Abstract
There is mounting evidence that prospective memory (PM) is impaired during HIV infection despite treatment. In this prospective study, 66 adults (43 HIV+ and 23 HIV negative) underwent PM assessment and cerebrospinal fluid (CSF) examination. HIV+ participants had significantly lower PM but significantly higher CSF concentrations of CXCL10 and quinolinic acid (QUIN). Higher CSF phosphorylated Tau (pTau) was associated with worse PM. In a secondary analysis excluding outliers, higher QUIN correlated with higher pTau. CSF QUIN is thus elevated during HIV infection despite antiretroviral therapy and could indirectly contribute to impaired PM by influencing the formation of pTau.
Keywords: Human Immunodeficiency Virus, Acquired Immunodeficiency Syndrome, Neurocognitive disorder, Cerebrospinal fluid, Tau proteins, Tryptophan
Graphical Abstract
1. Introduction
HIV-associated neurocognitive disorders (HAND) are persistently common in the combination antiretroviral (cART) era. Up to 40% of HIV+ adults with undetectable plasma HIV RNA levels and minimal neuropsychological comorbidities have at least mild neurocognitive impairment (Heaton et al., 2010). Biological evidence of ongoing neuronal damage during cART comes from magnetic resonance spectroscopy studies as well as studies of neuronal injury biomarkers such as neurofilament- light (Harezlak et al., 2011, Jessen Krut et al., 2014). Yet, the underlying pathogenesis of neuronal damage during cART remains poorly understood.
While inflammation persists during treated HIV and may be a cause of neuronal damage (Zayyad and Spudich, 2015), other processes could also contribute to HAND pathogenesis. Quinolinic acid (QUIN), for example, is a neurotoxin that is a product of the kynurenine pathway for tryptophan metabolism (Vecsei et al., 2013). CSF QUIN levels are elevated during HIV-associated dementia in the absence of cART (Heyes et al., 1991). The ratio of kynurenine to tryptophan (known at the K/T ratio) reflects increased activity of the kynurenine pathway and is associated with HIV-associated mortality when measured from the blood (Byakwaga et al., 2014). Similarly, the CSF Q/T ratio was found to be the earliest predictor of neurological disease in untreated simian immunodeficiency virus (SIV)- infected macaques, and kynurenine pathway metabolites in the brain do not normalize in the SIV-infected brain despite cART (Drewes et al., 2015).
There are also lingering questions as to whether HAND has any pathogenic similarities with Alzheimer’s disease (AD), the most common dementia worldwide. The tau protein, which is critical for the stabilization of microtubules and neuronal integrity, is hyperphosphorylated in AD and other dementias (Brunden et al., 2009). Several studies have attempted to determine whether HIV is also associated with abnormal Tau levels and might represent a “Tauopathy”. Studies in the pre-cART era focusing on total Tau (t-Tau) were small, and the results were conflicting (Andersson et al., 1999, Green et al., 2000). More recent studies have focused on phosphorylated Tau (pTau), which is more specific for Tauopathy-associated dementias. Again, some studies found a relationship between increased CSF pTau and HAND (Brew et al., 2005), while others did not (Clifford et al., 2009, Krut et al., 2016, Peterson et al., 2014).
