Abstract
Objective
High protein (particularly leucine-rich whey protein) intake is recommended to mitigate the adverse effect of weight loss on muscle mass. The effectiveness of this approach is unknown.
Methods
Seventy middle-aged (50–65 y old) postmenopausal women with obesity were randomized to: 1) weight maintenance (WM); or 2) weight loss and the Recommended Daily Allowance (RDA) for protein (0.8 g protein/kg/d; WL group); or 3) weight loss plus whey protein supplementation (total protein: 1.2 g/kg/d; WL-PS group). Thigh muscle volume and strength were assessed at baseline and after 5% and 10% weight loss in the weight loss groups and after matched time periods (~3 and 6 months, respectively) in the WM group.
Results
5% weight loss caused a greater decrease in thigh muscle volume in the WL than the WL-PS group (4.7±0.7% vs 2.8±0.8%, respectively; P<0.05). After 10% weight loss, there was no statistically significant difference in muscle mass loss in the two groups and the total loss was small in both groups (5.5±0.8% and 4.5±0.7%, respectively). The dietary interventions did not affect muscle strength.
Conclusion
Whey protein supplementation during diet-induced weight loss does not have clinically important therapeutic effects on muscle mass or strength in middle-aged postmenopausal women with obesity.
Keywords: diet, weight loss, muscle
Introduction
Obesity is associated with an increased risk of cardiometabolic abnormalities (1). Weight loss induced by consuming a hypocaloric diet can ameliorate or even completely resolve these co-morbidities (1, 2). However, diet-induced weight loss reduces total lean body and muscle mass (3–7), which could increase the risk of sarcopenia (defined as low muscle mass and function (8, 9)) in vulnerable populations, such as middle-aged, postmenopausal women and older adults (10–15). High protein intake (≥1.0 g per kg per day), particularly consumption of leucine-rich proteins such as whey protein, is recommended to prevent age-associated muscle loss (16–20) and to mitigate the adverse effect of diet-induced weight loss on muscle mass (18, 20, 21), because protein ingestion stimulates muscle protein synthesis in a dose-dependent manner (22), leucine ingestion augments the anabolic effect of protein consumption (23), and high protein intake blunts the weight loss-induced decline in lean body mass (24, 25). However, it is not known whether high protein intake during weight loss actually prevents the loss of skeletal muscle, because: i) the acute effect of protein ingestion on muscle protein synthesis might not predict the chronic effect of protein ingestion on muscle mass, which is determined by the balance between synthesis and breakdown; and ii) the weight loss-induced change in lean body mass (determined by using dual-energy X-ray absorptiometry [DXA]) is not a reliable surrogate for changes in muscle mass (determined by using computed tomography or magnetic resonance imaging (MRI)) (26).
We conducted a randomized controlled trial (RCT) to evaluate the effect of diet-induced weight loss with and without whey protein supplementation on muscle mass in middle-aged (50–65 y old) postmenopausal women with obesity. Subjects were randomized to one of three intervention groups: 1) a weight maintenance (WM) group; 2) a weight loss (WL) group, who consumed a hypocaloric diet containing the RDA of protein (0.8 g g/kg/d); and 3) a weight loss plus protein supplementation (WL-PS) group, who consumed a whey protein supplement in addition to a hypocaloric diet (daily protein intake: 1.2 g/kg/d). Thigh muscle volume was evaluated by MRI, and total fat-free mass (bone included), lean body mass (bone excluded) and leg lean mass (bone excluded) were evaluated by DXA before and after ~5% and ~10% weight loss in the two weight loss groups and after a matched duration of time (~3 and ~6 months, respectively) in the WM group. In addition, we measured one repetition maximum (1-RM) muscle strength and muscle force production capacity (peak isometric and isokinetic torque), and intermuscular adipose tissue (IMAT) content, which is associated with decreased muscle strength independent of muscle mass (27–30).
Methods
Human Subjects Research Compliance
The study was approved and monitored by the Human Research Protection Office at Washington University School of Medicine in St. Louis, MO, USA. Written informed consent was obtained from all subjects before their participation. Subject flow is shown in Figure S1.
Participants
Eighty-seven, middle-aged (50–65 y old) postmenopausal women with obesity were assessed for eligibility, and 75 were enrolled (Figure S1). All participants completed a comprehensive medical evaluation, which included a history and physical examination, a 75-g oral glucose tolerance test, and standard blood tests. Potential subjects were excluded if they met the following exclusion criteria: body mass index <30 or ≥50 kg/m2; unstable body weight (i.e., >2 kg change within 6 months of screening); engaged in ≥1.5 hours of exercise per week; serious chronic disease (e.g., neuromuscular, or cardiopulmonary, or chronic kidney disease, diabetes, cancer) or a condition that could interfere with body composition imaging (e.g., certain metal implants), or taking medications that could affect muscle mass and/or function (e.g., HMG-CoA reductase inhibitors, steroids) within one year before enrolling in the study. None of the subjects consumed tobacco products or reported regular consumption of >115 g alcohol per week or scored >2 points on the Michigan Alcohol Screening Test.
Study Design
Seventy subjects completed all baseline testing and were randomized to either the WM, or WL, or WL-PS group by using a computerized randomization scheme. In the WL and WL-PS groups, outcomes were assessed before starting the intervention and after subjects had lost ~5% and ~10% of their body weight. In the WM group, outcomes were assessed at baseline and after ~3 and ~6 months, to match the anticipated time to achieve ~5% and ~10% weight loss in the WL and WL-PS groups. The primary outcome measure was the change in thigh muscle volume after ~10% weight loss. Secondary outcomes included: 1) total fat-free mass, lean body mass, and leg lean mass, 2) 1-RM leg muscle strength (composite value for bilateral leg press, knee extension, and knee flexion), 3) unilateral (dominant leg) knee extension peak torque (composite value for isometric and 60 °/s and 180 °/s isokinetic exercises), 4) unilateral (dominant leg) knee flexion peak torque (composite value for isometric and 60 °/s and 180 °/s isokinetic exercises), and 5) thigh IMAT volume.
