Abstract
Each year, hundreds of thousands coronary bypass procedures are performed in the US, yet there currently exists no off-the-shelf alternative to autologous vessel transplant. In the present study, we investigated the use of mouse thrombospondin-2 knockout (TSP2 KO) cells, which secrete a non-thrombogenic and pro-migratory extracellular matrix (TSP2 KO ECM), to modify small diameter vascular grafts. To accomplish this, we first optimized the incorporation of TSP2 KO ECM on decellularized rat aortas. Because MMP levels are known to be elevated in TSP2 KO cell culture, it was necessary to probe the effect of the modification process on the graft's mechanical properties. However, no differences were found in suture retention, Young's modulus, or ultimate tensile strength between modified and unmodified grafts. Platelet studies were then performed to determine the time point at which the TSP2 KO ECM sufficiently reduced thrombogenicity. Finally, grafts modified by either TSP2 KO or WT cells or unmodified grafts, were implanted in an abdominal aortic interposition model in rats. After 4 weeks, grafts with incorporated TSP2 KO ECM showed improved endothelial and mural cell recruitment, and a decreased failure rate compared to control grafts. Therefore, our studies show that TSP2 KO ECM could enable the production of off-the-shelf vascular grafts while promoting reconstruction of native vessels.
Keywords: Decellularization, Vascular graft, Extracellular matrix, Genetically modified matrix
1. Introduction
Nearly 400,000 coronary artery bypass procedures are performed in the US each year [1]. These surgeries typically involve the saphenous vein or the internal mammary artery as the bypass conduit, but the use of autologous grafts is limited by their accessibility and availability, and their harvest increases the cost, time, and morbidity of the surgical procedure. Moreover, the outcomes of venous conduits are suboptimal compared to arterial grafts. The unmet need for an alternative has fueled extensive efforts to create functional replacement grafts [2–4]. Vascular tissue engineering promises to fulfill this need via the generation of native-like blood vessels, but despite progress in large-diameter, low pressure, and hemodialysis systems, has not yet succeeded [5,6]. Major obstacles to the production of engineered small-diameter (<6 mm) vascular grafts include thrombosis if the graft is to be cell-free, or production time and appropriate cell source, if the graft is to be implanted with cells to promote patency [7].
Ideal small-diameter vascular grafts would be “off-the-shelf.” They would be available for immediate use if necessary, but would also have the appropriate mechanical properties and present the opportunity for cell ingrowth and remodeling, with the ultimate goal of autologous tissue replacement of the implanted graft. Such a graft would therefore need to be initially cell-free and composed of some strong, flexible, and naturally modifiable material, all while promoting hemocompatibility. In order to create such a graft, several groups have begun to explore the use of extracellular matrix (ECM) proteins in order to enhance cell recruitment and mural remodeling. Specifically, whole ECM or proteins aiding endothelial cell (EC) adhesion, such as perlecan, collagen IV, and fibronectin, have been applied to experimental vascular grafts in an attempt to improve outcomes [8–11]. Particularly interesting was the finding that a fibronectin coating not only accelerated endothelialization, but also was found to enhance recruitment of smooth muscle cells (SMC) to the graft. While these results are encouraging, none of these coatings are inherently anti-thrombogenic. In fact, hemo-compatibility of collagen IV and fibronectin has been examined by other groups, and both were found to activate platelets in the absence of anti-platelet therapies such as hyaluronic acid or heparin [12,13].
Despite the imperfections, the use of ECM in vascular grafts continues to be studied. With the increasing popularity of decellularization, some groups have simply proposed the use of decellularized tissue as vascular grafts [14–17]. However, these grafts face the same pitfalls as ECM-coated grafts, and are susceptible to thrombosis [18]. Thus, there has been a corresponding increase in the study of decellularized tissue modification to enhance endothelialization and reduce thrombogenicity. In attempts to accelerate endothelialization, researchers have coated decellularized grafts with VEGF, VEGF hydrogels, integrin ligands, and cell adhesion peptides [19–23]. Other groups have chosen to focus on decreasing graft thrombogenicity. A variety of methods have been employed to incorporate heparin into decellularized grafts, including adsorption, layer-by-layer coating, covalent bonding via a collagen binding peptide or EDC chemistry, and “click” chemistry [24–29]. One group even went so far as to coat decellularized grafts with both heparin and thrombomodulin [30]. While examples of modifications of decellularized grafts that perform both functions have achieved some success, their creation is a complex, multistage process [31–34].
We have recently shown that, despite its similarities to wild-type (WT) ECM when analyzed by immunofluorescence and qPCR, thrombospondin-2 knock out (TSP2 KO) ECM resists platelet adhesion in denuded arteries in vivo and that this property is retained when TSP2 KO ECM is produced in vitro (Supplemental Fig. 1) [35]. This feature of TSP2 KO ECM presents a singular opportunity for the novel use of genetically modified ECM as a vascular graft enhancement. TSP2 is a matricellular protein known to interact with a variety of ECM proteins and cell-surface receptors, such as heparan sulfate proteoglycans and low density lipoprotein-related receptor protein (LRP) [36,37]. Much of what is known about the function of TSP2 has been gleaned from the study of TSP2 KO mice. It has been reported that tissues isolated from TSP2 KO mice have irregular collagen, and that collagen produced in vitro by cells isolated from TSP2 KO mice retain this trait [38,39]. Moreover, it has been reported that collagen fibrillogenesis is likely affected through decreased recycling of matrix metalloproteinases (MMPs) through LRP, a process that is facilitated by TSP2. This results not only in increased levels of MMPs, but also decreased levels of MMP targets, such as the collagen crosslinking protein tissue trans-glutaminase [40]. In addition to altering the thrombogenicity of TSP2 KO ECM, the abnormal collagen has proven more permissive for EC migration [39]. Taken together, these observations make TSP2 KO ECM an interesting candidate for vascular graft modification.
In the present study we have endeavored to take advantage of these properties of TSP2 KO ECM and apply them to small diameter vascular grafts. For our purposes, we have chosen to use decellularized rat aortas to serve as the base graft. This increasingly popular method of tissue scaffold preparation results in a scaffold with mechanical properties and structure as close as possible to the native tissue [41]. As a follow up to our most recent studies, the integration of TSP2 KO ECM in the lumen of a potential vascular graft has been optimized. It was confirmed that it detered platelet adhesion and activation when incorporated on decellularized rat aortas, and that TSP2 KO ECM modification did not affect graft mechanical properties. The promising results of this work prompted in vivo studies to determine whether this modification would improve graft outcomes. At 4 weeks post implantation when compared to unmodified grafts, a significant decrease in failure rate was noted in grafts incorporating TSP2 KO ECM. Moreover, the TSP2 KO ECM modification resulted in recruitment of cells expressing smooth muscle actin to the graft media, as well as increased endothelial cell population of the graft lumen.
