Cardiovascular disease (CVD) continues to be the leading cause of death and disability in the United States. Furthermore, over 40% of the US adult population is projected to have some form of CVD by 2030.1 Vascular smooth muscle cells (VSMCs) participate importantly in atherosclerosis, the major cause of myocardial infarction and stroke. MicroRNAs (miRNAs) are a class of small non-coding RNAs containing ~22 nucleotides. Studies using mouse models have shown that miRNAs are essential for cardiovascular development and function.2, 3 Furthermore, miRNAs are required for proliferation and differentiation of VSMCs during embryonic development 4 and for maintaining vascular contractile function, SMC contractile differentiation, and vascular remodeling in the postnatal stage.5 Numerous studies have linked altered miRNA expression to various diseases, indicating that miRNAs may play important roles in the pathogenesis of CVD.
In this issue of Circulation, Yang et al linked the function of miR-22 to VSMC phenotype switching. 6 They found that miR-22 expression is downregulated in different VSMC phenotype switching models, including injured femoral arteries (in vivo), explanted cultured thoracic aortic tissues (ex vivo) and late passages of cultured VSMCs (in vitro). The regulatory function of miR-22 in VSMC phenotype switching was further demonstrated via both gain- and loss-of-function experiments, in vitro and in vivo. miR-22 enhances the expression of VSMC marker genes and inhibits VSMC proliferation and migration without affecting VSMC apoptosis. Consistent with their prior report7, the authors identified Methyl CpG-binding Protein 2 (MECP2) as one of the downstream targets of miR-22 for regulating smooth muscle cell differentiation. Additionally, they linked the expression and function of HDAC4 (a histone deacetylase) and EVI1 (a transcriptional regulator and oncoprotein) to VSMC phenotype switching by showing that overexpression or knockdown of these miR-22 targets recapitulated miR-22-mediated regulation of VSMC phenotypes.
How do miR-22 and its downstream targets regulate the expression of genes related to SMC differentiation? Yang et al provided mechanistic insights and found that EVI1 binds to the promoter regions of several critical genes in VSMC phenotype switching, including SMαA, SM22α, SRF, and myocardin (Myocd), leading to H3K9me3 enrichment in these promoter regions, thereby regulating VSMC gene expression. Interestingly, EVI1 and MECP2, both transcriptional repressors, seem to share a similar molecular mechanism in regulating VSMC phenotype switching and smooth muscle cell differentiation by repressing the expression of VSMC differentiation genes. Together, this study uncovered an important regulatory function for miR-22 and its molecular mechanisms in VSMC. This study suggests that a miRNA may positively regulate gene expression by directly inhibiting the expression levels of transcriptional repressors and/or epigenetic silencers.
Probably one of the most exciting findings of the Yang study is the translational potential of miR-22 in vascular diseases. Using an arterial disease model of wire injury in mouse femoral arteries, they found that miR-22 agomiR (miRNA mimic) inhibits neointima formation in the injured arteries, while LNA-miR-22 (miRNA inhibitor) promotes neointimal hyperplasia. These in vivo data suggest that miR-22 promotes the switch from a proliferative, ‘synthetic’ state of VSMC to a contractile phenotype under pathological conditions. The functional significance of miR-22 in VSMC and arterial disease is further supported by the observation of decreased miR-22 expression, together with increased expression of its target genes, MECP2 and EVI1, in diseased human femoral arteries.
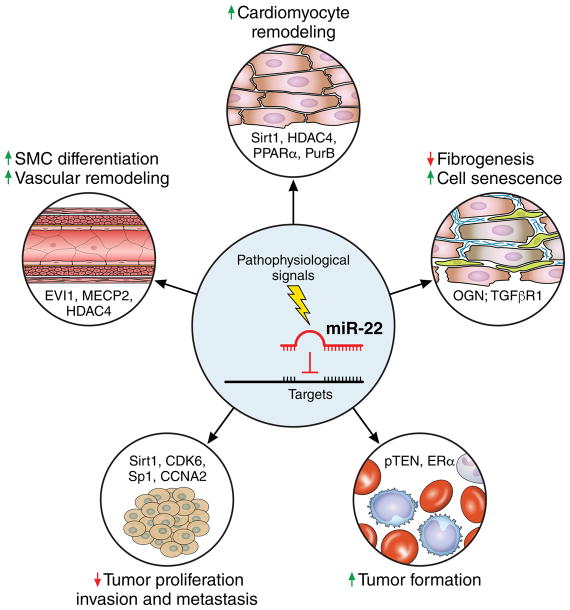
miR-22 has been shown to be ubiquitously expressed in multiple cell types and organs. In addition to playing a key role in VSMC remodeling, miR-22 is also an important regulator of cardiac function and remodeling (Figure). 8, 9 miR-22 null mice develop normally without displaying aberrant physiology under normal conditions. However, miR-22 null mice develop premature cardiac dilation and heart failure under cardiac stress conditions, indicating that miR-22 is indispensable for cardiac remodeling. This conclusion is further supported by studies conducted in cardiomyocyte-specific miR-22 knockout mice.8 Intriguingly, inhibition of miR-22 was reported to enhance fibrogenesis of cardiac fibroblasts, suggesting that miR-22 may also modulate cardiac function by regulating the function of non-cardiomyocytes in the heart. 10 Recently, miR-22 was demonstrated to be an important regulator of cardiac autophagy during aging.16 Inhibition of miR-22 after myocardial infarction activates cardiac autophagy and prevents post-infarction remodeling in old mice. Several target genes, including Sirt1, HDAC4, PPARα and PurB, are linked to the function of miR-22 in cardiomyocyte pathophysiology. 8, 9 These studies indicate that the primary function of miR-22 is involvement in the stress response and remodeling under pathophysiological conditions.
