Abstract
Rationale
Post-ischemic angiogenesis is critical to limit the ischemic tissue damage and improve the blood flow recovery. The regulation and the underlying molecular mechanisms of post-ischemic angiogenesis are not fully unraveled. Transcription factor-EB (TFEB) is emerging as a master gene for autophagy and lysosome biogenesis. However, the role of TFEB in vascular disease is less understood.
Objective
We aimed to determine the role of endothelial TFEB in post-ischemic angiogenesis and its underlying molecular mechanism.
Methods and Results
In primary human endothelial cells (ECs), serum starvation induced TFEB nuclear translocation. Vascular endothelial growth factor (VEGF) increased TFEB expression level and nuclear translocation. Utilizing genetically-engineered endothelial cell (EC) specific TFEB transgenic and knockout (KO) mice, we investigated the role of TFEB in post-ischemic angiogenesis in the mouse hind-limb ischemia model. We observed improved blood perfusion and increased capillary density in the EC-specific TFEB transgenic mice compared with the wild-type littermates. Furthermore, blood flow recovery was attenuated in EC-TFEB KO mice compared with control mice. In aortic ring cultures, the TFEB transgene significantly increased vessel sprouting, while TFEB deficiency impaired the vessel sprouting. In vitro, adenovirus-mediated TFEB overexpression promoted EC tube formation, migration and survival whereas small interfering RNA (siRNA)-mediated TFEB knockdown had the opposite effect. Mechanistically, TFEB activated AMP-activated protein kinase (AMPK)-α signaling and upregulated autophagy. Through inactivation of AMPKαor inhibition of autophagy, we demonstrated that the AMPKα and autophagy are necessary for TFEB to regulate angiogenesis in ECs. Finally, the positive effect of TFEB on AMPKα activation and EC tube formation was mediated by TFEB-dependent transcriptional upregulation of MCOLN1.
Conclusions
In summary, our data demonstrate that TFEB is a positive regulator of angiogenesis through activation of AMPKα and autophagy, suggesting that TFEB constitutes a novel molecular target for ischemic vascular disease.
Keywords: Endothelial cells, angiogenesis, ischemia, AMPKα, autophagy
Subject Terms: Angiogenesis, Animal Models of Human Disease, Endothelium/Vascular Type/Nitric Oxide, Vascular Disease
Introduction
Angiogenesis is critical to tissue regeneration or repair under conditions such as wound healing, muscle and bone repair, hypoxia, and chronic ischemia.1 Critical limb ischemia (CLI) is one of the most advanced peripheral arterial diseases (PAD) that links to a high risk of cardiovascular events including stroke and myocardial infarction2 and is associated with the main causes of mortality worldwide.3 The first in-human gene therapies for treatment of CLI were attempted in the late 1990s.4, 5 To date, besides endovascular treatment, few therapeutic alternatives to restore the blood flow in ischemic tissues are available.6 Therefore, it would be of significance to identify pivotal regulators of angiogenesis as therapeutic targets to promote blood flow recovery in vivo.7
Some transcription factors have been identified as essential for angiogenesis such as HIF1α, HoxD3 and COUP-TFII.8 TFEB has been demonstrated as a crucial regulator of lysosomal biogenesis and autophagy.9-11 Accumulated studies suggest that TFEB has beneficial effects on neurodegenerative disease such as Huntington's disease (HD)and Parkinson disease (PD)12, 13 and also regulates cell clearance in lysosomal storage disorders (LSDs).14 Recently, TFEB has been revealed to have an anti-apoptotic effect in the heart and to inhibit atherosclerosis.15-18
Noteworthy, TFEB deficient mice exhibit severe defects in placental vascularization and die during embryonic development.19 However, the role of TFEB in vascular biology still remains largely unknown. Consequently, the current study sought to define whether TFEB influences endothelial cell function and regulates post-ischemic angiogenesis. In the present work, utilizing genetically-engineered EC-specific TFEB transgenic mice, we found that TFEB functions as a positive regulator of angiogenesis with capability to modulate EC migration, tube formation, and apoptosis, and improve post-ischemic blood flow recovery. Mechanistically, TFEB activates AMPKα signaling and upregulates autophagy. Our findings uncovered a novel direct link between TFEB and post-ischemic angiogenesis and established a unique feedforward loop between TFEB and AMPKα.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
The data that support the findings of this study are available from the corresponding author upon reasonable request. Microarray data has been submitted to the NCBI Gene Expression Omnibus (GEO) database in MIAME format.
Results
TFEB is upregulated in ischemic skeletal muscle
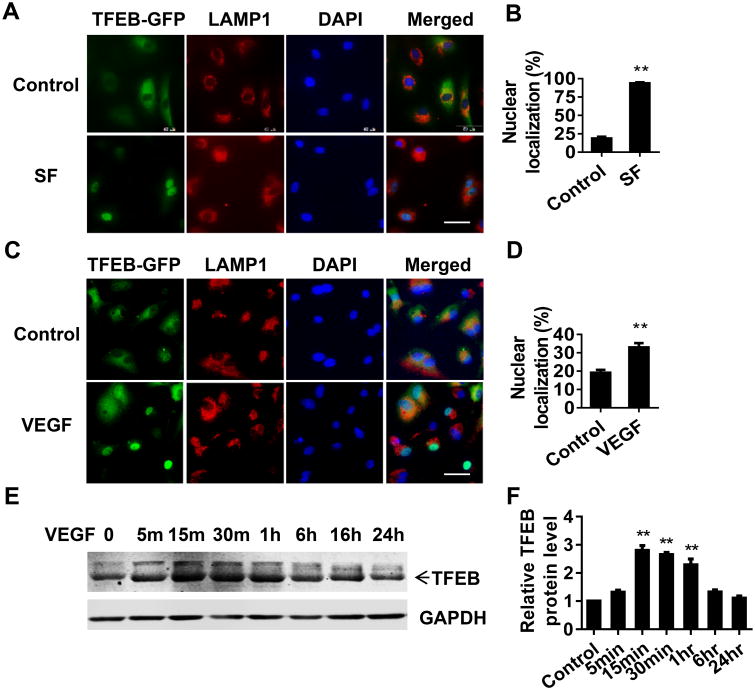
In response to ischemia, a cluster of anti-angiogenic and pro-angiogenic genes are dynamically downregulated or upregulated in mice.20 Autophagy as a critical cellular process may also be involved in the post-ischemic tissue regeneration. To determine whether TFEB, an autophagy master gene, is responsive to ischemia, we analyzed the expression of TFEB in the ischemic skeletal muscle at day 3 after ischemia. TFEB was upregulated to an average of 3.1-fold and 3-fold at the mRNA and protein levels, respectively, when compared to sham control (Online Figure IA-B). To further validate the expression of TFEB in ECs, we performed immunostaining for TFEB and CD31, a specific marker of mature EC. As shown in Online Figure IC, TFEB fluorescence intensity was stronger in the ischemic micro-environment in vivo and CD31 positive cells expressed higher TFEB protein. Nutrition depletion is a major component of ischemia in vivo. Of note, under normal growth conditions, TFEB was localized in the lysosome, but it translocated to the nucleus in serum-free medium (Figure 1A-B). LAMP1 was used as a lysosome marker. Interestingly, we observed increased TFEB translocation from the lysosomes to the nucleus in ECs upon VEGF stimulation (Figure 1C-D). VEGF treatment also increased TFEB protein expression in a rapid manner, even as short as 15min after VEGF treatment (Figure 1E-F). Taken together, our data suggest that TFEB is a nutrient-and pro-angiogenic factor-responsive transcription factor and, consequently, may play a role in the adaptation to the complicated ischemic micro-environmental conditions.