Diet intervention
Initial target energy intake in the weight loss groups was 70% of each person’s total daily energy expenditure (resting energy expenditure × an activity factor of 1.4 (31)); energy intake was then adjusted weekly as needed to achieve 0.5%–1.0% weight loss per week until 10% weight loss was achieved. In the WM group, each subject’s energy intake was adjusted as needed to maintain body weight within 2% of the initial body weight. Target protein intake was 0.8 g/kg/d for the WM and WL groups and 1.2 g/kg/d for subjects in the WL-PS group. This amount of protein (1.2 g/kg/d) is recommended to prevent sarcopenia (16–20) and was found to attenuate the loss of lean body mass associated with diet-induced weight loss (24, 25, 32). For breakfast, all subjects consumed two nutrition bars (NuGo Nutrition, Oakmont, PA) per day; for lunch and dinner, they were given a base diet of frozen entrees (eLiving meals, Morrison Healthcare, Atlanta, GA; Lean Cuisine, Nestlé USA, Solon, OH; and meals from Revel Kitchen, St. Louis, MO and our Clinical Research Unit metabolic kitchen). Subjects in the WL-PS group also consumed two servings of a whey protein isolate (Unjury®, ProSynthesis Laboratories, Inc, Reston, VA) per day (with breakfast and as a mid-afternoon snack) whereas subjects in the WL group consumed isocaloric foods that provided mostly carbohydrates and fat instead. Additional calories needed to meet each subject’s total energy and macronutrient requirements were consumed as fruits, vegetables, dairy products and starches. We used several strategies to ensure dietary compliance and to monitor dietary intake: i) all meals and the protein supplement were provided to our study subjects, ii) dietary intake was monitored by reviewing subjects’ daily diet records during weekly visits with the study dietician, and iii) blood urea nitrogen and, in a subset of participants, urinary urea nitrogen excretion were measured as objective markers of protein intake.
Outcomes Assessments
The following assessments were completed during outpatient visits to the Clinical Research Unit or the Center for Clinical Imaging Research at Washington University School of Medicine.
Body weight and body composition
Body weight was measured on a Seca 703 scale (Seca, Hanover, MD) to the nearest 0.1 kg. Total fat mass and fat-free mass, lean body mass, and bilateral leg lean mass were evaluated by using DXA (Lunar iDXA, GE Healthcare Lunar, Madison, WI). Thigh muscle and IMAT volumes were quantified by using magnetic resonance imaging (1.5-T superconducting magnet [Siemens, Iselin, NJ] and Matlab software [Mathworks, Natick, MA]); the region of interest constituted 22 consecutive 8 mm-thick bilateral T1-weighted axial images, which were acquired with and without fat saturation starting 10 cm proximal to the distal edge of the femur.
Muscle strength
1-RM muscle strength (i.e., the maximal amount of weight each participant was able to lift just once) was evaluated by using a Hoist multi-station weight machine (Hoist Fitness Systems, Poway, CA) for the following exercises (all bilateral): leg press, knee extension, and knee flexion. The goal was to attain the 1-RM for each exercise after ~5 incremental weight lifts; at every stage, subjects were allowed a second attempt if they were unable to lift an incremental weight the first time. Peak isometric and isokinetic (60 °/s and 180 °/s) torque of the knee extensors and flexors of the dominant leg were evaluated by using a Biodex 3 dynamometer (Biodex Medical Systems, Shirley, NY). Each exercise was repeated three times and the mean of the two highest torque recordings for each exercise was used for analysis. At baseline, subjects attended an orientation session to become familiar with the exercise equipment and testing procedures. After a median of 7 (quartile 1: 6; quartile 3: 13) days, all testing procedures were repeated to obtain each subject’s baseline values; subsequent testing sessions did not include further training.
Statistical analysis
SPSS version 24 for Windows (IBM, Armonk, NY) was used for statistical analyses. Our primary analysis was intention-to-treat (ITT) and included all subjects who completed baseline testing. Analysis of variance (normally distributed variables) or the Kruskal-Wallis test (skewed variables) were used to compare baseline subject characteristics, and total energy and macronutrient intake during the dietary intervention in the three groups. Diet-induced changes in body composition and strength were analyzed by using a linear mixed model with time and group as fixed factors. Analysis of covariance with the baseline value as covariate was used to compare the intervention-induced change in blood urea nitrogen concentration and urinary urea nitrogen excretion rate, which were assessed at baseline and after 10% weight loss only, in the three groups. We also performed a “Complete Case Analysis”, which included only subjects who completed all aspects of the study. Characteristics of subjects who did and did not complete the study were compared by using Student’s t-test. Relationships among variables of interest were evaluated by computing the coefficient of determination (R2). A P-value ≤0.05 was considered statistically significant. Baseline data are presented as mean ± SEM for normally distributed data sets and median (quartile1; quartile 3) for skewed data sets. Mean changes over time and their 95% confidence bounds are used to present intervention-induced changes in the three groups.
