Abstract
Background
Poor sleep is one of the most common problems reported during menopause, and is known to vary throughout the menopause transition. The objective of this study was to describe the dynamics of poor sleep among participants of the Midlife Women’s Health Study and to identify risk factors associated with poor sleep during the menopausal transition.
Methods
Annual responses to surveys that included questions about the frequency of sleep disturbances and insomnia were analyzed to determine the likelihood of persistent poor sleep throughout the menopausal transition and the correlation of responses to the different sleep-related questions, including frequency of restless sleep during the first year of the study. Responses to questions about a large number of potential risk factors were used to identify risk factors for poor sleep.
Results
Poor sleep in premenopause was not predictive of poor sleep in perimenopause, and poor sleep in perimenopause was not predictive of poor sleep in postmenopause. Frequencies of each of the measures of poor sleep were highly correlated. For all sleep outcomes, high frequency of depression was related to a high frequency of poor sleep. Vasomotor symptoms were also significantly related with a higher frequency of all poor sleep outcomes. A history of smoking was also associated with higher frequencies of insomnia and sleep disturbances.
Conclusions
The risk factors identified for poor sleep, depression and vasomotor symptoms, were consistently associated with poor sleep throughout the menopausal transition. The likelihood of these risk factors changed from premenopause, through perimenopause, and into postmenopause, however, which could explain changes in sleep difficulties across the menopausal transition. Treatment of these risk factors should be considered when addressing sleep difficulties in menopausal women.
Keywords: sleep problems, menopause, cohort study, depression, hot flashes
1. Introduction
One of the most common problems reported during menopause is poor sleep, with one-third to half of all women aged 40–64 reporting sleep problems [1]. Sleep problems seem to peak in late perimenopause and continue into postmenopause [2], with the odds of reporting severe sleep difficulty increased 2–3.5 fold during the menopausal transition [3,4]. While it is possible that these problems are due to aging [5,6], their clear variation across menopause stages [7] even when controlling for age [8] indicates that menopause itself plays a role in disrupting women’s sleep [9,10]. This may be due to direct physical impacts (changes in the hypothalamic-pituitary-ovarian hormones) or be related to emotional or behavioral responses to menopause (ie, stress or behavior changes) [9] or both [11]. However, other studies have found that the best predictor of poor sleep during menopause is poor sleep prior to menopause [12].
Although many studies have examined the role of different risk factors for poor sleep, reports have shown variable results due to heterogeneity in study design [9] and the fact that sleep is a complex outcome with many different functions (such as sleep efficiency [8], sleep architecture [5], sleep duration [13], night awakenings [14], circadian robustness [15], and polysomnography [15,16]), each of which can be affected by different risk factors [17]. Adding to the problems in determining the role of risk factors is the fact that many risk factor effects are likely bidirectional [9]; for instance, poor sleep is known to increase depression, anxiety, and stress, all of which increase rates of poor sleep [2,8,9,17,18].
Poor sleep includes insomnia, restless sleep, and sleep disturbances; the frequency of each of these outcomes was self-reported during the Midlife Women’s Health Study. The objective of this study was to describe the dynamics of poor sleep among participants of the Midlife Women’s Health Study and to identify risk factors associated with poor sleep during the menopausal transition.
2. Methods
2.1 Data collection
The Midlife Women’s Health Study was a cohort study of hot flashes among women 45–54 years of age conducted starting in 2006 among residents of Baltimore and its surrounding counties. All participants gave written informed consent according to procedures approved by the University of Illinois and Johns Hopkins University Institutional Review Boards. The study design for the parent study is described in detail elsewhere [19]. Briefly, women were recruited by mail, and were included if they were in the target age range, had intact ovaries and uteri, and were pre- or perimenopausal. Exclusion criteria consisted of pregnancy, a history of cancer, exogenous female hormone or herbal/plant substance, and no menstrual periods within the past year.
Participants made a baseline clinic visit, which included measurement of height and weight to calculate body mass index (BMI) and completion of a detailed 26-page baseline survey. Among the survey questions were the items “Please indicate how frequently you experienced sleep disturbances during the past year”, “Please indicate how frequently you experienced insomnia during the past year”, each on a five-point Likert scale (never, less than once per month, one-four times per month, two-four times per week, or more than five times per week); these questions resemble those of the MIDUS study, which have been validated [20]. Participants were also asked in the baseline survey to complete the statement “During the past week my sleep was restless” with a four-point Likert scale (rarely, some of the time, moderately, or most of the time). Such self-reporting of perceived sleep quality [9], although not necessarily correlating with actual sleep efficiency [1], has been found to be clinically relevant [21].
Participants were asked to complete a follow-up questionnaire during a clinic visit annually after the baseline visit. This questionnaire repeated previous questions about insomnia and sleep disturbances in the previous year as well as most other questions from the initial survey, excluding questions for which the answers would not change. The clinic visit was repeated weekly over four weeks, for a total of four visits in each year. Blood samples were collected at each scheduled clinic visit and stored until measurement of hormone levels as described below.