Figure 1. TFEB is responsive to pro-angiogenic stimuli.
A-B, TFEB nuclear translocation induced by serum deprivation. HUVECs were infected with Ad-TFEB-EGFP (10 MOI). Twenty hours later, the cells were cultured in 10% FBS DMEM (Control) or serum-free (SF) DMEM for 5 h. The subcellular location of TFEB was determined by GFP fluorescence. LAMP1 staining was used as marker of lysosomes. Representative images are shown in A and quantitatively analyzed in B. Scale bar= 50μm. C-D, TFEB translocation induced by VEGF. HCAECs were infected with Ad-TFEB-EGFP (10 MOI). Twenty hours later, the cells were treated with VEGF (10 ng/mL) or no treatment (control) in 10% FBS DMEM for 4 h. Representative images of TFEB-EGFP fluorescence and LAMP1 immunofluorescence are shown in C and TFEB nuclear translocation was quantitatively analyzed in D. Scale bar= 50 μm. E-F, VEGF induces TFEB protein expression. Under normal growth medium, HCAECs were stimulated with VEGF (10 ng/mL) for the indicated time. The expression of TFEB was detected by Western blot (E). Band intensity of TFEB was quantitatively analyzed and normalized against GAPDH (F). Three independent experiments were performed in all cases, and data are presented as mean ± SEM. ** P<0.01.
Increased angiogenesis and improved blood flow recovery in EC-TFEB transgenic mice after ischemic injury
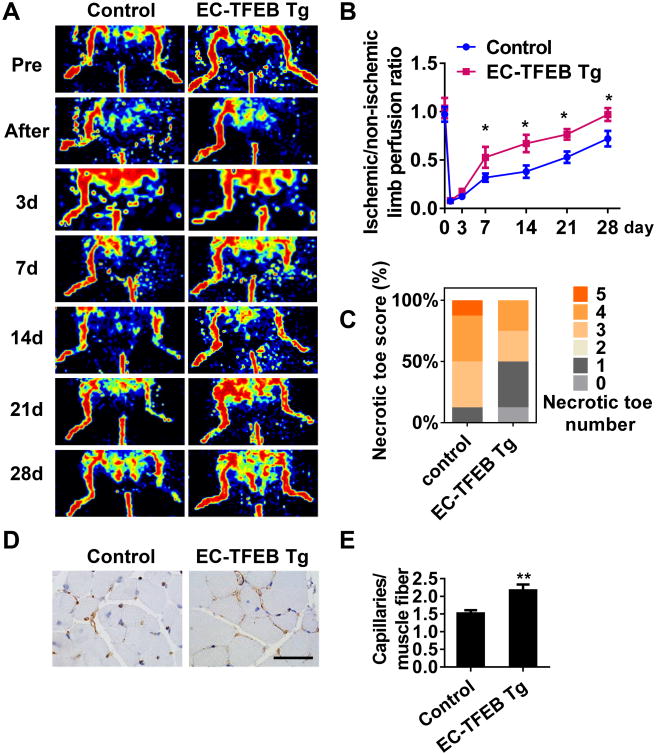
To determine the role of endothelial TFEB in angiogenesis in vivo, we generated an EC-specific TFEB transgenic (EC-TFEB Tg) mouse expressing the human TFEB transgene from a mouse Tie2 promoter. The genotyping of transgenic mice (Online Figure IIA), detection of TFEB transgene expression in both isolated mouse aortic endothelial cells (MAECs) in vitro (Online Figure IIB) and aortic ECs in vivo (Online Figure IIC), demonstrated successful transgenesis in the transgenic mouse model. No obviously histological changes were observed in the thoracic aorta and mesenteric artery from the transgenic mice when compared with control mice (Online Figure IID-G). No significant difference in capillary density was found in the gastrocnemius muscle between normal control mice and EC-TFEB Tg mice (Online Figure IIH-I) in basal conditions (before hind-limb ischemia), ruling out that the transgenic mice might have higher baseline capillary density. To reveal the effect of TFEB on ischemic angiogenesis, EC-TFEB Tg and control mice were subjected to hindlimb ischemia by ligation of the femoral artery. Using Laser Doppler perfusion imaging technology, we observed that the blood perfusion was significantly improved in EC-TFEB Tg mice at day 7, 14, 21, and 28 after ischemic injury compared with control mice (Figure 2A), with no appreciable differences at day 3 (Figure 2B). The necrotic degree reflected by necrotic toe number was considerably less in EC-TFEB Tg mice than that in control mice at day 14 after artery ligation with 12.5% of animals with 5 necrotic toes in the control mice and 12.5% showing no necrotic toes in the EC-TFEB Tg mice (Figure 2C). Consistent with these results, the capillary density in skeletal muscle at day 14 after ischemic injury was an average of 1.44-fold higher in EC-TFEB Tg mice when compared to control mice (Figure 2D-E). To determine whether TFEB affects arteriogenesis under ischemic conditions, we performed micro computed tomography (CT) as described previously21-23 at 14 days after hindlimb ischemia in EC-TFEB Tg and control mice. The EC-TFEB Tg mice showed no significant difference in vascular volume, vessel number and vascular volume to vessel number ratio in the ischemic thigh when compared with control mice (Online Figure III).
Figure 2. EC-specific TFEB transgene improves blood flow recovery after ischemic injury.
EC-TFEB Tg and Control mice were subjected to hindlimb ischemia. A, Representative images showing blood flow reperfusion monitored by Doppler Laser Ultrasound at the indicated time after ischemic injury. B, The ratio of ischemic/non-ischemic perfusion was quantitatively analyzed at the indicated time points, n=8 for each group. C, Necrotic toe score was determined at day 14 after ischemic injury. n=8 for each group. D, Representative images showing CD31 immunostaining in gastrocnemius muscle at 2 weeks after artery ligation. Scale bar =50 μm. E, The capillary density was evaluated as the ratio of capillary number per muscle fiber number, n=5 for each group. Data are presented as mean ± SEM. * P< 0.05; ** P<0.01.
Decreased angiogenesis and attenuated blood flow recovery in EC-TFEB KO mice after ischemic injury
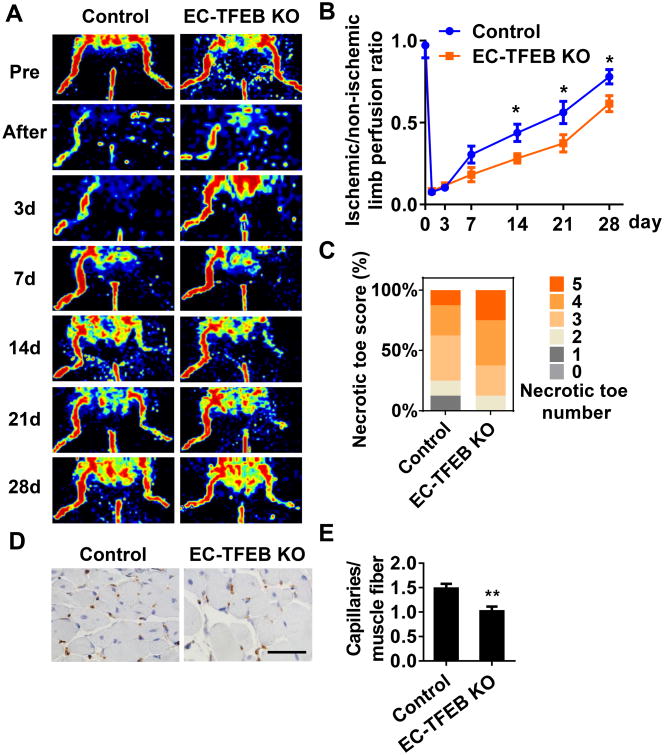
We further determined the role of endothelial TFEB in angiogenesis in EC-TFEB KO (floxed-TFEB homozygous/VE-Cad Cre+) mice. The TFEB deficiency in the EC-TFEB KO mice was validated by genotyping (Online Figure IVA-B) and detection of TFEB expression in both isolated MAECs in vitro (Online Figure IVC) and aortic ECs in vivo (Online Figure IVD). No obvious histological changes were observed in the thoracic aorta and mesenteric artery in the EC-TFEB KO mice when compared with the control mice (Online Figure IVE-H). In the hindlimb ischemic model, blood perfusion was significantly attenuated in EC-TFEB mice at day 14, 21, and 28 after ischemic injury compared with control mice (Figure 3A), with no appreciable differences at day 3 (Figure 3B). The EC-TFEB KO mice exhibited more severe toe necrotic score with 25% of animals having 5 necrotic toes compared to control mice in which 12.5% had 5 necrotic toes (Figure 3C). The capillary density in skeletal muscle at day 14 after ischemic injury was an average of 0.68-fold lower in EC-TFEB mice when compared to control mice (Figure 3D-E). Similarly, we further performed micro CT to assess arteriogeneis in EC-TFEB KO mice at 14 days after hindlimb ischemia. The EC-TFEB KO mice showed no significant difference in arteriogenesis (Online Figure V). Collectively, our data indicate that endothelial TFEB plays a critical role in post-ischemic angiogenesis.