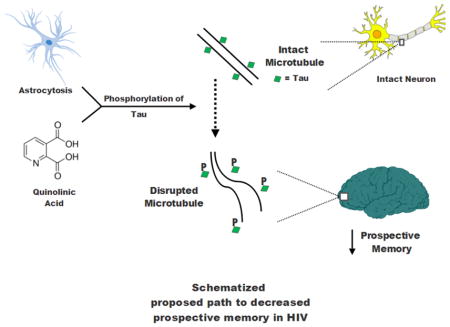
A study published in 2006 from the HIV Neurobehavioral Research Program (HNRP) at the University of California at San Diego focused on the relationship between t-Tau and the specific cognitive domain of prospective memory (PM) (Woods et al., 2006). PM relates to the execution of a future intention, also known as “remembering to remember”. Diminished PM is common among persons with HIV disease and is strongly associated with dependence in a wide range of activities of daily living in both older adults and in HIV-infected individuals (Woods et al., 2008a, Woods et al., 2012). The earlier HRNP study demonstrated that CSF t-Tau levels correlated inversely with PM. In the current study, we examined the relationship between PM and the more specific pTau in a new cohort of HIV-infected individuals. QUIN has been shown to induce the phosphorylation of Tau in vitro and co-localizes with hyperphosphorylated Tau in cortical neurons of the brain during AD (Rahman et al., 2009). Therefore, we also measured CSF kynurenine metabolites including QUIN, as well as other selected markers that have been associated with HIV and HAND.
2. Methods
2.1 Assessment of Participants
A cohort of HIV-infected (HIV+) and HIV-uninfected (HIVnegative) adults were prospectively recruited at the HNRP. Exclusion criteria were: 1) Positive urine drug screen (except cannabis) or breath test for alcohol; 2) Current drug or alcohol dependence within the past 30 days as determined by the Composite International Diagnostic Interview (CIDI version 2.1) (WorldHealthOrganization, 1998) using DSM-IV-TR criteria (AmericanPsychiatricAssociation, 1994); 3) A diagnosis of schizophrenia, psychosis, or clinically significant neurological disease including seizures and traumatic brain injury with loss of consciousness >15 minutes; or 4) A verbal intelligence quotient (IQ) estimate ≤70 on the Wechsler Test of Adult Reading (WTAR; Psychological Corporation, 2001). Only HIV+ participants who had virologic control on cART with paired plasma and CSF HIV RNA levels < 50 copies/ml were included for the current study. The study was approved by the Institutional Review Board and informed consent was obtained from all participants.
The Memory for Intentions Screening Test (MIST) (Woods et al., 2008b), a standardized 30-minute assessment composed of 8 PM items, was administered to all participants. The MIST contains equally balanced PM items that use a delay of either 2 minutes or 15 minutes. Cues were either time-based (e.g., “In 2 minutes, ask me what time this session ends.”) or event-based (e.g., “When I hand you a postcard, self-address it.”) Response modalities were either verbal (e.g., “Tell me the following”) or physical (e.g., “Perform the following action”). A series of word search puzzles served as ongoing distraction tasks that separated PM trials. Raw MIST summary scores were used as the primary PM criterion in all statistical analyses (range = 0 – 48, with higher scores reflecting better PM performance).
Participants also underwent comprehensive neuropsychological testing for the assessment of HAND according to Frascati criteria (Antinori et al., 2007). The following neurocognitive domains were assessed:
Retrospective memory with a) Long-delay discriminability index of the California Verbal Learning Test–Second Edition (CVLT–II; Delis, Kramer, Kaplan, and Ober, 2000) (Delis et al., 2000) and b) Logical Memory II subtest of the Wechsler Adult Intelligence Scale-Memory Scales–III (Wechsler, 1997).
Attention/working memory with a) Digit Span subtest of the Wechsler Adult Intelligence Scale–Third Edition and b) Trial 1 from the CVLT–II;
Executive function with a) total move score from the Tower of London–Drexel test (Culbertson and Zillmer, 1998) and b) Trail Making Test (TMT) Part B; (Reitan and Wolfson, 1985)
Speed of information processing with a) Digit Symbol subtest of the WAIS–III and b) TMT Part A;
Learning with a) CVLT–II Trials 1–5 total and b) Logical Memory I (LM–I);
Verbal fluency with the Action Fluency test (Woods et al., 2005); and
Motor skill with Grooved Pegboard Test (Klove, 1963).
Normative values that accounted for age, sex, race, and educational level were used to generate Z scores for each domain. A global clinical rating score was generated from results across these seven domains with a range of 1 (above average) to 9 (severely impaired). Global clinical rating scores ≥5 identified neurocognitive impairment and were used in HAND diagnosis (Woods et al., 2004).