Menopausal status was defined as follows: premenopausal women were those who experienced their last menstrual period within the past three months and reported 11 or more periods within the past year; perimenopausal women were those who experienced: their last menstrual period within the past year, but not within the past three months; their last menstrual period within the past three months and experienced 10 or fewer periods within the past year. Postmenopausal women were those women who had not experienced a menstrual period within the past year. Follow-up was discontinued for women if they reported hormone therapy, an oophorectomy, or a cancer diagnosis. At the year four visit, follow-up was discontinued for women determined to be postmenopausal. Recruitment and follow-up were completed in late June 2015, with women followed for one to seven years, based on time of enrollment and menopause status at year four.
Serum extracted from the collected blood samples was used to measure estradiol levels in each sample using commercially available, previously validated enzyme-linked immunosorbent assay (ELISA) kits (DRG, Springfield, New Jersey, USA) [22–25]. The minimum detection limits and intra-assay coefficients of variation were seven pg/ml and 3.3 ± 0.17%, respectively. The average inter-assay coefficient of variation for all assays was less than five. For estradiol concentrations among all women enrolled in the study, the number of values below the limit of detection during year one were: visit 1, n=7; visit 2, n=3; visit 3, n=13; visit 4, n=6. In the case of values lower than the detection limits for the assay, we used the limit of detection as the hormone value. Each sample was measured in duplicate within the same assay. Totals from the four samples in each year were averaged to account for variability in day of menstrual cycle, as participants were not expected to be able to schedule initial clinic visits on a particular day of their menstrual cycle due to the unpredictability of cycles during the menopausal transition. Estradiol levels were log-transformed to meet normality assumptions.
2.2 Dynamics of Sleep Outcomes
To determine the persistence of poor sleep through the menopausal transition, we calculated the probability of insomnia or sleep disturbances persisting from premenopause to perimenopause (pre-peri analysis) and the probability of insomnia or sleep disturbances persisting from perimenopause to postmenopause (peri-post analysis). Participants were assigned a menopause status for each year of the study. Women who transitioned from premenopause to perimenopause during the study were included in the pre-peri analysis, and women who transitioned from perimenopause to postmenopause during the study were included in the peri-post analysis. For each woman-stage combination, the worst responses for each sleep outcome (the most frequent occurrences of insomnia and sleep disturbances) were determined. The probability of insomnia or sleep disturbances persisting between stages was calculated using a proportional odds logistic regression model, where the outcome variable was the Likert value at the later stage and the predictor variable was the Likert value at the earlier stage. The predictor variable was considered as a linear variable, a categorical variable, or an ordinal variable. All models were fit with the polr function in the MASS package [26] in R 3.4.1 [27].
2.3 Interactions Among Sleep Outcomes
To determine the degree of correspondence among the three sleep outcomes (insomnia, sleep disturbances, and restless sleep) at the baseline clinic visit, Kendall’s tau was calculated for each two-way comparison using the Kendall package [28] in R 3.4.1 [27].
2.4 Risk Factors for Poor Sleep
To determine the risk factors for poor sleep outcomes, ordinal logistic regression models were fit to each of the three outcomes. For sleep disturbance and insomnia, all years of data were included by using random effects for repeated measures within women by year of study; for restless sleep, only the first year of data was included and the analysis was cross-sectional in nature. First, univariable models were fit for the variables menopause status, age, race, BMI, income level, health status, hot flashes, night sweats, hot flash severity, hot flash frequency, depression frequency, serum estradiol, education level, frequency of alcohol consumption, smoking status, and leisure activity levels. Race was limited to black and white, as the number of individuals of other races in the study was low. Income level was defined as low (<$35,000/year), moderate ($35,000–$75,000/year), and high (>$75,000/year). Health status was defined as response to “in general, how would you describe your health at present?”. Hot flashes were defined as response to “have you experienced hot flashes in the last year?” (for which the answers were yes, no, or don’t know). Night sweats were defined as response to “on average, how many hot flashes do you experience every night (between 9:00 p.m. and 6:00 a.m.)?” and were considered as both a binary variable (any hot flashes at night vs. no hot flashes at night) and a categorical variable for the number of hot flashes at night (none, one, two, three, four or more, or don’t know). Hot flash severity was defined as response to “how would you describe the majority of your hot flashes?” and was categorized as none, mild (sensation of heat without sweating), moderate (sensation of heat with sweating), and severe (sensation of heat with sweating that disrupts your usual activity). Hot flash frequency was defined as response to “generally, how often do you experience hot flashes?” and was categorized as none, monthly, weekly, daily, or don’t know. Depression frequency was categorized as never, rarely (<one/month), sometimes (one-four/month), frequently (two-four/week), and regularly (>five/week); responses to this question at baseline were validated against the CES-D scale [29]. Education level was categorized as no college attendance, some college or technical school attendance, graduated from college or technical school, and/or at least some graduate school attendance. Frequency of alcohol consumption was a linear variable, representing the number of days drinking alcohol in the last year; those reporting that they did not consume at least 12 drinks in the last year and declining to answer the number of days drinking alcohol were assumed to have had zero drinks in the last year. Smoking was categorized as current, former, or never. Leisure activity levels were categorized as compared to other women in the age group (much less, less, as much, more, and much more).