Figure 3. EC-specific TFEB KO attenuates blood flow recovery after ischemic injury.
EC-TFEB KO and control mice were subjected to hindlimb ischemia. A, Representative images of Doppler Laser Ultrasound showing blood flow re-perfusion monitored at the indicated time after artery injury. B, The ratio of ischemic/non-ischemic perfusion was quantitatively analyzed at the indicated time points, n=8 for each group. C, Necrotic toe score was determined at day 14 after ischemic injury. n=8 for each group. D, Representative images showing CD31 immunostaining in gastrocnemius muscle at 2 weeks after artery ligation, scale bar =50 μm. E, the capillary density was evaluated as the ratio of capillary number per muscle fiber number. n=5 for each group. Data are presented as mean ± SEM. * P<0.05; ** P<0.01.
TFEB promotes endothelial tube formation and migration
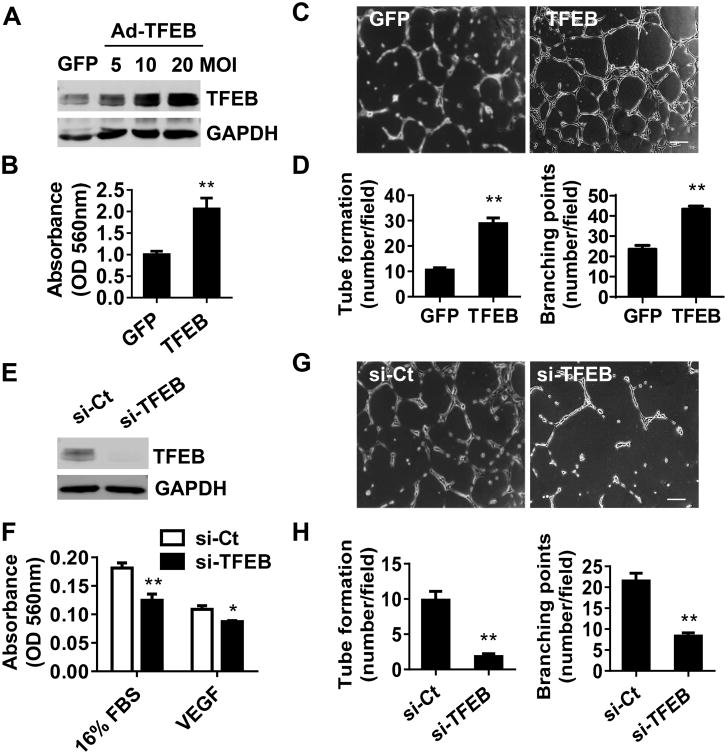
Endothelial cell proliferation, migration and tube formation are critical processes for angiogenesis, in which the formation of capillary-like tubes by ECs is a crucial step for blood flow recovery in vivo. We assessed the contribution of TFEB to EC biological functions by gain- and loss-of-function approaches. Adenovirus-mediated overexpression of TFEB was assessed by Western blot (Figure 4A). TFEB overexpression significantly promotes EC migration (Figure 4B). Tube formation assay showed that that TFEB significantly increased tube number and branching points to an average of 270% and 184%, respectively (Figure 4C-D). The knockdown efficiency of TFEB in ECs was determined by Western blot (Figure 4E). Consistent with the gain-of-function study, siRNA-mediated TFEB knockdown significantly inhibited EC migration (Figure 4F) and reduced the tube number and branching points by an average of 79.7% and 61.4%, respectively, compared with siRNA-control (Figure 4G-H). Moreover, TFEB has an inhibitory effect on EC apoptosis. Caspase 3 and PARP cleavage were reduced by TFEB overexpression (Online Figure VIA-B) and consistently enhanced by TFEB knockdown in the staurosporine (Stsp)-treated ECs (Online Figure VIC-D). TUNEL staining showed that the EC-TFEB transgene attenuated cell apoptosis (Online Figure VIIA), and consistently, EC-TFEB deficiency enhanced apoptosis in ischemic gastrocnemius muscle and the small arteries in ischemic skeletal muscle (Online Figure VIIB). However, TFEB overexpression did not significantly affect EC proliferation as measured by MTT (Online Figure VIIIA) and BrdU incorporation assays (Online Figure VIIIB). Taken together, our findings indicate that TFEB regulates EC biological functions essential for angiogenesis, including enhanced migration and tube formation and inhibition of apoptosis.
Figure 4. TFEB promotes tube formation and migration.
A, HUVECs were infected with Ad-GFP or Ad-TFEB (10 MOI). Forty-eight hours later, the expression of TFEB was detected by Western blot. B, HUVECs overexpressing TFEB or GFP were serum starved (0.5% FBS M199) for 24 h, and then incubated with 16% FBS M199. Migration was assessed with Boyden chamber assays. Three independent experiments were performed in triplicate. C-D, HUVECs overexpressing TFEB were plated onto Matrigel for 18h. Representative images of tube formation are shown in (C). Tube number/field and branching points/field in 6 random microscopic fields per group were measured by NIH imageJ and statistically analyzed. Three independent experiments were performed (D).E, HUVECs were transfected with siRNA targeting TFEB (20 nmol/L) or siRNA control (si-Ct) for 72 h and the expression of TFEB was detected by Western blot. F, Migration was determined in the TFEB knockdown and control ECs in the presence of 16% FBS or VEGF (10 ng/mL). Three independent experiments were performed in triplicate. G-H, TFEB knockdown ECs were plated onto Matrigel for 18 h. Representative images of tube formation are shown (G) and the tube formation in 6 random microscopic fields per group were measured and statistically analyzed. Three independent experiments were performed (H). In all cases, data are presented as mean ± SEM. * P<0.05; ** P<0.01.
