Abstract
The oral microbiome has been linked to a number of chronic inflammatory conditions, including obesity, diabetes, periodontitis, and cancers of the stomach and liver. These conditions disproportionately affect Mexican American women, yet few studies have examined the oral microbiota in this at-risk group. We characterized the 16S rDNA oral microbiome in 369 non-smoking women enrolled in the MD Anderson Mano a Mano Mexican American Cohort Study. Lower bacterial diversity, a potential indicator of oral health, was associated with increased age and length of US residency among recent immigrants. Grouping women by overarching bacterial community type (e.g., “Streptococcus,” “Fusobacterium,” and “Prevotella” clusters), we observed differences across a number of acculturation-related variables, including nativity, age at immigration, time in the US, country of longest residence, and a multi-dimensional acculturation scale. Participants in the cluster typified by higher abundance of Streptococcus spp. exhibited the lowest bacterial diversity and appeared the most acculturated as compared to women in the “Prevotella” group. Computationally-predicted functional analysis suggested the Streptococcus-dominated bacterial community had greater potential for carbohydrate metabolism while biosynthesis of essential amino acids and nitrogen metabolism prevailed among the Prevotella-high group. Findings suggest immigration and adaption to life in the US, a well-established mediator of disease risk, is associated with differences in oral microbial profiles in Mexican American women. These results warrant further investigation into the joint and modifying effects of acculturation and oral bacteria on the health of Mexican American women and other immigrant populations. The oral microbiome presents an easily accessible biomarker of disease risk, spanning biological, behavioral, and environmental factors.
Introduction
Hispanics/Latinos comprise nearly 18% of the US population and more than 35% of the state populations of Texas, New Mexico, and California [1]. The majority of US Latinos are first and second generation immigrants of Mexican origin [2,3]. Nearly half of Mexican American women are obese, and 27% do not have health insurance [4]. Hence, it is not surprising that Mexican American women suffer from disproportionate rates of a number of prominent health conditions related to obesity and/or chronic infection, including diabetes [4], uncontrolled hypertension [4], and cancers of the uterine cervix, stomach, and liver [5,6]. Acculturation, the process by which immigrants adapt to a new culture through changes in beliefs and behaviors, is associated with increased rates of obesity [7–9], diabetes [10,11], and cardiovascular risk factors [12,13]—all of which contribute to health disparities and chronic disease burden in this population.
The oral microbiome and its role in health and disease is a rapidly progressing research area with the potential to transform approaches to major public health problems currently facing Mexican American women; however, few studies have investigated the oral microbiota in this at-risk group. Emerging evidence links poor oral health to increased risk of cardiovascular disease [14,15], rheumatoid arthritis [16], and several cancers [17]. While the specific role of the oral microbiota remains unclear, research suggests bacteria in the oral cavity may influence disease by modulating inflammation and genome stability [18]. The oral microbiome is relatively stable throughout adult life [19–21], but inter- and intra-individual variations have been linked to tobacco use [22], diet [23], and oral hygiene [24–26]. Differences by geography and culture, which may reflect variation in diet, host genetics, or other factors, have also been observed [27–29]. Hence, the oral microbiome is poised to become a promising and easily accessible biomarker, potentially reflecting the intersection of biological, behavioral, and environmental risk factors that we cannot comprehensively measure or quantify in human epidemiologic studies.
We characterized the 16S oral microbiome in a group of 369 first and second generation Mexican American women enrolled in the MD Anderson Mano a Mano Mexican American Cohort Study (MACS) [30], a large prospective study of the genetic, social, and behavioral risk factors that contribute to cancer and chronic disease risk in the Mexican American population of Houston, Texas. We investigated the oral microbiota with respect to baseline demographic, acculturation, and health-related risk factors. Understanding these relationships will improve our knowledge of the oral microbiome and its potential as a biomarker of exposures and/or subsequent disease in Mexican American women.
Materials and methods
Study population and sample collection
The MD Anderson MACS cohort is an ongoing (enrollment 2001-) prospective, population-based study of predominantly low income, first generation immigrants of Mexican origin residing in the greater Houston metropolitan area [30]. All procedures in the current study as well as the parent Cohort were approved by the University of Texas MD Anderson Institutional Review Board and carried out in accordance with the appropriate regulations. Cohort participants provided written informed consent in the language of their choosing, English or Spanish. Upon enrollment, a baseline in-home interview consisting of questionnaires, measurement of height and weight, and biospecimen collection was conducted. Linguistic acculturation was measured using eight items from the Bidimensional Acculturation Scale for Hispanics [31]. Specifically, participants were asked how frequently they spoke, read, watched television programs, and listened to radio programs in English and Spanish. Responses were scored for each dimension. Over 85% of participants in the current study were classified as having high Hispanic acculturation; hence, analysis was limited to acculturation in the English dimension only. A query on food acculturation from the Cultural Life Style Inventory [32] was added to the baseline questionnaire in 2006. Physical activity was measured using an instrument derived from the California Teachers Study survey [33]. Further details of the MACS study can be found elsewhere [30].
For the current study, we randomly selected 375 adult women participating in the MACS cohort who met the following criteria: never smoker, not currently pregnant, and age ≥20 years at enrollment. Selection was further refined to those with complete data across key variables of interest. Current and former tobacco users were excluded to focus on associations with other, less known factors. Participants in the current study were characteristically similar to all women in the overall cohort [30], over 80% of whom are never smokers. Participant demographic data are available in S1 File.
Oral mouthwash samples, originally collected and processed at baseline for the primary purpose of human genetic association studies, were used for microbial analysis. Briefly, participants were asked to swish an alcohol-based mouthwash for 30 seconds, after which samples were collected and transported on ice to the laboratory where cellular content was isolated and resuspended in TE buffer. Samples were frozen at -80°C, where they remained until the point of processing for microbial 16S rDNA sequencing.