Multivariable models were fit to each of the outcomes using additive stepwise model selection based on the Wald test statistic. Variables were added to the model if the p-value of the Wald statistic was <0.05, with the order of addition determined by the smallest p-value. Multivariable model selection was repeated for each outcome based on subsets of the dataset representing premenopause, perimenopause, and postmenopause. Models for sleep disturbance and insomnia were fit using the OrdLorGee function in the multgee package [30] and models for restless sleep were fit using the polr function in the MASS package [26] in R 3.4.1 [27].
3. Results
3.1 Data collection
A total of 776 women provided data for this analysis. Of these, 191 provided one year of data, 104 provided two years of data, 91 provided three years of data, 231 provided four years of data, and the remaining 159 provided between five and seven years of data, for a total of 2,479 observations. During the study, 436 women transitioned from premenopausal to perimenopausal, and 219 women transitioned from perimenopausal to postmenopausal. In total, 51 women did not respond to the question about the frequency of sleep disturbances; the distribution among those answering the question across all years of the study was: never (395), rarely or <one per month (520), sometimes or one-four per month (726), frequently or two-four per week (517), and regularly or >five per week (270). A total of 56 women did not respond to the question about the frequency of insomnia; the distribution among those answering the question across all years of the study was: never (717), rarely or <one per month (619), sometimes or one-four per month (573), frequently or two-four per week (347), and regularly or >five per week (167). During the baseline survey, seven women did not respond to the question about the frequency of restless sleep; the distribution among those answering was: rarely (307), some of the time (254), moderately (117), and most of the time (91).
3.2 Dynamics of Sleep Outcomes
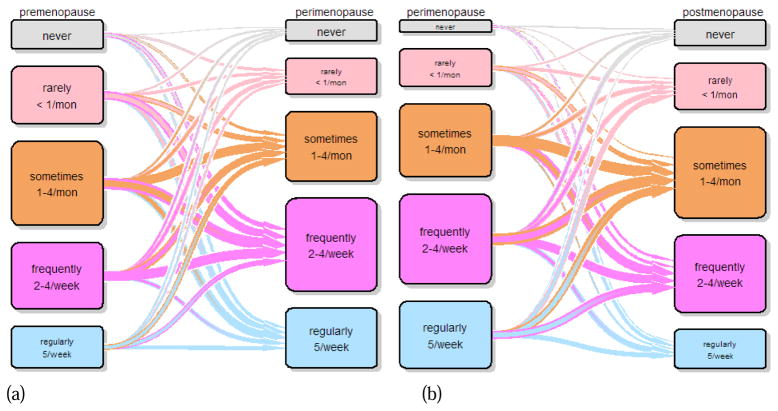
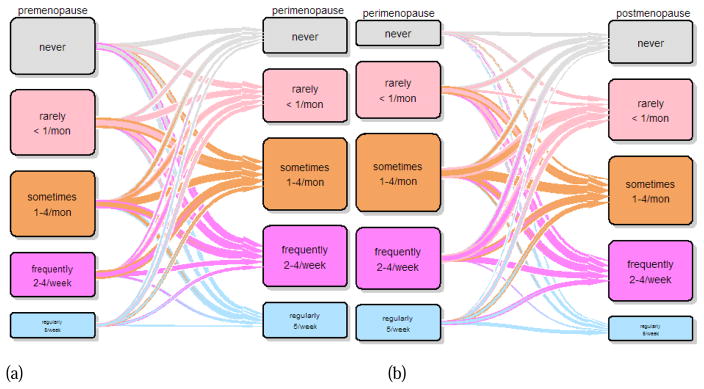
Sleep disturbance level in premenopause was not predictive of sleep disturbance level in perimenopause, nor was sleep disturbance level in perimenopause predictive of sleep disturbance level in postmenopause (Figure 1). Insomnia level in premenopause was not predictive of insomnia level in perimenopause (Figure 2). Women reporting insomnia regularly (more than five times a week) during perimenopause were significantly more likely to report higher levels of insomnia during postmenopause when perimenopausal insomnia levels were reported as factors (OR=3.6, 95% CI=1.29–10.36, p=0.01) or as ordinal variables (OR=2.9, 95% CI=1.30–6.57, p=0.01), but the effect was not noted with linear variables or the logistic regression analysis.