Sample size determination and power calculation
We chose our sample size based on the expected change in our primary outcome, thigh muscle volume. Using the same MRI method we employed in our study, our former colleagues at Washington University School of Medicine have reported a 6.9 ± 3.4% (mean ± SD) decrease in thigh muscle volume in overweight adults who completed a hypocaloric diet therapy to achieve 10% weight loss (6). Using a two-sided test, and a 0.05 α-level of significance, we estimated that the sample size required to detect this difference in either the WL or WL-PS compared with the WM groups with a power of 0.80 is five per group. To detect a 3.0, 4.0, or 5.0 percentage point difference in the pre/post change value for thigh muscle volume between the WL and WL-PS groups (e.g., a 6.9% change in the WL group compared to a 3.9%, 2.9% or 1.9% change in the WL-PS group, respectively) with a power ≥0.80 would require sample sizes of n=22, n=13, and n=9 per group, respectively. Differences less than that were considered clinically insignificant.
Results
Subject characteristics, dietary compliance, and changes in body weight
Baseline characteristics of subjects in the WM, WL, and WL-PS groups in both the ITT (n = 70; Tables 1 and 2) and Complete Case Analysis (n = 53; Tables S1 and S2) cohorts were not different. Characteristics of subjects who did and did not complete the study were also not different (data not shown).
Table 1.
Subjects’ body mass and composition at baseline and after ~5% and ~10% weight loss1
| WM (n=18) |
WL (n=27) |
WL-PS (n=25) |
|
|---|---|---|---|
| Body mass (kg) | |||
| Baseline | 100.9 ± 3.1 | 97.6 ± 2.5 | 94.8 ± 2.6 |
| After 5% weight loss2 | 101.0 ± 3.0 | 92.2 ± 2.4* | 89.8 ± 2.5* |
| After 10% weight loss2 | 101.4 ± 2.8 | 88.6 ± 2.3*† | 85.9 ± 2.4*† |
| Δ after 5% weight loss2 | 0.34 (−0.37, 1.06) | −5.38 (−6.02, −4.75)‡ | −5.20 (−5.81, −4.59)‡ |
| Δ after 10% weight loss2 | 0.64 (−0.12, 1.40) | −9.03 (−9.81, −8.24)‡ | −9.02 (−9.72, −8.32)‡ |
| Body fat (%) | |||
| Baseline | 49.2 ± 1.0 | 49.9 ± 0.8 | 49.5 ± 0.8 |
| After 5% weight loss2 | 49.8 ± 1.0* | 47.9 ± 0.8* | 47.6 ± 0.9* |
| After 10% weight loss2 | 49.9 ± 1.0 | 46.3 ± 0.9*† | 45.8 ± 0.9*† |
| Δ after 5% weight loss2 | 0.61 (0.06, 1.17) | −1.97 (−2.48, −1.45)‡ | −1.86 (−2.34, −1.39)‡ |
| Δ after 10% weight loss2 | 0.66 (−0.25, 1.57) | −3.53 (−4.52, −2.54)‡ | −3.67 (−4.56, −2.79)‡ |
| Total body fat-free mass (kg) | |||
| Baseline | 50.3 ± 1.2 | 48.2 ± 1.0 | 46.9 ± 1.0 |
| After 5% weight loss2 | 50.2 ± 1.2 | 47.1 ± 1.0* | 46.1 ± 1.0* |
| After 10% weight loss2 | 49.9 ± 1.2 | 46.7 ± 1.0* | 45.8 ± 1.0* |
| Δ after 5% weight loss2 | 0.05 (−0.70, 0.79) | −1.41 (−2.20, −0.61)‡ | −0.85 (−1.51, −0.18) |
| Δ after 10% weight loss2 | −0.14 (−0.92, 0.64) | −1.80 (−2.68, −0.92)‡ | −1.30 (−2.01, −0.59)‡ |
| Total body lean mass (kg) | |||
| Baseline | 47.7 ± 1.2 | 45.7 ± 0.9 | 44.4 ± 1.0 |
| After 5% weight loss2 | 47.7 ± 1.2 | 44.7 ± 1.0* | 43.7 ± 1.0* |
| After 10% weight loss2 | 47.4 ± 1.2 | 44.2 ± 1.0* | 43.3 ± 1.0* |
| Δ after 5% weight loss2 | 0.05 (−0.71, 0.81) | −1.42 (−2.12, −0.72)‡ | −0.94 (−1.59, −0.28) |
| Δ after 10% weight loss2 | −0.15 (−0.92, 0.61) | −1.81 (−2.52, −1.09)‡ | −1.32 (−2.06, −0.58)‡ |
| Leg lean mass (kg) | |||
| Baseline | 16.7 ± 0.5 | 15.6 ± 0.4 | 15.2 ± 0.4 |
| After 5% weight loss2 | 16.6 ± 0.5 | 15.1 ± 0.4* | 15.1 ± 0.4 |
| After 10% weight loss2 | 16.7 ± 0.5 | 15.0 ± 0.4* | 14.7 ± 0.4*† |
| Δ after 5% weight loss2 | −0.02 (−0.28, 0.33) | −0.66 (−0.93, −0.39)‡ | −0.18 (−0.49, 0.13)§ |
| Δ after 10% weight loss2 | 0.09 (−0.24, 0.42) | −0.61 (−0.92, −0.30)‡ | −0.55 (−0.84, −0.26)‡ |
Abbreviations: WL, weight loss; WL-PS, weight loss and protein supplementation; WM, weight maintenance. Values at baseline and after 5 and 10% weight loss and a matched time period in the weight maintenance group are expressed as mean ± SEM. Change values are expressed as adjusted means and 95% confidence bounds.