Laboratory methods/16S sequencing
DNA was extracted from the oral mouthwash cellular matter using the MoBio PowerSoil DNA isolation kit following manufacturer’s instructions. 16S rDNA sequencing was performed using Illumina MiSeq with barcoded primers targeting the V4 region: GGACTACHVGGGTWTCTAAT and GTGCCAGCMGCCGCGGTAA [34]. Raw sequences were merged and quality filtered using USEARCH [35]. Parameters for merging included minimum overlap of 50 base pairs, zero mismatches, and truncation quality value of 5. Quality filtering allowed for a maximum expected error rate of 0.05. Illumina PhiX control sequences were removed with Bowtie2 [36]. All remaining sequences were subsequently clustered into operational taxonomic units (OTUs), with chimera removal using UPARSE [37]. Taxonomy assignment was performed closed-reference against the SILVA database (release 123) at 97% identity, resulting in 7,083,883 total reads (median 18,190 reads/sample). Samples that produced fewer than 4,000 reads were excluded from subsequent analysis. The remaining samples (n = 369) were rarefied to 7,600 reads/sample. UPARSE centroid OTU sequences were queried via Basic Local Alignment Search Tool (BLAST) [38] to identify likely representative bacterial species. A rarefied OTU table with the associated centroid sequences is available in S2 File. Bacterial functional capabilities were imputed using Tax4Fun [39], an algorithm using phylogenetic relationships to predict gene content, ultimately assigning functional pathways using the Kyoto Encyclopedia of Genes and Genomes [40,41].
Statistical analysis
Bacterial alpha diversity was measured using observed OTU (total number of unique OTUs per sample), Chao1 index, and Shannon diversity index. Differences in alpha diversity by demographic and health behavior variables were analyzed by ANOVA or linear regression. Beta diversity was measured using Bray-Curtis dissimilarity distance and analyzed via permutational multivariate analysis of variance. Sparse Correlations for Compositional data (SparCC) [42], which accounts for the compositional nature of 16S sequencing, was used to identify bacterial co-occurrence using false discovery rate Q = 0.01. Microbiota-derived clustering was performed using Dirichlet multinomial mixtures (DMM) modeling [43]. To ensure cluster consistency, the DMM algorithm was repeated in 20 separate datasets at various levels of rarefaction (3,000–15,000 reads/sample). Seventy percent of datasets indicated three as the optimum number of clusters; thus, we generated three clusters for the dataset analyzed here. Differences in demographic risk factors across bacterial cluster were tabulated via contingency table and assessed via Pearson’s chi-square. Differentially abundant bacterial taxa and putative functional content of clusters were determined using Linear Discriminant Analysis (LDA) Effect Size (LEfSe) [44], applying the one-against-all strategy with a minimum logarithmic LDA score (i.e., biomarker effect size) of 2.5 and α = 1E-5. Statistical analyses were performed using SAGE Microbiome Explorer [45], R [46], or STATA 14 (StataCorp LP; College Station, TX), as appropriate.
Results
Participant characteristics
Baseline characteristics of the 369 non-smoking Mexican American women are detailed in Table 1. Median age was 39 years (range 20–78 years; birth years 1929–1989), more than 75% were currently married, and 50% had less than a high school education. The majority (80%) were born in Mexico, but of these, 52% had lived in the US for 15 years or more (immigration year range 1959–2009 for all participants). Rates of overweight and obesity were 36% and 47%, respectively—similar to those of the overall MACS cohort [30] and Mexican American women nationally [4].
Table 1. Observed OTUs by demographic characteristics in Mexican American women (N = 369 unless otherwise indicated).
| Unadjusted | Adjusted for age | Adjusted for age & education | ||||||
|---|---|---|---|---|---|---|---|---|
| N (%)a | Mean (SE) | P | Mean (SE) | P | Mean (SE) | P | ||
| Age (years) | ||||||||
| 20–29 | 47 (12.7) | 106.1 (3.9) | ||||||
| 30–39 | 148 (40.1) | 98.3 (2.2) | ||||||
| 40–49 | 94 (25.5) | 98.5 (2.8) | <0.01 | |||||
| ≥50 | 80 (21.7) | 87.3 (3.0) | ||||||
| Education level | ||||||||
| < High school | 183 (49.6) | 98.3 (2.0) | 0.62 | 99.0 (2.0) | 0.32 | |||
| High school diploma or equivalent | 87 (23.6) | 95.1 (2.9) | 94.3 (2.9) | |||||
| > High school | 97 (26.3) | 95.9 (2.8) | 95.4 (2.7) | |||||
| Marital status | ||||||||
| Married | 279 (75.6) | 97.6 (1.6) | 0.31 | 96.9 (1.6) | 0.91 | 96.2 (1.7) | 0.99 | |
| Not married | 89 (24.1) | 94.3 (2.9) | 96.5 (2.9) | 96.1 (2.9) | ||||
| Country of birth | ||||||||
| Mexico | 294 (79.7) | 97.7 (1.6) | 0.28 | 97.6 (1.6) | 0.33 | 96.8 (1.7) | 0.48 | |
| US | 75 (20.3) | 93.9 (3.1) | 94.3 (3.1) | 94.3 (3.1) | ||||
| Country of longest residence | ||||||||
| Mexico | 216 (58.5) | 99.7 (1.8) | 0.02 | 98.9 (1.8) | 0.11 | 97.9 (2.0) | 0.21 | |
| US | 153 (41.5) | 93.0 (2.2) | 94.3 (2.2) | 94.2 (2.2) | ||||
| Age of immigration (years)b | ||||||||
| 0–18 | 71 (23.7) | 92.1 (3.2) | 0.27 | 88.6 (3.3) | 0.02 | 88.5 (3.4) | 0.02 | |
| 19–24 | 95 (31.7) | 98.5 (2.8) | 96.5 (2.8) | 96.1 (2.9) | ||||
| 25–29 | 60 (20.0) | 100.9 (3.5) | 102.4 (3.5) | 101.8 (3.6) | ||||
| ≥30 | 74 (24.7) | 98.2 (3.2) | 102.8 (3.4) | 102.2 (3.5) | ||||