2. Materials and methods
2.1. Animal use
TSP2 KO and age-matched wild type (WT) C57/BL6 mice aged 3–4 months were used to isolate dermal fibroblasts (DFs) for ECM production. Fisher F344 rats (Charles River Laboratories, Boston, MA) were used for in vivo implantation experiments. Graft donors and recipients were age and sex matched. All animal studies were performed as procedures approved by the Institutional Care and Use Committee of Yale University.
2.2. Vessel decellularization
Donor rats were euthanized and thoracic aortas were harvested for decellularization. Upon isolation, fresh aortas were placed in a salt wash (1 M NaCl, 0.1 M NaOH, 25 mM EDTA) for 4 h with shaking at room temperature. After washing overnight in PBS +100 mM EDTA, aortas were placed in a Benzonase wash (2 U/mL) for 6 h with shaking at room temperature, and once again washed overnight in PBS + 100 mM EDTA. On the third day, vessels were placed in a CHAPS buffer as described previously, before extensive washing with PBS [16]. Decellularized vessels were stored in PBS at 4 °C until ready for use. Segments of porcine aorta were obtained and decellularized as described previously [28].
2.3. Preparation of TSP2 KO ECM
DFs from TSP2 KO and WT mice were isolated as described previously [35]. Briefly, dorsal skin was isolated from mice, washed in an antibiotic solution and placed in a trypsin solution dermis side down overnight at 4 °C. Excess fat was removed from the dermis by scraping before cutting the skin into small pieces for ultimate digestion by collagenase (0.25% in DMEM, Sigma). After allowing the skin to be digested in collagenase solution for 3 h at 37 °C, cells were collected by centrifugation, washed, and plated for tissue culture. Cells between passages 1 and 3 were used to seed decellularized aortas for this study. To ensure complete cell and matrix coverage of the aortas, 50 μl of cells (at a concentration of 3 million cells/mL) were pipetted into the lumen of decellularized aortas thrice, with 30 min and a 1/3 rotation of aortas performed between seedings. Aortas were kept at 37 °C for the duration of seeding. After seeding, cells on aortas were cultured to encourage the production of ECM as described previously [39]. Secondary decellularization was also performed as described previously with the exception that it was performed for 10 min, and with the additional step of DNase (100 U/mL, Roche) treatment for 1 h at 37 °C, before washing with PBS and storage (4 °C, PBS) until use [35]. Supplemental Fig. 2 includes representative images of dermal fibroblast-seeded and secondarily decellularized grafts.
2.4. In vitro studies
2.4.1. Fluorescence
Immunofluorescence examination of samples was performed as described previously [35]. Briefly, after fixation (4% paraformaldehyde [PFA]) and washing, samples were blocked (1% bovine serum albumin [BSA] in PBS), incubated with anti-fibronectin antibody (Abcam) overnight at 4 °C, washed (PBS), and incubated with secondary antibody (Invitrogen) in order to ensure the TSP2 KO ECM was retained during the experiment. In addition, samples from platelet studies were stained with rhodamine-phalloidin (Molecular Probes) according to standard procedures before mounting for fluorescence.
2.4.2. Electron microscopy
After fixation (2% PFA in 0.1 M cacodylate buffer), samples were dehydrated through a gentle ethanol gradient and placed in hexamethyldisilazane overnight. Samples were then air-dried and sputter coated with chromium for viewing via scanning electron microscopy (SEM) (Hitachi SU-70).
2.5. Mechanical testing
Suture strength testes were performed as described previously [42]. Uniaxial testing was performed using an INSTRON 5848 as described previously [28].
2.6. Platelet studies
Platelet studies on unmodified, TSP2 KO ECM modified, or WT ECM modified decellularized aortas were performed as described previously [35]. Briefly, PRP plasma was isolated from WT mice and allowed to interact with the unmodified or modified surfaces of the decellularized aortas with shaking for 30 min at 37 °C before washing with PBS to remove non-adherent platelets. Samples were then bisected and one half was fixed for examination by fluorescence, while the other was fixed for SEM.
2.7. Graft implantation
To evaluate the effect of ECM incorporation on decellularized grafts in vivo, grafts were implanted as abdominal interposition grafts. Briefly, rats were anesthetized with isofluorane and a midline incision was performed under standard sterile conditions. The infra-renal abdominal aorta was exposed, cross-clamped, and divided between the renal and inferior mesenteric arteries. A segment of unmodified, TSP2 KO ECM modified, or WT ECM modified decellularized aorta (5–7 mm in length, n = 9 per group) was inserted end-to-end into the aorta using a 10-0 monofilament nylon suture. After de-clamping, graft flow and hemostasis were confirmed before the incision was closed. After recovery from surgery, animals were maintained without postoperative anticoagulation treatment.
2.8. Assessment of in vivo grafts
At 1, 2, and 4 weeks post implantation, grafts were examined using a Vevo 770 micro-ultrasound system (VisualSonics) to monitor graft patency and blood flow (Supplemental Fig. 3). At 4 weeks post implantation, grafts were explanted and fixed (4% PFA) for histological examination. Grafts were divided into anastomotic and mid-graft portions, paraffin embedded, sectioned and hematoxylin and eosin (H&E) and elastic van-Gieson (EVG) stains were performed. Immunofluorescence was also performed on paraffin-embedded sections. Briefly, slides were deparaffinized, rehydrated, blocked, incubated with primary antibodies against either, α-smooth muscle actin (α-SMA), calponin, CD68 or CD45 (Abcam) at 4 °C overnight. After washing and incubation with a secondary antibody (Invitrogen), samples were mounted for fluorescence microscopy. Immunofluorescence staining for von Willebrand factor (vWF) was performed in a similar manner except that a FITC-tagged primary antibody (Invitrogen) was used.
2.9. Image analysis
Percent area covered by platelets and fibronectin was determined by analysis with Metamorph. Number of cells infiltrating the media, percentage vWF positive perimeter, outer graft diameter, and percent lumen loss were analyzed using ImageJ. Failure was defined as >75% occlusion, in accordance with the literature [43].