All subjects who completed baseline testing were included in this intention-to-treat analysis. Differences in absolute values were analyzed by using a linear mixed model. Change values were analyzed by using a linear mixed model with the baseline values as a covariate. The multiple imputation technique was used to account for missing values.
Testing performed at a matched time period in the WM group.
Value significantly different from the corresponding value at baseline, P < 0.05.
Value significantly different from the corresponding value after 5% weight loss, P < 0.05.
Value significantly different from the corresponding value in the WM group, P < 0.05.
Value significantly different from the corresponding value in the WL group, P < 0.05.
Table 2.
Subjects’ muscle function at baseline and after ~5% and ~10% weight loss1
| WM (n=18) |
WL (n=27) |
WL-PS (n=25) |
|
|---|---|---|---|
| Sum 1-RM strength (kg)2 | |||
| Baseline | 182 ± 8 | 163 ± 6 | 170 ± 6 |
| After 5% weight loss3 | 182 ± 8 | 161 ± 6 | 170 ± 7 |
| After 10% weight loss3 | 189 ± 7*† | 164 ± 6 | 173 ± 6 |
| Δ after 5% weight loss3 | 1 (−4, 6) | −2 (−7, 2) | 0 (−5, 5) |
| Δ after 10% weight loss3 | 8 (1, 15) | 0 (−6, 7) | 3 (−4, 9) |
| Sum 1-RM strength/muscle volume (g/cm3) | |||
| Baseline | 47.6 ± 1.7 | 44.2 ± 1.3 | 45.8 ± 1.4 |
| After 5% weight loss3 | 47.9 ± 1.7 | 45.6 ± 1.4 | 47.2 ± 1.4 |
| After 10% weight loss3 | 49.2 ± 1.6 | 47.3 ± 1.3*† | 48.6 ± 1.4*† |
| Δ after 5% weight loss3 | 0.6 (−1.1, 2.3) | 1.3 (−0.2, 2.8) | 1.5 (−0.2, 3.1) |
| Δ after 10% weight loss3 | 2.0 (0.0, 4.0) | 2.9 (1.1, 4.8) | 2.8 (0.9, 4.8) |
| Sum knee extension peak torque (Nm)4 | |||
| Baseline | 333 ± 16 | 305 ± 13 | 326 ± 14 |
| After 5% weight loss3 | 324 ± 17 | 288 ± 15* | 318 ± 15 |
| After 10% weight loss3 | 335 ± 16 | 303 ±13† | 309 ± 13 |
| Δ after 5% weight loss3 | −7 (−23, 8) | −18 (−33, −4) | −7 (−22, 7) |
| Δ after 10% weight loss3 | 4 (−16, 24) | −6 (−24, 13) | −14 (−30, 2) |
| Sum knee extension peak torque/muscle volume (Nm/cm3 × 103) | |||
| Baseline | 88.1 ± 4.0 | 82.6 ± 3.1 | 87.7 ± 3.3 |
| After 5% weight loss3 | 84.9 ± 4.3 | 81.3 ± 3.5 | 88.2 ± 3.7 |
| After 10% weight loss3 | 86.9 ± 3.9 | 87.2 ± 3.3† | 87.6 ± 3.3 |
| Δ after 5% weight loss3 | −2.6 (−7.1, 1.9) | −1.7 (−6.2, 2.7) | 0.7 (−3.6, 4.9) |
| Δ after 10% weight loss3 | −1.1 (−6.7, 4.6) | 3.8 (−2.1, 9.7) | −0.1 (−5.1, 5.0) |
| Sum knee flexion peak torque (Nm)4 | |||
| Baseline | 192 ± 9 | 178 ± 7 | 188 ± 7 |
| After 5% weight loss3 | 188 ± 8 | 167 ± 6* | 181 ± 6 |
| After 10% weight loss3 | 188 ± 8 | 177 ± 7 | 183 ± 6 |
| Δ after 5% weight loss3 | −1 (−11, 8) | −13 (−22, −5) | −6 (−15, 2) |
| Δ after 10% weight loss3 | −1 (−12, 10) | −3 (−13, 7) | −5 (−15, 5) |
| Sum knee flexion peak torque/muscle volume (Nm/cm3 × 103) | |||
| Baseline | 51.6 ± 2.3 | 48.6 ± 1.8 | 51.1 ± 1.8 |
| After 5% weight loss3 | 50.5 ± 2.0 | 48.2 ± 1.7 | 50.6 ± 1.7 |
| After 10% weight loss3 | 50.2 ± 2.0 | 51.1 ± 1.7 | 52.1 ± 1.7 |
| Δ after 5% weight loss3 | −0.6 (−3.4, 2.1) | −1.0 (−3.7, 1.7) | −0.1 (−2.7, 2.4) |
| Δ after 10% weight loss3 | −0.7 (−3.7, 2.2) | 2.2 (−0.7, 5.1) | 1.3 (−1.4, 4.1) |
Abbreviations: 1-RM, 1-repetition maximum; WL, weight loss; WL-PS, weight loss and protein supplementation; WM, weight maintenance. Values at baseline and after 5 and 10% weight loss and a matched time period in the weight maintenance group are expressed as mean ± SEM. Change values are expressed as adjusted means and 95% confidence bounds.
All subjects who completed baseline testing were included in this intention-to-treat analysis. Differences in absolute values were analyzed by using a linear mixed model. Change values were analyzed by using a linear mixed model with the baseline values as a covariate. The multiple imputation technique was used to account for missing values.
Sum of bilateral leg press, knee extension, and knee flexion exercises.
Testing performed at a matched time period in the WM group.