| Time in US (years)b | ||||||||
| <5 | 23 (7.7) | 110.9 (5.6) | 0.01 | 110.0 (5.8) | 0.10 | 109.3 (5.9) | 0.14 | |
| 5–9 | 48 (16.0) | 99.5 (3.9) | 98.9 (4.0) | 98.6 (4.0) | ||||
| 10–14 | 72 (24.0) | 101.4 (3.2) | 101.1 (3.2) | 100.5 (3.4) | ||||
| 15–19 | 70 (23.3) | 97.0 (3.2) | 96.6 (3.3) | 95.9 (3.4) | ||||
| 20–24 | 28 (9.3) | 93.0 (5.1) | 93.5 (5.1) | 93.3 (5.2) | ||||
| ≥25 | 59 (19.7) | 88.1 (3.5) | 89.5 (4.0) | 89.5 (4.1) | ||||
| English acculturation score | ||||||||
| 1–1.75 | 152 (41.2) | 99.1 (2.2) | 0.20 | 99.6 (2.2) | 0.15 | 98.6 (2.5) | 0.27 | |
| 2–2.75 | 109 (29.5) | 98.0 (2.6) | 97.5 (2.6) | 97.6 (2.6) | ||||
| 3–4 | 106 (28.7) | 93.1 (2.6) | 93.0 (2.6) | 92.9 (2.6) | ||||
| Food acculturationc | ||||||||
| Only Mexican foods | 55 (16.5) | 101.2 (3.5) | 0.14 | 100.9 (3.4) | 0.16 | 99.4 (3.6) | 0.28 | |
| Mostly Mexican foods | 117 (35.1) | 97.4 (2.4) | 97.3 (2.4) | 96.7 (2.4) | ||||
| Mix /Mostly American/Other | 161 (48.3) | 93.5 (2.0) | 93.6 (2.0) | 93.3 (2.0) | ||||
| History of alcohol consumption | ||||||||
| No | 310 (84.0) | 97.9 (1.5) | 0.12 | 98.0 (1.5) | 0.08 | 97.3 (1.6) | 0.12 | |
| Yes | 59 (16.0) | 91.9 (3.5) | 91.4 (3.5) | 91.3 (3.5) | ||||
| History of farm work | ||||||||
| No | 284 (77.0) | 97.5 (1.6) | 0.49 | 97.2 (1.6) | 0.70 | 96.6 (1.6) | 0.61 | |
| Yes | 85 (23.0) | 95.2 (3.0) | 96.0 (2.9) | 94.9 (3.0) | ||||
| BMI (kg/m2) | ||||||||
| Lean (<25) | 63 (17.1) | 101.0 (3.4) | 0.34 | 99.6 (3.4) | 0.33 | 99.5 (3.4) | 0.37 | |
| Overweight (25–29) | 132 (35.8) | 96.0 (2.4) | 96.2 (2.3) | 95.4 (2.4) | ||||
| Obese class I (30–34) | 104 (28.2) | 94.1 (2.7) | 93.9 (2.6) | 93.2 (2.7) | ||||
| Obese class II+ (≥35) | 70 (19.0) | 99.4 (3.3) | 100.6 (3.2) | 99.2 (3.3) | ||||
| Physical activity level (tertiles) | ||||||||
| Light | 132 (35.8) | 96.3 (2.4) | 97.3 (2.3) | 96.2 (2.4) | ||||
| Moderate | 145 (39.3) | 97.7 (2.3) | 0.83 | 97.4 (2.2) | 0.72 | 96.7 (2.3) | 0.74 | |
| Heavy | 88 (23.9) | 95.7 (2.9) | 94.7 (2.9) | 94.0 (2.9) | ||||
Abbreviations: BMI, body mass index; OTU, operational taxonomic unit; SE, standard error.
a Totals may not add up to 100% due to missing responses in <3% of participants.
b Those not born & raised in US; N = 300.
c Variable not assessed prior to 2006; N = 333 for 2006–2011 enrollment.
A core oral microbiome
A total of 511 unique OTUs, a proxy for bacterial species, were detected in participant mouthwash samples using 16S rRNA gene sequencing (average 97 OTUs/sample). Assigning OTUs at the genus level, we identified a core oral microbiome defined by 18 genera detected in 98% or more of samples (Fig 1A). Consistent with other large-scale studies of the oral microbiota [28,29,47], Streptococcus was the most abundant genus, with mean (SD) relative abundance of 37% (12%). Prevotella_7 (11%), Haemophilus (10%), Veillonella (6%), and Neisseria (5%) completed the top five. Other core bacteria included, in order of relative abundance, Gemella, Rothia, Fusobacterium, Prevotella, Alloprevotella, Actinomyces, Porphyromonas, Granulicatella, Leptotrichia, Bergeyella, Campylobacter, Capnocytophaga, and Oribacterium. Current classification using the SILVA rRNA gene database differentiates Prevotella from Prevotella_7 by OTU content. Taxa abundance was marked by considerable inter-individual variability (Fig 1A and 1B), with core genera accounting for 65.2%-99.7% of sequences/sample in all but one person.
Fig 1. Core oral genera of Mexican American women (Houston, TX, 2004–2011) exhibit wide variation in relative abundance.
(A) Box plots indicate log relative abundance of 18 core taxa, defined as those genera detected in ≥ 98% of study subjects. (B) Stacked bar plot shows the contribution of all core genera across each study participant.
Host-microbiome relationships: Bacterial alpha diversity
We evaluated bacterial alpha diversity with respect to several key demographic and health behavior variables. Notably, age was inversely associated with taxonomic richness as measured by observed OTU and Chao index (P<0.01) (Table 1 and S1 Table). This relationship appeared more pronounced in those having lived longer in the US than Mexico, regardless of country of birth (S1 Fig). However, the magnitudes of correlation were modest (observed OTU, r = -0.28; Chao, r = -0.32) and not observed with Shannon diversity (S2 Table and S1 Fig).
Among women born and/or raised in Mexico, taxonomic richness was also inversely correlated with years lived in the US (Table 1). Adjustment for age at sample collection attenuated this association but strengthened the relationship between observed OTU and age at immigration. Taxonomic richness was 16% higher among those immigrating at or after age 30 compared with those arriving at age 18 or prior (88.6 vs 102.8 OTUs, P = 0.02). Additional adjustment for educational attainment, a proxy for socioeconomic status, did not meaningfully change these relationships. Of the remaining variables examined, including educational attainment, marital status, country of birth, history of farm work, alcohol consumption, body mass index (BMI), physical activity, and two acculturation metrics representing English linguistic acculturation [31] and the type of food typically eaten at home [32], none were associated with alpha diversity in univariate or multivariate analyses.