2.2 Laboratory testing
HIV RNA levels from plasma and CSF were measured with a commercial assay (Roche Amplicor v.1.5 with lower limit of detection 50 copies/ml). CSF biomarkers of inflammation and astrocytosis that have been associated with HAND were also measured. These included: CXCL10, an interferon- induced chemokine shown to promote HIV replication in lymphocytes and macrophages (Liu et al., 2011); CCL2, a chemokine responsible for monocyte migration (Dhillon et al., 2008), and s100β, a protein expressed by astrocytes that has an autocrine effect of astrocyte apoptosis (Sen and Belli, 2007). CXCL10 was measured via electrochemiluminescence (Mesoscale Discovery). pTau was measured by Luminex bead array (Invitrogen). S100β was measured by enzyme linked immunosorbent assay (Diasorin). Tryptophan (TRP), Kynurenine (KYN), Picolinic acid (PIC), and QUIN were measured with methods previously described (Jones et al., 2015, Lim et al., 2017). Briefly, TRP and KYN were measured using ultra high performance liquid chromatography (UHPLC), while QUIN and PIC were measured using gas chromatography- mass spectrometry (GC-MS).
2.3 Statistical analyses
For the statistical analysis, variable distributions were inspected for skewness and outliers. As done in previous studies by our group and others, skewness was reduced by natural log transformation to enable use of parametric tests (Anderson et al., 2015, Jessen Krut et al., 2014). Comparisons of HIV+ and negative subjects on continuous variables were performed using t-tests. Biomarker comparisons were made without and with adjustment for significant imbalances in demographic profiles of the two groups using linear regression. Comparisons for categorical variables were performed using chi-square tests. Transformation did not substantially improve the distribution of CSF red blood cell (RBC) count so the non-parametric Wilcoxon rank sum test was used to test differences between HIV+ and negative groups. Pearson correlation coefficients were used to test associations between CSF biomarkers and PM as well as CSF biomarkers and pTau. Linear regression was also performed to compare PM and pTau as dependent variables to independent variables that were statistically significant in univariate correlations. We hypothesized that there would be significant relationships between between QUIN, pTau, and PM. Comparisons were two-tailed and alpha for statistical significance was set at <0.05.
3. Results
3.1 Neuropsychological and biomarker results
A total of 66 participants were assessed (43 HIV+ and 23 HIV-negative, see Table 1 for demographic/disease characteristics). HIV+ participants were older and more likely to be men. HIV+ participants were infected for a median of 15 years, median current CD4+ T cell count was 559 cells/μL, and median nadir CD4+ T-cell count was 199 cells/μL. The median duration of the current cART regimen was 17 months and median central nervous system penetration (CPE) score was 7 (interquartile range 6–9). The most commonly prescribed cART regimens were: efavirenz/tenofovir/emtricitabine (n=5), ritonavir-boosted atazanavir/tenofovir/emtricitabine (n=5), and ritonavir-boosted lopinavir/tenofovir/emtricitabine (n=4). There was no significant difference in CSF RBC count between HIV+ and negative groups (median RBC 6 versus 2 respectively, p=0.8). The proportion of participants with neurocognitive impairment was not significantly different between groups (27.9% versus 17.4%, p=0.34). However, the MIST summary score was significantly lower in the HIV+ group (mean 39.8 in HIV+ versus 43.2 in HIV-negative, p=0.039). Mean time-based (6.07 versus 6.78, p= 0.062) and event-based (7.21 versus 7.61, p= 0.12) PM subscores also tended to be lower in HIV+ participants.
Table 1.