TFEB regulates autophagy in ECs
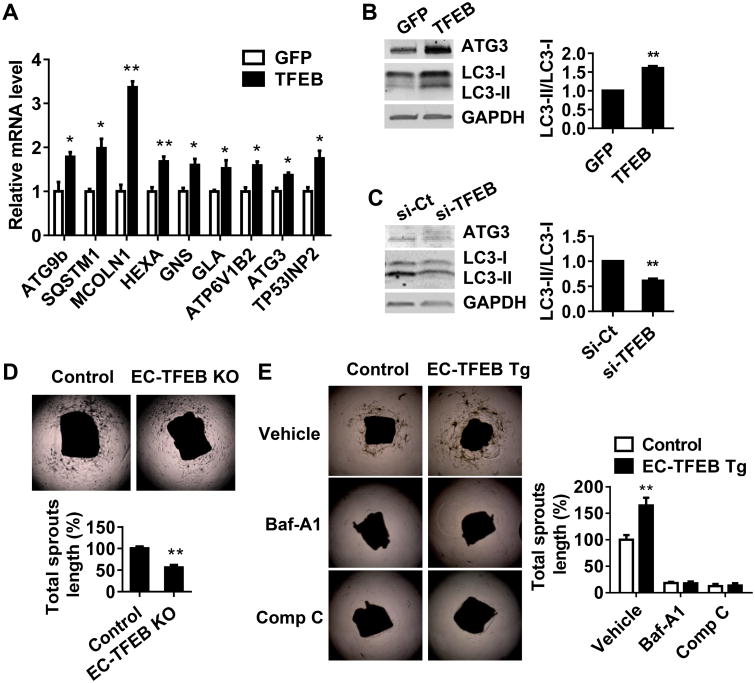
Next, we addressed the molecular mechanism that mediates the pro-angiogenic effect of TFEB in ECs. TFEB, as an autophagy master regulator, upregulates autophagy and lysosome biogenesis in various cell types.9 Our microarray analysis suggest that TFEB upregulates numerous autophagy-related genes (Online Figure IXA). TFEB overexpression significantly increased the expression of autophagy genes such as MCOLN1 and ATG9b at the mRNA level (Figure 5A). LC3-II/LC3-I ratio was significantly increased in the TFEB overexpressing ECs and consistently decreased in the TFEB deficient ECs (Figure 5B-C). The mouse aortic ring sprouting assay is widely used to quantify the development of microvessels and assess salient steps in angiogenesis.24 This Ex vivo study suggests that EC-TFEB deficiency significantly decreases the aortic ring sprouting (Figure 5D), which reinforces the results that EC-TFEB KO impairs post-ischemic angiogenesis in vivo. Consistently, the EC-TFEB transgene increased the aortic ring sprouting (Figure 5E). We applied bafilomycin A, an autophagy inhibitor, to assess the contribution of autophagy to TFEB-induced aortic sprouting. Inhibition of autophagy significantly attenuated the pro-angiogenic effect of TFEB (Figure 5E). AMPKα is a well-recognized key regulator of autophagy25, angiogenesis26 and energy homeostasis.27 We observed that inhibition of AMPKα by antagonist Compound C decreased aortic ring sprouting. In the presence of Compound C, TFEB upregulation cannot induce sprout outgrowth (Figure 5E), suggesting that AMPKα is critical for TFEB-regulated pro-angiogenic effect. Our microarray data also revealed that TFEB regulates angiogenesis-related genes (Online Figure IXB).
Figure 5. TFEB upregulates autophagy in ECs.
A-B, HUVECs were infected with Ad-GFP or Ad-TFEB (10 MOI). The expression of autophagy and lysosome biogenesis related genes was determined by Real-time PCR. Three independent experiments were performed (A). LC3 and ATG3 expression was detected by Western blot. Representative blots are shown in (B) and the ratio of LC3-II/LC3-I was quantitatively analyzed. Data are from 3 independent experiments. C, HUVECs were transfected with si-Ct or si-TFEB (20 nmol/L) for 72 h. The ratio of LC3-II/LC3-I was quantitatively analyzed. Data are from three independent experiments. D, Sprouts grown from aortic rings of Control and EC-TEB KO mice were measured and quantitatively analyzed at day 5, n=5 for each group. E, Aortic rings isolated from control or EC-TFEB Tg mice were embedded into Matrigel in the presence of bafilomycin-A1 (200 nmol/L) or Compound C (10 μmol/L). Five days later, the sprouts grown from the corresponding aortic rings were measured and quantitatively analyzed, n=4 for each group. In all cases, data are presented as mean ± SEM. * P<0.05; ** P<0.01.
Autophagy and AMPKα are required for TFEB to regulate tube formation
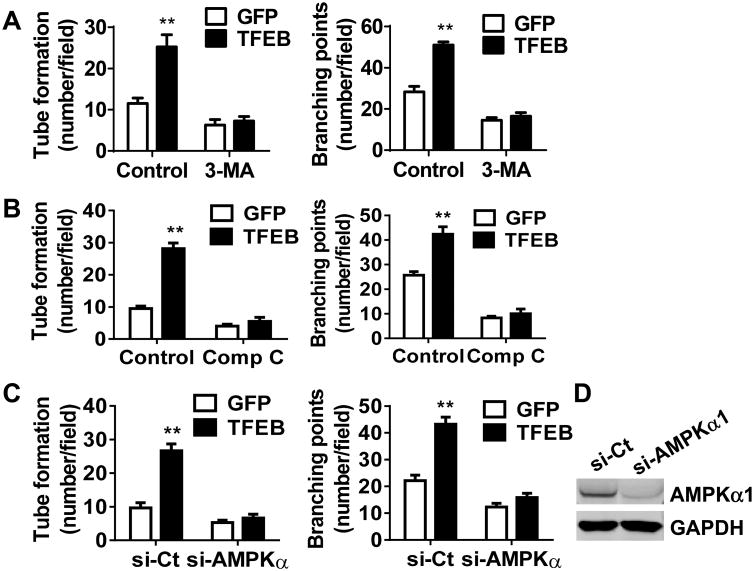
Besides the finding that autophagy mediates the effect of TFEB on aortic ring sprouting, we demonstrated that 3-Methyladenine (3-MA), an autophagy antagonist that inhibits class III PI3K, attenuates the positive effect of TFEB on tube formation (Figure 6A). To determine whether AMPKα signaling is essential to mediate TFEB effects on tube formation, we antagonized AMPKα activity by either pharmacological inactivation or siRNA-mediated knockdown. TFEB overexpression failed to increase tube formation when compared with GFP control in the presence of Compound C (Figure 6B). In the AMPKα-knockdown cells, there was no significant difference in both tube number and branching points between TFEB and GFP groups (Figure 6C), even upon 80% downregulation in AMPKα protein levels (Figure 6D). Moreover, the pro-angiogenic capacity of the TFEB transgene was significantly attenuated in the Matrigel plugs containing Compound C or 3-MA (Online Figure X). AMPKα activates autophagy through inhibition of mTOR signaling pathway.25, 28 We determined the phosphorylation of mTOR downstream effectors, 4E-BP1 and S6 ribosomal protein. The phosphorylation of both proteins was significantly attenuated in the TFEB overexpressing ECs (Online Figure XI). Taken together, our findings suggest that AMPKα and autophagy are critical signaling pathways mediating the TFEB pro-angiogenic effects in ECs.
Figure 6. TFEB requires autophagy and AMPKα activity for efficient tube formation.
A-B, HUVECs were infected with Ad-GFP or Ad-TFEB (10 MOI). Twenty-four hours later the cells were treated with 3-MA (5 mmol/L) (A) or compound C (10 μmol/L) (B) for another 16 h. The EC tube number/field and branching points/field in 6 random microscopic fields per group were measured and statistically analyzed. C, HUVECs were transfected with siRNA targeting AMPKα or siRNA control (20 nmol/L). Twenty-four hours later, the ECs were infected with Ad-GFP or Ad-TFEB (10 MOI) for another 48 h. The EC tube formation in 6 random microscopic fields per group were measured and statistically analyzed. D, The knockdown efficiency of AMPKα was determined by Western blot. Three independent experiments were performed in all cases and data are presented as mean ± SEM. ** P<0.01.