Host-microbiome relationships: Bacterial communities
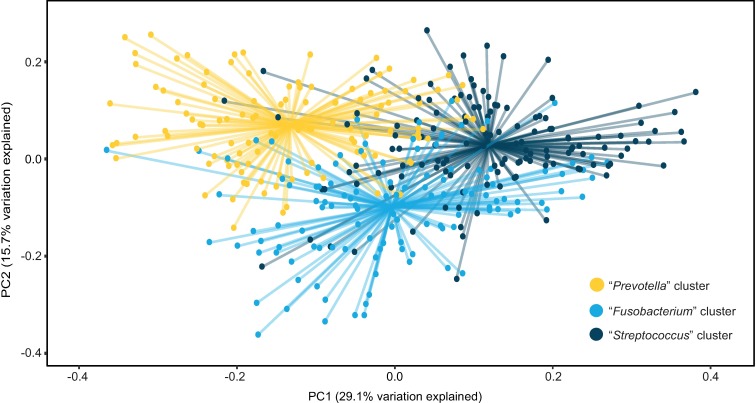
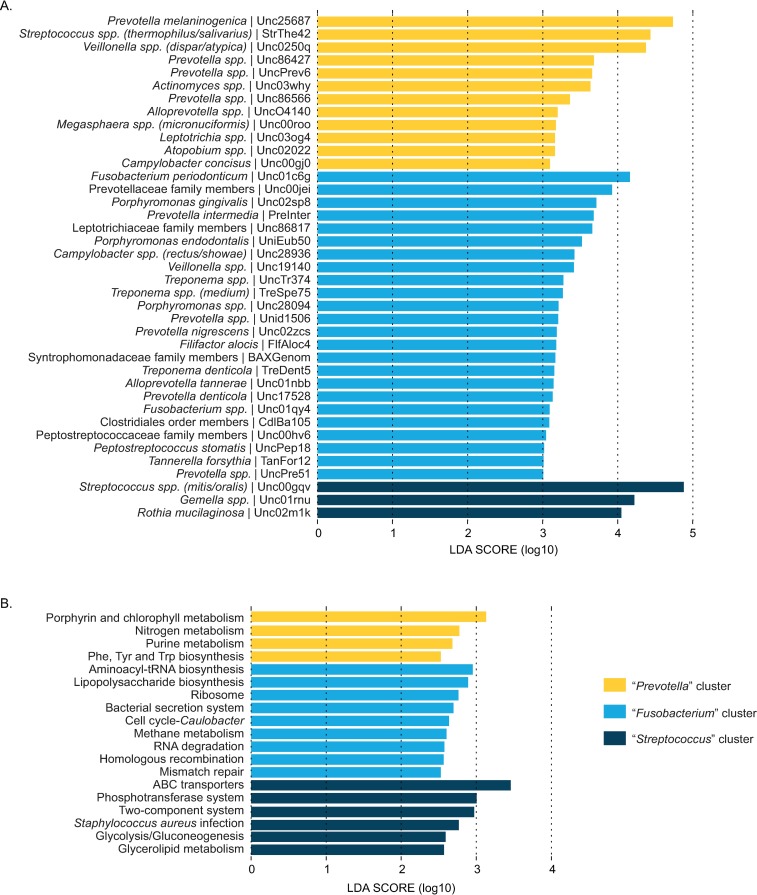
SparCC bacterial correlation analysis [42] indicated strong potential for differential bacterial community structure (S2 Fig). Hence, we used DMM modeling [43] to cluster samples on the basis of their oral microbiota into three oral community types. Beta diversity assessment indicated overlapping yet distinct bacterial conent in each community (Fig 2). Communities were named “Streptococcus,” “Fusobacterium,” or “Prevotella” based on the foremost differentially abundant OTU as identified by LEfSe [44] (Fig 3A and S3 Table). Bacterial alpha diversity varied significantly between clusters, with observed OTU and Shannon diversity highest among the “Fusobacterium” group followed by the “Prevotella” and “Streptococcus” clusters (Table 2). Consistent with reports associating high bacterial diversity with poor oral health [48], OTUs representing Fusobacterium periodonticum, Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia—all known oral pathogens [49]—were more abundant among “Fusobacterium” women (Fig 3A).
Fig 2. Beta diversity analysis indicates overlapping yet distinct bacterial community clusters as determined by DMM modeling.
Bray-Curtis dissimilarity distance varied significantly by DMM cluster (P<0.01) and was visualized using principal coordinates analysis. Lines connect samples to cluster centroids. DMM, Dirichlet multinomial mixtures.
Fig 3.
LEfSe analysis indicates differentially abundant taxa (A) and Tax4Fun-imputed functional pathways (B) by DMM cluster. Clusters were named for the single OTU with the greatest effect size. LEfSe analyses were conducted using α = 1E-05 and minimum LDA score = 2.5. Due to space limitations, only taxa with a minimum LDA score of 3.0 are shown. A complete list of taxa with minimum LDA score of 2.5 is included in supporting information. DMM, Dirichlet multinomial mixtures; LDA, linear discriminant analysis; LEfSe, LDA effect size.