2.10. Statistical analysis
All data are expressed as means ± the standard error of the mean. Fisher's exact test was used to compare rates of failure in the grafts. Other statistical differences were determined by one-way analysis of variance. A p-value of p < 0.05 was considered to be significant.
3. Results
3.1. Successful incorporation of TSP2 KO ECM on vascular grafts
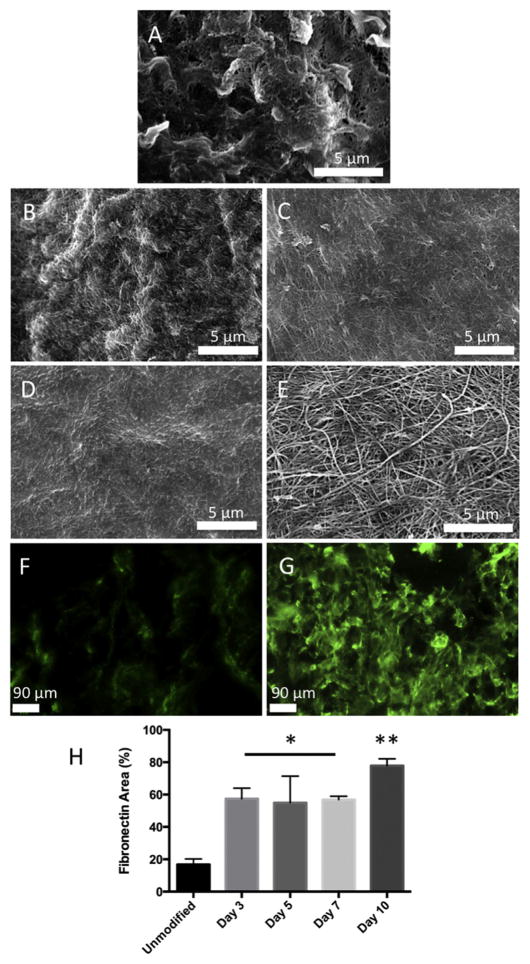
We have previously reported that TSP2 KO ECM can be deposited in tissue culture wells while retaining its non-thrombogenic properties [35,39]. In order to determine whether TSP2 KO ECM could be incorporated on a potential vascular graft, DFs were isolated from TSP2 KO mice and seeded onto decellularized rat aortas. DFs were cultured on aortas for 3, 5, 7, or 10 days before decellularization and en face visualization by SEM. TSP2 KO ECM could clearly be visualized on the luminal surface of aortas after 3 days, however, the modification was not thick enough to conceal the undulating ultrastructure of the native vessel (Fig. 1B and A, respectively). By day 5, the lumen of the vessel no longer exhibited the native ultrastructure, indicating a thicker, more complete modification (Fig. 1 C). This was also observed after 7 and 10 days, though the ECM fibrils were more mature after 10 days in culture (Fig. 1D and E respectively). In addition, ECM incorporation was observed by en fas immunofluorescence detection of fibronectin, and quantification of these images showed significantly increased levels on ECM-modified grafts (Fig. 1F–H).
Fig. 1. TSP2 KO ECM is successfully incorporated in the lumen of vascular grafts.
Rat aortas were decellularized and the lumens were seeded with TSP2 KO fibroblasts and cultured before a second decellularization. SEM images show an unmodified graft (A) compared to TSP2 KO ECM in the lumen after 3 (B), 5 (C), 7 (D), and 10 (E) days in culture (Hitachi). While a complete, but thin ECM can be found as early as 3 days, the most mature modification is found after 10 days. Presence of TSP2 KO ECM was confirmed by immunofluorescent detection of fibronectin. Very little fibronectin was detectable on unmodified grafts (F, green color), while fibroncetin convered a significant portion of the TSP2 KO ECM modified grafts at 10 days (G). Quantification of fibronectin coverage at all time points was performed, showing significantly higher fibronectin coverage on ECM-modified grafts, with the highest percent coverage found at 10 days (H). *p < 0.05, **p < 0.01 compared to unmodified. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Mechanical properties of ECM modified grafts
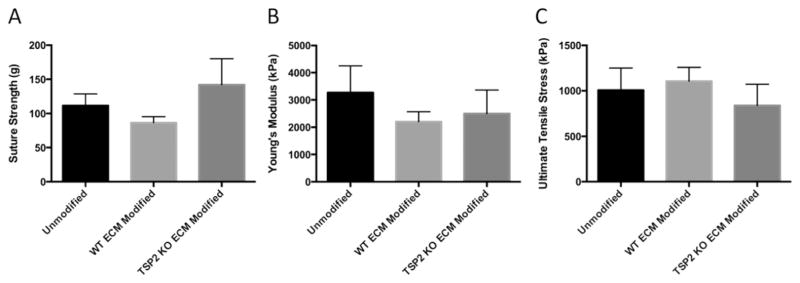
Previous studies have shown that MMP-2 levels are elevated in TSP2 KO DF culture [44]. Because the chosen graft is composed of natural ECM, it was important to confirm that the modification process would not affect the mechanical integrity of the graft. In order to probe this question, TSP2 KO ECM was incorporated on decellularized aortas for the maximum time period (10 days) before decellularization. In order to determine whether any potential differences were the result of general ECM modification or specific to TSP2 KO ECM, WT ECM was also incorporated on a group of vessels for 10 days. Segments of ECM modified and unmodified aortas were then subjected to suture retention and uniaxial mechanical testing. No differences were observed among the groups (Fig. 2).
Fig. 2. ECM modification does not alter graft mechanical properties.
Decellularized, unmodified and ECM modified (10 days) grafts were subjected to suture strength and INSTRON testing. There were no differences found among the groups for suture strength (n = 3), Young's modulus or ultimate tensile strength (n = 6).
