Summary
The regulatory mechanisms that control neural stem cell (NSC) activation in the adult ventricular-subventricular zone (V-SVZ) stem cell niche have been the focus of intense investigation, yet how the niche first develops and organizes is poorly understood. Here, we examined matrix metalloproteinases (MMPs) for potential roles in V-SVZ stem cell niche development. MMP12 was found to promote appropriate niche cellular arrangements, the formation of specialized niche extracellular matrix, and the translational planar cell polarity of ependymal cells that surround and support niche NSCs. Surprisingly, ependymal cells were found to have an intracellular pool of MMP12 that promoted ependymal cell ciliogenesis by upregulating FOXJ1. In addition, both extracellular and intracellular MMP12 were found to regulate V-SVZ niche output by promoting NSC quiescence. These findings reveal that extracellular and intracellular MMP12 have both unique and overlapping roles that help orchestrate the development of the adult V-SVZ stem cell niche.
Keywords: neural stem cell niche, ependymal cell, matrix metalloproteinase, ventricular-subventricular zone, V-SVZ
Graphical Abstract
Highlights
-
•
Both extracellular and intracellular MMP12 function in the V-SVZ stem cell niche
-
•
Extracellular MMP12 promotes cellular and extracellular V-SVZ niche organization
-
•
Intracellular MMP12 is sufficient to enhance ependymal FOXJ1 and ciliogenesis
-
•
Both MMP12 forms suppress V-SVZ neural stem cell proliferation
Shan et al. report that matrix metalloproteinase 12 (MMP12) is required for the appropriate development of the V-SVZ neural stem cell niche, with secreted MMP12 promoting niche organization and function, including the regulation of neural stem cell quiescence. An unexpected intracellular pool of a truncated yet functional MMP12 was also identified, which has a distinct role in promoting ependymal ciliogenesis.
Introduction
Stem cells often reside in specialized microenvironments, or niches, where resident support cells, growth factors, and extracellular matrix (ECM) proteins regulate stem cell behaviors, including quiescence, proliferation, and differentiation (Brizzi et al., 2012). One such niche resides in the adult ventricular zone (VZ) of the mammalian brain, consisting of adult neural stem cells (NSCs) whose apical processes are surrounded by a “pinwheel” of multiciliated ependymal cells (ECs) (Mirzadeh et al., 2008), whose specialized cell-cell contacts help to regulate NSC functions (Lehtinen and Walsh, 2011, McClenahan et al., 2016, Paez-Gonzalez et al., 2011). In addition, ECM aggregates, or hubs, are localized by the ECM receptor dystroglycan at the junctions between ECs and NSCs in the apical VZ (McClenahan et al., 2016, Shen et al., 2008). Beneath the EC pinwheels, in the subventricular zone (SVZ), NSC basal processes contact vascular basal lamina via α6-containing integrins (Shen et al., 2008). Together, these and other cell-cell and cell-ECM arrangements in the ventricular-subventricular zone (V-SVZ) are thought to be critical for postnatal stem cell activation and regulated postnatal neurogenesis and gliogenesis (McClenahan et al., 2016, Mercier et al., 2002, Relucio et al., 2012, Shen et al., 2008).
The adult V-SVZ niche emerges postnatally in rodents. Shortly after birth, radial glial cells, which serve as embryonic NSCs, begin to transition into both adult NSCs and ECs. ECs achieve a planar cell polarity whereby the basal bodies of their motile cilia polarize on their apical surfaces, which is essential for the coordinated ciliary movements that direct cerebrospinal fluid (CSF) circulation and regulate neuroblast migration (Mirzadeh et al., 2010b, Sawamoto et al., 2006). During this time, ECs and NSCs arrange into cellular pinwheels, and diffusely distributed ECM on ECs transitions into aggregated ECM hubs at the interface of ECs and NSCs (McClenahan et al., 2016).
Given the dramatic reorganization of cellular and extracellular structures that take place in the postnatal V-SVZ, we hypothesized that matrix metalloproteinases (MMPs), a family of extracellular endopeptidases, may be important in this process. The 24 mammalian MMPs are mostly secreted or membrane-tethered proteins that cleave ECM proteins, cell adhesion molecules, and growth factors (Nagase et al., 2006). Due to the wide range of substrate selectivity, their expression, secretion, and activation are tightly controlled in a cellular and developmental fashion (Page-McCaw et al., 2007). MMPs have been shown to regulate the development and function of other stem cell niches, e.g., the hematopoietic stem cell niche and the mammary gland epithelial stem cell niche (Heissig et al., 2002, Kessenbrock et al., 2013, Nishida et al., 2012). In the adult V-SVZ, MMP24 (MT5-MMP) has been found to co-localize with NSCs and control their quiescence, in both physiological and pathological states, by cleaving N-cadherin (Porlan et al., 2014). However, whether MMPs regulate the postnatal development of the V-SVZ NSC niche itself remains largely unknown.
In this study, we sought to understand how MMPs contribute to the development and function of the postnatal V-SVZ stem cell niche. We characterized MMP family expression during the NSC-to-EC transition and found MMP12 to be highly upregulated during this process, and that ECs can express an intracellular MMP12. Using an Mmp12 mutant mouse with a selective loss of the secreted, extracellular MMP12, we explored the functions of both extracellular and intracellular MMP12 during V-SVZ niche establishment. Our study reveals that extracellular MMP12 regulates the cellular and ECM rearrangements needed to build a mature niche, whereas intracellular MMP12 has a distinct function in regulating EC ciliogenesis, with both extracellular and intracellular MMP12 forms promoting NSC quiescence and thus regulating niche output.
Results
Identifying MMP12 as a Possible Regulator of Postnatal V-SVZ Niche Development
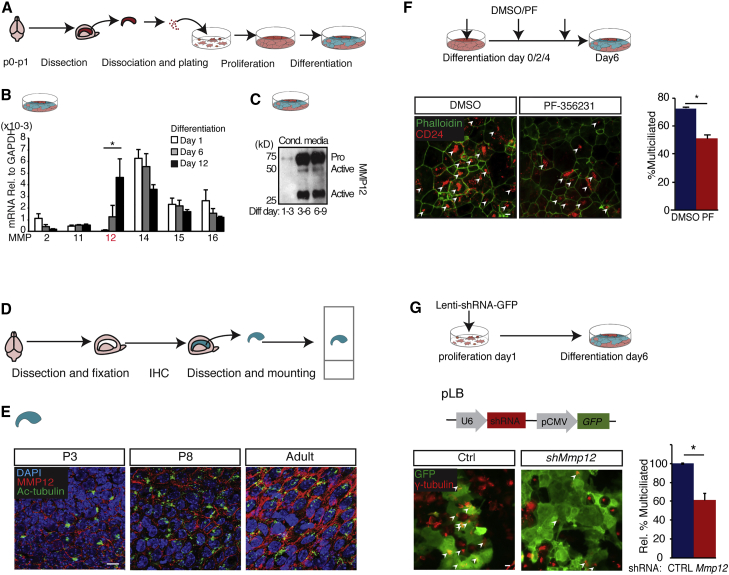
To explore a potential role for MMPs in regulating V-SVZ niche development, we applied a broad-spectrum MMP inhibitor, GM6001, to V-SVZ EC cultures (see Figure 1A), and observed a significant block in EC maturation as judged by the decrease in multiciliated (γ-tubulin+) cells and FoxJ1 promoter activity (Figures S1A and S1B). To determine the MMP(s) potentially responsible for this phenotype, we collected total mRNA from the EC cultures at days 1, 6, and 12 of differentiation and analyzed Mmp gene mRNA levels using qRT-PCR. Of the 24 Mmps and their splicing variants, only Mmp2, 11, 12, 14, 15, and 16 were highly expressed (>5 × 10−4 relative to Gapdh). Among these, Mmp12 was unique in being strongly upregulated during EC differentiation (Table S1 and Figure 1B). We validated the presence of MMP12 protein, both pro- (≥55 kDa) and active (22–45 kDa) forms, in western blots of conditioned media from differentiating ECs (Figure 1C). We next examined MMP12 in vivo using whole-mount immunohistochemistry (IHC) (Figure 1D), and identified MMP12 immunoreactivity associated with multiciliated ECs (visualized using acetylated α-tubulin immunoreactivity) that appeared to increase during V-SVZ niche development (Figure 1E).
Figure 1.
MMP Expression in the Developing V-SVZ Stem Cell Niche
(A) Schematic of ependymal cell (EC) cultures.
(B) Time course to assess mRNA levels of the most highly expressed Mmp family members in differentiating ECs reveals Mmp12 is upregulated during differentiation (∗p < 0.05, day 1 versus day 12, n = 3 independent experiments, one-way ANOVA with Tukey-Kramer correction).
(C) MMP12 western blotting of conditioned media from ECs at differentiation days 1–3, 3–6, and 6–9 (representative blot of 3 repeats).
(D) Schematic of V-SVZ whole-mount IHC.