Table 2. Demographic characteristics of Mexican American women by DMM cluster; values indicate N (%) unless otherwise indicated.
| Streptococcus Cluster (S)a | Fusobacterium Cluster (F)a | Prevotella Cluster (P)a | Pairwise P | |||
|---|---|---|---|---|---|---|
| S vs F | S vs P | F vs P | ||||
| N, total | 133 | 120 | 116 | |||
| Age (years) | ||||||
| 20–29 | 17 (12.8) | 22 (18.3) | 8 (6.9) | <0.01d | 0.25 | <0.01d |
| 30–39 | 54 (40.6) | 51 (42.5) | 43 (37.1) | |||
| 40–49 | 26 (19.5) | 36 (30.0) | 32 (27.6) | |||
| ≥50 | 36 (27.1) | 11 (9.2) | 33 (28.4) | |||
| Education level | ||||||
| < High school | 65 (48.9) | 63 (52.5) | 55 (47.4) | 0.84 | 0.36 | 0.27 |
| High school diploma or equivalent | 29 (21.8) | 24 (20.0) | 34 (29.3) | |||
| > High school | 38 (28.6) | 32 (26.7) | 27 (23.3) | |||
| Marital status | ||||||
| Married | 98 (73.7) | 91 (75.8) | 90 (77.6) | 0.61 | 0.48 | 0.84 |
| Not married | 35 (26.3) | 28 (23.3) | 26 (22.4) | |||
| Country of birth | ||||||
| Mexico | 98 (73.7) | 94 (78.3) | 102 (87.9) | 0.39 | <0.01d | 0.05 |
| US | 35 (26.3) | 26 (21.7) | 14 (12.1) | |||
| Country of longest residence | ||||||
| Mexico | 64 (48.1) | 73 (60.8) | 79 (68.1) | 0.04 | <0.01d | 0.24 |
| US | 69 (51.9) | 47 (39.2) | 37 (31.9) | |||
| Age of immigration (years)b | ||||||
| 0–18 | 34 (33.3) | 21 (22.1) | 16 (15.5) | 0.13 | 0.01d | 0.66 |
| 19–24 | 33 (32.3) | 28 (29.5) | 34 (29.3) | |||
| 25–29 | 19 (18.6) | 20 (21.1) | 21 (33.0) | |||
| ≥30 | 16 (15.7) | 26 (27.4) | 32 (31.1) | |||
| Time in US (years)b | ||||||
| <5 | 3 (2.9) | 14 (14.7) | 6 (5.8) | <0.01d | 0.19 | 0.12 |
| 5–9 | 14 (13.7) | 18 (18.9) | 16 (15.5) | |||
| 10–14 | 18 (17.6) | 24 (25.3) | 30 (29.1) | |||
| 15–19 | 27 (26.5) | 22 (23.2) | 21 (20.4) | |||
| 20–24 | 14 (13.7) | 7 (57.4) | 7 (6.8) | |||
| ≥25 | 26 (25.5) | 10 (10.5) | 23 (22.3) | |||
| English acculturation score | ||||||
| 1–1.75 | 48 (36.1) | 53 (44.2) | 51 (44.0) | 0.20 | <0.01d | 0.10 |
| 2–2.75 | 32 (24.1) | 33 (27.5) | 44 (37.9) | |||
| 3–4 | 51 (38.3) | 34 (28.3) | 21 (18.1) | |||
| Food acculturationc | ||||||
| Only Mexican foods | 15 (12.4) | 24 (22.2) | 16 (15.4) | 0.13 | 0.11 | 0.13 |
| Mostly Mexican foods | 39 (32.2) | 33 (30.6) | 45 (43.3) | |||
| Mix /Mostly American/Other | 67 (55.4) | 51 (47.2) | 43 (41.3) | |||
| History of alcohol consumption | ||||||
| No | 109 (82.0) | 100 (83.3) | 101 (87.1) | 0.77 | 0.27 | 0.42 |
| Yes | 24 (18.0) | 20 (16.7) | 15 (12.9) | |||
| History of farm work | ||||||
| No | 104 (78.2) | 101 (84.2) | 79 (68.1) | 0.23 | 0.07 | <0.01d |
| Yes | 29 (21.8) | 19 (15.8) | 37 (31.9) | |||
| BMI (kg/m2) | ||||||
| Lean (<25) | 22 (16.5) | 22 (18.3) | 19 (16.4) | 0.31 | 0.60 | 0.94 |
| Overweight (25–29) | 50 (37.6) | 41 (34.2) | 41 (35.3) | |||
| Obese class I (30–34) | 42 (31.6) | 30 (25.0) | 32 (27.6) | |||
| Obese class II+ (≥35) | 19 (14.3) | 27 (22.5) | 24 (20.7) | |||
| Physical activity level (tertiles) | ||||||
| Light | 48 (36.1) | 42 (35.0) | 42 (36.2) | 0.99 | 0.21 | 0.28 |
| Moderate | 49 (36.8) | 44 (36.7) | 52 (44.8) | |||
| Heavy | 36 (27.1) | 31 (25.8) | 21 (18.1) | |||
| Shannon diversity, Mean (SD) | 2.14 (0.33) | 283 (0.38) | 2.59 (0.28) | <0.01d | <0.01d | <0.01d |
| Observed OTUs, Mean (SD) | 73.9 (15.4) | 125.5 (17.0) | 93.8 (18.0) | <0.01d | <0.01d | <0.01d |
Abbreviations: BMI, body mass index; DMM, Dirichlet multinomial mixtures; SD, standard deviation.
a Totals may not add up to 100% due to missing responses in <3% of participants.
b Those not born & raised in US; N = 300.
c Variable not assessed prior to 2006; N = 333 for 2006–2011 enrollment.
d P-value significant following Sidak correction for multiple comparisons.
The relative abundance of core genera also varied by DMM cluster (S3 Fig). Specifically, the “Streptococcus” cluster exhibited the highest levels of Streptococcus, Haemophilus and Gemella; the “Fusobacterium” group contained higher amounts of Fusobacterium, Porphyromonas, Alloprevotella, and Prevotella; and “Prevotella” women exhibited greater abundance of Prevotella_7, Actinomyces and Veillonella.
Differences in predicted functional pathways were also observed by bacterial cluster (Fig 3B), with greater potential for essential amino acid biosynthesis and nitrogen metabolism among the “Prevotella” bacterial community type and carbohydrate metabolism and transport among the “Streptococcus” group. The “Fusobacterium” cluster indicated putative functional differences in DNA replication and repair as well as bacteria-host interactions.