3.3. Decreased platelet activation and aggregation on TSP2 KO ECM modified grafts
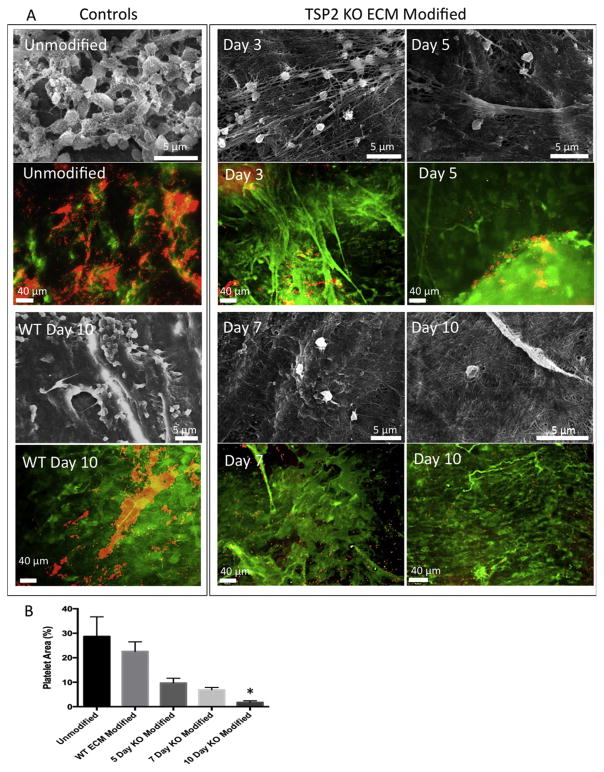
With the ultimate goal of creating an off-the-shelf vascular graft, it was important to perform optimization experiments to determine the minimum time after which incorporated TSP2 KO ECM is non-thrombogenic. In order to determine this threshold, platelet studies were performed. Fluorescence images showed significant aggregation of platelets on control grafts, as well as on grafts on which a TSP2 KO ECM had been incorporated for 3 days (Fig. 3A). However, at later time points, TSP2 KO ECM modified grafts appeared to have fewer adherent platelets. In addition, SEM images of platelets on grafts indicated less activation (less spread, less ruffied appearance) as modification duration increased. Quantification of immunofluorescence showed that while there was a trend toward decreased platelet adhesion on TSP2 KO ECM modified over time compared to both unmodified and WT ECM modified grafts, significance was not reached until the TSP2 KO ECM had been incorporated for 10 days (Fig. 3B).
Fig. 3. Platelet activation and aggregation are decreased after 10 day modification with TSP2 KO ECM.
(A) Representative images of platelets interacting with unmodified, WT ECM modified, and TSP2 KO ECM modified (multiple durations) vascular grafts. SEM images show activated (ruffied, spread) platelet morphology on unmodified and WT ECM-modified grafts, as well as decreasing levels of activated morphology on TSP2 KO ECM as modified duration increases (Hitachi). Representative images of platelets visualized by rhodamine-phalloidin (red color) on unmodified, WT ECM modified, and TSP2 KO ECM modified (multiple durations) vascular grafts are also shown. Detection of fibronectin (green color) confirms the retention of ECM during the duration of the experiment (Zeiss). (B) Quantification of platelet area by Image J showed reduced platelet aggregation on day 10 TSP2 KO ECM modified grafts. n = 5, *p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Increased endothelial population of TSP2 KO ECM modified grafts
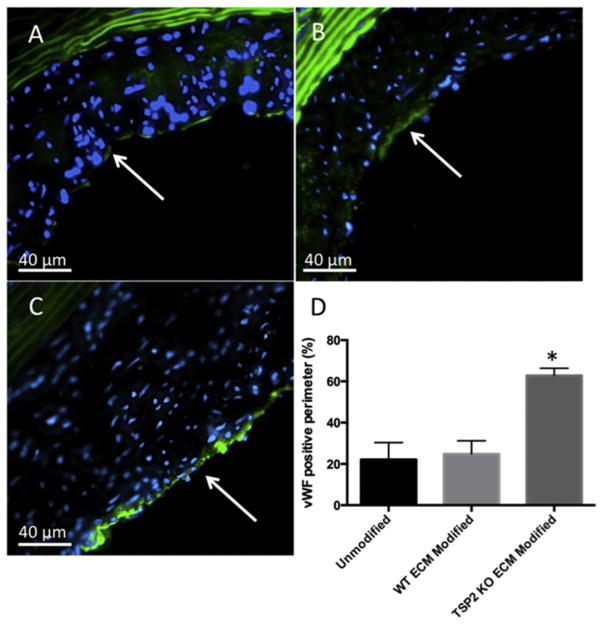
After explant at four weeks post-implantation, it was important to evaluate the host response to unmodified and ECM modified grafts. Because ECs play an important role in the hemostasis of healthy vessels, one measure of success is the extent of endothelial coverage of the graft lumen. To probe this metric, vWF was detected by immunohistochemical analysis of mid-graft sections of un-modified (Fig. 4A), WT ECM modified (Fig. 4B), and TSP2 KO ECM modified (Fig. 4C) grafts and the percentage of the lumen perimeter staining positive was quantified. The extent of endothelial-positive lumen was found to be higher in the grafts incorporating TSP2 KO ECM compared to control grafts (Fig. 4D). This result was also confirmed via immunohistochemistry for CD31 (Supplemental Fig. 4).
Fig. 4. TSP2 KO ECM modified grafts have increased endothelialization after 4 weeks in vivo.