(E) Representative images of V-SVZ whole-mount IHC at P3, P8, and P60 (adult). MMP12 is associated with multiciliated ECs (acetylated tubulin, Ac-tubulin), with MMP12 levels increasing during development.
(F) EC cultures treated with DMSO (vehicle) or PF-356231 (5 μM) at differentiation days 0, 2, and 4. The percentage of multiciliated ECs (CD24, EC marker co-localizing with cilia) is decreased by PF-356231 (arrowheads point to multiciliated ECs; error bars denote SEM; ∗p < 0.05, t test, n = 3 independent experiments).
(G) Upper: EC cultures were transduced with virus containing control shRNA (Ctrl) or Mmp12 shRNA. Middle: lentiviral construct pLB. Lower: Mmp12 shRNA significantly reduces the percentage of multiciliated ECs (arrowheads point to multiciliated GFP+ cells; error bars denote SEM; ∗p < 0.05, t test, n = 3 independent experiments).
Scale bars, 10 μm.
To assess MMP12 function, we used a MMP12-specific inhibitor, PF-356231, and lentivirus-delivered Mmp12 short hairpin RNA (shRNA), to specifically target MMP12 activity and expression in EC cultures (Figures 1F, 1G, S1C, and S1D for shRNA validation). The percentage of ECs that were multiciliated (CD24+, with visible cilia patches) at day 6 was significantly decreased by 5 μM PF-356231 treatment (Figure 1F). Additional scoring of multiciliated ECs using γ-tubulin immunoreactivity resulted in a similar decrease in multiciliated cells by PF-356231 (vehicle: 53.4% ± 2.8%, n = 3; PF: 27.7% ± 4.2%, n = 3; p = 0.003). EC cultures were next transduced with lentivirus co-expressing shRNA and GFP (Figure 1G and Supplemental Experimental Procedures). Here, significantly fewer multiciliated ECs (γ-tubulin+) were observed in lentivirus transduced cells (GFP+) with Mmp12 shRNA compared with control shRNA (Figure 1G).
A Functional Intracellular MMP12 Is Expressed in Mmp12 Mutant Ependymal Cells
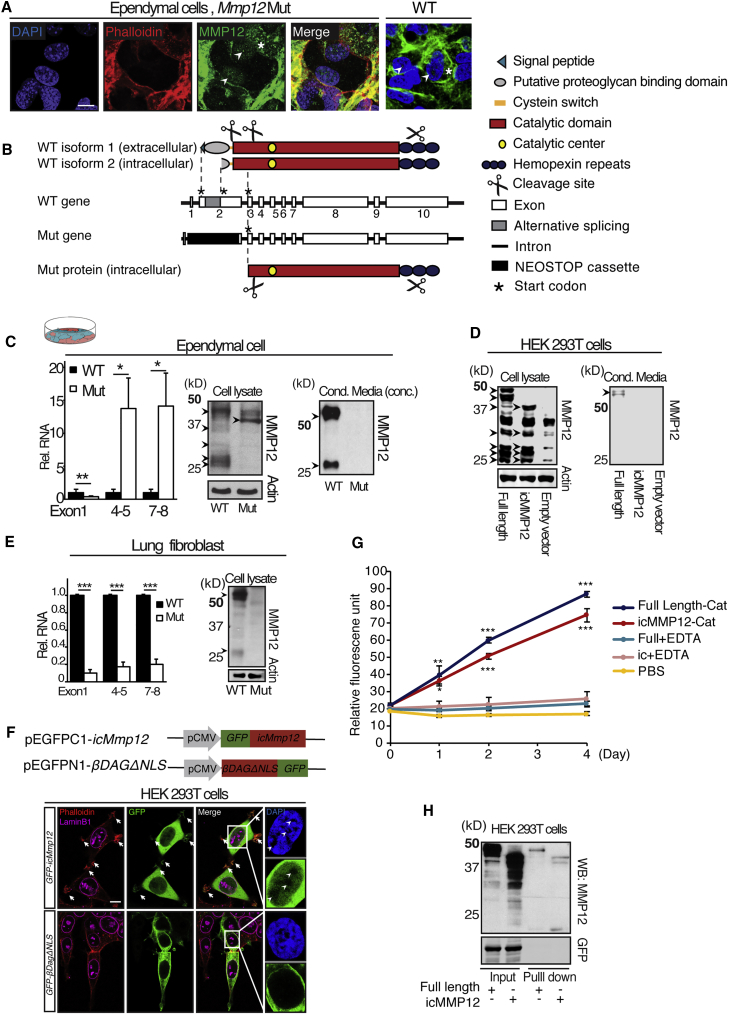
To further assess the function of MMP12 during V-SVZ niche development, we analyzed Mmp12 mutant mice, B6.129X-Mmp12tm1Sds/J (Shipley et al., 1996). However, using EC cultures prepared from Mmp12 mutant mice, we noticed MMP12 immunoreactivity in cell cortices and nuclei that was similar to that in wild-type (WT) (Figure 2A). When examining the transgenic strategy of the Mmp12 mutant, we noted that an in-frame start codon was preserved after replacing most of exon 2 with a flipped neomycin-STOP cassette (Figure 2B, mutant gene). If expressed, this gene product could produce a protein that lacks the signal peptide but preserves most of the catalytic domain and the hemopexin repeats, and would therefore be predicted to be a functional intracellular protein (Figure 2B, mutant protein).
Figure 2.
An Intracellular MMP12 Persists in Mmp12 Mutant Ependymal Cells
(A) IHC of Mmp12 mutant (Mut) and wild-type (WT) EC cultures at differentiation day 12, highlighting MMP12 in the cell cortex (asterisk, overlapping with phalloidin) and nucleus (arrowheads, DAPI).
(B) Mmp12 gene and MMP12 protein domains in WT and Mut mice. Splicing variations in exon2 lead to alternative Mmp12 transcripts in WT cells (WT isoform 2).
(C) MMP12 expression in WT and Mut ECs. Left: qPCR analysis with primers targeting different Mmp12 exons. Note the high levels of Mmp12 mRNA in Mut ECs only from primers targeting downstream exons (WT, n = 5; Mut, n = 6; 5 independent experiments; ∗p < 0.05, ∗∗p < 0.01, t test). Middle: western blots of cell lysates to detect intracellular MMP12 (icMMP12). An ∼40-kDa band is detected in Mut. Right: western blots of conditioned media to detect extracellular MMP12. No MMP12 is detected in Mut.
(D) Western blots of HEK293T cells transfected with constructs containing full-length or icMMP12 cDNA. Left: cell lysates. Right: conditioned media. Note that full-length and icMMP12 show similar patterns (arrowheads) as WT and Mut, respectively, in (C).
(E) MMP12 expression in WT and Mut lung fibroblast cells. Left: qRT-PCR. Mmp12 transcript is diminished at all tested exons (n = 3 independent experiments, ∗∗∗p < 0.001, t test). Right: western blots of cell lysates. No 40-kDa band is detected in Mut, as compared with (C).
(F) Confocal images of GFP IHC in HEK293T cells transfected with GFP-icMMP12 or βDAGΔNLS-EGFP. In contrast to βDAGΔNLS-EGFP, GFP-icMMP12 co-localizes with DAPI-stained nuclear DNA. Arrowheads indicate that DAPI− areas are also negative for GFP-icMMP12. GFP-icMMP12 also co-localizes with phalloidin at filopodia (arrows).
(G) MMP12 enzymatic activity assay on purified full-length or mutant icMMP12 catalytic domain proteins. Both proteins when incubated with FRET peptide substrate upregulated 480/530 nm fluorescent signal, compared with 50 mM EDTA-treated negative control or PBS (full-length catalytic domain, n = 3; icMMP12 catalytic domain, n = 4; full-length or icMMP12 + EDTA, n = 3; PBS, n = 5; 3 independent experiments, one-way ANOVA with Tukey-Kramer correction; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001).
(H) Streptavidin-agarose pull-down assay on HEK293T cells transfected with full-length MMP12 and icMMP12. Both full-length-MMP12 and icMMP12 were pulled down with a 5′ biotinylated DNA 50mer known to bind MMP12 (representative blot for 3 independent experiments).
Error bars denote SEM. Scale bars, 10 μm.