Figure 1.
Transition plot for frequency of sleep disturbance from (a) premenopause to perimenopause and (b) perimenopause to postmenopause
Figure 2.
Transition plot for frequency of insomnia from (a) premenopause to perimenopause and (b) perimenopause to postmenopause
3.3 Interactions Among Sleep Outcomes
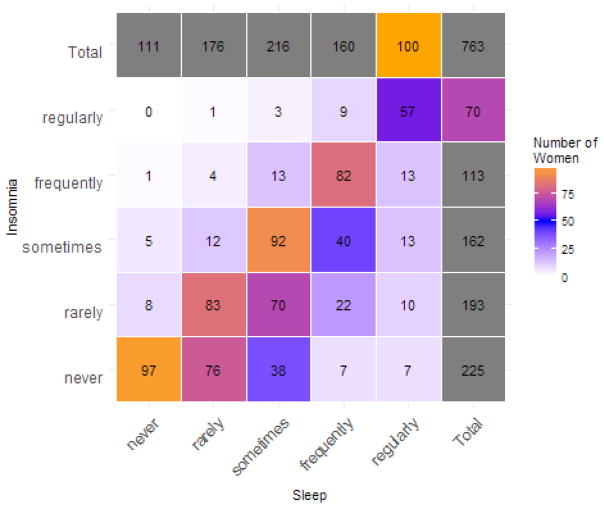
We found a high degree of correlation between the three sleep variables (insomnia, sleep disturbances, and restless sleep). The correlation values were as follows: between insomnia and sleep disturbances (τ = 0.636), insomnia and restless sleep (τ = 0.591), and sleep disturbances and restless sleep (τ = 0.614). The cross-tabulation of responses by individual at baseline for sleep disturbances and insomnia are shown in Figure 3 as an example. All two-way interactions were significant (p < 10−16 for all comparisons), showing that responses to all questions about sleep were significantly correlated with each other.
Figure 3.
Cross-tabulation of answers to questions about frequency of sleep problems at baseline in the Midlife Women’s Health Study, between sleep disturbances and insomnia
3.4 Risk Factors for Poor Sleep
The description of risk factors at baseline are shown in Table 1.
Table 1.
Description of the study population at baseline.
| Categorical Variable | Level | Number of Women |
|---|---|---|
| Insomnia | never | 228 |
| rarely (<one/month) | 193 | |
| sometimes (one-four/month) | 163 | |
| frequently (two-four/week) | 113 | |
| regularly (≥five/week) | 70 | |
| Sleep Disturbance | never | 111 |
| rarely (<one/month) | 177 | |
| sometimes (one-four/month) | 216 | |
| frequently (two-four/week) | 162 | |
| regularly (≥five/week) | 100 | |
| Restless Sleep | rarely | 307 |
| some of the time | 254 | |
| moderately | 117 | |
| most of the time | 91 | |
| Menopause Status | pre | 358 |
| peri | 238 | |
| post | 1 | |
| Hot flashes in last year | no | 330 |
| don’t know | 9 | |
| yes | 258 | |
| Number of hot flashes at night | none | 403 |
| don’t know | 38 | |
| one | 82 | |
| two | 37 | |
| three | 25 | |
| four or more | 12 | |
| Any hot flashes at night | no | 561 |
| yes | 205 | |
| Severity of hot flashes | none | 339 |
| mild | 90 | |
| moderate | 141 | |
| severe | 27 | |
| Frequency of hot flashes | none | 339 |
| dont know | 20 | |
| monthly | 107 | |
| weekly | 70 | |
| daily | 61 | |
| Frequency of depression | never | 206 |
| < one/mon | 214 | |
| one-four/mon | 122 | |
| two-four/week | 30 | |
| > five/week | 25 | |
| Education | < college | 52 |
| some college | 145 | |
| graduated college | 181 | |
| graduate level | 219 | |
| Smoking status | Never | 308 |
| Former | 225 | |
| Current | 64 | |
| Leisure activity level | much less | 44 |
| less | 148 | |
| as much | 189 | |
| more | 146 | |
| much more | 70 | |
| Race | black | 137 |
| white | 427 | |
| Income | low | 63 |
| moderate | 149 | |
| high | 352 | |
| Health | poor | 5 |
| fair | 44 | |
| good | 179 | |
| very good | 209 | |
| excellent | 127 | |
| Continuous Variable | Median | Range |
| Age | 50 | 45, 61 |
| Estradiol | 49.8 | 5.6, 766.4 |
| Number of days consuming alcohol in last year | 3 | 0, 31 |
| BMI | 27.2 | 15.1, 66.3 |
Due to the high level of correlation between results, only results for insomnia are presented here. Results for sleep disturbances and restless sleep are available in the supplementary material. The univariable analysis results of the potential risk factors for insomnia are shown in Table 2. The frequency of sleep disturbances and insomnia were significantly increased in peri- and post-menopausal women by hot flashes, including increasing severity, frequency, and number of night sweats; by increasing frequency of depression; by smoking; and by increasing age. The frequency of sleep disturbances was also significantly increased by decreasing leisure activity levels, and the frequency of insomnia was increased by decreasing estradiol levels. The frequency of restless sleep at baseline was significantly increased in peri-menopausal women, by hot flashes, including increasing severity, frequency, and number of night sweats; by increasing frequency of depression; by smoking; and by increasing age.