Results are reported as either mean (standard deviation) or number (percent), NA= Not applicable; PM= Prospective Memory; K= Kynurenine; T= Tryptophan Biomarkers are reported as natural log transformed values
| Variable | HIV + (n=43) | HIV− (n=23) | 2 group p-value | Adjusted p-value* |
|---|---|---|---|---|
| Age (Years) | 46.1 (9.1) | 37.1 (12.0) | 0.003 | NA |
| Sex (Male) | 37 (86.0%) | 11 (47.8%) | 0.001 | NA |
| Race (White) | 31 (72.1%) | 11 (47.8%) | 0.051 | NA |
| PM summary score | 39.8 (6.2) | 43.2 (6.1) | 0.039 | 0.412 |
| CSF biomarkers | ||||
| CXCL10 | 6.41 (0.50) | 5.46 (0.53) | < 0.001 | < 0.001 |
| S100β | −0.09 (0.60) | −0.12 (0.41) | 0.859 | 0.781 |
| CCL2 | 6.31 (0.36) | 6.21 (0.31) | 0.290 | 0.582 |
| pTau | 3.93 (0.53) | 3.53 (0.55) | 0.004 | 0.086 |
| Tryptophan | 0.52 (0.40) | 0.69 (0.24) | 0.070 | 0.041 |
| Kynurenine | −2.07 (0.95) | −2.79(0.89) | 0.004 | 0.058 |
| Picolinic Acid | 3.96 (0.86) | 4.43 (0.44) | 0.004 | 0.039 |
| Quinolinic Acid | 3.40 (0.86) | 2.83 (0.43) | 0.001 | 0.025 |
| K/T ratio | −2.58 (1.07) | −3.48 (0.90) | 0.001 | 0.016 |
Denotes linear regression adjusted values based on imbalances in age, gender, and race.
Several CSF biomarkers differed between HIV+ and HIV-negative groups, the majority of which persisted in adjusted analyses (Table 1). CXCL10 and QUIN were significantly higher in HIV+ participants (Figures 1a and 1b). CSF pTau was also higher in the HIV+ group in univariate analysis but this difference weakened in multivariate analysis. In contrast, CSF levels of TRP and PIC were lower in HIV+ participants. As a result, CSF KYN/TRP ratio was nearly one log higher in HIV+ participants (Figure 1c).
Figure 1.
Biomarker values are natural log transformed. Horizontal line= median. QUIN= Quinolinic Acid. K= Kynurenine. T= Tryptophan
3.2 Outcome variable: Prospective memory
Correlations between CSF biomarkers and PM for the entire cohort are shown in Table 2. PM correlated negatively with both pTau (r= − 0.357, p= 0.003), and CCL2 (r= − 0.280, p= 0.024). Additionally, both time-based and event-based PM sub-scores correlated negatively with pTau (r= − 0.302, p= 0.014 and r= − 0.307, p= 0.012 respectively). The only significant marker in the multiple linear regression model with PM as the outcome variable (R2 = 0.17, p=0.0032) was pTau (T ratio= −2.61, p= 0.011).
Table 2.
Correlations between CSF markers and prospective memory (PM) Sorted in descending order of correlation strength
| Domain | Biomarker | Correlation(r) for n=66 | P value |
|---|---|---|---|
| PM | pTau | −0.357 | 0.003* |
| PM | CCL2 | −0.280 | 0.024* |
| PM | CXCL10 | −0.175 | 0.160 |
| PM | s100B | −0.156 | 0.210 |
| PM | QUIN | 0.072 | 0.568 |
Correlations with p value < 0.05 denoted by *
Quin= quinolinic acid; pTau= phosphorylated Tau
(See text for multivariate model results for PM)
3.3 Outcome variable: pTau
Analyses comparing QUIN to pTau identified 4 substantial outliers (Figure 2). The four outliers were all HIV+ and had better PM than other HIV+ participants (p=0.0037) but did not differ in other demographic or disease characteristics. They did tend to be more likely to take abacavir (50.0% vs. 10.3%, p=0.06) but not other cART drugs, suggesting that abacavir may alter the relationship between QUIN and pTau. For this reason, stratified analyses were performed with: 1) the entire cohort (n=66) and 2) excluding these 4 outliers (n=62). For the entire cohort, pTau correlated with s100β (r= 0.359, p= 0.003) and CXCL10 (r= 0.307, p=0.012) (Table 3). Without the 4 outliers, these two correlations remained statistically significant and the correlation between pTau and QUIN substantially strengthened (r= 0.381, p= 0.002, see Table 3 and Figures 2 and 3). For correlates of pTau, multiple linear regression for the entire cohort showed a significant association with s100β (t ratio= 2.79, p= 0.007 in Table 4) which remained significant when the outliers were excluded. This regression analysis of the subgroup also showed that the relationship between QUIN and pTau strengthened to near statistical significance (t ratio= 1.86, p= 0.068).