While MMPs are conventionally known as extracellular proteins, intracellular functions were recently discovered in many MMPs (e.g., MMPs 2, 9, 12, and 14) (Houghton et al., 2009, Jobin et al., 2017, Lovett et al., 2012, Marchant et al., 2014, Shimizu-Hirota et al., 2012, Zhang et al., 2015). For MMP12 in particular, its hemopexin domain has been found to have an antibacterial function intracellularly in macrophages (Houghton et al., 2009). Furthermore, extracellular MMP12 was reported to be transported back into cells, where it enzymatically cleaves intracellular proteins as well as translocates into the nucleus and regulates transcription (Marchant et al., 2014). In addition, the NCBI Gene database describes two transcriptional variants of mouse Mmp12 without a signal peptide sequence (NCBI Gene: NM_001320076.1, NM_001320077.1), based on RIKEN's functional annotation of the mammalian genome (e.g., Figure 2B, WT isoform 2). To date, the expression and function of these potential intracellular MMP12s remains uncharacterized. We used PCR primers targeting regions of the Mmp12 gene that varied among Mmp12 isoforms, and found a transcript consistent with Mmp12 WT isoform 2, which is predicted to translate into an intracellular MMP12 (icMMP12, Figures S2A–S2D). To test whether a truncated, icMMP12-encoding mRNA is expressed in Mmp12 mutant ECs, we used primers targeting different exons (Figure 2B, WT gene) to quantify Mmp12 cDNA with qRT-PCR (Figure 2C). As expected exon 1-containing transcripts were significantly reduced in Mmp12 mutant cells. In contrast, exons 4,5- and exons 7,8-containing transcripts were present in Mmp12 mutant cells (and even upregulated compared with WT). The 5′ end of this potential mRNA in Mmp12 mutant ECs was confirmed with 5′ RACE (rapid amplification of cDNA ends), with the predicted ATG being the only possible in-frame start codon (Figures S2E and S2F).
To determine whether icMMP12-encoding mRNA was translated into protein, we used cell lysates (to detect intracellular MMP12) and conditioned media (to detect extracellular MMP12) from WT and Mmp12 mutant EC cultures for western blotting. Full-length MMP12 is a 55-kDa protein. Typically, the pro-domain at the N terminus is first cleaved off to generate a 45-kDa active enzyme, then processed on both N and C termini, producing several intermediate products as well as a mature 25-kDa protein, presumably consisting only of the catalytic domain (illustrated in Figure 2B) (Shapiro et al., 1992, Shapiro et al., 1993) (TopFIND: P34960). In WT cell lysates, we found that full-length MMP12 can be detected as a 45-kDa activated protein band, a few intermediate bands, and an ∼25-kDa mature band, which is consistent with the known protein band pattern for MMP12 (Figure 2C). Furthermore, the >55-kDa species observed in conditioned media cannot be detected in cell lysates (for an example of an entire blot see Figure S1C). On the other hand, in Mmp12 mutant lysates, an ∼40-kDa band was observed (Figure 2C), indicating the presence of the mutant icMMP12 predicted by mRNA analysis. Conditioned media from WT cells contained a >50-kDa band and an ∼25-kDa band (Figure 2C). In contrast, the conditioned media from Mmp12 mutant cells lack any detectable MMP12 (Figure 2C), consistent with a complete loss of secreted MMP12, as reported by Shipley et al. (1996).
To validate that the bands observed in Mmp12 mutant lysates were indeed MMP12 proteins, we made expression constructs of full-length and truncated Mmp12, each preserving their native Kozak sequence. HEK293T cell lysates expressing the full-length or truncated recombinant MMP12s showed banding patterns similar to those of lysates from WT and Mmp12 mutant EC cultures, respectively (Figure 2D, cell lysate). Consistent with EC cultures, only full-length Mmp12 cDNA expressed secreted MMP12 (Figure 2D). Based on 5′ RACE (Figures S2E and S2F) and western blotting (Figures 2C and 2D), we inferred that translation of the icMMP12 in Mmp12 mutant ECs starts from the predicted ATG site.
The B6.129X-Mmp12tm1Sds/J mouse line had been used as a complete Mmp12 knockout, with no intracellular MMP12 being reported (Houghton et al., 2009, Jobin et al., 2017, Shipley et al., 1996); therefore, we sought to determine whether mutant icMMP12 was found in other cells. In lung fibroblasts, which express high levels of MMP12, we found that, unlike ECs, all primer sets detected diminished levels of Mmp12 cDNA in Mmp12 mutant lung fibroblasts (Figure 2E). In WT lung fibroblasts the predominant MMP12 protein was ∼55 kDa (Figure 2E), similar to what was detected in conditioned media from full-length Mmp12 cDNA, with no detectable 40-kDa band as found in ECs (Figure 2C).
To assess mutant icMMP12 subcellular localization, we cloned icMmp12 cDNA into pEGFP-C1 and transfected HEK293T cells (Figure 2F). GFP immunocytochemistry indicated that GFP-icMMP12 was cytosolic and nuclear. Nuclear icMMP12 co-localized with DAPI, consistent with previous observations that MMP12 can enter the nucleus and associate with DNA (Marchant et al., 2014). As a negative control, we transfected pEGFPN1-βDAGΔNLS whose protein product is excluded from the nucleus (Figure 2F). We also transfected GFP-icMMP12 into both the V-SVZ (using neonatal ventricle electroporation) and EC cultures, and detected nuclear immunoreactivity in V-SVZ cells (Figures S2G and S2H).
Given that the mutant icMMP12 catalytic domain is slightly truncated, we wanted to confirm its biological activities. To test for enzymatic activity, we transfected 293T cells with full-length or icMMP12 catalytic domain tethered with a C-terminal 7xHis tag (pCDNA3.1+-FLMmp12Cat-7His, pCDNA3.1+-icMmp12Cat-7His) and prepared recombinant protein with Ni-nitrilotriacetic acid agarose beads under native conditions. Using an MMP12-specific enzymatic activity assay, full-length MMP12 and icMMP12 were both found to be enzymatically active (Figure 2G). Streptavidin-agarose pull-down assays were performed to assess DNA-binding ability (Figure 2H). After co-transfecting HEK293T cells with pEF1-mmp12-V5His and pMAX-GFP, or, with pEF1-icmmp12-V5His and pMAX-GFP, we incubated cell lysates with a 5′-biotinylated DNA oligo described to bind to MMP12 (Marchant et al., 2014). Streptavidin-agarose beads pulled down both full-length (45 kDa) and icMMP12 (40 kDa), but not co-transfected GFP, suggesting that icMMP12 retains DNA-binding activity.
Extracellular MMP12 Regulates V-SVZ Neural Stem Cell Niche Pinwheel Organization
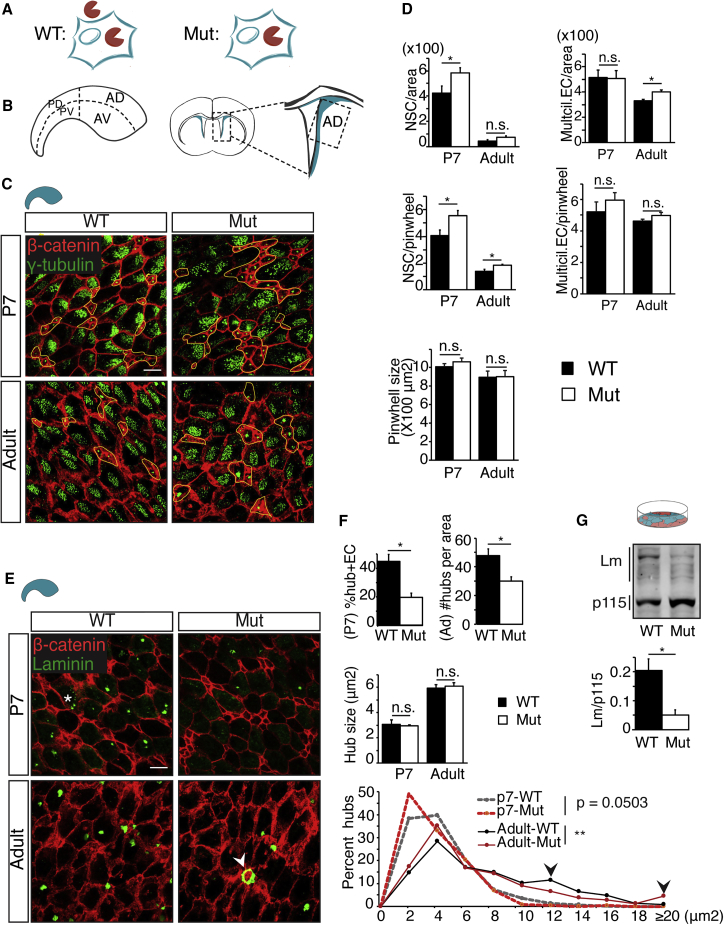
Given that Mmp12 mutant ECs retain a functional icMMP12, we were able to use this mouse to explore the functions of extracellular MMP12 in postnatal V-SVZ development (Figure 3A). We focused our analysis within the anterior-dorsal V-SVZ as it contains the most typical ECs and pinwheels (Figure 3B), performing whole-mount IHC on WT and Mmp12 mutant mice with β-catenin antibodies to label cell junctions and γ-tubulin antibodies to label ciliary basal bodies (Figure 3C). NSCs were defined by their small apical surface area and single cilium basal body, and ECs by their larger apical surface area and patches of ciliary basal bodies. At postnatal day 7 (P7) the Mmp12 mutant V-SVZ had more NSCs per area than in WT (Figure 3D, top left), as well as more NSCs per pinwheel in P7 and adult (8-week) mice, although overall pinwheel sizes were not affected (Figure 3D, middle and bottom). In contrast, the adult Mmp12 mutant V-SVZ contained slightly more ECs (Figure 3D, top right), although the number of ECs per pinwheel were not significantly affected (Figure 3D, middle right). Considering V-SVZ regional heterogeneities, we also examined the anterior-ventral (AV) and posterior-dorsal (PD) V-SVZ, areas known to contain niche pinwheels (Shook et al., 2012). We found similar increases in NSCs per pinwheel and NSCs per area in the AV, but not PD, V-SVZ (Figures S3A and S3B). Together, these results indicate that the lack of extracellular MMP12 leads to disturbances in V-SVZ niche pinwheel organization in the anterior V-SVZ, although EC maturation appears to proceed with relative normality.