Figure.
miR-22 as a key regulator of cardiac and vascular remodeling, tumor suppressor or oncogene in a context- and target-dependent manner. Multiple miR-22 targets, including Sirt1, HDAC4, PPARα and PurB in cardiomyocytes8,9, EVI1, MECP2 and HDAC4 in SMCs7, OGN and TGFβR1 in cardiac fibroblasts10,11, Sirt1, CDK6, Sp1 and CCNA2 in most of cancer cells12,13, and pTEN and ERα in cancer cells of chronic lymphocytic leukemia and HBV-related liver cancer14,15, have been reported.
While the Yang et al study has provided compelling evidence to link the function of miR-22 to two downstream targets, MECP2 and EVI1, via regulation of histone methylation and consequent control of genes required for VSMC phenotype switching, additional investigation could further strengthen this functional and mechanistic connection. For example, it will be important to test whether restoring the expression of MECP2 and EVI1 is able to fully/partially correct the transcriptome and rescue the phenotype in miR-22 gain-of-function VSMCs. Furthermore, it remains unclear how MECP2 and EVI1 regulate histone methylation, especially H3K9me3, at VSMC gene promoters. An unbiased Chip-seq could be applied to investigate how miR-22 affects H3K9me3 in gene promoter regions genome-wide. Along with RNA-seq analysis, this will help us to better dissect the role of miR-22 in epigenetic regulation of gene expression in VSMC phenotype switching, which will allow a deeper understanding of how the specificity of epigenetic and transcriptional regulation is determined. Whole-genome transcriptome analyses in miR-22 overexpressed and knockdown VSMCs will provide an overview of how miR-22 alters gene expression and signaling pathways during VSMC remodeling. In the Yang et al study, a model of wire-induced injury in femoral arteries was used to define the function of miR-22 in vascular disease. Given that atherosclerosis is often caused by high cholesterol, mouse models with high cholesterol in the blood, such as ApoE mutant mice, will be essential to confirm the observations reported here.
Other than in the cardiovascular system, miR-22 has also been extensively studied in tumorigenesis.17 The expression and function of this miRNA in cancer is quite complicated. MiR-22 has been reported to be either a tumor suppressor or an oncomiR in different cancers/tumors (Figure). One of the main targets of miR-22 in tumorigenesis is pTEN, a repressor of the PI3K/AKT signaling pathway.14 Interestingly, pTEN is also a miR-22 target during cardiac hypertrophy.18 Studies have shown that pTEN plays an important role in VSMC biology by inhibiting cell proliferation and migration, which is similar to the function of miR-22 in VSMCs. Future studies are warranted to determine the relationship between miR-22 and pTEN in regulating VSMC phenotype switching. Although a single miRNA may exert different biological functions in different cell populations, miR-22 inhibits cell proliferation and migration in both VSMCs and cancer cells. Whether this miR-22 function is mediated by similar targets and/or signaling pathways in VSMCs and cancer cells remains to be answered.
The current study provides an attractive target, miR-22, for therapeutic application in coronary atherosclerosis. MiR-22-coated stent could potentially prevent in-stent restenosis, which is an unsolved problem for current clinical treatment. This strategy restricts miRNA to local smooth muscle cells and largely avoids potential side effects from non-specific uptake of miRNA into other cell populations/organs, since miR-22 can also serve as an oncogene. Future studies are warranted to demonstrate proof-of-concept evidence for this strategy and determine the lowest effective dosage for therapeutic application. In addition, unbiased approaches must be used to investigate transcriptome changes and the dynamic changes in histone modifications when miR-22 is overexpressed in VSMCs. These data will provide us a chance to more finely define the molecular mechanism of miR-22 in regulating VSMC phenotype switching and explore the potential side effects of miR-22 gain-of-function in VSMCs, which is a prerequisite for therapeutic application of miR-22 in CVD. With whole genome data, we could identify the “unwanted” dysregulated signaling pathways that mediate undesirable side effects of miR-22. Therefore, an improved strategy for the future could incorporate specific siRNAs to target “unwanted” signaling pathways, mitigating the side effects of miR-22 therapy without affecting its efficacy.
Acknowledgments
We thank members of the Wang laboratory for discussions.
Sources of Funding: Research was supported by the American Heart Association, Muscular Dystrophy Association and the NIH (HL085635, HL116919, HL125925) (Wang). Z-P Huang is supported by the Hundred Talents Program of Sun Yat-sen University and an American Heart Association Scientist Development Grant.
