al., 2015). It remains to be determined whether engrafted myonuclear number can be further augmented by additional modifications or higher transplanted cell dosage, but the data presented in this study suggest that it may be possible to perform experiments or clinical applications using small amounts of starting human muscle tissue that can regenerate and expand in vivo with additional stimulation.
Experimental Procedures
Human Muscle Procurement
This study was conducted under the approval of the Committee on Human Research at The University of California, San Francisco (UCSF). Biopsies were obtained from individuals undergoing surgery at UCSF. Written informed consent was obtained from all subjects.
Animal Care and Transplantation Studies
All mice were bred and housed in a pathogen-free facility at UCSF. All procedures were approved and performed in accordance with the UCSF Institutional Animal Care and Use Committee. All experiments were unblinded and performed in 8- to 12-week-old NSG mice (The Jackson Laboratory) and NSG mice crossed with MDX mice (The Jackson Laboratory), creating NSG/MDX mice. Mice were randomized to all experimental groups by sex and littermates and were pretreated with 18 Gy on the day before transplantation. HuSCs were injected along with 50 μL of 0.5% bupivacaine directly into the TA muscle of one leg in a single injection or multiple injections as indicated.
CXCR4+/CD29+/CD56+ Satellite Cell Sorting
Freshly harvested human muscle was either immediately digested or stored in DMEM with 30% FBS at 4°C. Muscle was digested, erythrocytes lysed, and hematopoietic and endothelial cells depleted with magnetic column depletion. Viable cells were depleted for CD31-, CD34-, and CD45-expressing cells. Cells that remained after depletion were sorted for CXCR4+/CD29+/CD56+ and collected for further experimentation.
PAX7 Immunostaining of Cells from Digested Muscle
Sorted cells were collected in 20% FBS-DMEM with 10 mM ROCKi and plated directly into wells of BioCoated laminin-coated chamber slides (BD Biosciences). Slides were stained with monoclonal rabbit anti-PAX7 antibody (1:500, Abcam) (see Figure S6 for antibody controls). Immunostaining antibodies are listed in Table S4.
Satellite Cell Cryopreservation
Satellite cells were suspended in DMEM/F12 with 20% FBS, 1× ITS, 1× glutamine, 1× gentamicin, and 10% DMSO, frozen at a cooling rate of −1°C/min overnight and then moved to storage in vapor-phase nitrogen. In this study, satellite cells were thawed and used in experiments after 5 months of cryostorage.
qRT-PCR Analysis
Relative expression of individual genes compared with control groups was calculated by the ΔΔ-Ct (delta-delta cycle threshold) method with GAPDH or RPS13 as the housekeeping gene. qRT-PCR primer sequences are available in Table S5.
Statistical Analysis
Normality of the data was checked utilizing the Shapiro-Wilk normality test in GraphPad Prism. Means between or across groups were compared using two-tailed t tests for experiments involving two groups, or one-way ANOVA with post hoc Tukey multiple comparisons when comparisons were made across three or more groups to determine significance (p < 0.05) between test conditions and controls, and multiple groups. Multivariate regression was utilized as indicated for comparing satellite cell yield per gram controlling for age, gender, and muscle type. All human muscle samples collected over the past year and processed within 12 hr after biopsy were used for data analyses in Figure 1. At least three mice per group were used for all transplantation experiments. At least three biological replicates for each experiment were performed unless otherwise noted, with exact n values listed in each figure legend. All error bars are depicted as SEM. p values in figures are indicated by asterisks (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
Author Contributions
S.M.G. designed and performed experiments, analyzed data, and wrote the manuscript. S.T. designed and performed experiments, analyzed data, and edited the manuscript. A.W. performed experiments and edited the manuscript. S.L. performed statistical analyses and the qPCR of Figure 5 and edited the manuscript. A.J. designed and performed flow-cytometry experiments. J.D. and G.K. performed experiments optimizing satellite cell isolation. J.H.P., H.S., R.S., P.D.K., C.H., W.R., E.K., S.H., and W.Y.H. provided human muscle tissue and provided ongoing comments. J.H.P. designed and oversaw the research, analyzed the data, and wrote the manuscript.