Figure 3.
Extracellular MMP12 Regulates V-SVZ Niche Cellular and ECM Organization
(A) The presence or absence of extracellular and intracellular MMP12s in WT and Mmp12 mutant (Mut) cells.
(B) V-SVZ areas analyzed in this study. Left: whole-mount V-SVZ. Right: an anterior brain coronal section. AD, anterior dorsal; AV, anterior ventral; PD, posterior dorsal; PV, posterior ventral.
(C) Representative images of WT and Mut whole-mount IHC to indicate cell junctions (β-catenin) and ciliary basal bodies (γ-tubulin). Neural stem cell (NSC) clusters are highlighted in yellow.
(D) Quantification of NSCs, ECs, and pinwheels. NSCs/area at P7, multiciliated ECs/area at adult, and NSCs/pinwheel at P7 and adult are increased in Mut (P7 WT, n = 6 mice; P7 Mut, n = 6 mice; adult WT, n = 4 mice; adult Mut, n = 3 mice; ∗p < 0.05, t test).
(E) Representative images of WT and Mut whole-mount IHC to indicate cell junctions (β-catenin) and ECM hubs (laminin). Adult ECM hubs are often abnormal in size in Mut (arrowheads) (∗p < 0.05, t test).
(F) The percentage of ECs with ECM at P7 and the ECM hubs per area in adults are both lower in Mut V-SVZ (∗p < 0.05, t test; P7 WT, n = 4 mice; Mut, n = 4 mice; adult WT, n = 5 mice; Mut, n = 6 mice). In adults, Mut hub size distribution is different than in WT (∗∗p < 0.01 by Kolmogorov-Smirnov test; P7 WT, n = 674 hubs; Mut, n = 273, from 4 mice each; adult WT, n = 883, 5 mice; Mut, n = 587, 6 mice).
(G) Mut EC cultures at differentiation day 12 have a significantly lower level of laminin (WT, n = 4; Mut, n = 3; 3 independent experiments; ∗p < 0.05, t test).
Error bars denote SEM. Scale bars, 10 μm.
To determine whether extracellular MMP12-regulated ECM hubs, aggregated ECM structures near the ventricle surface (McClenahan et al., 2016), we performed whole-mount IHC with a polyclonal laminin-111 antibody that detects the majority of laminin subtypes. At P7 ECM hubs were not fully formed, so we measured the percentage of ECs with ECM aggregates (Figure 3E, asterisk), while in the adult V-SVZ we assessed ECM hub density and ECM hub size. We found that Mmp12 mutants had significantly fewer ECM-associated ECs at P7 and fewer ECM hubs per area in adulthood (Figure 3F, top). Although the mean ECM hub sizes were similar in WT and Mmp12 mutant (Figure 3F, middle), ECM hubs in Mmp12 mutants had altered size distributions, with fewer medium-sized hubs and more abnormally large ones (Figure 3E, arrowhead; Figure 3F, bottom, arrowheads). Mmp12 mutant EC cultures had less laminin protein (Figure 3G); however, no obvious changes in laminin cleavage products were observed in cell lysates (not shown). Together these data indicate an aberrant V-SVZ ECM in mice that lack extracellular MMP12.
Since laminin can be a substrate of MMP12, it was surprising that eliminating extracellular MMP12 would lead to less laminin. As all laminin subunit mRNA transcripts were at normal levels in Mmp12 mutant ECs (not shown), MMP12 likely regulates laminin at the post-transcriptional level. One possibility is an upregulation of other MMPs or downregulation of TIMPs (tissue inhibitors of metalloproteinases), leading to enhanced laminin digestion. Out of all the Mmps and Timps, we found that only Mmp2 mRNA levels were modestly upregulated in Mmp12 mutant EC cultures (Figures S4A and S4C). However, we detected similar levels of the MMP2 pro-enzyme and no activated MMP2 protein from either WT or Mmp12 mutant cells by gelatin zymography (Figure S4B) We also found no change in protein levels of β-dystroglycan and α6-integrin, two laminin receptors whose loss could affect cell association with laminin, and no change in mRNA levels of β1 and β4, the integrin β subunits that associate with α6 (Figure S4D).
Extracellular MMP12 Regulates Translational Planar Cell Polarity of V-SVZ ECs
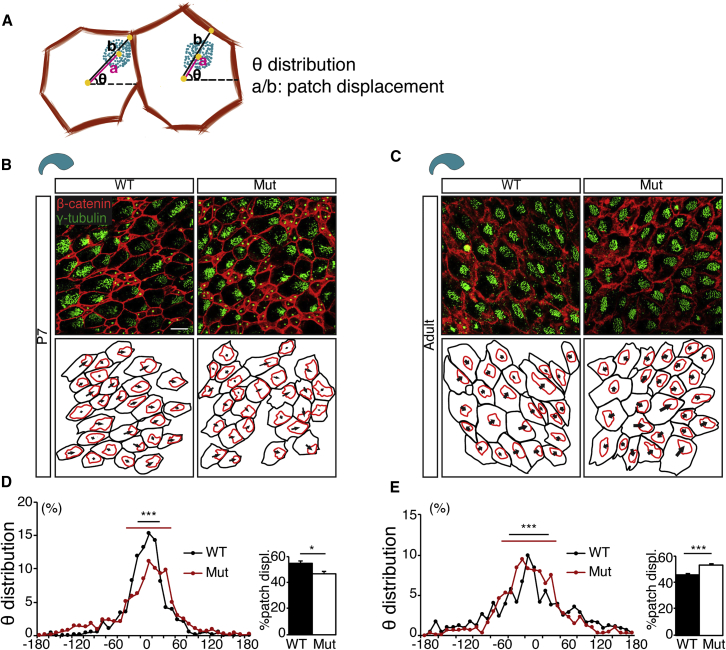
To determine whether extracellular MMP12 regulates V-SVZ translational planar cell polarity, we analyzed whole mounts for basal body patch displacement and angle distribution (Figure 4A), as described by Mirzadeh et al. (2010b). We found that V-SVZ translational polarity was disturbed in both P7 (Figures 4B and 4D [left]) and adult Mmp12 mutant mice (Figures 4C and 4E [left]). Basal body patches were less displaced in Mmp12 mutants at P7 (Figure 4D, right), indicative of decreased EC maturity, but more displaced in adults (Figure 4E, right). The abnormality in patch displacement was not a result of alterations of basal body patch sizes (P7: WT = 29.61 ± 1.45 μm2, n = 4 mice; Mut = 30.54 ± 1.76 μm2, n = 5; p = 0.68; adult: WT = 35.47 ± 1.59 μm2, n = 4; Mut = 33.26 ± 0.57 μm2, n = 3; p = 0.30). The AV and PD V-SVZ of P7 Mmp12 mutant mice also had dispersed patch angle distributions, but no significant changes in patch displacement (Figure S3C).
Figure 4.
Extracellular MMP12 Regulates EC Translational Planar Cell Polarity
(A) Planar cell polarity analysis. Cell boundaries (red) and ciliary basal body (green) patches were traced to calculate their respective centers (yellow). The distance between the centers (a) and distance between cell center and cell border (b) are used to calculate basal body patch displacement (a/b). The distribution of line angles (θ) throughout the field was measured.
(B and C) Representative images of WT and Mmp12 mutant (Mut) whole-mount IHC (upper) and tracings (lower) at P7 (B) and adult (C). Arrows indicate displacement of basal body patches from cell centers.
(D and E) Quantification of θ distribution and patch displacement. θ distributions are disrupted in Mut at P7 and adult (∗∗∗p < 0.001, Watson's U2). Mut patch displacements are decreased at P7 (∗p < 0.05, t test) and increased in adults (∗∗∗p < 0.001, t test). Four or more mice were analyzed in each group. Cells traced: P7 WT, n = 571 cells; P7 Mut, n = 1,354; adult WT, n = 960; adult Mut, n = 814.
Error bars denote SEM. Scale bars, 10 μm.