Table 2.
Description of variables and their univariable association with insomnia throughout the Women’s Midlife Health Study, based on an ordinal logistic regression model
| Categorical Variable | Level | Number of Women | Coefficient | p-value | Wald p-value |
|---|---|---|---|---|---|
| Menopause Status | pre | 1117 | 0 | base | <0.001 |
| peri | 981 | 0.379 | <0.001 | ||
| post | 340 | 0.449 | <0.001 | ||
| Hot flashes in last year | no | 1101 | 0 | base | <0.001 |
| don’t know | 128 | 0.087 | 0.509 | ||
| yes | 1215 | 0.362 | <0.001 | ||
| Number of hot flashes at night | none | 1514 | 0 | base | <0.001 |
| don’t know | 153 | 0.361 | 0.007 | ||
| one | 361 | 0.319 | 0.001 | ||
| two | 214 | 0.554 | <0.001 | ||
| three | 118 | 0.559 | <0.001 | ||
| four or more | 69 | 0.602 | 0.007 | ||
| Any hot flashes at night | no | 1667 | 0 | base | <0.001 |
| yes | 762 | 0.37 | <0.001 | ||
| Severity of hot flashes | none | 1228 | 0 | base | <0.001 |
| mild | 445 | 0.243 | 0.003 | ||
| moderate | 670 | 0.467 | <0.001 | ||
| severe | 89 | 0.587 | 0.005 | ||
| Frequency of hot flashes | none | 1228 | 0 | base | <0.001 |
| don’t know | 68 | 0.223 | 0.227 | ||
| monthly | 422 | 0.215 | 0.012 | ||
| weekly | 349 | 0.485 | <0.001 | ||
| daily | 367 | 0.538 | <0.001 | ||
| Frequency of depression | never | 981 | 0 | base | <0.001 |
| < one/mon | 812 | 0.537 | <0.001 | ||
| one-four/mon | 459 | 1.012 | <0.001 | ||
| two-four/week | 113 | 1.371 | <0.001 | ||
| > five/week | 68 | 1.571 | <0.001 | ||
| Education | < college | 233 | 0 | base | 0.733 |
| some college | 618 | 0.269 | 0.26 | ||
| graduated college | 757 | 0.201 | 0.389 | ||
| graduate level | 862 | 0.192 | 0.403 | ||
| Smoking status | Never | 1323 | 0 | base | 0.001 |
| Former | 892 | 0.329 | 0.004 | ||
| Current | 229 | 0.574 | 0.001 | ||
| Leisure activity level | much less | 92 | 0 | base | 0.069 |
| less | 334 | 0.158 | 0.381 | ||
| as much | 446 | −0.05 | 0.794 | ||
| more | 298 | −0.059 | 0.768 | ||
| much more | 132 | −0.367 | 0.126 | ||
| Race | black | 728 | 0 | base | 0.13 |
| white | 1653 | −0.198 | 0.13 | ||
| Income level | low | 296 | 0 | base | 0.322 |
| moderate | 663 | 0.14 | 0.277 | ||
| high | 1436 | 0.21 | 0.135 | ||
| Health status | poor | 21 | 0 | base | <0.001 |
| fair | 179 | 1.158 | 0.076 | ||
| good | 769 | 1.494 | 0.017 | ||
| very good | 993 | 1.762 | 0.005 | ||
| excellent | 475 | 1.915 | 0.002 | ||
| Continuous Variable | Median | Range | Coefficient | p-value | |
| Age | 50 | 45, 61 | 0.036 | 0.011 | |
| Estradiol | 49.8 | 5.6, 766.4 | −0.002 | 0.037 | |
| Number of days consuming alcohol in last year | 3 | 0, 31 | 0.011 | 0.039 | |
| BMI | 27.2 | 15.1, 66.3 | −0.019 | 0.018 | |
The final multivariable model for insomnia (Table 3) included health status, depression frequency, menopause status, experience of night sweats, and smoking status. There was again a clear trend for increasing risk of insomnia as depression frequency increased; women with regular depression were 4.8 times as likely to have more frequent insomnia as women without depression. Both perimenopausal and postmenopausal women were approximately 1.4 times as likely to have more frequent insomnia as premenopausal women. Women who had night sweats were 1.3 times as likely to have more frequent insomnia as women who did not have insomnia, and smokers were 1.2–1.4 times as likely to have more frequent insomnia as women who never smoked. When only postmenopausal women were included, only depression frequency remained in the final model for insomnia, with a slightly larger coefficient value than in the full model, indicating that in these women the risk of insomnia is more strongly correlated with depression than in perimenopausal women. When only perimenopausal women were included, the effects of depression were similar to the model based on all women. In addition, the odds of higher levels of insomnia increased with frequency of night sweats, with women having four or more night sweats per week 4.3 times as likely to have more frequent insomnia as women with no night sweats. When only premenopausal women were included, the effects of depression were slightly increased; in addition, the overall frequency of hot flashes was added to the model, with an increased risk of more frequent insomnia as the frequency of hot flashes increased.