Detection of vWF (green color) was performed after 4 weeks implantation on mid-graft sections of unmodified (A), WT ECM modified (B), and TSP2 KO ECM modified (C) grafts to visualize endothelial cells (Zeiss). (D) Quantification by Image J showed increased vWF-positive percentage of the lumen perimeter on TSP2 KO ECM modified grafts. n = 4, *p < 0.05. Arrows indicate luminal surface. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Increased cell recruitment to the media of TSP2 KO ECM modified grafts
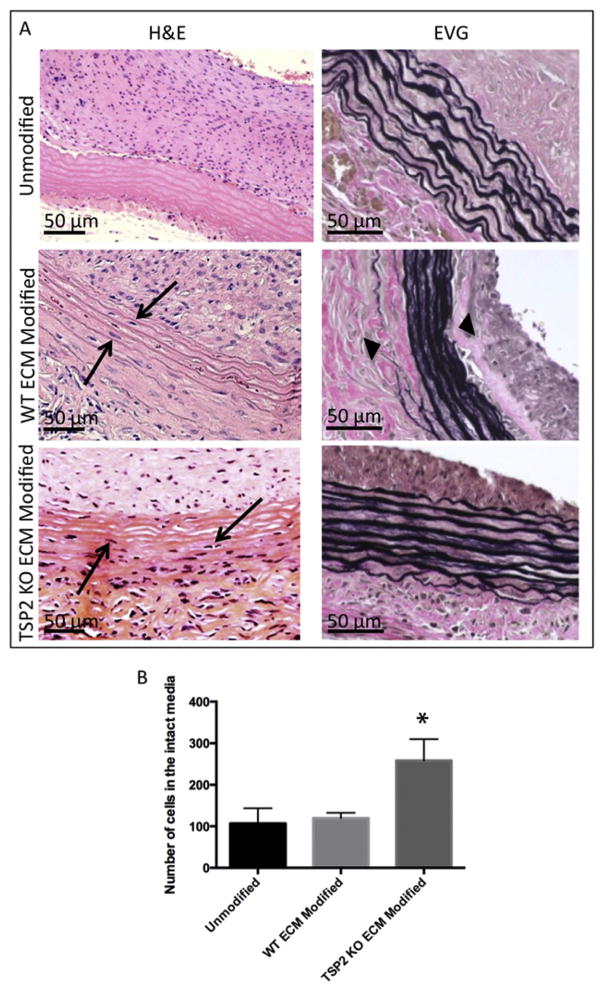
While endothelialization is important for hemostasis, ingrowth of cells into the graft media is also necessary for long-term stability and function. Mid-graft sections stained with H&E showed nuclei in the media of both ECM modified grafts, while few nuclei were found in the media of unmodified grafts (Fig. 5A). However, further staining with EVG showed that the outer laminae of WT ECM modified grafts were not intact, suggesting some graft damage (Fig. 5A). When cell recruitment to the intact media was quantified, differences in cellularization were only found to be significant in TSP2 KO ECM modified grafts (Fig. 5B).
Fig. 5. TSP2 KO ECM modified grafts have increased cell recruitment into the media after 4 weeks in vivo.
(A) H&E images of unmodified, WT ECM modified, and TSP2 KO ECM modified grafts reveal cell recruitment into the media of ECM-coated grafts (arrows). However, EVG images show elastic laminae of WT ECM modified grafts were found to be disrupted (arrowheads). (B) Quantification by Image J showed increased cell recruitment in the intact media in TSP2 KO ECM modified grafts. n = 6, *p < 0.05.
3.6. SMC-like cells are preferentially recruited to TSP2 KO ECM graft media
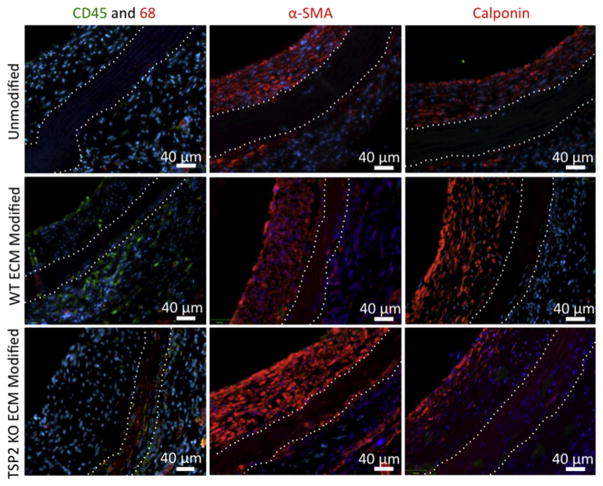
The increased cell recruitment to the media of the TSP2 KO ECM modified grafts was encouraging. However, it was important to determine whether the cell types present would support healthy graft remodeling. In order to accomplish this, sections of grafts were stained for SMC markers (α-SMA, calponin), as well as for immune cell markers (CD45 for lymphocytes, CD68 for macrophages). Immunofluorescence images showed that the majority of cells in the media of ECM modified vessels are SMC-like (α-SMA positive), which could be either SMCs or myofibroblasts. In addition, immune cell infiltration was present but to a lesser extent. Of note, grafts modified with WT ECM showed more pronounced CD45 immunoreactivity (Fig. 6).
Fig. 6. TSP2 KO ECM modified grafts have increased smooth muscle and immune cell infiltration into the media 4 weeks in vivo.
Representative images of immunohisto-chemistry (CD45, CD68, α-SMA, and calponin) show cells invading the media in ECM modified grafts are immune and smooth muscle cells (Zeiss). All sections were counterstained with DAPI for nuclei (blue color). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.7. Lower rates of failure in TSP2 KO ECM modified grafts
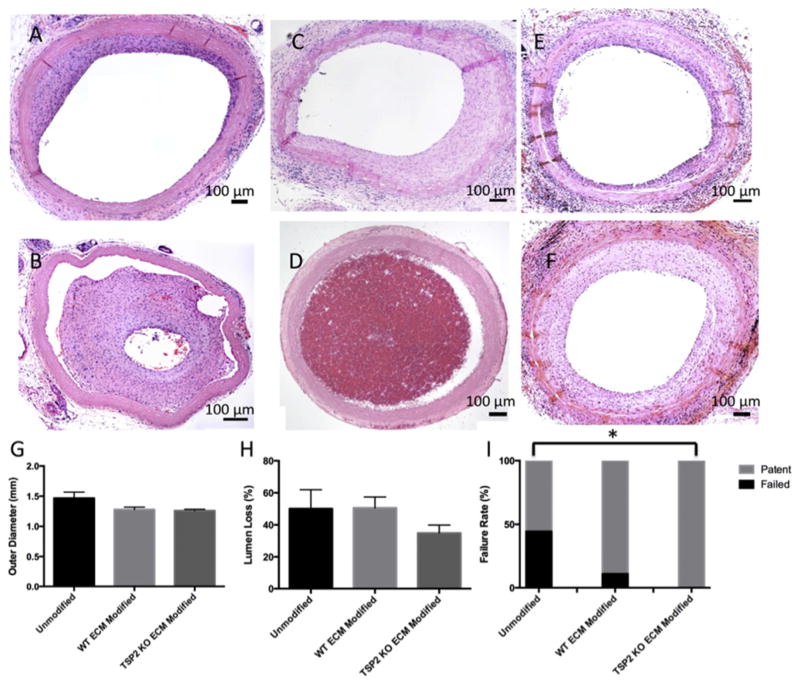
Gross histological evaluation of explants indicated a range of responses to unmodified (Fig. 7A and B), WT ECM modified (Fig. 7C and D), and TSP2 KO ECM modified (Fig. 7E and F) grafts. Image analysis of mid-graft sections showed that outer graft diameter did not vary among the groups (Fig. 7G). Moreover, outer graft diameter measured from grafts that were never implanted or only briefly implanted due to surgical failure ranged from 1.1 to 1.4 mm (not shown), which were similar to the diameters of the grafts explanted after 4 weeks. However, while the decrease in lumen loss was not significant (Fig. 7H), a decreased rate of failure was found in grafts incorporating TSP2 KO ECM compared to unmodified grafts (Fig. 7I).