Figure 2.
Positive correlation between QUIN and pTau, with and without QUIN outliers. Asterisks represent the four outliers. Blue area represents regression line and 95% confidence interval without QUIN outliers.
Table 3.
Correlations between CSF markers and phosphorylated Tau (pTau) Groups are n=66 (includes QUIN outliers) and n=62 (excludes QUIN outliers) Sorted in descending order of correlation strength
| Biomarker | Biomarker | Correlation(r) for n=66 | P value | Correlation(r) for n=62 | P value |
|---|---|---|---|---|---|
| pTau | s100B | 0.359 | 0.003* | 0.295 | 0.020* |
| pTau | CXCL-10 | 0.307 | 0.012* | 0.388 | 0.002* |
| pTau | CCL-2 | 0.223 | 0.074 | 0.226 | 0.080 |
| pTau | QUIN | 0.104 | 0.41 | 0.381 | 0.0023* |
QUIN= quinolinic acid
Correlations with p value < 0.05 denoted by *; Correlation in bold becomes <0.05 after exclusion of QUIN outliers
Figure 3.
Significant univariate correlations between CSF biomarkers and phosphorylated Tau (pTau) in group (n=62) that excludes QUIN outliers.
Green arrows denote positive relationships QUIN= quinolinic acid
Table 4.
Linear regression table for best model explaining phosphorylated (pTau) for n=66 and n=62 groups when including biomarkers from table 3 with p<0.05
| Biomarker (n=66) | T ratio | P Value | Biomarker (n=62) | T ratio | P Value |
|---|---|---|---|---|---|
| S100β | 2.79 | 0.007 | S100β | 2.06 | 0.044 |
| CXCL10 | 1.92 | 0.060 | QUIN | 1.86 | 0.068 |
| QUIN | 0.06 | 0.822 | CXCL10 | 1.52 | 0.134 |
QUIN= quinolinic acid
R2= 0.20, p= 0.0034 for n=66 and R2= 0.21, p= 0.0008 for n= 62
4. Discussion and conclusions
HAND in the cART era continues to be common (Heaton et al., 2010). However, it is not clear if all markers that were associated with HAND in the pre-cART era remain pertinent in the cART era. We acknowledge that the HIV+ and HIV-negative groups were not well matched in this study (particularly with regards to sex and age). This may have influenced the finding that biomarker concentrations differed between the two groups. For example, differences in cytokine secretion have been observed based on sex in previous studies involving ex vivo models of sepsis (Asai et al., 2001). If the groups were better matched in our study, then multivariate analyses may not have been needed. The HIV-infected participants reflect the HIV clinic population at UCSD, which is mostly white and male. While some key demographics differed between the groups, CXCL10 and QUIN remained higher in the HIV+ group even after adjusting for these differences. Blood levels of CXCL10 have previously been found to be elevated during treated HIV infection (Wada et al., 2015), and the current study demonstrates that this elevation also exists in the central nervous system (CNS). The kynurenine/tryptophan (K/T) ratio, a reflection of indoleamine- 2,3-dioxygenase (IDO) activity, has recently been linked to poor CD4+ recovery and mortality during cART (Byakwaga et al., 2014). The current analysis suggests that this pathway is also upregulated in the CNS during HIV infection despite virologic suppression. QUIN is a product of this pathway and is a known neurotoxin (Vecsei et al., 2013). Therefore the finding of higher QUIN levels despite complete virologic suppression is important. The contrast of higher CSF QUIN and lower CSF PIC found in this study suggests that the non-enzymatic conversion of 2-amino-3-carboxymuconate-semialdehyde, a late stage catabolite of the kynurenine pathway, predominates in the CNS during HIV. This is the likely cause of higher QUIN, which given its neurotoxic properties could contribute to neuronal injury and neuropsychological impairment. While our multivariate analyses confirmed several biomarker concentration differences between HIV+ and negative groups, a study in which participants were matched for demographics may have been more conclusive. Future investigations should incorporate matching for age and sex.