TFEB activates the AMPKα signaling pathway
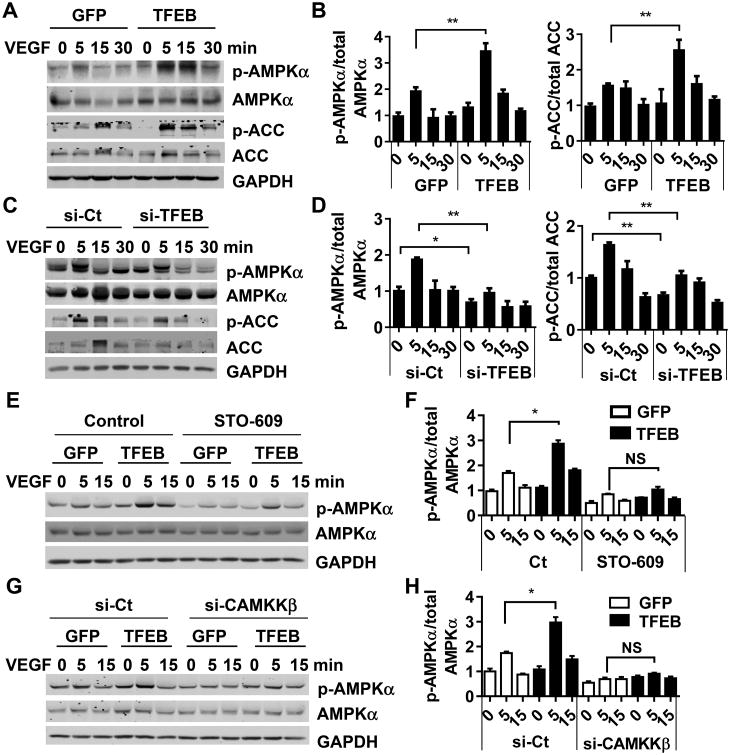
Next, we demonstrated that the AMPKα signaling pathway was enhanced by TFEB in ECs. Phosphorylation of AMPKα at Thr172 and acetyl-CoA carboxylase (ACC), a downstream substrate of AMPKα, at Ser79 were significantly increased by TFEB overexpression in the presence of VEGF. At 5 min after VEGF stimulation, the phosphorylation at AMPKα at Thr172 was increased by 1.76-fold and ACC at Ser79 was up by 1.72-fold (Figure 7A-B). In the absence of VEGF, TFEB could increase AMPKα activation at a dose of 20 MOI or higher (Online Figure XIIA-B). Consistently, TFEB knockdown decreased phosphorylation of both proteins both under basal conditions and upon VEGF activation (Figure 7C-D). Furthermore, we isolated mouse aortic ECs from control and EC-TFEB Tg mice. The phosphorylation of AMPKα was enhanced in the EC-TFEB transgenic ECs (Online Figure XIIC-D), in agreement with the in vitro data observed in primary human ECs.
Figure 7. TFEB activates AMPKα signaling.
A-B, HUVECs were infected with Ad-GFP or Ad-TFEB (10 MOI). Twenty-four hours later, the cells were treated with VEGF (10 ng/mL) for the indicated time. C-D, HUVECs were transfected with si-TFEB or si-Ct (20 nmol/L) for 3 days, and then treated with VEGF (10 ng/mL) for the indicated time. Phosphorylated and total AMPKα and ACC were detected by Western blot (A and C). Three independent experiments were performed. Band intensities of phosphorylated AMPKα and ACC were quantitatively analyzed and normalized against total AMPKα or ACC, respectively (B and D). Data are presented as mean ± SEM. * P<0.05; ** P<0.01. E-F, HUVECs were infected with Ad-GFP or Ad-TFEB (10 MOI). Forty-eight hours later, the cells were pretreated with STO-609 (5 μmol/L) for 30 min, and then stimulated with VEGF (10 ng/mL) for the indicated time. G-H, HUVECs were transfected with si-CAMKKβ (20 nmol/L) or si-Ct. Twenty-four hours later, the cells were infected with Ad-GFP or Ad-TFEB for 48 h followed by VEGF (10 ng/mL) stimulation. Phosphorylated and total AMPKα were detected by Western blot (E and G). Three independent experiments were performed. The band intensity of phosphorylated AMPKα was quantitatively analyzed and normalized against total AMPKα (F and H) Data are presented as mean ± SEM. * P<0.05.
Next, we set to understand how TFEB activates AMPKα.AMPKα is activated by multiple well-recognized regulators such as LKB1 and CAMKKβ.26 However, our data showed that the phosphorylation of LKB1 at Ser428 was not significantly changed by TFEB, implying that LKB1 may not a mediator in the TFEB-dependent effects on AMPKα (Online Figure XIII). We used STO-609 (5 μmol/L), a CAMKKβ inhibitor, to antagonize the CAMKKβ activity. Enhanced phosphorylation of AMPKα by TFEB overexpression was significantly attenuated in the presence of STO-609 (Figure 7E-F). We further inactivated CAMKKβ by siRNA, and observed a similar phenotype in the CAMMKβ-knockdown ECs (Figure 7G-H). Collectively, our data suggest that CAMKKβ mediates the TFEB-dependent modulation of AMPKα signaling.
MCOLN1 mediates the pro-angiogenic effect of TFEB in ECs
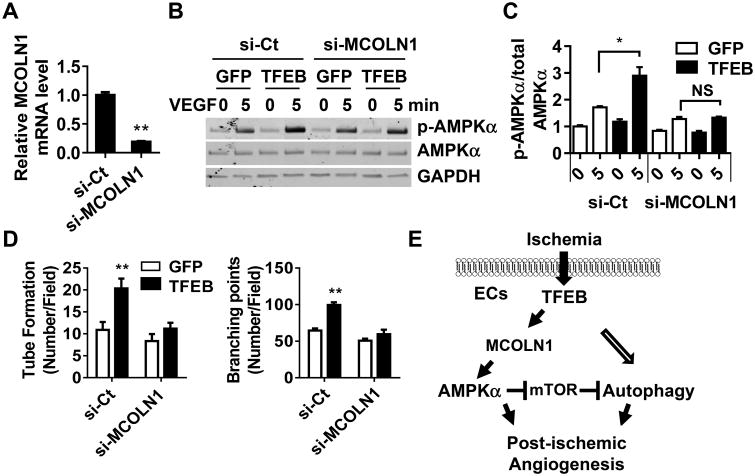
As a transcription factor, translocation of TFEB to the nucleus could result in transcriptional regulation of its target genes.9, 29, 30 MCOLN1, also known as TRPML1 or ML1, is an endosomal and lysosomal calcium channel. TFEB increased the expression of MCOLN1 to an average of 3.3-fold at the mRNA level (Online Figure XIVA). Furthermore, we demonstrated that TFEB bound to the MCOLN1 promoter as determined by CHIP assay (Online Figure XIVB). TFEB overexpression induced an average of 2.98-fold increase in its binding to the MCOLN1 promoter. A previous study demonstrated that TFEB elevated the intracellular calcium concentration in HeLa cells, an immortal human cervical cancer cell line.31 We found that TFEB could significantly increase the intracellular Ca2+ in ECs as well, which may contribute to the activation of CAMKKβ by TFEB (Online Figure XIVC-D). In MCOLN1 knockdown ECs (Figure 8A), the TFEB-induced elevation of intracellular calcium (Online Figure XIVC-D), AMPKα activation (Figure 8B-C) and tube formation (Figure 8D) were significantly attenuated. These data indicate that MCOLN1 is critical in the TFEB-dependent pro-angiogenic phenotype.
Figure 8. MCOLN1 mediates the TFEB activation of AMPKα.