Fig. 7. TSP2 KO ECM modified grafts have an improved patency rate at 4 weeks in vivo.
A range of representative H&E images of unmodified (A,B) WT ECM modified (C,D) and TSP2 KO ECM (E,F) modified grafts after 4 weeks implanted in rat aortas. Quantification of the outer diameters of the grafts using Image J revealed no significant differences among groups (G). Quantification of the percentage of the area occluded by tissue ingrowth using Image J showed a trend toward decreased percent occlusion in TSP2 KO ECM modified grafts (H). In addition, TSP2 KO ECM modified grafts showed a decreased rate of failure (I). n = 9, *p < 0.05.
4. Discussion
In the present study, we have utilized a novel surface enhancement composed of genetically modified whole ECM to improve the outcomes of implanted small diameter vascular grafts. We have shown that not only is it feasible to incorporate TSP2 KO ECM on a vascular graft, but also that it improved implantation outcomes when compared to unmodified and WT ECM modified counterparts. Specifically, we have shown that after 10 days in cell culture, TSP2 KO ECM had formed a non-thrombogenic surface on the lumen of the decellularized aorta, and that this reduced platelet adhesion and activation compared to control grafts. Importantly, mechanical analysis of ECM modified grafts revealed no differences between the groups, which suggests that exposure of grafts TSP2 KO cell culture and thus higher levels of MMPs does not significantly alter their mechanical properties.
The promising results of the in vitro studies prompted us to examine the effect of the TSP2 KO ECM modification on implantation outcomes in vivo. At 4 weeks post-implantation, sections of explanted grafts revealed increased luminal endothelial cell presence in grafts incorporating TSP2 KO ECM, as evidenced by immunofluorescence for vWF. This result is encouraging because increased EC presence in the graft correlates with increased hemocompatibility, as ECs function to inhibit coagulation in healthy vessels. The reason for this success may be twofold. In addition to our previous studies showing that TSP2 KO ECM is more permissive for EC migration, more recent studies have shown that ECM stiffness also effects EC phenotype. We have previously shown that TSP2 KO ECM has a stiffness of approximately 2 kPa [35,39]. Culture of ECs on matrices of similar compliances has been shown to increase elongation, promote the formation of tighter cell junctions, and increase nitric oxide production [45]. Therefore, it is possible that the incorporated ECM not only enhances EC migration, expediting the formation of a complete endothelium on the graft, but it may also promote a healthier EC phenotype. While we find our results encouraging, it has been shown that trans-anastomotic endothelialization is minimal in humans [46]. Therefore, further analysis in a more comparable model, such as a canine model, would be necessary to determine the true pro-migratory effects of TSP2 KO ECM on ECs. In addition, performing an isolation loop-graft study would elucidate the capacity of TSP2 KO ECM coated grafts to be endothelialized in a manner similar to that observed in humans.
While the formation of an endothelial lining is important for hemostasis, long-term outcomes of the grafts will also be affected by the recruitment of other cells, such as SMCs, to re-form an intact vascular media. The current work shows that cell recruitment to the media is increased in TSP2 KO ECM modified grafts compared to control grafts. Other researchers, while examining a fibronectin coating of a similar vascular graft noted that those grafts also saw significant cell ingrowth [9]. They proposed that fibronectin mediated this phenomenon by stimulating the expression of the integrin α5β1 and MMP-2 in SMCs, resulting in cell attraction to the site and in remodeling mediating cell migration, respectively [9]. Moreover, fibronectin has also been shown to increase expression of MMPs in fibroblasts, which also populate the vessel adventitia [47]. The ECM involved in this report contained enough fibronectin that it was used as an indicator of ECM incorporation. Thus it is reasonable to conclude that the fibronectin in the TSP2 KO ECM could be responsible for a response similar to the one previously reported. Additionally, because the TSP2 KO cell culture environment is known to contain higher MMP levels, this could precondition the grafts for infiltration more than coating with fibronectin or even WT ECM [44]. While the effects of TSP2 KO ECM thus far have been promising, it is important to consider the possibility that the addition of altered ECM could disrupt the balance between degradation and production of ECM by repopulating cells. Ongoing studies are examining the effect of TSP2 KO ECM on WT cells, as well as further elucidating the differences between TSP2 KO and WT ECM via proteomics.
Increased cell recruitment to the media of TSP2 KO ECM modified grafts suggests possible additional benefits. Indubitably, the majority of recruited cells were SMC-like, and ideally SMCs would populate the media in order to form a graft that resembles the native vasculature. In addition, it was found that some of the cells stained positive for immune cell markers, such as CD68. While these cells do not typically constitute a large portion of the media in healthy vasculature, there presence is not necessarily problematic. In fact, some researchers purposefully recruit CD68+ monocytes using bone marrow mononuclear cells or a stromal cell-derived factor 1α in grafts in order to take advantage of their ability to induce SMC and EC incorporation in the graft [48,49]. Therefore, it may be advantageous to have a certain level of immune cell recruitment to the graft.
Finally, the current work has shown that over-all outcomes are improved in grafts containing TSP2 KO ECM. TSP2 KO ECM modified grafts were found to be less likely to fail than unmodified grafts. In fact, the TSP2 KO ECM group was the only group in which no grafts failed. More qualitative analysis of the graft histology also revealed an interesting phenomenon – the neo-intima of grafts incorporating TSP2 KO ECM appeared to more circumferentially uniform, while the neo-intima of control grafts was often asymmetrically deposited around the graft lumen. A recent study has shown that early vein graft thrombi may serve as a niche for the establishment of SMCs in the media due to the rich pool of chemoattractants found there. Such niches may result in the thicker regions of SMC growth, and thus the occurrence of asymmetrical neointima [50]. Therefore, it is possible that the symmetry found in the TSP2 KO ECM modified grafts is a further indication that a non-thrombogenic surface is maintained in the early stages of implantation until endothelialization can occur.