Table 3.
Final multivariable ordinal logistic regression model for insomnia frequency throughout the Midlife Women’s Health Study
| Variable | level | Estimate | SE | Z | P-value |
|---|---|---|---|---|---|
| Health status (compared to poor) | fair | −0.953 | 0.537 | 1.775 | 0.076 |
| good | −1.272 | 0.498 | 2.557 | 0.011 | |
| very good | −1.292 | 0.497 | 2.598 | 0.009 | |
| excellent | −1.453 | 0.514 | 2.827 | 0.005 | |
| Depression frequency (compared to none) | < one/mon | 0.660 | 0.131 | −5.043 | <0.001 |
| one-four/mon | 1.221 | 0.158 | −7.745 | <0.001 | |
| two-four/week | 1.779 | 0.256 | −6.944 | <0.001 | |
| five/week | 1.409 | 0.315 | −4.475 | <0.001 | |
| Menopause status (compared to premenopause) | perimenopause | 0.406 | 0.119 | −3.423 | 0.001 |
| postmenopause | 0.366 | 0.170 | −2.154 | 0.031 | |
| Any hot flashes at night | 0.285 | 0.120 | −2.371 | 0.018 | |
| Smoking status (compared to never) | Former smoker | 0.340 | 0.139 | −2.450 | 0.014 |
| Current smoker | 0.132 | 0.209 | −0.632 | 0.528 | |
4. Discussion
This study shows that the risk factors for poor sleep are consistent and stable throughout the menopausal transition. However, as the most consistent of these risk factors are often time-varying, the experience of poor sleep does not appear to be consistent as women transition from premenopause, through perimenopause, and into postmenopause. The one exception is women with frequent insomnia (more than five times a week) during perimenopause, who were more likely to experience higher levels of insomnia in postmenopause; which corresponds with previous findings that poor sleep during the menopausal transition is associated with poor sleep prior to menopause [12]. The lack of association for all but the most frequent insomnia suggests that it is the change in risk factors, rather than the direct biological effects of menopause, that are responsible for most sleep difficulties during the menopausal transition.
It is important to note that the correlations between outcome variables (sleep disturbances, insomnia, and restless sleep) were large and significant. Women who did not report sleep disturbances were unlikely to report insomnia or restless sleep. In contrast, regular sleep disturbances were often reported in conjunction with regular insomnia or restless sleep. It is understandable that self-reported sleep quality measures are highly correlated, either due to shared underlying causes or to failure of women to distinguish between types of poor sleep.
One risk factor was found to be consistently and significantly associated with all sleep outcomes in all multivariable models: frequency of depression. Many other studies have also found that poor sleep is associated with depression [3,8,9,17], including during the menopausal transition [7,11]. The strength and consistency of this relationship suggest that poor sleep in menopausal women is highly correlated with self-reported feelings of depression. This supports current clinical recommendations, which suggest that psychological treatment should play a more long-term role in treatment of sleep disorders than medication, which can be habit forming [21].
Hot flashes and, especially, night sweats were also found to be highly associated with poor sleep. While other studies have also found relationships between vasomotor symptoms and sleep difficulties [3,4,7,9,11–14,17,31], we have found that night sweats were more consistently associated with these outcomes, despite the high level of correlation between the frequencies of hot flashes and night sweats. This is potentially a direct effect of discomfort during night sweats preventing or disturbing sleep, but may be related to a common mechanism underlying hot flashes and arousal. Further research should determine if treatment of the underlying problem (vasomotor symptoms) might be effective for preventing or treating sleep difficulties.