HCAECs were transfected with si-MCOLN1 (20 nmol/L) or si-Ct for 48 h, and then were infected with Ad-GFP or Ad-TFEB (10 MOI) for another 48 h. A, The knockdown efficiency of MCOLN1 was determined by Real-time PCR. B-C, ECs were treated with VEGF (10 ng/mL) for the indicated time. Phosphorylated and total AMPKα were detected by Western blot. Three independent experiments were performed. Band intensity of phosphorylated AMPKα was quantitatively analyzed and normalized against total AMPKα. C-D, The EC tube number/field and branching points/field in 6 random microscopic fields per group were measured and statistically analyzed. Three independent experiments were performed. Data are presented as mean ± SEM. E, Schema for the TFEB-dependent regulation of post-ischemic angiogenesis. TFEB upregulates MCOLN1-AMPKα and autophagy axis to promote angiogenesis. In all cases, data are presented as mean ± SEM. * P<0.05, ** P<0.01.
Discussion
Although TFEB has been demonstrated to be a pivotal regulator of autophagy and lysosome biogenesis, the role of TFEB in vascular biology remains largely unknown. Our previous study demonstrated that TFEB inhibits EC inflammation and reduces atherosclerosis in mice.17 TFEB global knockout mice are embryonic lethal with impaired vascularization,19 but the availability and viability of EC-selective TFEB knockout and Tie2-driven TFEB transgenic mice provided valuable tools to investigate its specific function in ECs. Here, using these EC-specific genetically engineered transgenic mice, we identified TFEB as a novel factor promoting angiogenesis, which defines TFEB as a potential therapeutic target relevant to the treatment of ischemic vascular disease.
The patterns of gene expression after acute hindlimb ischemia revealed that pro-angiogenic factors such as VEGF, PIGF, HDGF and MCP1 were upregulated at early stages of ischemia (3-7 days).20, 32 We found that TFEB was increased in the ischemic skeletal muscle at day 3 after vascular injury in vivo and TFEB subcellular localization was responsive to nutrient deprivation in vitro. The elevated expression or nuclear translocation of TFEB in response to ischemia or nutrient deprivation implies an adaptive and potentially protective effect of TFEB on ECs. TFEB can be phosphorylated by mTOR, and retained at the lysosome membrane.10, 29, 30 However, in response to adverse environmental cues, TFEB is dephosphorylated and translocates to the nucleus to transcriptionally regulate gene expression of its targets.10, 29, 30 Consistent with these data, we found that TFEB clearly accumulated in the nucleus (Figure 1A) in response to serum deprivation. Consequently, it is possible that TFEB could mediate mTOR dependent signal transduction under conditions of starvation in ECs. On the other hand, TFEB suppressed mTOR activity in ECs (Online Figure XI), revealing an interplay between TFEB and mTOR signaling. As a novel finding, we observed that TFEB is responsive to VEGF in ECs, which expands our insight in the molecular regulation of TFEB including gene expression and nuclear translocation. Inhibition of VEGF receptor 2 (VEGFR2) or AMPKα prevented decorin-induced TFEB upregulation and nuclear translocation in ECs.33 Follow-up studies are warranted to determine whether VEGFR2 and AMPKα mediate the VEGF-dependent regulation of TFEB in ECs.
Compared to the regulation of angiogenesis in response to ischemia, the control of arteriogenesis in general and collateral formation in particular remains unclear at large.34 Arteriogenesis can occur at sites of arterial occlusion where ischemia is not prominent but hemodynamic factors such as shear stress forces are changed.35 Our data suggest that neither EC-TFEB transgene nor EC-TFEB KO have appreciable effects on arteriogenesis in the ischemic thigh as assessed by micro CT (Figures 2 and 3). We investigated the entire plethora of biological features of EC function such as proliferation, migration, apoptosis and other signal transduction pathways potentially involved in the pro-angiogenic phenotype elicited by increased TFEB expression. Our data suggest that tube formation, migration and survival are key processes by which TFEB critically modulates angiogenesis, a conclusion supported by our in vivo data. Indeed, a growing number of studies suggest that TFEB can inhibit apoptosis in various cell types and tissues. Hepatic TFEB gene transfer decreased detrimental activation of liver apoptosis in the alpha-1-anti-trypsin deficient (AATD) mice36 and multiple sulfatase deficient (MSD) mice.31 TFEB had a robust protective effect and enhanced cell survival in midbrain dopamine neurons12 and cardomyctes.15, 16
Mechanistically, TFEB activates AMPKα and autophagy in ECs. TFEB is a master gene that regulates autophagy and lysosome biogenesis.10, 11, 29, 30 Autophagy is crucial in maintaining vessel wall homeostasis and autophagic dysregulation may induce vascular aging and associated pathological process.37 Autophagy may protect ECs from glucose, angiotensin II and oxygen-glucose deprivation (OGD)-induced endothelial dysfunction.37 Accumulated studies indicate that proper autophagy promotes EC tube formation and migration in vitro38-42 and angiogenesis in vivo,42 depending on the cellular and molecular microenvironment. A recent study demonstrated that endothelial-specific deletion of raptor (an essential component of the mTORC1 signaling complex) protects against hindlimb ischemia in diabetic mice via activation of autophagy.43 In the current study, we systematically addressed the critical role of TFEB in post-ischemic restoration and biological processes in ECs, and revealed an essential role of autophagy in TFEB-induced tube formation, aortic sprouting and angiogenesis. Although our data suggest that AMPKα and autophagy mediate the effect of TFEB on angiogenesis, we cannot exclude the potential mediator role of other pro-angiogenic factors in the TFEB regulation of angiogenesis.
AMPKα is a critical regulator for autophagy25 and angiogenesis under hypoxia.26 Through inhibition of AMPKα in tube formation assay in vitro (Figure 4), aortic ring sprouting assay ex vivo (Figure 5) and Matrigel plug in vivo (Online Figure X), we determined that AMPKα is a novel signaling pathway that mediates the pro-angiogenic effect of TFEB in ECs. It has been demonstrated that AMPKα inhibits mTOR signaling.25, 28 One of our major findings in the current study is that TFEB activates AMPKα and simultaneously inhibits mTOR signaling in ECs (Online Figure XI), suggesting a TFEB-AMPKα-mTOR axis in ECs, which probably further contributes to lower level of phosphorylated TFEB (higher transcriptional activity). Thus, TFEB and AMPKα may form a positive feedback loop in ECs (Figure 8E). Indeed, besides regulating autophagy, AMPKα is a multi-functional kinase affecting metabolism and cytoskeletal signaling28 that may also mediate autophagy-independent positive effects on angiogenesis. Whether AMPKα can further promote angiogenesis in an autophagy-independent fashion in the context of TFEB upregulation is still unclear and warrants follow-up studies.
In our experimental system, we observed that TFEB did not alter the LKB1 phosphorylation, an important upstream regulator of AMPKα.27 However, we demonstrated that CAMKKβ is required for the TFEB-dependent regulation of AMPKα. It has been reported that CAMKKβ was critical for VEGF to activate AMPKα44 Recent molecular studies of endolysosomal Ca2+ physiology revealed that Ca2+ is critical in controlling diverse cellular processes and diseases.45 MCOLN1 plays a critical role in calcium homeostasis and could control the intracellular Ca2+ concentration.31 In our study, we demonstrated that TFEB upregulates MCOLN1 and increases intracellular Ca2+. Lysosomal Ca2+ activates calcinuerin, which induces TFEB nuclear translocation by binding and dephosphorylating TFEB.46 Thus, a self-sustaining positive feedback loop between TFEB and calcium signaling may exist as well in ECs. In the current study, our data suggest that autophagy and AMPKα activation are, at the very least, required for TFEB to regulate tube formation. Yet, we must acknowledge that TFEB may also cross-talk with other angiogenic signaling pathways, and may regulate angiogenesis under other specific conditions such as development, diabetes and tumor formation, which remain to be investigated. Interestingly, our data suggest that TFEB is required for angiogenesis and for activation of autophagy even in basal conditions in vitro, thus opening the possibility that TFEB is a homeostatic regulator of angiogenesis in the absence of EC dysfunction.