Acknowledgments
This work was supported by the CIRM New Faculty Physician Scientist award RN3-06504 to J.H.P., the UCSF PROF-PATH program via NIH R25MD006832 to S.M.G., UCSF Research Allocation Program for trainees to S.L., and the Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research fellowship to A.W. Support from the UCSF Department of Surgery is also acknowledged. The authors would like to thank Pamela Derish for editorial comments and Lauren Byrnes for helpful discussions and would like to express their thanks for the cooperation of Donor Network West and all of the organ and tissue donors and their families for giving the gift of life and the gift of knowledge by their generous donation.
Published: February 22, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, six figures, and five tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.01.022.
Supplemental Information
References
- Alexander M.S., Rozkalne A., Colletta A., Spinazzola J.M., Johnson S., Rahimov F., Meng H., Lawlor M.W., Estrella E., Kunkel L.M. CD82 is a marker for prospective isolation of human muscle satellite cells and is linked to muscular dystrophies. Cell Stem Cell. 2016;19:800–807. doi: 10.1016/j.stem.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareja A., Holt J.A., Luo G., Chang C., Lin J., Hinken A.C., Freudenberg J.M., Kraus W.E., Evans W.J., Billin A.N. Human and mouse skeletal muscle stem cells: convergent and divergent mechanisms of myogenesis. PLoS One. 2014;9:e90398. doi: 10.1371/journal.pone.0090398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp J.R., Heslop L., Yu D.S., Tajbakhsh S., Kelly R.G., Wernig A., Buckingham M.E., Partridge T.A., Zammit P.S. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimah K., Ehrhardt J., Mouly V., Butler-Browne G.S., Partridge T.A., Morgan J.E. Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum. Gene Ther. 2004;15:1109–1124. doi: 10.1089/hum.2004.15.1109. [DOI] [PubMed] [Google Scholar]
- Bulfield G., Siller W.G., Wight P.A., Moore K.J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc. Natl. Acad. Sci. USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni A., Hettmer S., Lynes M.D., Rao T.N., Tchessalova D., Sinha I., Lee B.T., Tseng Y.H., Wagers A.J. Isolation of progenitors that exhibit myogenic/osteogenic bipotency in vitro by fluorescence-activated cell sorting from human fetal muscle. Stem Cell Reports. 2014;2:92–106. doi: 10.1016/j.stemcr.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charville G.W., Cheung T.H., Yoo B., Santos P.J., Lee G.K., Shrager J.B., Rando T.A. Ex vivo expansion and in vivo self-renewal of human muscle stem cells. Stem Cell Reports. 2015;5:621–632. doi: 10.1016/j.stemcr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirieleison S.M., Feduska J.M., Schugar R.C., Askew Y., Deasy B.M. Human muscle-derived cell populations isolated by differential adhesion rates: phenotype and contribution to skeletal muscle regeneration in Mdx/SCID mice. Tissue Eng. Part A. 2012;18:232–241. doi: 10.1089/ten.tea.2010.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.A., Olsen I., Zammit P.S., Heslop L., Petrie A., Partridge T.A., Morgan J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Cooper R.N., Irintchev A., Di Santo J.P., Zweyer M., Morgan J.E., Partridge T.A., Butler-Browne G.S., Mouly V., Wernig A. A new immunodeficient mouse model for human myoblast transplantation. Hum. Gene Ther. 2001;12:823–831. doi: 10.1089/104303401750148784. [DOI] [PubMed] [Google Scholar]
- Darabi R., Arpke R.W., Irion S., Dimos J.T., Grskovic M., Kyba M., Perlingeiro R.C. Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell. 2012;10:610–619. doi: 10.1016/j.stem.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMario J.X., Uzman A., Strohman R.C. Fiber regeneration is not persistent in dystrophic (MDX) mouse skeletal muscle. Dev. Biol. 1991;148:314–321. doi: 10.1016/0012-1606(91)90340-9. [DOI] [PubMed] [Google Scholar]