Sum of unilateral (dominant leg) isometric (0 °/s) and isokinetic (at 60 °/s and 180 °/s) exercises.
Value significantly different from the corresponding value at baseline, P < 0.05.
Value significantly different from the corresponding value after 5% weight loss, P < 0.05.
Subjects who completed the study in both the WL and WL-PS groups achieved the targeted ~5% and ~10% weight loss at ~3 and ~6 months, respectively (Figure 1). Protein intake (assessed by food records) closely matched the prescribed amounts of 0.8 g/kg/d in the WM and WL groups and 1.2 g/kg/d in the WL-PS group (Table 3). The additional amount of protein (0.4 g/kg/d more in the WL-PS than the WL group) did not markedly alter overall diet composition (Table 3). Blood urea nitrogen concentration and urinary urea nitrogen excretion rate, which are biomarkers of dietary protein intake, were not different among the three groups at baseline but were greater (P <0.001) in the WL-PS than the WL and WM groups at the end of the dietary intervention (Table 3).
Figure 1. Changes in body mass during the dietary interventions in subjects who completed all study visits.
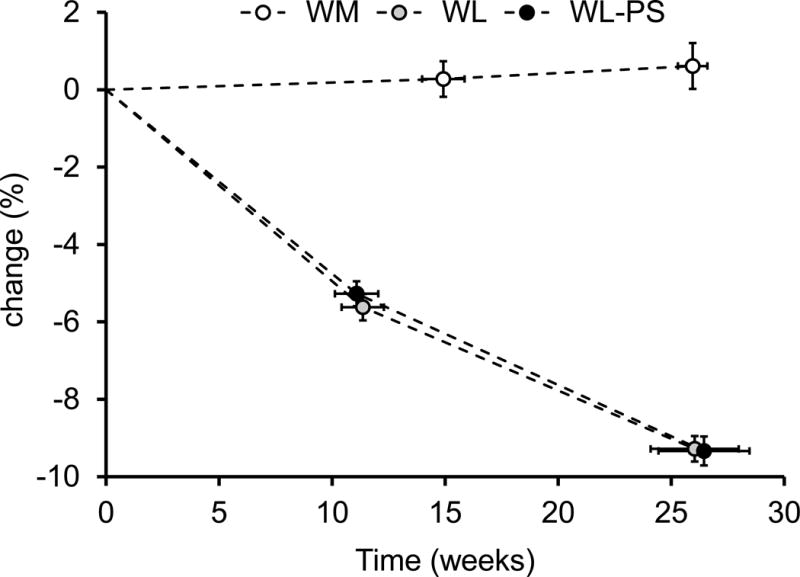
Data are expressed as mean ± SEM. WL: weight loss (n=18); WL-PS: weight loss and protein supplementation (n=19); WM: weight maintenance (n=16).
Table 3.
Reported energy and macronutrient intake and biomarkers of protein intake
| WM | WL | WL-PS | |
|---|---|---|---|
| Energy (kJ/day) | 7,435 ± 545 | 5,676 ± 208* | 5,681 ± 180* |
| Carbohydrates (% total energy) | 47 ± 1 | 50 ± 1† | 44 ± 1 |
| Carbohydrates (% non-protein energy intake) | 58 ± 1 | 64 ± 1 | 65 ± 2* |
| Fat (% total energy) | 34 ± 1 | 28 ± 1* | 24 ± 1* |
| Fat (% non-protein energy intake) | 42 ± 1 | 36 ± 1 | 35 ± 2* |
| Protein | |||
| % total energy | 19 ± 1 | 22 ± 1*† | 31 ± 1* |
| grams/day | 82 ± 8 | 74 ± 3† | 105 ± 3* |
| grams/kg body weight/day | 0.78 ± 0.06 | 0.86 ± 0.03† | 1.22 ± 0.03* |
| Blood urea nitrogen concentration (mg/dl) | |||
| Baseline | 13.6 ± 0.6 | 14.8 ± 0.8 | 12.7 ± 0.7 |
| Δ after 10% weight loss1 | 0.2 (−2.1, 1.7) | −1.6 (−3.3, 0.2)† | 3.4 (1.6, 5.1)* |
| Urinary urea nitrogen excretion rate (mg/kg/d)2 | |||
| Baseline | 97 ± 12 | 121 ± 11 | 107 ± 6 |
| Δ after 10% weight loss1 | −16 (−64, 33) | 1 (−39, 42)† | 67 (27, 106)* |
Abbreviations: WL, weight loss; WL-PS, weight loss and protein supplementation; WM, weight maintenance. Baseline values are expressed as mean ± SEM. Change values are expressed as adjusted means and 95% confidence bounds.
Testing performed at a matched time period in the WM group.
Represents data from a subset of subjects (WM, n=7; WL, n=10; WL-HP, n=10) who performed 24-h urine collections before and after weight loss or a matched time period in the WM group. Assuming 6.25% nitrogen in dietary protein and 90% of nitrogen loss occurs in urine, protein intake after weight loss was 0.80 ± 0.06 g/kd/day in the WL group and 1.22 ± 0.21 g/kg/day in the WL-PS group.
Value significantly different from the corresponding value in the WM group, P < 0.05.
Value significantly different from the corresponding value in the WL-PS group.