The limitation of our study is the unavailability of EC-specific inactivation of AMPKα in our EC-TFEB mouse models. Pharmacological inhibition of AMPKα in vivo may introduce confounding factors from systemic effects on other tissues, and complicate the interpretation of the data and elucidation of the mechanism.27 EC-TFEB KO/EC-ATG5 KO and EC-TFEB KO/EC-AMPKα KO mice would be ideal tools to determine the mediating role of autophagy and AMPKα, respectively, in TFEB regulation of angiogenesis in vivo. Another limitation is that Tie2 and VE-Cad-driven Cre recombinase are expressed, albeit at very low levels, in blood monocytes and bone marrow-derived hematopoietic cells 47, 48, respectively. However, our previous study demonstrated that TFEB protein abundance was not significantly increased in peritoneal or bone marrow-derived macrophages from EC-TFEB Tg mice.17
In conclusion, we provide evidence that TFEB is critical for angiogenesis and, consequently, for improving blood flow reperfusion under ischemic conditions. Based on our findings, we uncovered a molecular mechanism underlying the positive effect of TFEB on angiogenesis. Our findings have relevance for the treatment of chronic ischemia and define TFEB as a new potential therapeutic target for ischemic vascular injury. Understanding how to best exploit this novel molecular target could contribute to therapeutic strategies aimed at rescuing ischemic tissues at large.
Supplementary Material
Novelty and Significance.
What Is Known?
TFEB is a lysosome biogenesis and autophagy master gene that modulates cell and tissue function. TFEB-deficient mouse is embryonic lethal with impaired placental vascularization.
Autophagy is crucial in maintaining vessel wall homeostasis and autophagic dysregulation may induce vascular pathological process.
AMPKα is a key metabolic sensor and critical in regulation of autophagy and angiogenesis in ECs.
What New Information Does This Article Contribute?
TFEB is upregulated in ischemic conditions in ECs.
Endothelial TFEB promotes post-ischemic angiogenesis and improves blood flow recovery.
Autophagy and AMPKα signaling mediate the pro-angiogenic effect of TFEB in ECs
MCOLN1 is a novel TFEB target gene that is critical for TFEB-dependent pro-angiogenic effect.
Post-ischemic angiogenesis is critical for tissue regeneration and blood flow recovery. Through gain- and loss-fo function strategies in vivo, we found that endothelial TFEB promotes angiogenesis after hindlimb ischemia and thus confers a beneficial effect on blood flow recovery in post-ischemic conditions. Mechanistically, TFEB activates AMPKα and enhances autophagy, which mediates the TFEB-dependent regulation of angiogenesis. These findings expand our knowledge on the role of endothelial TFEB to maintain vascular homeostasis and define TFEB as a potential therapeutic target for treatment of ischemic vascular disease.
Acknowledgments
We would like to thank Dr. Monica Justice at the Hospital for Sick Children, University of Toronto, Canada for providing the floxed-Tfeb mice.
Sources of Funding: This work was partially supported by NIH grants HL068878, HL105114, and HL117491 (Y.E.C.), HL138094 (Y.F.), HL138139 (J.Z.), NIH/NCRR S10RR026475, NIH contract U42OD011174, and American Heart Association grants 14SDG19880014 (Y.F.)15SDG24470155 (Y.G.) and 17PRE33400179 (H.L.).
Nonstandard Abbreviations and Acronyms
- ECs
endothelial cells
- TFEB
transcription factor-EB
- AMPK
AMP-activated protein kinase
- ACC
acetyl-CoA Carboxylase
- CAMKKβ
Ca2+/calmodulin-dependent protein kinase kinase-beta
- LKB1
serine/threonine kinase 11
- VEGF
vascular endothelial growth factor
- HIF1α
hypoxia-inducible factor 1-alpha
- KDR
kinase inserts domain receptor/VEGFR2
- Tie2
endothelial-specific receptor tyrosine kinase
- MCOLN1
Mucolipin-1
Footnotes
Disclosure: None.
References
- 1.Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends in biochemical sciences. 1997;22:251–256. doi: 10.1016/s0968-0004(97)01074-8. [DOI] [PubMed] [Google Scholar]
- 2.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the framingham offspring study. American heart journal. 2002;143:961–965. doi: 10.1067/mhj.2002.122871. [DOI] [PubMed] [Google Scholar]
- 3.Schanzer A, Conte MS. Critical limb ischemia. Current treatment options in cardiovascular medicine. 2010;12:214–229. doi: 10.1007/s11936-010-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isner JM, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes JF. Clinical evidence of angiogenesis after arterial gene transfer of phvegf165 in patient with ischaemic limb. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 5.Laitinen M, Makinen K, Manninen H, Matsi P, Kossila M, Agrawal RS, Pakkanen T, Luoma JS, Viita H, Hartikainen J, Alhava E, Laakso M, Yla-Herttuala S. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Human gene therapy. 1998;9:1481–1486. doi: 10.1089/hum.1998.9.10-1481. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R, Tongers J, Losordo DW. Human studies of angiogenic gene therapy. Circulation research. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Group TIW. Inter-society consensus for the management of peripheral arterial disease (tasc ii) Journal of vascular surgery. 2007;45(S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Hamik A, Wang B, Jain MK. Transcriptional regulators of angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1936–1947. doi: 10.1161/01.ATV.0000232542.42968.e3. [DOI] [PubMed] [Google Scholar]
- 9.Ballabio A. The awesome lysosome. EMBO Mol Med. 2016;8:73–76. doi: 10.15252/emmm.201505966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A. Tfeb links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, Di Malta C, Donaudy F, Embrione V, Polishchuk RS, Banfi S, Parenti G, Cattaneo E, Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 12.Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A. Tfeb-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E1817–1826. doi: 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RA, Lazarowski ER, Damian VA, Masliah E, La Spada AR. Pgc-1alpha rescues Huntington's disease proteotoxicity by preventing oxidative stress and promoting tfeb function. Science translational medicine. 2012;4:142ra197. doi: 10.1126/scitranslmed.3003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spampanato C, Feeney E, Li L, Cardone M, Lim JA, Annunziata F, Zare H, Polishchuk R, Puertollano R, Parenti G, Ballabio A, Raben N. Transcription factor eb (tfeb) is a new therapeutic target for pompe disease. EMBO Mol Med. 2013;5:691–706. doi: 10.1002/emmm.201202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma X, Godar RJ, Liu H, Diwan A. Enhancing lysosome biogenesis attenuates bnip3-induced cardiomyocyte death. Autophagy. 2012;8:297–309. doi: 10.4161/auto.18658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X, Liu H, Murphy JT, Foyil SR, Godar RJ, Abuirqeba H, Weinheimer CJ, Barger PM, Diwan A. Regulation of the transcription factor eb-pgc1alpha axis by beclin-1 controls mitochondrial quality and cardiomyocyte death under stress. Molecular and cellular biology. 2015;35:956–976. doi: 10.1128/MCB.01091-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu H, Fan Y, Qiao C, Liang W, Hu W, Zhu T, Zhang J, Chen YE. Tfeb inhibits endothelial cell inflammation and reduces atherosclerosis. Sci Signal. 2017;10 doi: 10.1126/scisignal.aah4214. [DOI] [PubMed] [Google Scholar]