- Dumont N.A., Wang Y.X., von Maltzahn J., Pasut A., Bentzinger C.F., Brun C.E., Rudnicki M.A. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 2015;21:1455–1463. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada S., Higuchi S., Segawa M., Koda K., Yamamoto Y., Tsujikawa K., Kohama Y., Uezumi A., Imamura M., Miyagoe-Suzuki Y. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp. Cell Res. 2004;296:245–255. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Garcia S.M., Tamaki S., Xu X., Pomerantz J.H. Human satellite cell isolation and xenotransplantation. Methods Mol. Biol. 2017;1668:105–123. doi: 10.1007/978-1-4939-7283-8_8. [DOI] [PubMed] [Google Scholar]
- Goudenege S., Lebel C., Huot N.B., Dufour C., Fujii I., Gekas J., Rousseau J., Tremblay J.P. Myoblasts derived from normal hESCs and dystrophic hiPSCs efficiently fuse with existing muscle fibers following transplantation. Mol. Ther. 2012;20:2153–2167. doi: 10.1038/mt.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E.P., Morgan J.E., Watkins S.C., Partridge T.A. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J. Neurol. Sci. 1990;99:9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latil M., Rocheteau P., Châtre L., Sanulli S., Mémet S., Ricchetti M., Tajbakhsh S., Chrétien F. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat. Commun. 2012;3:903. doi: 10.1038/ncomms1890. [DOI] [PubMed] [Google Scholar]
- Marg A., Escobar H., Gloy S., Kufeld M., Zacher J., Spuler A., Birchmeier C., Izsvak Z., Spuler S. Human satellite cells have regenerative capacity and are genetically manipulable. J. Clin. Invest. 2014;124:4257–4265. doi: 10.1172/JCI63992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Chun S., Asfahani R., Lochmuller H., Muntoni F., Morgan J. Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol. Ther. 2014;22:1008–1017. doi: 10.1038/mt.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.G., Sharma K.R., Pavlath G.K., Gussoni E., Mynhier M., Lanctot A.M., Greco C.M., Steinman L., Blau H.M. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Montarras D., Morgan J., Collins C., Relaix F., Zaffran S., Cumano A., Partridge T., Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- Partridge T. Myoblast transplantation. Neuromuscul. Disord. 2002;12(Suppl 1):S3–S6. doi: 10.1016/s0960-8966(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Pisani D.F., Dechesne C.A., Sacconi S., Delplace S., Belmonte N., Cochet O., Clement N., Wdziekonski B., Villageois A.P., Butori C. Isolation of a highly myogenic CD34-negative subset of human skeletal muscle cells free of adipogenic potential. Stem Cells. 2010;28:753–764. doi: 10.1002/stem.317. [DOI] [PubMed] [Google Scholar]
- Sacco A., Doyonnas R., Kraft P., Vitorovic S., Blau H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood R.I., Christensen J.L., Conboy I.M., Conboy M.J., Rando T.A., Weissman I.L., Wagers A.J. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Silva-Barbosa S.D., Butler-Browne G.S., de Mello W., Riederer I., Di Santo J.P., Savino W., Mouly V. Human myoblast engraftment is improved in laminin-enriched microenvironment. Transplantation. 2008;85:566–575. doi: 10.1097/TP.0b013e31815fee50. [DOI] [PubMed] [Google Scholar]
- Skuk D., Goulet M., Roy B., Chapdelaine P., Bouchard J.P., Roy R., Dugre F.J., Sylvain M., Lachance J.G., Deschenes L. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J. Neuropathol. Exp. Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Skuk D., Paradis M., Goulet M., Chapdelaine P., Rothstein D.M., Tremblay J.P. Intramuscular transplantation of human postnatal myoblasts generates functional donor-derived satellite cells. Mol. Ther. 2010;18:1689–1697. doi: 10.1038/mt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub V., Rafael J.A., Chamberlain J.S., Campbell K.P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A., Nakatani M., Ikemoto-Uezumi M., Yamamoto N., Morita M., Yamaguchi A., Yamada H., Kasai T., Masuda S., Narita A. Cell-surface protein profiling identifies distinctive markers of progenitor cells in human skeletal muscle. Stem Cell Reports. 2016;7:263–278. doi: 10.1016/j.stemcr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wilschut K.J., Kouklis G., Tian H., Hesse R., Garland C., Sbitany H., Hansen S., Seth R., Knott P.D. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Reports. 2015;5:419–434. doi: 10.1016/j.stemcr.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.