The role of phosphorylated Tau (pTau) in HAND has been the subject of controversy. A recent study found no significant difference in CSF pTau concentration between individuals with HIV on cART and HIV seronegative controls (Krut et al., 2017). CSF pTau was significantly higher in the HIV+ group in our study, but this difference became statistically non-significant after adjustment for demographics. Strict matching may be needed to more definitively determine differences in pTau between HIV+ individuals on cART and HIV-negative individuals. We found that multiple markers were associated with elevated pTau, which suggests that the formation of this abnormal protein may result from multiple processes in the CNS during HIV (see graphical abstract). In the multivariate model, s100β (a marker of astrocytosis) was significantly associated with pTau, while there was a strong association trend between QUIN and pTau. These findings might be explained by evidence that QUIN can induce phosphorylation of Tau (Rahman et al., 2009), and that astrocytosis may contribute to tauopathies (Leyns and Holtzman, 2017). However, given that our analysis included HIV-negative participants, further research will be needed to more definitively evaluate whether s100β and QUIN are independently associated with pTau in a cohort limited to HIV+ individuals.
Prospective memory (PM) has been consistently linked to problems with activities of daily living during HIV (Woods et al., 2008b). Previous studies by our group showed that PM is diminished in HIV+ individuals with mixed profiles of virologic control (Carey et al., 2006, Morgan et al., 2012). The current analysis shows that PM is significantly worse in HIV+ adults despite virologic suppression in both plasma and CSF during cART. However, we acknowledge that the difference between the two groups became less significant in multivariate analysis. There is a reliable effect of age on prospective memory (PM) in the general population, such that older adults show worse PM than younger adults. (Henry et al., 2004) In the setting of HIV disease, we tend to see additive effects of HIV and aging on PM as measured in the laboratory such that HIV-associated PM deficits are exaggerated in older versus younger samples. (Avci et al., 2016, Woods et al., 2010) In the current data, we see a similar effect, whereby we observe a large, significant adverse effect of HIV on our summary measure of PM in the older adults (using a median split) (p =0.003, Cohen’s d = −1.1). In our younger adults, HIV is associated with a small, non-significant effect (p =0.51, Cohen’s d = −0.2). There is no reliable evidence that sex affects PM in either healthy samples or in the setting of HIV disease. For example, recent data from our laboratory using a large HIV+ cohort found no significant effects of sex on the summary measure of PM used in this study. (Avci et al., 2017) Similarly, in the present study, sex was not significantly associated with PM in either the HIV− or HIV+ groups (p values > 0.05).
Larger studies are indicated to determine the overall prevalence of HIV-associated PM impairment during effective cART and to examine its relationship to other clinical outcome measures such as mortality. Lastly, the finding of a significant relationship between higher pTau and decreased PM in multivariate analysis indicates that this protein may have a detrimental role with regards to this particular cognitive domain. Again, a study limited to HIV-infected individuals will be needed to show an HIV-specific effect. While we found that pTau was the only marker that was significantly associated with diminished PM, we did not measure all markers that have been associated with HAND. It is possible that the incorporation of other HAND-associated markers would have resulted in a weaker association between pTau and PM. While we acknowledge that the relationship between higher pTau and lower PM does not prove causation, the results justify more research into all domains of cognition during HIV and the factors that may influence them.