- 18.Godar RJ, Ma X, Liu H, Murphy JT, Weinheimer CJ, Kovacs A, Crosby SD, Saftig P, Diwan A. Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy. 2015;11:1537–1560. doi: 10.1080/15548627.2015.1063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steingrimsson E, Tessarollo L, Reid SW, Jenkins NA, Copeland NG. The bhlh-zip transcription factor tfeb is essential for placental vascularization. Development. 1998;125:4607–4616. doi: 10.1242/dev.125.23.4607. [DOI] [PubMed] [Google Scholar]
- 20.Lee CW, Stabile E, Kinnaird T, Shou M, Devaney JM, Epstein SE, Burnett MS. Temporal patterns of gene expression after acute hindlimb ischemia in mice: Insights into the genomic program for collateral vessel development. Journal of the American College of Cardiology. 2004;43:474–482. doi: 10.1016/j.jacc.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Zhang MJ, Sansbury BE, Hellmann J, Baker JF, Guo L, Parmer CM, Prenner JC, Conklin DJ, Bhatnagar A, Creager MA, Spite M. Resolvin d2 enhances postischemic revascularization while resolving inflammation. Circulation. 2016;134:666–680. doi: 10.1161/CIRCULATIONAHA.116.021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol. 2004;287:H302–310. doi: 10.1152/ajpheart.00928.2003. [DOI] [PubMed] [Google Scholar]
- 23.Moraes F, Paye J, Mac Gabhann F, Zhuang ZW, Zhang J, Lanahan AA, Simons M. Endothelial cell-dependent regulation of arteriogenesis. Circulation research. 2013;113:1076–1086. doi: 10.1161/CIRCRESAHA.113.301340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons M, Alitalo K, Annex BH, Augustin HG, Beam C, Berk BC, Byzova T, Carmeliet P, Chilian W, Cooke JP, Davis GE, Eichmann A, Iruela-Arispe ML, Keshet E, Sinusas AJ, Ruhrberg C, Woo YJ, Dimmeler S American Heart Association Council on Basic Cardiovascular S, Council on Cardiovascular S, Anesthesia. State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: A scientific statement from the american heart association. Circulation research. 2015;116:e99–132. doi: 10.1161/RES.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alers S, Loffler AS, Wesselborg S, Stork B. Role of ampk-mtor-ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Molecular and cellular biology. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisslthaler B, Fleming I. Activation and signaling by the amp-activated protein kinase in endothelial cells. Circulation research. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 27.Towler MC, Hardie DG. Amp-activated protein kinase in metabolic control and insulin signaling. Circulation research. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 28.Mihaylova MM, Shaw RJ. The ampk signalling pathway coordinates cell growth, autophagy and metabolism. Nature cell biology. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A. A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mtor and tfeb. The EMBO journal. 2012;31:1095–1108. doi: 10.1038/emboj.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pena-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, Xie XJ, Corey DR, Brugarolas J. Regulation of tfeb and v-atpases by mtorc1. The EMBO journal. 2011;30:3242–3258. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Developmental cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rissanen TT, Vajanto I, Hiltunen MO, Rutanen J, Kettunen MI, Niemi M, Leppanen P, Turunen MP, Markkanen JE, Arve K, Alhava E, Kauppinen RA, Yla-Herttuala S. Expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 (kdr/flk-1) in ischemic skeletal muscle and its regeneration. The American journal of pathology. 2002;160:1393–1403. doi: 10.1016/S0002-9440(10)62566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neill T, Sharpe C, Owens RT, Iozzo RV. Decorin-evoked paternally expressed gene 3 (peg3) is an upstream regulator of the transcription factor eb (tfeb) in endothelial cell autophagy. J Biol Chem. 2017;292:16211–16220. doi: 10.1074/jbc.M116.769950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eitenmuller I, Volger O, Kluge A, Troidl K, Barancik M, Cai WJ, Heil M, Pipp F, Fischer S, Horrevoets AJ, Schmitz-Rixen T, Schaper W. The range of adaptation by collateral vessels after femoral artery occlusion. Circulation research. 2006;99:656–662. doi: 10.1161/01.RES.0000242560.77512.dd. [DOI] [PubMed] [Google Scholar]
- 35.Pipp F, Boehm S, Cai WJ, Adili F, Ziegler B, Karanovic G, Ritter R, Balzer J, Scheler C, Schaper W, Schmitz-Rixen T. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24:1664–1668. doi: 10.1161/01.ATV.0000138028.14390.e4. [DOI] [PubMed] [Google Scholar]
- 36.Pastore N, Blomenkamp K, Annunziata F, Piccolo P, Mithbaokar P, Maria Sepe R, Vetrini F, Palmer D, Ng P, Polishchuk E, Iacobacci S, Polishchuk R, Teckman J, Ballabio A, Brunetti-Pierri N. Gene transfer of master autophagy regulator tfeb results in clearance of toxic protein and correction of hepatic disease in alpha-1-anti-trypsin deficiency. EMBO Mol Med. 2013;5:397–412. doi: 10.1002/emmm.201202046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nussenzweig SC, Verma S, Finkel T. The role of autophagy in vascular biology. Circulation research. 2015;116:480–488. doi: 10.1161/CIRCRESAHA.116.303805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du J, Teng RJ, Guan T, Eis A, Kaul S, Konduri GG, Shi Y. Role of autophagy in angiogenesis in aortic endothelial cells. Am J Physiol Cell Physiol. 2012;302:C383–391. doi: 10.1152/ajpcell.00164.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang HJ, Zhang D, Tan YZ, Li T. Autophagy in endothelial progenitor cells is cytoprotective in hypoxic conditions. Am J Physiol Cell Physiol. 2013;304:C617–626. doi: 10.1152/ajpcell.00296.2012. [DOI] [PubMed] [Google Scholar]
- 40.Hartmann P, Zhou Z, Natarelli L, Wei Y, Nazari-Jahantigh M, Zhu M, Grommes J, Steffens S, Weber C, Schober A. Endothelial dicer promotes atherosclerosis and vascular inflammation by mirna-103-mediated suppression of klf4. Nature communications. 2016;7:10521. doi: 10.1038/ncomms10521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang CY, Chen PH, Lu SC, Hsieh MC, Lin CW, Lee HM, Jawan B, Kao YH. Propofol-enhanced autophagy increases motility and angiogenic capacity of cultured human umbilical vascular endothelial cells. Life Sci. 2015;142:49–59. doi: 10.1016/j.lfs.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Lu Q, Yao Y, Hu Z, Hu C, Song Q, Ye J, Xu C, Wang AZ, Chen Q, Wang QK. Angiogenic factor aggf1 activates autophagy with an essential role in therapeutic angiogenesis for heart disease. PLoS Biol. 2016;14:e1002529. doi: 10.1371/journal.pbio.1002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan W, Han D, Sun Z, Ma S, Gao L, Chen J, Li X, Li X, Fan M, Li C, Hu D, Wang Y, Cao F. Endothelial deletion of mtorc1 protects against hindlimb ischemia in diabetic mice via activation of autophagy, attenuation of oxidative stress and alleviation of inflammation. Free Radic Biol Med. 2017;108:725–740. doi: 10.1016/j.freeradbiomed.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Stahmann N, Woods A, Spengler K, Heslegrave A, Bauer R, Krause S, Viollet B, Carling D, Heller R. Activation of amp-activated protein kinase by vascular endothelial growth factor mediates endothelial angiogenesis independently of nitric-oxide synthase. J Biol Chem. 2010;285:10638–10652. doi: 10.1074/jbc.M110.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan AJ, Platt FM, Lloyd-Evans E, Galione A. Molecular mechanisms of endolysosomal ca2+ signalling in health and disease. The Biochemical journal. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- 46.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and tfeb. Nature cell biology. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. Ve-cadherin-cre-recombinase transgenic mouse: A tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- 48.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.