Footnotes
Disclosure: None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, Rojas M, Hammond SM, Schneider MD, Selzman CH, Meissner G, Patterson C, Hannon GJ, Wang DZ. Targeted deletion of dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Nat Acad Sci USA. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang ZP, Chen JF, Regan JN, Maguire CT, Tang RH, Dong XR, Majesky MW, Wang DZ. Loss of micrornas in neural crest leads to cardiovascular syndromes resembling human congenital heart defects. Arterioscler thromb vasc biol. 2010;30:2575–2586. doi: 10.1161/ATVBAHA.110.213306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. Micrornas are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler thromb vasc biol. 2010;30:1118–1126. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernandez-Hernando C, Offermanns S, Miano JM, Sessa WC. Smooth muscle mirnas are critical for post-natal regulation of blood pressure and vascular function. PloS one. 2011;6:e18869. doi: 10.1371/journal.pone.0018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang F, Chen Q, He S, Yang M, Maguire EM, An W, Afzal TA, Luong LA, Zhang L, Xiao Q. Mirna-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.027799. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, Wen G, Huang Y, Yu X, Chen Q, Afzal TA, Luong le A, Zhu J, Ye S, Zhang L, Xiao Q. Microrna-22 regulates smooth muscle cell differentiation from stem cells by targeting methyl cpg-binding protein 2. Arterioscler thromb vasc biol. 2015;35:918–929. doi: 10.1161/ATVBAHA.114.305212. [DOI] [PubMed] [Google Scholar]
- 8.Huang ZP, Chen J, Seok HY, Zhang Z, Kataoka M, Hu X, Wang DZ. Microrna-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ Res. 2013;112:1234–1243. doi: 10.1161/CIRCRESAHA.112.300682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurha P, Abreu-Goodger C, Wang T, Ramirez MO, Drumond AL, van Dongen S, Chen Y, Bartonicek N, Enright AJ, Lee B, Kelm RJ, Jr, Reddy AK, Taffet GE, Bradley A, Wehrens XH, Entman ML, Rodriguez A. Targeted deletion of microrna-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation. 2012;125:2751–2761. doi: 10.1161/CIRCULATIONAHA.111.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong Y, Cao H, Wang Q, Ye J, Sui L, Feng J, Cai X, Song H, Zhang X, Chen X. Mir-22 may suppress fibrogenesis by targeting tgfbetar i in cardiac fibroblasts. Cell physiol biochem. 2016;40:1345–1353. doi: 10.1159/000453187. [DOI] [PubMed] [Google Scholar]
- 11.Jazbutyte V, Fiedler J, Kneitz S, Galuppo P, Just A, Holzmann A, Bauersachs J, Thum T. Microrna-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age. 2013;35:747–762. doi: 10.1007/s11357-012-9407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi RU, Takata T, Shimamoto A, Ochiya T, Tahara H. Mir-22 represses cancer progression by inducing cellular senescence. J cell biol. 2011;193:409–424. doi: 10.1083/jcb.201010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, Hu Y, Liu HX, Wan YJ. Mir-22-silenced cyclin a expression in colon and liver cancer cells is regulated by bile acid receptor. J biolog chem. 2015;290:6507–6515. doi: 10.1074/jbc.M114.620369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palacios F, Abreu C, Prieto D, Morande P, Ruiz S, Fernandez-Calero T, Naya H, Libisch G, Robello C, Landoni AI, Gabus R, Dighiero G, Oppezzo P. Activation of the pi3k/akt pathway by microrna-22 results in cll b-cell proliferation. Leukemia. 2015;29:115–125. doi: 10.1038/leu.2014.158. [DOI] [PubMed] [Google Scholar]
- 15.Jiang R, Deng L, Zhao L, Li X, Zhang F, Xia Y, Gao Y, Wang X, Sun B. Mir-22 promotes hbv-related hepatocellular carcinoma development in males. Clin cancer res. 2011;17:5593–5603. doi: 10.1158/1078-0432.CCR-10-1734. [DOI] [PubMed] [Google Scholar]
- 16.Gupta SK, Foinquinos A, Thum S, Remke J, Zimmer K, Bauters C, de Groote P, Boon RA, de Windt LJ, Preissl S, Hein L, Batkai S, Pinet F, Thum T. Preclinical development of a microrna-based therapy for elderly patients with myocardial infarction. J Am Coll Cardiol. 2016;68:1557–1571. doi: 10.1016/j.jacc.2016.07.739. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Li Y, Ding M, Zhang H, Xu X, Tang J. Molecular mechanisms and clinical applications of mir-22 in regulating malignant progression in human cancer (review) Internat j oncol. 2017;50:345–355. doi: 10.3892/ijo.2016.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu XD, Song XW, Li Q, Wang GK, Jing Q, Qin YW. Attenuation of microrna-22 derepressed pten to effectively protect rat cardiomyocytes from hypertrophy. J cell physiol. 2012;227:1391–1398. doi: 10.1002/jcp.22852. [DOI] [PubMed] [Google Scholar]