Intracellular MMP12 Promotes Ependymal Ciliogenesis via FOXJ1
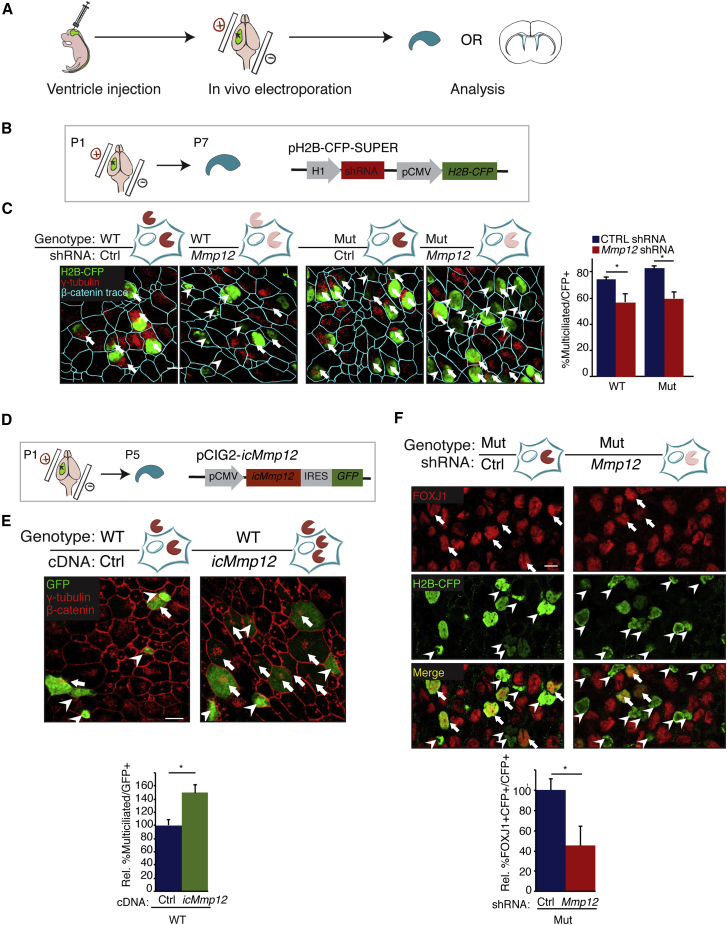
In contrast to MMP12 pharmacological inhibition or Mmp12 shRNA knockdown with which EC ciliogenesis was inhibited (Figures 1F and 1G), Mmp12 mutant mice, which serve as an extracellular MMP12 knockout, had normal EC ciliogenesis both in vivo (Figures 3C and 3D) and in vitro (not shown). Because the MMP12 inhibitor PF-356231 and Mmp12 shRNA are predicted to suppress both extracellular and intracellular MMP12, we hypothesized that the ciliogenic defects in these experiments could reflect separate functions of intracellular MMP12 (icMMP12). To manipulate potential icMMP12 expression in the developing V-SVZ, we used neonatal ventricle electroporation to introduce plasmids to the V-SVZ at P1 followed by an analysis of EC ciliogenesis (Figure 5A). First, we electroporated both WT and Mmp12 mutant mice with an Mmp12 shRNA plasmid co-expressing histone H2B-CFP (pH2B-CFP-SUPER), then analyzed V-SVZ whole mounts at P7 (Figure 5B). In WT mice, Mmp12 shRNA prevents both extracellular and intracellular MMP12 expression, whereas in Mmp12 mutant mice, Mmp12 shRNA prevents icMMP12 expression in cells that already lack extracellular MMP12, therefore revealing potential functions of icMMP12 (Figure 5C, schematics). Mmp12 shRNA reduced the percentage of multiciliated cells that emerged from transfected CFP+ VZ cells in both WT and Mmp12 mutant mice (Figure 5C). We also found no cleaved-caspase-3+ cells in the EC layer, indicating that cell death is unlikely to be contributing to the decrease in mature ECs (Figure S5A). If icMMP12 is required for ciliogenesis, then a prediction is that overexpressing icMMP12 may promote ciliogenesis. We therefore electroporated an icMMP12 construct into P1 WT mice (Figure 5D), and evaluated the whole mount at P5 to better catch a potential enhancement of EC ciliogenesis. Indeed, icMMP12 overexpression increased the percentage of multiciliated ECs that formed by P5 (Figure 5E). To determine whether icMMP12 affected ECM hub formation, we overexpressed icMMP12 in WT neonates through ventricle electroporation and analyzed whole mounts using laminin IHC. However, we did not observe significant changes in the percentage of ECM hub+ cells on the ventricular surface (control, 7.8% ± 1.1%, n = 5; icMMP12, 10.6% ± 1.8%, n = 4, p = 0.21), which suggests that icMMP12 is not instrumental for ECM hub formation.
Figure 5.
Intracellular MMP12 Regulates EC Ciliogenesis
(A) Schematic of neonatal in vivo electroporation.
(B) In vivo electroporation of WT and Mmp12 mutant (Mut) mice with scrambled shRNA or Mmp12 shRNA.
(C) Left: representative images of whole-mount IHC at P7. Arrows, multiciliated H2B-CFP+ cells. Arrowheads, non-multiciliated H2B-CFP+ cells. Right: in both WT and Mut mice, Mmp12 shRNA significantly reduced the percentage of multiciliated H2B-CFP+ cells (WT, n = 4 mice; Mut, n = 3 mice; ∗p < 0.05, t test).
(D) In vivo electroporation of WT mice with icMmp12 cDNA co-expressing GFP, followed by whole-mount IHC at P5.
(E) Representative IHC images at P5. Arrows, multiciliated GFP+ cells. Arrowheads, non-multiciliated GFP+ cells. The relative percentage of multiciliated GFP+ cells was increased by icMMP12 overexpression (Ctrl, n = 5 mice; icMmp12, n = 6 mice; ∗p < 0.05, t test).
(F) Same experimental scheme as in (B). Upper: Representative images of FOXJ1, GFP co-IHC (arrows, FOXJ1+CFP+ cells; arrowheads, FOXJ1−CFP+ cells). Lower: The percentage of FOXJ1+ cells within the CFP+ cell population was decreased by Mmp12 shRNA (ctrl shRNA, n = 5 mice; Mmp12 shRNA, n = 4 mice; ∗p < 0.05, t test).
Error bars denote SEM. Scale bars, 10 μm.
FOXJ1 has been shown to be a master regulator of ciliogenesis in ECs as well as in other multiciliated cells (Jacquet et al., 2009). As GM6001 (a pan-MMP inhibitor) blocked FoxJ1 transcription in EC cultures (Figures S1A and S1B), we hypothesized that icMMP12 may support ciliogenesis by regulating FOXJ1 levels. To test this, we electroporated Mmp12 mutant mice at P1 with control or Mmp12 shRNA, as above (Figure 5B), and analyzed whole mounts at P7 to determine the percentage of FOXJ1+ cells within the CFP+ cells. We found that Mmp12 shRNA on the background of Mmp12 mutant mice significantly decreased the percentage of FOXJ1+ cells, indicating that icMMP12 regulates EC ciliogenesis at the level of, or upstream of, FOXJ1 (Figure 5F).
Extracellular and Intracellular MMP12 Both Promote Neural Stem Cell Quiescence
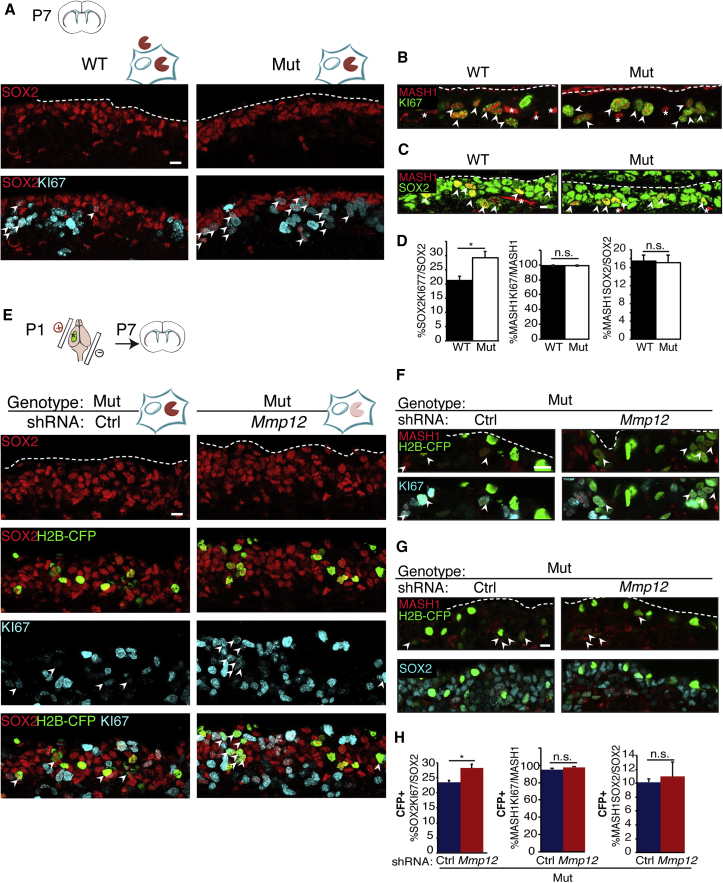
To investigate potential roles for extracellular and intracellular MMP12 in niche function, we analyzed the proliferation of NSCs (SOX2+MASH1−) and transient amplifying progenitors (TAPs, SOX2+Mash1+) in the Mmp12 mutant and WT littermate V-SVZ (Figure 6A), as well as in the mmp12 mutant V-SVZ following either Mmp12 or control shRNA (Figure 6E). Coronal sections were assessed by IHC for Ki67 to detect proliferating cells, and SOX2 to detect a combination of NSCs and TAPs. At P7, V-SVZ in Mmp12 mutant mice had a higher percentage of proliferating SOX2+ cells than WT mice (Figures 6A and 6D [left]). shRNA-mediated loss of icMMP12 in the Mmp12 mutant V-SVZ also increased proliferation (Figures 6E and 6H [left]). To distinguish NSCs and TAPs within the SOX2+ cell pool, we co-labeled Mash1, a TAP marker (Lee et al., 2012), and Ki67, and found no changes in proliferative TAPs (Figures 6B, 6D [middle], 6F, and 6H [middle]). Finally, considering that >90% of TAPs were proliferative, a shift in the %TAPs within the total SOX2+ pool could also contribute to the increase in SOX2+ cell proliferation. However, we found no change in the %Mash1+ out of the total SOX2+ cell pool (right-hand panels of Figures 6C, 6D, 6G, and 6H), indicating that the increase in proliferation in SOX2+ cells reflects increased NSC activation.