While our focus has been on the rat model, we have also confirmed that our modification can be incorporated in potential vascular grafts from larger animals (pig, Supplemental Fig. 5). In addition, it is not unreasonable to expect that this kind of graft might be available from human sources. Other researchers have even begun to look at human umbilical cord and placenta for appropriately sized vessels for decellularization and subsequent implantation [16,17]. Taken together, the results of this study show that TSP2 KO ECM is a uniquely useful modification for small-diameter vascular grafts. This innovative use of a genetically modified ECM is a single strategy that could render experimental or commercially available small diameter grafts non-thrombogenic, and pro-migratory for ECs, all while facilitating cell infiltration of the graft media. In short, TSP2 KO ECM could allow for the production of off-the-shelf vascular grafts that approximate the properties of native vessels.
Supplementary Material
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.biomaterials.2017.06.025.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke Statistics-2016 update: a report from the american heart association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Cleary MA, Geiger E, Grady C, Best C, Naito Y, Breuer C. Vascular tissue engineering: the next generation. Trends Mol Med. 2012;18(7):394–404. doi: 10.1016/j.molmed.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Peck M, Gebhart D, Dusserre N, McAllister TN, L'Heureux N. The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs. 2012;195(1–2):144–158. doi: 10.1159/000331406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzpatrick LE, McDevitt TC. Cell-derived matrices for tissue engineering and regenerative medicine applications. Biomater Sci. 2015;3(1):12–24. doi: 10.1039/C4BM00246F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139(2):431–436. 436 e431–432. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 6.Lawson JH, Glickman MH, Ilzecki M, et al. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet. 2016;387(10032):2026–2034. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krawiec JT, Weinbaum JS, Liao HT, et al. In Vivo functional evaluation of tissue-engineered vascular grafts fabricated using human adipose-derived stem cells from high cardiovascular risk populations. Tissue Eng Part A. 2016;22(9–10):765–775. doi: 10.1089/ten.tea.2015.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heo Y, Shin YM, Lee YB, Lim YM, Shin H. Effect of immobilized collagen type IV on biological properties of endothelial cells for the enhanced endothelialization of synthetic vascular graft materials. Colloids Surf B Bio-interfaces. 2015;134:196–203. doi: 10.1016/j.colsurfb.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Assmann A, Delfs C, Munakata H, et al. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials. 2013;34(25):6015–6026. doi: 10.1016/j.biomaterials.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Kidd KR, Patula VB, Williams SK. Accelerated endothelialization of interpositional 1-mm vascular grafts. J Surg Res. 2003;113(2):234–242. doi: 10.1016/s0022-4804(03)00210-5. [DOI] [PubMed] [Google Scholar]
- 11.Lord MS, Yu W, Cheng B, Simmons A, Poole-Warren L, Whitelock JM. The modulation of platelet and endothelial cell adhesion to vascular graft materials by perlecan. Biomaterials. 2009;30(28):4898–4906. doi: 10.1016/j.biomaterials.2009.05.063. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Zhang K, Ma W, et al. Investigation of enhanced hemocompatibility and tissue compatibility associated with multi-functional coating based on hyaluronic acid and Type IV collagen. Regen Biomater. 2016;3(3):149–157. doi: 10.1093/rb/rbv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutiongco MF, Anderson DE, Hinds MT, Yim EK. In vitro and ex vivo hemocompatibility of off-the-shelf modified poly(vinyl alcohol) vascular grafts. Acta Biomater. 2015;25:97–108. doi: 10.1016/j.actbio.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancuso L, Gualerzi A, Boschetti F, Loy F, Cao G. Decellularized ovine arteries as small-diameter vascular grafts. Biomed Mater. 2014;9(4):045011. doi: 10.1088/1748-6041/9/4/045011. [DOI] [PubMed] [Google Scholar]
- 15.Dahan N, Zarbiv G, Sarig U, Karram T, Hoffman A, Machluf M. Porcine small diameter arterial extracellular matrix supports endothelium formation and media remodeling forming a promising vascular engineered biograft. Tissue Eng Part A. 2012;18(3–4):411–422. doi: 10.1089/ten.TEA.2011.0173. [DOI] [PubMed] [Google Scholar]
- 16.Gui L, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A. 2009;15(9):2665–2676. doi: 10.1089/ten.tea.2008.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider KH, Aigner P, Holnthoner W, et al. Decellularized human placenta chorion matrix as a favorable source of small-diameter vascular grafts. Acta Biomater. 2016;29:125–134. doi: 10.1016/j.actbio.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Xiong Y, Chan WY, Chua AW, et al. Decellularized porcine saphenous artery for small-diameter tissue-engineered conduit graft. Artif Organs. 2013;37(6):E74–E87. doi: 10.1111/aor.12014. [DOI] [PubMed] [Google Scholar]
- 19.Iijima M, Aubin H, Steinbrink M, et al. Bio-active coating of decellularized vascular grafts with a temperature-sensitive VEGF-conjugated hydrogel accelerates autologous endothelialization in vivo. J Tissue Eng Regen Med. 2016:1–12. doi: 10.1002/term.2321. http://dx.doi.org/10.1002/term.2321. [DOI] [PubMed]
- 20.Mahara A, Somekawa S, Kobayashi N, et al. Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Bio-materials. 2015;58:54–62. doi: 10.1016/j.biomaterials.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Smith RJ, Jr, Koobatian MT, Shahini A, Swartz DD, Andreadis ST. Capture of endothelial cells under flow using immobilized vascular endothelial growth factor. Biomaterials. 2015;51:303–312. doi: 10.1016/j.biomaterials.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boer U, Spengler C, Jonigk D, et al. Coating decellularized equine carotid arteries with CCN1 improves cellular repopulation, local biocompatibility, and immune response in sheep. Tissue Eng Part A. 2013;19(15–16):1829–1842. doi: 10.1089/ten.TEA.2012.0558. [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Lee K, Moon SH, et al. Mussel-inspired cell-adhesion peptide modification for enhanced endothelialization of decellularized blood vessels. Macromol Biosci. 2014;14(8):1181–1189. doi: 10.1002/mabi.201400052. [DOI] [PubMed] [Google Scholar]