Comparing demographics and health behaviors by DMM cluster, we found the “Streptococcus” cluster to be more acculturated, particularly as compared to the “Prevotella” group: “Streptococcus” participants reported higher English linguistic acculturation scores, were more likely to have been born in the US, or if born in Mexico, to have immigrated at an earlier age (Table 2). In contrast, the “Prevotella” group was more likely to report a history of farm work. Both the “Streptococcus” and “Prevotella” groups were significantly older than the cluster driven by Fusobacterium (mean (SD) of 42.2 (1.0) and 43.9 (1.1) vs 37.8 (1.0) years, respectively; P<0.01). None of the other variables tested, including BMI and physical activity, differed by cluster assignment.
Discussion
We examined the oral microbiome in a group of non-smoking Mexican American women from the Houston, Texas, metropolitan area. To our knowledge, this is the first study to characterize the oral microbiota in a large group of first and second generation Mexican American women. We identified a core microbiome of 18 taxa common to 98% or more of women and three microbial community types. Women in the three microbiome clusters varied by age and a variety of acculturation-related variables, including country of birth, country of longest residence, age at immigration, years lived in the US, and acculturation score. Among first generation immigrants, we further observed that time in the US and younger age at immigration were inversely associated with taxonomic richness. Collectively, these results support the potential mutability of the oral microbiota in response to cultural adaptation associated with immigration. They further provide a baseline profile for future studies to investigate relationships between oral bacteria and prospective disease in at-risk Mexican American women.
The oral microbiome is dominated by a handful of genera—Streptococcus, Prevotella, and Haemophilus, to name a few. We observed these and other well-known oral bacteria in our participants, with relative abundance varying by bacterial community type. Of particular note, the “Prevotella” and “Streptococcus”-defined clusters differed in the relative abundance of nearly all core taxa identified in our study. These clusters also differed demographically, with “Prevotella” individuals more likely to have been born in Mexico, to have resided longest in Mexico, and to have immigrated to the US at a later age compared to the “Streptococcus” group. Together, these differences support the potential for a microbial transition associated with immigration and adaptation whereby a “Prevotella” community dominates in recent Mexican immigrants but transitions to the “Streptococcus” signature over time.
The concept of a microbial transition parallels that of acculturation, a likely contributor to any bacterial shift. Observed differences in English linguistic acculturation score [31,32] support this hypothesis, with the “Streptococcus” signature dominating among more acculturated women. Furthermore, recent evidence indicates acculturation occurs more rapidly in younger immigrants [50], which is consistent with the earlier US arrival observed among women in the “Streptococcus” cluster. Several studies have reported positive relationships between dental care and use of the English language among Hispanics [51–53]. While dental history was not available for the current study, Streptococcus species linked with better oral health, including S. mitis and S. oralis [49,54,55], were significantly more abundant in the “Streptococcus” community type. By comparison, the “Prevotella” cluster exhibited higher levels of S. salivarius and Veillonella spp., both of which have been linked to dental caries [54,56]. These observations suggest differences in oral health and/or oral health determinants (e.g., access to dental care) contribute to the relationships observed here. Frequency of dentist visits was only recently incorporated into the MACS questionnaire but appears low; nonetheless, the relationship between dental visits and the oral microbiome could be explored in future studies. Importantly, our work examines the oral microbiota at just a single time point in women enrolled between 2004–2011. The influence of birth or immigration cohort effects cannot be excluded.
Inter-individual variation of the microbiota has previously been observed by geography and culture. Comparing Germans, native Alaskans, and Africans, Li and colleagues observed differential abundances across many common and highly abundant oral bacteria, including Prevotella, Veillonella, and Haemophilus [29]. Takeshita et al. reported similar taxonomic differences in a study comparing the salivary microbiome of South Koreans to the Japanese [28]—two populations with arguably fewer differences in diet and host genetic variation. These studies support the idea of a core human oral microbiome that, despite differences in relative taxa abundance, provides functional consistency and stability across cultures. Data from the Human Microbiome Project support this assertion, with the taxonomic composition of the buccal mucosa exhibiting far more variability than its microbial metabolic pathway content [47]. Hence, while our data suggest acculturation in Mexican American women may be accompanied by compositional changes of the oral microbiota, differences in the overarching function of these communities are likely small. Our own analysis supports this hypothesis as the variation in putative functional pathways between our bacterial community clusters was more modest than their observed taxonomic differences. Consequently, many roads may lead to a “healthy” oral microbiome.
In addition to the “Prevotella” and “Streptococcus” clusters, we observed a third group of women defined by higher levels of moderate- to high-risk periodontal pathogens. Presence of pathogens was further reflected in putative functional differences, with bacteria-host interaction and cellular turnover/repair pathways dominating the “Fusobacterium” cluster. One-third of our participants presented with this bacterial signature, suggesting periodontal disease or risk thereof may be widespread within our cohort. History of periodontitis was not assessed in the MACS study, but prevalence of this inflammatory disease is higher among Hispanics/Latinos according to national surveys [57]. Consistent with these observations, Mason et al. recently reported greater abundance of Porphyromonas, Treponema, and Fusobacterium spp. in the subgingival microbiota of Latinos as compared to non-Hispanic whites and non-Hispanic blacks [58]. These disparities may in part be due to chronic inflammation as a result of the physical, psychosocial, and cultural stressors that accompany immigration. Notably, Miranda and Matheny found that acculturative stress was inversely related to time in the US among adult Latinos [59], and women in the “Fusobacterium” cluster were more likely to report living in the US less than 10 years.
Interest in the oral microbiome is rising as scientists identify ever more associations between bacteria of the mouth and complex disease. Many of these associations stem from links to periodontitis and associated diseases, which may reflect an underlying susceptibility or avenue toward systemic inflammation. Although the directionality of these relationships is often unclear, large cohorts such as MACS provide the opportunity to elucidate the timing of such relationships and explore the oral microbiome as a potential biomarker or etiologic underpinning of disease. Moreover, our study shows that MACS mouthwash samples, initially collected for human genetic studies and kept in frozen storage for up to 15 years, produce high quality microbial sequencing data; hence, studies to examine the oral microbiome and incident disease are underway. Characterizing the oral microbiome in an effort to identify those at highest disease risk will help us better target health interventions to those with greatest need. As a putative comprehensive assessment of biological, behavioral, and environmental risk factors, the oral microbiome may prove to be one of the most informative and easily accessible biomarkers for research in low income, resource poor populations.