Figure 6.
Extracellular and Intracellular MMP12 Both Suppress NSC Proliferation
(A–C) IHC in coronal sections from WT and Mmp12 mutant (Mut) mice at P7. Arrowheads, double-positive cells; asterisks, non-specific vascular staining.
(D) Quantification of (A), (B), and (C) (left to right). The %Ki67+ cells within the SOX2+ cell population is significantly increased in Mut (WT, n = 6 animals; Mut, n = 9; ∗p < 0.05, t test). The %Ki67+ cells within MASH1+ cells, or the %MASH1+ cells within SOX2+ cells are not changed (WT, n = 4 mice; Mut, n = 4 mice; p > 0.05, t test).
(E–G) IHC in coronal sections from P7 Mut mice electroporated with control (Ctrl) or Mmp12 shRNA. (E) SOX2, H2B-CFP, Ki67 co-IHC. (F) MASH1, H2B-CFP, Ki67 co-IHC. (G) MASH1, H2B-CFP, SOX2 co-IHC. Arrowheads point to triple-positive cells.
(H) Quantification of (E), (F), and (G) (left to right). Within H2B-CFP+ cells, the percentage of SOX2+ cells that are Ki67+ is significantly increased by Mmp12 shRNA (Ctrl shRNA, n = 3 mice; Mmp12 shRNA, n = 3 mice; ∗p < 0.05, t test). The %Ki67+ cells among MASH1+ cells or the %MASH1+ cells among SOX2+ cells are not changed by Mmp12 knockdown (WT, n = 4 mice; Mut, n = 4 mice; p > 0.05, t test).
Error bars denote SEM. Dashed lines indicate ventricular surfaces. Scale bars, 10 μm.
Mmp12 mutant mice (lacking extracellular MMP12) were previously reported to have delayed myelination in the absence of any change in oligodendrocyte progenitors (Larsen et al., 2006), but it remained unclear whether icMMP12 influences neurogenesis and oligodendrogenesis. We therefore introduced Mmp12 shRNA by ventricle electroporation into Mmp12 mutant mice and assessed neuronal (DCX+) and oligodendroglial (OLIG2+) progenitors in whole mounts. The fractions of neuronal and oligodendroglial progenitors were similar to those of Mmp12 and control shRNAs (Figure S5B) suggesting that icMMP12 does not affect NSC fate decisions. To determine whether MMP12 influenced rostral migration of neuroblasts, we compared the olfactory bulb (OB) in WT and Mmp12 mutants or performed icMMP12 knockdown in the Mmp12 mutant V-SVZ. After electroporating constructs at P1 and assessing coronal sections at P7 (Figure S5C), we found more H2B-CFP+ cells in the OB when icMMP12 was silenced (Figures S5C–S5F), indicating that icMMP12 may influence the ability of V-SVZ neuroblasts to reach their OB target.
Discussion
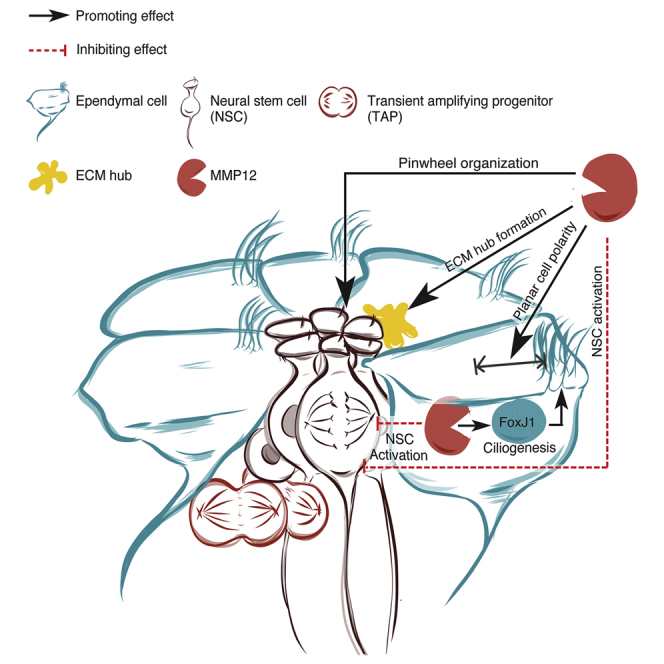
We investigated the potential role of the MMP family in the development of the V-SVZ NSC niche and found that MMP12 is not only highly expressed in developing ECs but regulates EC maturation and, ultimately, V-SVZ niche output. While MMPs have been found to regulate other stem cell niches (Heissig et al., 2002, Kessenbrock et al., 2013, Nishida et al., 2012), in the V-SVZ only one other MMP, MMP24 (MT5-MMP), has been linked to V-SVZ stem cell niche function, where it is enriched in adult NSC cell junctions and has been shown to regulate adult V-SVZ stem cell quiescence (Porlan et al., 2014). Since it is currently unknown whether Mmp24 plays a role in the development of the V-SVZ NSC niche, our finding that MMP12 regulates EC and V-SVZ niche development is the first to report this role for any MMP.
Surprisingly, we found that Mmp12 mutant ECs express a truncated yet functional intracellular MMP12 protein, which we referred to as icMMP12, whose expression may be cell type specific. We further found that the loss of the extracellular MMP12 had no effect on EC ciliogenesis, while the loss of icMMP12 attenuated EC ciliogenesis, demonstrating that icMMP12 is sufficient to support MMP12's role in EC ciliogenesis. Although MMPs are well known as extracellular proteases, a recent landmark study has suggested that extracellular MMP12 can be taken up by cells and then function intracellularly, both as a protease and as a transcriptional regulator (Marchant et al., 2014). In addition, two variant transcripts have been predicted to produce an intracellular-only MMP12. Indeed, we found that one of these alternative transcripts is likely to be present in WT ECs. Therefore ECs may contain a unique pool of MMP12 whose sole function is intracellular. A better understanding of the expression patterns of the different Mmp12 isoforms, both developmentally and across different cell types, may help to reveal new insights into the putative roles of intracellular MMP12.
The distinct cellular locations of extracellular and intracellular MMP12s indicated that they may have unique functions. By comparing the V-SVZ in WT and Mmp12 mutant mice, which solely lack the secreted, extracellular MMP12, we concluded that extracellular MMP12 regulates the development of the V-SVZ niche pinwheel organization and associated ECM. Since a main function of MMPs is as a protease that can cleave the ECM, it was surprising that Mmp12 mutant mice had fewer ECM hubs and decreased laminin levels, in contrast to the laminin accumulation in alveoli previously reported in Mmp12 mutant mice (Warner et al., 2004). This indicates that the ECM disturbances in the V-SVZ of Mmp12 mutant mice likely do not occur directly through altered laminin degradation by MMP12. We therefore explored the possibility that alterations in other MMPs, TIMPs, α6-containing integrins, or dystroglycan may lead to the ECM phenotypes, but found no obvious differences. This does not eliminate the possibility that other MMPs and proteases may be responsible for the altered ECM and cellular arrangements found in the Mmp12 mutant V-SVZ, which could occur via changes in their translation, secretion, or activation state.
ECM hubs first appear in the V-SVZ at P6–P7 on surfaces of ECs and are thought to be important for regulating niche NSCs by capturing growth factors found in the CSF (Mercier, 2016). ECM hubs are thought to be anchored by integrin and dystroglycan ECM receptors, which may serve to initiate signaling cascades influential to NSC behavior (McClenahan et al., 2016, Shen et al., 2008). While the mechanism by which ECM hubs are produced and organized remains unknown, possible mechanisms include spatially targeted ECM deposition or clearance, or aggregating/relocating existing ECM. While ECM hubs appear to form while associated with ECs, VZ microglia may also contribute to hub development, as VZ microglia express several ECM proteins and proteases including MMP12 (Shigemoto-Mogami et al., 2014). The discovery that MMP12 regulates VZ ECM hubs may help to shed light on the mechanism(s) by which ECM hub development proceeds.