- 24.Gong W, Lei D, Li S, et al. Hybrid small-diameter vascular grafts: anti-expansion effect of electrospun poly epsilon-caprolactone on heparin-coated decellularized matrices. Biomaterials. 2016;76:359–370. doi: 10.1016/j.biomaterials.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 25.Tao Y, Hu T, Wu Z, et al. Heparin nanomodification improves biocompatibility and biomechanical stability of decellularized vascular scaffolds. Int J Nanomedicine. 2012;7:5847–5858. doi: 10.2147/IJN.S37113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang B, Suen R, Wertheim JA, Ameer GA. Targeting heparin to collagen within extracellular matrix significantly reduces thrombogenicity and improves endothelialization of decellularized tissues. Biomacromolecules. 2016;17(12):3940–3948. doi: 10.1021/acs.biomac.6b01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai WW, Gu YJ, Wang XN, Chen CZ. Heparin coating of small-caliber decellularized xenografts reduces macrophage infiltration and intimal hyperplasia. Artif Organs. 2009;33(6):448–455. doi: 10.1111/j.1525-1594.2009.00748.x. [DOI] [PubMed] [Google Scholar]
- 28.Dimitrievska S, Cai C, Weyers A, et al. Click-coated, heparinized, decellularized vascular grafts. Acta Biomater. 2015;13:177–187. doi: 10.1016/j.actbio.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang B, Suen R, Wang JJ, Zhang ZJ, Wertheim JA, Ameer GA. Mechano-compatible polymer-extracellular-matrix composites for vascular tissue engineering. Adv Healthc Mater. 2016;5(13):1594–1605. doi: 10.1002/adhm.201501003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glynn JJ, Hinds MT. Bioactive anti-thrombotic modification of decellularized matrix for vascular applications. Adv Healthc Mater. 2016;5(12):1439–1446. doi: 10.1002/adhm.201600020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koobatian MT, Row S, Smith RJ, Jr, Koenigsknecht C, Andreadis ST, Swartz DD. Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials. 2016;76:344–358. doi: 10.1016/j.biomaterials.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Wang A, Tang Z, et al. The effect of stromal cell-derived factor-1alpha/heparin coating of biodegradable vascular grafts on the recruitment of both endothelial and smooth muscle progenitor cells for accelerated regeneration. Biomaterials. 2012;33(32):8062–8074. doi: 10.1016/j.biomaterials.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou M, Liu Z, Wei Z, et al. Development and validation of small-diameter vascular tissue from a decellularized scaffold coated with heparin and vascular endothelial growth factor. Artif Organs. 2009;33(3):230–239. doi: 10.1111/j.1525-1594.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 34.Conklin BS, Wu H, Lin PH, Lumsden AB, Chen C. Basic fibroblast growth factor coating and endothelial cell seeding of a decellularized heparin-coated vascular graft. Artif Organs. 2004;28(7):668–675. doi: 10.1111/j.1525-1594.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 35.Kristofik N, Calabro NE, Tian W, et al. Impaired von Willebrand factor adhesion and platelet response in thrombospondin-2 knockout mice. Blood. 2016;128(12):1642–1650. doi: 10.1182/blood-2016-03-702845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hui Chen DKS, Deane F, Mosher Properties of recombinant mouse thrombospondin 2 expressed in spodoptera cells. J Biol Chem. 1994;269(51):32226–32232. [PubMed] [Google Scholar]
- 37.Hui Chen DKS, Deane F, Mosher Metabolism of Thrombospondin 2: binding and Degradation by 3T3 cells and glycosaminoglycan-variant Chinese hamster ovary cells. J Biol Chem. 1996;271(27):15993–15999. doi: 10.1074/jbc.271.27.15993. [DOI] [PubMed] [Google Scholar]
- 38.Kyriakides TR, Zhu YH, Smith LT, et al. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140(2):419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krady MM, Zeng J, Yu J, et al. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am J Pathol. 2008;173(3):879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agah A, Kyriakides TR, Bornstein P. Proteolysis of cell-surface tissue trans-glutaminase by matrix Metalloproteinase-2 contributes to the adhesive defect and matrix abnormalities in thrombospondin-2-null fibroblasts and mice. Am J Pathol. 2005;167(1):81–88. doi: 10.1016/S0002-9440(10)62955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsimpoulas M, Morticelli L, Michalopoulos E, et al. Investigation of the biomechanical integrity of decellularized rat abdominal aorta. Transpl Proc. 2015;47(4):1228–1233. doi: 10.1016/j.transproceed.2014.11.061. [DOI] [PubMed] [Google Scholar]
- 42.Gui L, Dash BC, Luo J, et al. Implantable tissue-engineered blood vessels from human induced pluripotent stem cells. Biomaterials. 2016;102:120–129. doi: 10.1016/j.biomaterials.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee YU, Mahler N, Best CA, et al. Rational design of an improved tissue-engineered vascular graft: determining the optimal cell dose and incubation time. Regen Med. 2016;11(2):159–167. doi: 10.2217/rme.15.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhantao Yang TRK, bornstein Paul. Matricellular proteins as modulators of cell–matrix interactions: adhesive defect in thrombospondin 2- null fibro-blasts is a consequence of increased levels of matrix Metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohn JC, Zhou DW, Bordeleau F, et al. Cooperative effects of matrix stiffness and fluid shear stress on endothelial cell behavior. Biophys J. 2015;108(3):471–478. doi: 10.1016/j.bpj.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennel T, Zilla P, Bezuidenhout D. Differentiating transmural from trans-anastomotic prosthetic graft endothelialization through an isolation loop-graft model. J Vasc Surg. 2013;58(4):1053–1061. doi: 10.1016/j.jvs.2012.11.093. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Lin Z, Foolen J, et al. Disentangling the multifactorial contributions of fibronectin, collagen and cyclic strain on MMP expression and extracellular matrix remodeling by fibroblasts. Matrix Biol. 2014;40:62–72. doi: 10.1016/j.matbio.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A. 2010;107(10):4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muylaert DE, van Almen GC, Talacua H, et al. Early in-situ cellularization of a supramolecular vascular graft is modified by synthetic stromal cell-derived factor-1alpha derived peptides. Biomaterials. 2016;76:187–195. doi: 10.1016/j.biomaterials.2015.10.052. [DOI] [PubMed] [Google Scholar]
- 50.Blaas I, Heinz K, Wurtinger P, et al. Vein graft thrombi, a niche for smooth muscle cell colonization - a hypothesis to explain the asymmetry of intimal hyperplasia. J Thromb Haemost. 2016;14(5):1095–1104. doi: 10.1111/jth.13295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.