Conclusions
First and second generation Mexican American women face a number of health issues modulated by acculturation and adaptation to life in the US. The oral microbiota, itself linked to many of these conditions, also appears to differ by factors associated with immigration among Mexican American women and has the potential to impact host health via a multitude of functions. Whether and how differences in oral health or oral health care contribute to these relationships warrant further research and could have broad implications for how we target public health problems in this burgeoning population.
Supporting information
Bacterial richness as measured by observed OTU (A) and Chao index (C) is inversely associated with age in women who have lived >50% of their life in the US (observed OTU: r = -0.28, P<0.01; Chao: r = -0.32, P<0.01) versus Mexico (observed OTU: r = -0.07, P = 0.31; Chao: r = -0.05, P = 0.46). Among women who resided longer in the US, country of birth did not affect this relationship (US vs Mexico, P = 0.56 for observed OTU and P = 0.87 for Chao) (B & D). (E) Shannon diversity did not vary with age, irrespective of country of longest residence (US, P = 0.14; Mexico, P = 0.12). Among women residing longer in the US, the relationship between Shannon diversity and age was not modified by country of birth (US vs Mexico, P = 0.22) (F). OTU, operational taxonomic unit.
(PDF)
OTU co-occurrence relationships among Mexican American women identified by SparCC correlation analysis. Analysis was restricted to OTUs detected at ≥0.1% relative abundance in at least one-third of samples. Positive correlations (co-occurrence) are shown in shades of blue and negative correlations (co-exclusion) in red. Correlation strength is indicated by circle size, with larger circles depicting stronger associations. Only significant relationships are shown (FDR Q = 0.1). Black rectangles demarcate results of hierarchical clustering.
(PDF)
Relative abundance of “core” genera in Mexican American women by DMM cluster. (A) Stacked bar graphs indicate the proportion of each sample represented by each core genus. (B) Box-plots of core taxa by DMM cluster. Post-multiple comparison adjustment, pairwise P<0.05: a “Streptococcus” cluster vs “Fusobacterium” cluster; b “Streptococcus” cluster vs “Prevotella” cluster; c “Fusobacterium” cluster vs “Prevotella” cluster.
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Joan Dong and Tulin Ayvaz for technical assistance. This study would not have been possible without the generous support and cooperation of the MACS participants, as well as the dedicated Cohort study team.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Cancer Institute at the National Institutes of Health [CA016672 to Ronald Depinho, MD; R25CA057730 to Shine Chang, PhD]. The Cohort additionally receives funds collected pursuant to the Comprehensive Tobacco Settlement of 1998 and appropriated by the 76th Legislature to the University of Texas MD Anderson Cancer Center; from the Caroline W Law Fund for Cancer Prevention; and from the Dan Duncan Family Institute for Risk Assessment and Cancer Prevention. Study content is solely the responsibility of the authors and does not necessarily represent the official views of any funding agency. Funders did not contribute to the design or conduct of the study; the collection, analysis, or interpretation of data; or the preparation, review, or approval of the manuscript.
References
- 1.U.S. Census Bureau, Population Division. Annual estimates of the resident population by sex, age, race, and Hispanic origin for the United States and states: April 1, 2010 to July 1, 2015; 2016. [cited 2016 Sep 9]. Database: American FactFinder [Internet]. Available from: http://www.factfinder.census.gov.
- 2.Generational differences. Report No. 7054. Pew Hispanic Center. 2004. Available from: http://www.pewhispanic.org/files/2011/10/13.pdf.
- 3.U.S. Census Bureau. American Community Survey 1-year estimates; 2016. [cited 2016 Sep 9]. Database: American FactFinder [Internet]. Available from: http://www.factfinder.census.gov.
- 4.National Center for Health Statistics. Health, United States, 2015: with special feature on racial and ethnic health disparities. Report No. 2016–1232. Hyattsville, MD: US Department of Health and Human Services; 2016. [PubMed]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66: 7–30. doi: 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer facts & figures for Hispanics/Latinos 2015–2017. Report No. 862315. Atlanta, GA: American Cancer Society; 2015.
- 7.Albrecht SS, Diez Roux AV, Aiello AE, Schulz AJ, Abraido-Lanza AF. Secular trends in the association between nativity/length of US residence with body mass index and waist circumference among Mexican-Americans, 1988–2008. Int J Public Health. 2013; 58: 573–581. doi: 10.1007/s00038-012-0414-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia L, Gold EB, Wang L, Yang X, Mao M, Schwartz AV. The relation of acculturation to overweight, obesity, pre-diabetes and diabetes among U.S. Mexican-American women and men. Ethn Dis. 2012; 22: 58–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Barcenas CH, Wilkinson AV, Strom SS, Cao Y, Saunders KC, Mahabir S, et al. Birthplace, years of residence in the United States, and obesity among Mexican-American adults. Obesity (Silver Spring). 2007; 15: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien MJ, Alos VA, Davey A, Bueno A, Whitaker RC. Acculturation and the prevalence of diabetes in US Latino adults, National Health and Nutrition Examination Survey 2007–2010. Prev Chronic Dis. 2014; 11: E176 doi: 10.5888/pcd11.140142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson C, Zhao H, Daniel CR, Hromi-Fiedler A, Dong Q, Elhor Gbito KY, et al. Acculturation and diabetes risk in the Mexican American Mano a Mano Cohort. Am J Public Health. 2016; 106: 547–549. doi: 10.2105/AJPH.2015.303008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012; 308: 1775–1784. doi: 10.1001/jama.2012.14517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daviglus ML, Pirzada A, Durazo-Arvizu R, Chen J, Allison M, Aviles-Santa L, et al. Prevalence of low cardiovascular risk profile among diverse Hispanic/Latino adults in the United States by age, sex, and level of acculturation: The Hispanic Community Health Study/Study of Latinos. J Am Heart Assoc. 2016; 5: e003929 doi: 10.1161/JAHA.116.003929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008; 23: 2079–2086. doi: 10.1007/s11606-008-0787-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95: 559–569. doi: 10.1067/moe.2003.107 [DOI] [PubMed] [Google Scholar]