Highlights.
Study of prospective memory (PM) and cerebrospinal fluid (CSF) during HIV infection
Prospective memory was diminished in HIV+ compared to HIV negative
CSF levels of CXCL10 and quinolinic acid (QUIN) were higher in HIV+ participants
Higher phosphorylated Tau was associated with worse PM
Secondary analysis showed that higher CSF QUIN was associated with higher pTau
Acknowledgments
Funding sources:
NIH K23MH095679
NIH K24MH097673
NIH R01MH073419
Footnotes
Conflicts of interest:
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- unaids.org. Nov, 2016. UNAIDS fact sheet. [Google Scholar]
- AmericanPsychiatricAssociation. Diagnostic and statistical manual of mental disorders. 1994. [Google Scholar]
- Anderson AM, Fennema-Notestine C, Umlauf A, Taylor MJ, Clifford DB, Marra CM, et al. CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. Journal of neurovirology. 2015;21:559–67. doi: 10.1007/s13365-015-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L, Blennow K, Fuchs D, Svennerholm B, Gisslen M. Increased cerebrospinal fluid protein tau concentration in neuro-AIDS. Journal of the neurological sciences. 1999;171:92–6. doi: 10.1016/s0022-510x(99)00253-1. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 2001;16:340–3. doi: 10.1097/00024382-200116050-00003. [DOI] [PubMed] [Google Scholar]
- Avci G, Loft S, Sheppard DP, Woods SP Group HIVNRP. The effects of HIV disease and older age on laboratory-based, naturalistic, and self-perceived symptoms of prospective memory: does retrieval cue type and delay interval matter? Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2016;23:716–43. doi: 10.1080/13825585.2016.1161001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci G, Sheppard DP, Tierney SM, Kordovski VM, Sullivan KL, Woods SP. A systematic review of prospective memory in HIV disease: from the laboratory to daily life. Clin Neuropsychol. 2017:1–33. doi: 10.1080/13854046.2017.1373860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–2. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Trojanowski JQ, Lee VM. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nat Rev Drug Discov. 2009;8:783–93. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byakwaga H, Boum Y, 2nd, Huang Y, Muzoora C, Kembabazi A, Weiser SD, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. The Journal of infectious diseases. 2014;210:383–91. doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I Group HIVNRC. Prospective memory in HIV-1 infection. Journal of clinical and experimental neuropsychology. 2006;28:536–48. doi: 10.1080/13803390590949494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–7. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. The Tower of London (DX): a standardized approach to assessing executive functioning in children. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 1998;13:285–301. [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test. 2. 2000. [Google Scholar]
- Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP -1 in development of HIV-dementia. Front Biosci. 2008;13:3913–8. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes JL, Meulendyke KA, Liao Z, Witwer KW, Gama L, Ubaida-Mohien C, et al. Quinolinic acid/tryptophan ratios predict neurological disease in SIV-infected macaques and remain elevated in the brain under cART. Journal of neurovirology. 2015;21:449–63. doi: 10.1007/s13365-015-0334-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AJ, Giovannoni G, Hall-Craggs MA, Thompson EJ, Miller RF. Cerebrospinal fluid tau concentrations in HIV infected patients with suspected neurological disease. Sex Transm Infect. 2000;76:443–6. doi: 10.1136/sti.76.6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. Aids. 2011;25:625–33. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychol Aging. 2004;19:27–39. doi: 10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, et al. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Annals of neurology. 1991;29:202–9. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, et al. Biomarker evidence of axonal injury in neuroasymptomatic HIV-1 patients. PloS one. 2014;9:e88591. doi: 10.1371/journal.pone.0088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J, et al. Expression of the Kynurenine Pathway in Human Peripheral Blood Mononuclear Cells: Implications for Inflammatory and Neurodegenerative Disease. PloS one. 2015;10:e0131389. doi: 10.1371/journal.pone.0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klove H. Clinical neuropsychology. In: Forster FM, editor. Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- Krut JJ, Price RW, Zetterberg H, Fuchs D, Hagberg L, Yilmaz A, et al. No support for premature central nervous system aging in HIV-1 when measured by cerebrospinal fluid phosphorylated tau (p-tau) Virulence. 2016:1–6. doi: 10.1080/21505594.2016.1212155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krut JJ, Price RW, Zetterberg H, Fuchs D, Hagberg L, Yilmaz A, et al. No support for premature central nervous system aging in HIV-1 when measured by cerebrospinal fluid phosphorylated tau (p-tau) Virulence. 2017;8:599–604. doi: 10.1080/21505594.2016.1212155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns CEG, Holtzman DM. Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener. 2017;12:50. doi: 10.1186/s13024-017-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CK, Bilgin A, Lovejoy DB, Tan V, Bustamante S, Taylor BV, et al. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep. 2017;7:41473. doi: 10.1038/srep41473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine & growth factor reviews. 2011;22:121–30. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Weber E, Rooney AS, Grant I, Woods SP Hiv Neurobehavioral Research Program Hnrp G. Longer ongoing task delay intervals exacerbate prospective memory deficits in HIV-associated neurocognitive disorders (HAND) Journal of clinical and experimental neuropsychology. 2012;34:416–27. doi: 10.1080/13803395.2012.654764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J, Gisslen M, Zetterberg H, Fuchs D, Shacklett BL, Hagberg L, et al. Cerebrospinal fluid (CSF) neuronal biomarkers across the spectrum of HIV infection: hierarchy of injury and detection. PloS one. 2014;9:e116081. doi: 10.1371/journal.pone.0116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PloS one. 2009;4:e6344. doi: 10.1371/journal.pone.0006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead–Reitan Neuropsycholgical Test Battery: Therapy and clinical interpretation. 1985 [Google Scholar]
- Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci Res. 2007;85:1373–80. doi: 10.1002/jnr.21211. [DOI] [PubMed] [Google Scholar]
- Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. Aids. 2015;29:463–71. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3. New York: The Psychological Corporation; 1997. Manual. [Google Scholar]
- Woods SP, Dawson MS, Weber E, Grant I Group HIVNRC. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. Journal of clinical and experimental neuropsychology. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I, et al. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008a;22:110–7. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moran LM, Dawson MS, Carey CL, Grant I Group HIVNRC. Psychometric characteristics of the memory for intentions screening test. Clin Neuropsychol. 2008b;22:864–78. doi: 10.1080/13854040701595999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Morgan EE, Marquie-Beck J, Carey CL, Grant I, Letendre SL, et al. Markers of macrophage activation and axonal injury are associated with prospective memory in HIV-1 disease. Cogn Behav Neurol. 2006;19:217–21. doi: 10.1097/01.wnn.0000213916.10514.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of clinical and experimental neuropsychology. 2004;26:759–78. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Troster AI, et al. Action (verb) fluency: test-retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc. 2005;11:408–15. [PubMed] [Google Scholar]
- Woods SP, Weinborn M, Velnoweth A, Rooney A, Bucks RS. Memory for intentions is uniquely associated with instrumental activities of daily living in healthy older adults. J Int Neuropsychol Soc. 2012;18:134–8. doi: 10.1017/S1355617711001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WorldHealthOrganization. Composite international diagnostic interview (CIDI, version 2.1) Geneva, Switzerland: 1998. [Google Scholar]
- Zayyad Z, Spudich S. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND) Current HIV/AIDS reports. 2015;12:16–24. doi: 10.1007/s11904-014-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]