While Mmp12 mutant mice have disturbances in the translational planar cell polarity of VZ ECs, ciliogenesis itself remained unchanged. Yet, surprisingly, inhibiting MMP12 activity with a membrane permeable compound caused ciliogenic defects in EC cultures, suggesting that icMMP12 is involved in ciliogenesis. We performed shRNA knockdown or overexpression of icmmp12 in vivo to test this hypothesis, and furthermore found that icMMP12 regulates EC ciliogenesis through FOXJ1, a transcription factor required for multiciliogenesis (Jacquet et al., 2009). As MMP12 has been reported to act both as an intracellular protease and transcription regulator, it will be interesting to determine whether icMMP12 regulates FOXJ1 levels through its proteolytic activities or through a transcriptional-modulatory mechanism.
Stem cell niches and their support cells tightly control stem cell behaviors (Li and Xie, 2005). In the current study, we found that either selective loss of extracellular MMP12 (comparing WT with Mmp12 mutants) or loss of icMMP12 via knockdown strategies led to a significant upregulation of NSC proliferation. As Mmp12 mRNA was virtually non-detectable in NSCs in vitro, we inferred that enhanced NSC proliferation in response to MMP12 loss was most likely an indirect consequence of disturbed niche support cells. For example, secreted MMP12 originating from ECs may normally influence surface proteins on NSCs, or translocate into NSCs to regulate NSC activation state. Consequently, loss of extracellular MMP12, originating from ECs, could affect NSC proliferation by disturbing NSC-intrinsic mechanisms. On the other hand, changes in NSC proliferation seen following the loss icMMP12 in the Mmp12 mutant V-SVZ, may occur due to dysregulated timing of EC differentiation that in conjunction alters the activation dynamics of the postnatal NSC pool. Recent evidence indicates that adult neurogenesis is important in neuron-circuit plasticity (Obernier et al., 2014) and is required for olfactory-dependent behaviors (Gheusi et al., 2000, Sakamoto et al., 2011). As increased NSC proliferation may lead to early depletion of the NSC pool, it will be important to investigate how MMP12 can affect the aging V-SVZ and related neuron-circuit homeostasis.
The early postnatal V-SVZ undergoes complex organizational changes in a short span of time to generate the adult NSC niche, in a process that is only partly understood. This study demonstrates that MMP12 is involved in multiple aspects of V-SVZ niche development, leading to changes in this stem cell niche's function, and additionally reveals a unique function of intracellular MMP12 in EC maturation. These discoveries provide new insight into how MMPs can act to sculpt both the form and function of developing stem cell niches, with implications for homeostasis in adult and aging brains.
Experimental Procedures
Animals
Mice were housed and cared for according to NIH and IACUC guidelines. C57/bl6 mice and mutant mice of C57/bl6 background (B6.129X-Mmp12tm1Sds/J) (Shipley et al., 1996) were from The Jackson Laboratory. FoxJ1-promoter GFP-transgenic mice were a gift from Dr. Ken-Ichi Takemaru (Ostrowski et al., 2003).
Image Acquisition and Analysis
Confocal images were acquired from Zeiss LSM510 or LeicaSP8X confocal laser-scanning microscopes. Selected fluorescent images for cell culture were acquired using a Zeiss Axiovert 200M epifluorescent microscope. Image analysis was done with Fiji software, and data processing for planar cell polarity was done with MATLAB software (Supplemental Experimental Procedures).
Statistical Analysis
Statistical analyses (one-way ANOVA followed by multiple comparisons with Tukey-Kramer corrections, Watson's U2, and Kolmogorov-Smirnov test) were performed using MATLAB. t Tests were performed with Excel. p < 0.05 was considered statistically significant.
Antibodies and Dyes
Please refer to Supplemental Experimental Procedures for full details.
Primers, DNA Oligos, and Sequencing
DNA sequencing was carried out at Stony Brook Genomics Core Facility. Please refer to Supplemental Experimental Procedures for a list of primers.
MMP12 Enzymatic Activity Assay
Proteolytic activity of MMP12 was analyzed with SensoLyte 520 MMP-12 assay kit. Sodium azide (0.02%) was added to all reactions to prevent microbe growth during incubation. Reactions were incubated in the dark at 37°C for 0, 1, 2, and 4 days before fluorescence reading with a Fluoroskan Ascent at excitation/emission of 480/530 nm.
Ependymal Cell Culture
EC cultures were adapted from a previous study (Paez-Gonzalez et al., 2011). All media contained L-glutamine and 1% penicillin/streptomycin. Minimum essential medium with HEPES was used for dissections. DMEM with 10% fetal bovine serum (FBS) was used to stimulate cell proliferation. DMEM with 2% FBS was used to induce EC differentiation. Papain solution, containing 1.2 μg/mL papain, 0.24 mg/mL L-cysteine, and 30 μg/mL DNaseI type IV dissolved in dissection medium was activated at 37°C for 30 min followed by sterile filtration. Tissue culture dishes were coated with 5 μg/mL poly-D-lysine at room temperature for at least 1 hr before being washed twice with sterile water. Lateral ventricular walls were dissected from P0–P1 mice in dissection medium and placed in a vial with 1 mL of dissection medium. Activated papain solution (200 μL) was added to each vial and incubated at 37°C for 20 min. The reaction was stopped by adding 2 mL of proliferation media. Tissue was mechanically dissociated with a fire polished Pasteur pipet in dissection medium. Dissociated cells were then switched into proliferation medium, and cells were plated at the density of 0.08 brains/cm2 tissue culture surface. Cells reached confluence in 6–7 days and were switched to differentiation medium, in which cells differentiate into ECs for 6–12 days.
Whole-Mount V-SVZ Immunohistochemistry
Whole-mount V-SVZ immunohistochemistry was adapted from a previous protocol (Mirzadeh et al., 2010a). In brief, mouse brains were dissected to expose the lateral ventricular walls. The tissue was submerged in 1% paraformaldehyde (PFA) in PBS and fixed at 4°C overnight. The tissue was subject to additional fixation with pre-chilled methanol-acetone (1:1) on ice for 10 min or heat-mediated antigen retrieval in sodium acetate buffer (pH 6), depending on the target antigen. Tissue was then washed with PBS and blocked in blocking solution (PBS containing 0.1% Triton X-100, 10% donkey serum) at room temperature for 1 hr, followed by primary antibody incubation overnight at 4°C, and secondary antibody incubation at room temperature for 2 hr. Antibodies were diluted in blocking solution. After washing, tissue was stained with DAPI for 10 min, and lateral walls were further dissected and mounted on glass slides with Slowfade Gold and coverslipped.
Streptavidin-Agarose Pull-Down Assay
The streptavidin-agarose pull-down assay was adapted from an existing protocol (Wu, 2006). In brief, 5′ biotinylated single-stranded oligos were dissolved to 100 mM in NaCl-Tris-EDTA buffer (pH 8.0). Complementary oligos were combined at 1:1 and incubated in a thermocycler at 95°C for 5 min, followed by −1.0°C per min for 70 min. HEK293T cells were transfected with XtremeGene HP transfection reagent. After 48 hr, cells were lysed on ice in 250 μL non-denaturing lysis buffer (25 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1% Triton X-100, 1 mM PMSF) and cleared by centrifugation. The lysates were quantified with DC protein assay and adjusted to 8 mg/mL. Streptavidin-agarose beads were blocked in 4% BSA for 1 hr and 50 μL of the beads was used to pre-clear the lysate for 0.5 hr at room temperature. Pre-cleared cell lysate (250 μL) was then combined with 750 μL of PBS 1 mM with PMSF, 0.5 nM annealed oligo, and 50 μL of streptavidin-agarose beads, and incubated with gentle rotation at room temperature for 2 hr. The beads were then washed three times with PBS with 1 mM PMSF and boiled for 3 min with 50 μL 4× LDS sample buffer with 12% 2-mercaptoethanol. The eluate (25 μL) was used for western blotting.
Neonatal Ventricle Electroporation
Neonatal ventricle electroporation was adapted from a previous protocol (Feliciano et al., 2013). In brief, DNA constructs were purified with EndoFree Plasmid Maxi Kit and quantified with NanoDrop to ensure concentration >2 μg/μL, 260/230 > 2, and 1.8 < 260/280 < 2. P1 mice with milk spots were anesthetized using hypothermia. A 10-μL Hamilton syringe with 32-gauge needle was used for injection of 1 μL of DNA into the left lateral ventricle, at the middle point between the left eye and the center of lambda suture, and at the depth of 2–3 mm. The heads were then held with a tweezer electrode wet with PBS and subjected to electroporation at 5 square pulses, 100 V, 50 ms per pulse with 950-ms intervals, using an ECM 830 Generator. Mice recovered on a heating pad until resuming activity before being returned to their mothers.
Author Contributions
H.C. and X.S. designed experiments, analyzed data, and wrote the manuscript. X.S., L.T., and Q.Y. conducted experiments.