Changes in total fat-free mass, lean body mass, leg lean mass, thigh muscle and IMAT volumes, and muscle strength
Weight loss caused a decrease in total fat-free and lean body mass, leg lean mass and thigh muscle volume in both the WL and WL-PS groups. After ~5% weight loss, the decreases in total fat-free and lean body mass, leg lean mass, and thigh muscle volume in the WL-PS group were approximately half that in the WL group, whereas after ~10% body weight loss, the decrease from baseline tended to be lower in the WL-PS than the WL group but the difference between the groups was very small and not statistically significantly different (P ≥ 0.31 in the ITT analysis shown in Table 1 and Figure 2, and P ≥0.24 in the Complete Case Analysis shown in Table S1 and Figure S2). Although the relationships between the changes in thigh muscle volume and the changes in total fat free mass, lean body mass, and leg lean mass were statistically significant (all p < 0.05), the correlations between each of these pairs of outcome measures were weak (R2 = 0.116, R2 = 0.210, and R2 = 0.059, respectively).
Figure 2. Changes in thigh muscle and intermuscular adipose tissue volumes during the dietary interventions in all subjects who completed baseline testing (intention-to-treat analysis).

The left panel shows thigh muscle and intermuscular adipose tissue volumes expressed as mean ± SEM at baseline (black bars) and after 5% (grey bars) and 10% (white bars) weight loss and a matched time period in the weight maintenance group. The right panel shows the corresponding relative changes from baseline expressed as adjusted means with 95% confidence bounds. Differences in the left panel were analyzed by using a linear mixed model. Change values in the right panel were analyzed by using a linear mixed model with the baseline values as a covariate. The multiple imputation technique was used to account for missing values. IMAT: intermuscular adipose tissue. WL: weight loss (n=27); WL-PS: weight loss and protein supplementation (n=25); WM: weight maintenance (n=18). * Value significantly different from the corresponding value at baseline, P < 0.05. † Value significantly different from the corresponding value after 5% weight loss. ‡ Value significantly different from the corresponding value in the WM group, P < 0.05. § Value significantly different from the corresponding value in the WL group, P < 0.05.
Weight loss reduced IMAT volume in both the WL and WL-PS groups, and the decrease was not different between the two groups (Figures 2 and S2). Neither 1-RM thigh muscle strength nor peak torque were altered by the dietary interventions (Tables 2 and S2).
Discussion
We conducted a RCT to evaluate the effect of dietary whey protein supplementation on thigh muscle and IMAT volumes and muscle function after ~5% and ~10% weight loss in middle-aged, postmenopausal women with obesity. Participants consumed either a standard-protein (0.8 g protein/kg/d) weight loss diet or the same diet in which part of breakfast and an afternoon snack were replaced with isocaloric whey protein supplements that provided an additional 0.4 g protein/kg/d. We found that protein supplementation during weight loss blunted the initial decline in thigh muscle volume after 5% weight loss, and tended to decrease the reduction in thigh muscle volume after 10% weight loss. The decline in thigh muscle volume after 10% weight loss in both groups was very small, representing less than a 6% (~200 cm3) decrease in bilateral thigh muscle volume, which is consistent with the results reported previously in middle-aged and older adults (5, 6). Moreover, the decline in muscle volume in both the_WL or WL-PS groups was not associated with a decrease in muscle strength. Weight loss caused the same decrease in IMAT volume in both the WL and WL-PS groups. These data demonstrate that 10% weight loss, induced by consuming a hypocaloric diet containing the RDA for protein (0.8 g/kg/day), does not have clinically important adverse effects on muscle mass and strength in middle-aged, postmenopausal women with obesity. Moreover, increasing daily protein intake by 50% above the RDA attenuates muscle loss, but the effect on muscle mass is very small, and does not translate into an improvement in muscle strength.
Our data are consistent with the results from previous studies, which included young and older adult men and women, and found that high protein intake caused a small, but statistically significant, attenuation in the decline in lean body mass after moderate weight loss in studies that lasted up to ~6 months (24, 25), but had no effect on the amount of total body mass or body composition in studies that lasted 12 months (33). However, we are not aware of any previous studies that evaluated the effect of high protein intake or protein supplementation during weight loss on muscle mass or volume in people with obesity. It has been reported that weight loss-induced changes in DXA-derived total lean body mass do not accurately reflect changes in muscle mass (determined directly by using computed tomography or magnetic resonance imaging (MRI)) (26). Our results confirm this observation, because we found very weak correlations between the change in thigh muscle volume determined by MRI and the changes in total fat-free mass, lean body mass and leg lean mass determined by DXA. Together, these results demonstrate that high protein intake during diet-induced weight loss attenuates the decline in lean body mass and muscle mass, but the effect is small and does not cause a decrease in muscle strength. Moreover, weight loss causes a much greater decrease in body weight than muscle mass, so the ratio of muscle mass to body weight increased, which presumably contributes to improved physical function despite a reduction in muscle mass and no change in strength observed after weight loss in older adults with obesity (5). Additional studies are needed to evaluate the effect of short-term protein supplementation during weight loss on muscle mass after long-term weight maintenance or weight regain.
Increased IMAT content in people with obesity is associated with poor muscle function (27–30), and weight loss decreases IMAT content (3, 7, 34, 35). The results from our study are therefore consistent with and extend the findings from earlier studies by demonstrating that the weight loss-induced decrease in IMAT is not associated with an increase in muscle strength, and that increased protein intake does not affect the magnitude of the weight-loss induced change in IMAT content or affect strength. It is, however, possible that the change in IMAT content in our subjects was too small to affect muscle function or that the concomitant loss of muscle mass counteracted the potential benefit of reduced IMAT content on muscle function.