Smoking has been indicated previously as a risk factor for poor sleep during menopause [8,10]. We also found that smoking was associated with sleep disturbances and insomnia. Although we previously found an association between smoking and vasomotor symptoms in the same study population[32], the impact of smoking in this study remains even when accounting for the impact of hot flashes. Therefore, there is likely another pathway by which smoking leads to poor sleep. It has been suggested that smoking is related to anxiety [8], which could be related to sleep difficulties [9]. However, that relationship has not been tested, and the current study did not measure anxiety. Further research should examine this relationship, especially as the menopausal transition has potential to trigger anxiety.[33]
Other studies have suggested that exercise and alcohol intake may be confounders in studies of poor sleep. The potential for these variables to be confounders in this analysis was formally assessed by comparing the fitted coefficient of each final multivariable model with the fitted coefficients of the same model with either leisure activity level or frequency of alcohol consumption added. The addition of the potential confounders did not change the coefficients of any of the models by more than 14%, and in most cases the proportional change in the fitted coefficients was less than five%.
This study is limited by the use of self-reported sleep outcomes, rather than objective measures. However, self-reported measures are considered appropriate for determining clinically relevant outcomes [21], as they represent the experience of the individual. Validated questions were used when available, but the single questions to assess insomnia and depression would not have been as specific as the use of a validated scale; future research may be able to improve the rigor of the analysis by using the CES-D to assess depression and assessment tools such as the Pittsburgh Sleep Quality Index [34]. We were also limited in the number of post-menopausal women for analysis, as the study design called for enrollment only of premenopausal and perimenopausal women and for cessation of data collection from women who had become post-menopausal by year four. Further study of these effects in postmenopausal women should rely on adapted study designs to capture more observations of this group. Definition of menopause stage was also based on menstrual symptoms alone, rather than in combination with hormone levels, which may have decreased the specificity of our categorizations. In addition, many of the potential variables for the risk factor analysis were highly correlated; we used a forward stepwise model selection procedure in order to identify the variable that best explains the outcome within the final multivariable models.
Results were found to vary for time-based associations by assumptions on the structure of the sleep variables, whether ordinal or linear. Likert scales are often translated into linear variables, but this simplification is not recommended for two reasons: first, this assumes that the categories are equally spaced, which may not be true; and second, linear models assume a normal distribution in the residuals, which is most certainly not true for Likert scales. Our findings, in which an association between insomnia in perimenopause and postmenopause was only evident with categorical analyses, reinforces this technical recommendation.
Based on the results of this study, we recommend that women suffering from poor sleep during the menopausal transition be examined and treated for depression and vasomotor symptoms. Women should also be counseled that changes in sleep quality occur during the menopausal transition, but that poor sleep early in menopause is not correlated with poor sleep later in menopause.
Supplementary Material
Highlights.
Poor sleep in one menopause stage does not predict poor sleep later in menopause.
Depression and hot flashes are consistent risk factors for poor sleep in menopause.
Insomnia, sleep disturbances, and restless sleep commonly co-occur.
Acknowledgments
We acknowledge Lisa Gallicchio, Susan Miller, Judith Keifer, Teresa Greene and Howard Zacur for their help with recruitment on this study.
Funding: This work was supported by the National Institutes of Health (grant number R01 ES 026956, 2017) and the Carle Illinois Collaborative Research Seed Program (2017).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freedman RR. Menopause and sleep. Menopause. 2014;21:534–5. doi: 10.1097/GME.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 2.Polo-Kantola P. Sleep disturbances at the menopause. Maturitas. 2015;81:109. doi: 10.1016/j.maturitas.2015.02.025. [DOI] [Google Scholar]
- 3.Tom SE, Kuh D, Guralnik JM, Mishra G. Self-reported sleep difficulty during the menopausal transition: Results From a Prospective Cohort Study. Menopause. 2010;17:1128–35. doi: 10.1097/gme.0b013e3181dd55b0.Self-reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kravitz H, Zhao X, Bromberger J, Gold E. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep. 2008;31:979–90. [PMC free article] [PubMed] [Google Scholar]
- 5.Lampio L, Polo-Kantola P, Himanen S-L, Kurki S, Huupponen E, Engblom J, et al. Sleep During Menopausal Transition: A 6-Year Follow-Up. Sleep. 2017;40:165–72. doi: 10.1093/sleep/zsx090. [DOI] [PubMed] [Google Scholar]