Acknowledgments
We thank Dr. David Anderson (Caltech) for the Mash1 antibody, Dr. Ken-Ichi Takemaru (Stony Brook University [SBU]) for FoxJ1 reporter mice, Dr. Kevin Czaplinski (SBU) for electroporation equipment, technical advice, and critical reading of the manuscript, Dr. Cindy Leiton (SBU) for Mmp primers, Ms. Alexa Lampasona (SBU) for assistance with 5′ RACE and critical reading of the manuscript, Dr. Steve Winder (University of Sheffield) for the βDAGΔNLS-EGFP construct, Dr. Maya Shelly (SBU) for electroporation equipment, and Dr. David Talmage (SBU) for critical reading of the manuscript.
Published: March 1, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, five figures, and one table and can be found with this article online at https://doi.org/10.1016/j.stemcr.2018.01.038.
Supplemental Information
References
- Brizzi M.F., Tarone G., Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Feliciano D.M., Lafourcade C.A., Bordey A. Neonatal subventricular zone electroporation. J. Vis. Exp. 2013;72:50197. doi: 10.3791/50197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G., Cremer H., McLean H., Chazal G., Vincent J.D., Lledo P.M. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc. Natl. Acad. Sci. USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B., Hattori K., Dias S., Friedrich M., Ferris B., Hackett N.R., Crystal R.G., Besmer P., Lyden D., Moore M.A. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A.M., Hartzell W.O., Robbins C.S., Gomis-Ruth F.X., Shapiro S.D. Macrophage elastase kills bacteria within murine macrophages. Nature. 2009;460:637–641. doi: 10.1038/nature08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet B.V., Salinas-Mondragon R., Liang H., Therit B., Buie J.D., Dykstra M., Campbell K., Ostrowski L.E., Brody S.L., Ghashghaei H.T. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin P.G., Butler G.S., Overall C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta. 2017;1864(11 Pt A):2043–2055. doi: 10.1016/j.bbamcr.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Dijkgraaf G.J., Lawson D.A., Littlepage L.E., Shahi P., Pieper U., Werb Z. A role for matrix metalloproteinases in regulating mammary stem cell function via the Wnt signaling pathway. Cell Stem Cell. 2013;13:300–313. doi: 10.1016/j.stem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P.H., DaSilva A.G., Conant K., Yong V.W. Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and -12 null mice. J. Neurosci. 2006;26:2207–2214. doi: 10.1523/JNEUROSCI.1880-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Hu J., Ralls S., Kitamura T., Loh Y.P., Yang Y., Mukouyama Y.S., Ahn S. The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS One. 2012;7:e50501. doi: 10.1371/journal.pone.0050501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen M.K., Walsh C.A. Neurogenesis at the brain-cerebrospinal fluid interface. Annu. Rev. Cell Dev. Biol. 2011;27:653–679. doi: 10.1146/annurev-cellbio-092910-154026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Xie T. Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Lovett D.H., Mahimkar R., Raffai R.L., Cape L., Maklashina E., Cecchini G., Karliner J.S. A novel intracellular isoform of matrix metalloproteinase-2 induced by oxidative stress activates innate immunity. PLoS One. 2012;7:e34177. doi: 10.1371/journal.pone.0034177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant D.J., Bellac C.L., Moraes T.J., Wadsworth S.J., Dufour A., Butler G.S., Bilawchuk L.M., Hendry R.G., Robertson A.G., Cheung C.T. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat. Med. 2014;20:493–502. doi: 10.1038/nm.3508. [DOI] [PubMed] [Google Scholar]
- McClenahan F.K., Sharma H., Shan X., Eyermann C., Colognato H. Dystroglycan suppresses notch to regulate stem cell niche structure and function in the developing postnatal subventricular zone. Dev. Cell. 2016;38:548–566. doi: 10.1016/j.devcel.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F. Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell. Mol. Life Sci. 2016;73:4661–4674. doi: 10.1007/s00018-016-2314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F., Kitasako J.T., Hatton G.I. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J. Comp. Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z., Merkle F.T., Soriano-Navarro M., Garcia-Verdugo J.M., Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z., Doetsch F., Sawamoto K., Wichterle H., Alvarez-Buylla A. The subventricular zone en-face: wholemount staining and ependymal flow. J. Vis. Exp. 2010;39:1938. doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z., Han Y.G., Soriano-Navarro M., Garcia-Verdugo J.M., Alvarez-Buylla A. Cilia organize ependymal planar polarity. J. Neurosci. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nishida C., Kusubata K., Tashiro Y., Gritli I., Sato A., Ohki-Koizumi M., Morita Y., Nagano M., Sakamoto T., Koshikawa N. MT1-MMP plays a critical role in hematopoiesis by regulating HIF-mediated chemokine/cytokine gene transcription within niche cells. Blood. 2012;119:5405–5416. doi: 10.1182/blood-2011-11-390849. [DOI] [PubMed] [Google Scholar]
- Obernier K., Tong C.K., Alvarez-Buylla A. Restricted nature of adult neural stem cells: re-evaluation of their potential for brain repair. Front. Neurosci. 2014;8:162. doi: 10.3389/fnins.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski L.E., Hutchins J.R., Zakel K., O'Neal W.K. Targeting expression of a transgene to the airway surface epithelium using a ciliated cell-specific promoter. Mol. Ther. 2003;8:637–645. doi: 10.1016/s1525-0016(03)00221-1. [DOI] [PubMed] [Google Scholar]
- Paez-Gonzalez P., Abdi K., Luciano D., Liu Y., Soriano-Navarro M., Rawlins E., Bennett V., Garcia-Verdugo J.M., Kuo C.T. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron. 2011;71:61–75. doi: 10.1016/j.neuron.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porlan E., Marti-Prado B., Morante-Redolat J.M., Consiglio A., Delgado A.C., Kypta R., Lopez-Otin C., Kirstein M., Farinas I. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat. Cell Biol. 2014;16:629–638. doi: 10.1038/ncb2993. [DOI] [PubMed] [Google Scholar]
- Relucio J., Menezes M.J., Miyagoe-Suzuki Y., Takeda S., Colognato H. Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone. Glia. 2012;60:1451–1467. doi: 10.1002/glia.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M., Imayoshi I., Ohtsuka T., Yamaguchi M., Mori K., Kageyama R. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc. Natl. Acad. Sci. USA. 2011;108:8479–8484. doi: 10.1073/pnas.1018782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K., Wichterle H., Gonzalez-Perez O., Cholfin J.A., Yamada M., Spassky N., Murcia N.S., Garcia-Verdugo J.M., Marin O., Rubenstein J.L. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- Shapiro S.D., Griffin G.L., Gilbert D.J., Jenkins N.A., Copeland N.G., Welgus H.G., Senior R.M., Ley T.J. Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J. Biol. Chem. 1992;267:4664–4671. [PubMed] [Google Scholar]
- Shapiro S.D., Kobayashi D.K., Ley T.J. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J. Biol. Chem. 1993;268:23824–23829. [PubMed] [Google Scholar]
- Shen Q., Wang Y., Kokovay E., Lin G., Chuang S.M., Goderie S.K., Roysam B., Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y., Hoshikawa K., Goldman J.E., Sekino Y., Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 2014;34:2231–2243. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Hirota R., Xiong W., Baxter B.T., Kunkel S.L., Maillard I., Chen X.W., Sabeh F., Liu R., Li X.Y., Weiss S.J. MT1-MMP regulates the PI3Kdelta.Mi-2/NuRD-dependent control of macrophage immune function. Genes Dev. 2012;26:395–413. doi: 10.1101/gad.178749.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley J.M., Wesselschmidt R.L., Kobayashi D.K., Ley T.J., Shapiro S.D. Metalloelastase is required for macrophage-mediated proteolysis and matrix invasion in mice. Proc. Natl. Acad. Sci. USA. 1996;93:3942–3946. doi: 10.1073/pnas.93.9.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shook B.A., Manz D.H., Peters J.J., Kang S., Conover J.C. Spatiotemporal changes to the subventricular zone stem cell pool through aging. J. Neurosci. 2012;32:6947–6956. doi: 10.1523/JNEUROSCI.5987-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner R.L., Lukacs N.W., Shapiro S.D., Bhagarvathula N., Nerusu K.C., Varani J., Johnson K.J. Role of metalloelastase in a model of allergic lung responses induced by cockroach allergen. Am. J. Pathol. 2004;165:1921–1930. doi: 10.1016/S0002-9440(10)63244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.K. Analysis of protein-DNA binding by streptavidin-agarose pulldown. Methods Mol. Biol. 2006;338:281–290. doi: 10.1385/1-59745-097-9:281. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Amorosa L.F., Coyle S.M., Macor M.A., Lubitz S.E., Carson J.L., Birnbaum M.J., Lee L.Y., Haimovich B. Proteolytic cleavage of AMPKalpha and intracellular MMP9 expression are both required for TLR4-mediated mTORC1 activation and HIF-1alpha expression in leukocytes. J. Immunol. 2015;195:2452–2460. doi: 10.4049/jimmunol.1500944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.