The results from our study might not translate to other populations (e.g., younger women or men) or protein interventions. We studied postmenopausal women between 50 and 65 years of age and studied the effect of only a single type (whey) and dose (0.4 g/kg/d in addition to the RDA of 0.8 g/kg/d) of protein, because: i) the prevalence of obesity and future risk of sarcopenia in middle-aged postmenopausal women (10–15); ii) whey protein causes a greater stimulation of muscle protein synthesis than many other types of protein because of its high leucine content (16, 20); and iii) 1.5 times the RDA of protein (a total of 1.2 g protein/kg per day) is the amount recommended by a consortium of the International Association of Gerontology and Geriatrics, the International Academy on Nutrition and Aging, the European Union Geriatric Medicine Society, and the Australian and New Zealand Society for Geriatric Medicine, for people at risk of sarcopenia and people undergoing dietary weight loss therapy (16–21). In addition, we did not evaluate whether concomitant exercise training might have generated a beneficial effect of protein supplementation on muscle mass. However, the data from several recent systematic reviews and meta-analyses show little or no effect of increased protein/amino acid intake on exercise training-induced muscle hypertrophy in weight-stable middle-aged and older adults (36–41).
High protein intake during weight loss therapy is often recommended to facilitate both short-term and long-term weight loss because protein increases satiety and the thermogenic effect of feeding (21, 42–44). However, the results from the most recent systematic review and meta-analysis show that high protein intake does not cause greater weight loss than standard protein intake in older people with obesity who participated in a weight loss program (24). Furthermore, high protein intake after weight loss does not improve long-term weight maintenance of weight loss (45). In addition, we recently found that protein supplementation could have adverse metabolic effects by preventing the weight loss-induced improvement in insulin sensitivity (32). Therefore, the current recommendations of high protein intake during weight loss therapy should be reconsidered.
Conclusion
In summary, our findings demonstrate that in middle-aged, postmenopausal women with obesity, moderate 5%–10% weight loss does not have adverse effects on maximal muscle strength despite a small decrease in muscle mass. Moreover, a 50% increase in protein intake during weight loss therapy does not have clinically important therapeutic effects on muscle mass or muscle strength. These results indicate that increasing protein intake beyond the RDA during weight loss therapy is not necessary in middle-aged, postmenopausal women with obesity.
Supplementary Material
What is already known about this subject?
Diet-induced weight loss reduces total lean body and muscle mass.
High protein intake (≥1.0 g per kg per day) mitigates the adverse effect of diet-induced weight loss on lean body mass.
Leucine has unique muscle anabolic properties.
What does the study add?
A randomized controlled trial to evaluate the effect of diet-induced weight loss with and without leucine-rich protein supplementation on muscle mass in postmenopausal women with obesity who are at increased risk for sarcopenia.
The results from this study show that consuming 50% more protein than the current Recommended Daily Allowance during weight loss blunts the weight loss-associated muscle loss in postmenopausal women with obesity but the effect is small and clinically insignificant.
In addition, the data show that weight loss does not adversely affect muscle strength in postmenopausal women with obesity because the muscle mass loss associated with weight loss is small.
Acknowledgments
The authors thank Janet Winkelmann, Rachel Burrows, Sophie Julliand, Lynda Bowers, and Kathryn Gratza for help with subject recruitment, scheduling, and testing; the staff of the Clinical Research Unit and Metabolic Kitchen for their help in performing the studies, and the study subjects for their participation.
Funding: This publication was made possible by NIH grants DK 94483, DK 56341 (Nutrition and Obesity Research Center), DK 20579 (Diabetes Research Center), DK 52574 (Digestive Disease Research Center), and UL1 TR000448 (Clinical Translational Science Award, including KL2 sub-award TR 000450) and support from the Atkins and Pershing Square Foundations.
Footnotes
Disclosure: None of the authors had any potential conflicts of interest and no funding entity had any role in the design, implementation, analysis and interpretation of the data.
ClinicalTrials.gov number: NCT01538836
Author contributions: BM and SK designed the study; GIS, PKC, DNR, SK and BM conducted the study; GIS and BM analyzed the data; GIS and BM drafted the manuscript; PKC, DNR, and SK critically revised the manuscript for important intellectual content; DNR and SK provided medical supervision for the study; BM was responsible for overall study supervision and had primary responsibility for the final content. All authors read and approved the final manuscript.