- 6.Hachul H, Frange C, Bezerra AG, Hirotsu C, Pires GN, Andersen ML, et al. The effect of menopause on objective sleep parameters: Data from an epidemiologic study in São Paulo, Brazil. Maturitas. 2015;80:170–8. doi: 10.1016/j.maturitas.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: A community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Frías C, Figueroa-Vega NN, Malacara JM. Relationship of sleep alterations with perimenopausal and postmenopausal symptoms. Menopause. 2014;21:1017–22. doi: 10.1097/GME.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 9.Shaver JL, Woods NF. Sleep and menopause: a narrative review. Menopause. 2015;22:899–915. doi: 10.1097/GME.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q, Lang CP. Examining the relationship between subjective sleep disturbance and menopause. Menopause. 2014;21:1301–18. doi: 10.1097/GME.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 11.Pien GW, Sammel MD, Freeman EW, Lin H, DeBlasis TL. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31:991–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman EW, Sammel MD, Gross SA, Pien GW. Poor sleep in relation to natural menopause. Menopause. 2015;22:719–26. doi: 10.1097/GME.0000000000000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun D, Shao H, Li C, Tao M. Sleep disturbance and correlates in menopausal women in Shanghai. J Psychosom Res. 2014;76:237–41. doi: 10.1016/j.jpsychores.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Lampio L, Polo-Kantola P, Polo O, Kauko T, Aittokallio J, Saaresranta T. Sleep in midlife women. Menopause. 2014;21:1217–24. doi: 10.1097/GME.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Santos C, Saura CB, Lucas JAR, Castell P, Madrid JA, Garaulet M. Menopause status is associated with circadian- and sleep-related alterations. Menopause. 2016;23:682–90. doi: 10.1097/GME.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 16.Mirer AG, Young T, Palta M, Benca RM, Rasmuson A, Peppard PE. Sleep-disordered breathing and the menopausal transition among participants in the Sleep in Midlife Women Study. Menopause. 2017;24:157–62. doi: 10.1097/GME.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vousoura E, Spyropoulou AC, Koundi KL, Tzavara C, Verdeli H, Paparrigopoulos T, et al. Vasomotor and depression symptoms may be associated with different sleep disturbance patterns in postmenopausal women. Menopause. 2015;22:1053–7. doi: 10.1097/GME.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 18.de Zambotti M, Sugarbaker D, Trinder J, Colrain IM, Baker FC. Acute stress alters autonomic modulation during sleep in women approaching menopause. Psychoneuroendocrinology. 2016;66:1–10. doi: 10.1016/j.psyneuen.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziv-gal A, Smith RL, Gallicchio L, Miller SR, Zacur HA, Flaws JA. The Midlife Women’s Health Study – a study protocol of a longitudinal prospective study on predictors of menopausal hot flashes. 2017:1–11. doi: 10.1186/s40695-017-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlson CW, Gallagher MW, Olson CA, Hamilton NA. Insomnia symptoms and well-being: Longitudinal follow-up. Heal Psychol. 2013;32:311–9. doi: 10.1037/a0028186. [DOI] [PubMed] [Google Scholar]
- 21.Tal Z, Suh JA, Dowdle SL, Nowakowski CS. Treatment of Insomnia, Insomnia Symptoms, and Obstructive Sleep Apnea During and After Menopause: Therapeutic Approaches. n.d doi: 10.2174/1573400510666140929194848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cochran CJ, Gallicchio LM, Miller SR, Zacur H, Flaws JA. Cigarette Smoking, Androgen Levels, and Hot Flushes in Midlife Women. Obs Gynecol. 2008;112:1037–44. doi: 10.1097/AOG.0b013e318189a8e2.Cigarette. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallicchio LM, Schilling C, Romani WA, Miller SR, Zacur H, Flaws JA. Endogenous hormones, participant characteristics, and symptoms among midlife women. Maturitas. 2008;59:114–27. doi: 10.1016/j.maturitas.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallicchio LM, Visvanathan K, Miller SR, Babus JK, Lewis LM, Zacur HA, et al. Body mass, estrogen levels, and hot flashes in midlife women. Am J Obstet Gynecol. 2005;193:1353–60. doi: 10.1016/j.ajog.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Visvanathan K, Gallicchio LM, Schilling C, Babus JK, Lewis LM, Miller SR, et al. Cytochrome gene polymorphisms, serum estrogens, and hot flushes in midlife women. Obstet Gynecol. 2005;106:1372–81. doi: 10.1097/01.AOG.0000187308.67021.98. [DOI] [PubMed] [Google Scholar]
- 26.Venables WN, Ripley BD. Modern Applied Statistics with S. 4. New York: Springer; 2002. [Google Scholar]
- 27.The R Development Core Team. R: A Language and Environment for Statistical Computing. 2009. [Google Scholar]
- 28.McLeod AI. Kendall: Kendall rank correlation and Mann-Kendall trend test. 2011. [Google Scholar]
- 29.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 30.Touloumis A. GEE Solver for Correlated Nominal or Ordinal Multinomial Responses. 2014. [Google Scholar]
- 31.Pinkerton JV, Abraham L, Bushmakin AG, Cappelleri JC, Komm BS. Relationship between changes in vasomotor symptoms and changes in menopause-specific quality of life and sleep parameters. Menopause. 2016;23:1060–6. doi: 10.1097/GME.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 32.Smith RL, Flaws JA, Gallicchio LM. Does quitting smoking decrease the risk of midlife hot flashes? A longitudinal analysis. Maturitas. 2015;82:123–7. doi: 10.1016/j.maturitas.2015.06.029. doi: http://dx.doi.org/10.1016/j.maturitas.2015.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35:320–30. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, III, CFR, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.