References
- 1.Klein S, Burke LE, Bray GA, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E, Klein S. Fundamentals of cardiometabolic risk factor reduction: achieving and maintaining weight loss with pharmacotherapy or bariatric surgery. Clin Cornerstone. 2008;9:41–48. doi: 10.1016/s1098-3597(08)60027-7. discussion 49-51. [DOI] [PubMed] [Google Scholar]
- 3.Ryan AS, Harduarsingh-Permaul AS. Effects of weight loss and exercise on trunk muscle composition in older women. Clin Interv Aging. 2014;9:395–402. doi: 10.2147/CIA.S56662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santanasto AJ, Glynn NW, Newman MA, et al. Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;2011 doi: 10.1155/2011/516576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–491. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen MB. Changes in body composition at menopause–age, lifestyle or hormone deficiency? J Br Menopause Soc. 2002;8:137–140. doi: 10.1258/136218002100321974. [DOI] [PubMed] [Google Scholar]
- 12.Meema HE. Menopausal and aging changes in muscle mass and bone mineral content. A roentgenographic study. J Bone Joint Surg Am. 1966;48:1138–1144. [PubMed] [Google Scholar]
- 13.Aloia JF, McGowan DM, Vaswani AN, Ross P, Cohn SH. Relationship of menopause to skeletal and muscle mass. Am J Clin Nutr. 1991;53:1378–1383. doi: 10.1093/ajcn/53.6.1378. [DOI] [PubMed] [Google Scholar]
- 14.Tanko LB, Movsesyan L, Mouritzen U, Christiansen C, Svendsen OL. Appendicular lean tissue mass and the prevalence of sarcopenia among healthy women. Metabolism. 2002;51:69–74. doi: 10.1053/meta.2002.28960. [DOI] [PubMed] [Google Scholar]
- 15.Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci. 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- 16.Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Landi F, Calvani R, Tosato M, et al. Protein intake and muscle health in old age: from biological plausibility to clinical evidence. Nutrients. 2016;8 doi: 10.3390/nu8050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips SM, Chevalier S, Leidy HJ. Protein “requirements” beyond the RDA: implications for optimizing health. Appl Physiol Nutr Metab. 2016;41:565–572. doi: 10.1139/apnm-2015-0550. [DOI] [PubMed] [Google Scholar]
- 19.Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016;8 doi: 10.3390/nu8060359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. 2015;80(Suppl 1):A8–A15. doi: 10.1111/1750-3841.12802. [DOI] [PubMed] [Google Scholar]
- 21.Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101:1320S–1329S. doi: 10.3945/ajcn.114.084038. [DOI] [PubMed] [Google Scholar]
- 22.Moore DR, Churchward-Venne TA, Witard O, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 23.Murphy CH, Saddler NI, Devries MC, et al. Leucine supplementation enhances integrative myofibrillar protein synthesis in free-living older men consuming lowerand higher-protein diets: a parallel-group crossover study. Am J Clin Nutr. 2016;104:1594–1606. doi: 10.3945/ajcn.116.136424. [DOI] [PubMed] [Google Scholar]
- 24.Kim JE, O’Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev. 2016;74:210–224. doi: 10.1093/nutrit/nuv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:1281–1298. doi: 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 26.Pourhassan M, Schautz B, Braun W, et al. Impact of body-composition methodology on the composition of weight loss and weight gain. Eur J Clin Nutr. 2013;67:446–454. doi: 10.1038/ejcn.2013.35. [DOI] [PubMed] [Google Scholar]
- 27.Choi SJ, Files DC, Zhang T, et al. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71:557–564. doi: 10.1093/gerona/glv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 29.Therkelsen KE, Pedley A, Hoffmann U, Fox CS, Murabito JM. Intramuscular fat and physical performance at the Framingham Heart Study. Age. 2016;38:31. doi: 10.1007/s11357-016-9893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcus RL, Addison O, Dibble LE, et al. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629637. doi: 10.1155/2012/629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr. 1996;50:72–92. [PubMed] [Google Scholar]
- 32.Smith GI, Yoshino J, Kelly SC, et al. High-protein intake during weight loss therapy eliminates the weight loss-induced improvement in insulin action in obese postmenopausal women. Cell Rep. 2016;17:849–861. doi: 10.1016/j.celrep.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwingshackl L, Hoffmann G. Long-term effects of low-fat diets either low or high in protein on cardiovascular and metabolic risk factors: a systematic review and meta-analysis. Nutr J. 2013;12:48. doi: 10.1186/1475-2891-12-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura E, Kumahara H, Tobina T, et al. Aerobic exercise attenuates the loss of skeletal muscle during energy restriction in adults with visceral adiposity. Obes Facts. 2014;7:26–35. doi: 10.1159/000358576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teichtahl AJ, Wluka AE, Wang Y, et al. Associations of surgical and nonsurgical weight loss with knee musculature: a cohort study of obese adults. Surg Obes Relat Dis. 2016;12:158–164. doi: 10.1016/j.soard.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Finger D, Goltz FR, Umpierre D, et al. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med. 2015;45:245–255. doi: 10.1007/s40279-014-0269-4. [DOI] [PubMed] [Google Scholar]
- 37.Holm L, Nordsborg NB. Supplementing a normal diet with protein yields a moderate improvement in the robust gains in muscle mass and strength induced by resistance training in older individuals. Am J Clin Nutr. 2017;106:971–972. doi: 10.3945/ajcn.117.165860. [DOI] [PubMed] [Google Scholar]
- 38.Liao CD, Tsauo JY, Wu YT, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106:1078–1091. doi: 10.3945/ajcn.116.143594. [DOI] [PubMed] [Google Scholar]
- 39.Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. doi: 10.1136/bjsports-2017-097608. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reidy PT, Rasmussen BB. Role of ingested amino acids and protein in the promotion of resistance exercise-induced muscle protein anabolism. J Nutr. 2016;146:155–183. doi: 10.3945/jn.114.203208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc. 2016;959:e951–959. doi: 10.1016/j.jamda.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton EF, Bray GA, Burton JH, Smith SR, Redman LM. No evidence for metabolic adaptation in thermic effect of food by dietary protein. Obesity. 2016;24:1639–1642. doi: 10.1002/oby.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quatela A, Callister R, Patterson A, MacDonald-Wicks L. The energy content and composition of meals consumed after an overnight fast and their effects on diet induced thermogenesis: A systematic review, meta-analyses and meta-regressions. Nutrients. 2016;8 doi: 10.3390/nu8110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Astrup A, Raben A, Geiker N. The role of higher protein diets in weight control and obesity-related comorbidities. Int J Obes. 2015;39:721–726. doi: 10.1038/ijo.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kjolbaek L, Sorensen LB, Sondertoft NB, et al. Protein supplements after weight loss do not improve weight maintenance compared with recommended dietary protein intake despite beneficial effects on appetite sensation and energy expenditure: a randomized, controlled, double-blinded trial. Am J Clin Nutr. 2017;106:684–697. doi: 10.3945/ajcn.115.129528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