- 16.Mercado F, Marshall RI, Klestov AC, Bartold PM. Is there a relationship between rheumatoid arthritis and periodontal disease? J Clin Periodontol. 2000; 27: 267–272. [DOI] [PubMed] [Google Scholar]
- 17.Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008; 9: 550–558. doi: 10.1016/S1470-2045(08)70106-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bierne H, Hamon M, Cossart P. Epigenetics and bacterial infections. Cold Spring Harb Perspect Med. 2012; 2: a010272 doi: 10.1101/cshperspect.a010272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belstrom D, Holmstrup P, Bardow A, Kokaras A, Fiehn NE, Paster BJ. Temporal stability of the salivary microbiota in oral health. PLoS One. 2016; 11: e0147472 doi: 10.1371/journal.pone.0147472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaura E, Brandt BW, Teixeira de Mattos MJ, Buijs MJ, Caspers MP, Rashid MU, et al. Same exposure but two radically different responses to antibiotics: resilience of the salivary microbiome versus long-term microbial shifts in feces. MBio. 2015; 6: e01693–15. doi: 10.1128/mBio.01693-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David LA, Materna AC, Friedman J, Campos-Baptista MI, Blackburn MC, Perrotta A, et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 2014; 15: R89 doi: 10.1186/gb-2014-15-7-r89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016; 10:2435–2446. doi: 10.1038/ismej.2016.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato I, Vasquez A, Moyerbrailean G, Land S, Djuric Z, Sun J, et al. Nutritional correlates of human oral microbiome. J Am Coll Nutr. 2017; 36: 88–98. doi: 10.1080/07315724.2016.1185386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corby PM, Biesbrock A, Bartizek R, Corby AL, Monteverde R, Ceschin R, et al. Treatment outcomes of dental flossing in twins: molecular analysis of the interproximal microflora. J Periodontol. 2008; 79: 1426–1433. doi: 10.1902/jop.2008.070585 [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Yang F, Zeng X, Chen J, Li R, Wen T, et al. Preliminary characterization of the oral microbiota of Chinese adults with and without gingivitis. BMC Oral Health. 2011; 11: 33 doi: 10.1186/1472-6831-11-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F, Zeng X, Ning K, Liu KL, Lo CC, Wang W, et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012; 6: 1–10. doi: 10.1038/ismej.2011.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasidze I, Li J, Quinque D, Tang K, Stoneking M. Global diversity in the human salivary microbiome. Genome Res. 2009; 19: 636–643. doi: 10.1101/gr.084616.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeshita T, Matsuo K, Furuta M, Shibata Y, Fukami K, Shimazaki Y, et al. Distinct composition of the oral indigenous microbiota in South Korean and Japanese adults. Sci Rep. 2014; 4: 6990 doi: 10.1038/srep06990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Quinque D, Horz HP, Li M, Rzhetskaya M, Raff JA, et al. Comparative analysis of the human saliva microbiome from different climate zones: Alaska, Germany, and Africa. BMC Microbiol. 2014; 14: 316 doi: 10.1186/s12866-014-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow WH, Chrisman M, R Daniel C, Ye Y, Gomez H, Dong Q, et al. Cohort Profile: The Mexican American Mano a Mano Cohort. Int J Epidemiol; Forthcoming. doi: 10.1093/ije/dyv016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marin G. A new measurement of acculturation for Hispanics: the bidimensional acculturation scale for Hispanics (BAS). Hisp J Behav Sci. 1996; 18: 297–316. [Google Scholar]
- 32.Mendoza R. An empirical scale to measure type and degree of acculturation in Mexican-American adolescents and adults. J Cross-Cultural Psyschol. 1989; 20: 375–385. [Google Scholar]
- 33.Mai PL, Sullivan-Halley J, Ursin G, Stram DO, Deapen D, Villaluna D, et al. Physical activity and colon cancer risk among women in the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2007; 16: 517–525. doi: 10.1158/1055-9965.EPI-06-0747 [DOI] [PubMed] [Google Scholar]
- 34.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011; 108 Suppl 1: 4516–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010; 26: 2460–2461. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 36.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012; 9: 357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013; 10: 996–998. doi: 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Schwartz S, Wagner L, Miller W. A greedy algorithm for aligning DNA sequences. J Comput Biol. 2000; 7: 203–214. doi: 10.1089/10665270050081478 [DOI] [PubMed] [Google Scholar]
- 39.Asshauer KP, Wemheuer B, Daniel R, Meinicke P. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 2015; 31: 2882–2884. doi: 10.1093/bioinformatics/btv287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000; 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016; 44: D457–62. doi: 10.1093/nar/gkv1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedman J, Alm EJ. Inferring correlation networks from genomic survey data. PLoS Comput Biol. 2012; 8: e1002687 doi: 10.1371/journal.pcbi.1002687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes I, Harris K, Quince C. Dirichlet multinomial mixtures: generative models for microbial metagenomics. PLoS One. 2012; 7: e30126 doi: 10.1371/journal.pone.0030126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011; 12: R60 doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alkek Center for Metagenomics and Microbiome Research, Baylor College of Medicine. Sage microbiome explorer. 2015. Available from: http://sage.mx.
- 46.R Core Team, R Foundation for Statistical Computing. R: a language and environment for statistical computing. 2016. Available from: http://www.r-project.org.
- 47.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012; 486: 207–214. doi: 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012; 6: 1176–1185. doi: 10.1038/ismej.2011.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998; 25: 134–144. [DOI] [PubMed] [Google Scholar]
- 50.Cheung BY, Chudek M, Heine SJ. Evidence for a sensitive period for acculturation: younger immigrants report acculturating at a faster rate. Psychol Sci. 2011; 22: 147–152. doi: 10.1177/0956797610394661 [DOI] [PubMed] [Google Scholar]
- 51.Graham MA, Tomar SL, Logan HL. Perceived social status, language and identified dental home among Hispanics in Florida. J Am Dent Assoc. 2005; 136: 1572–1582. [DOI] [PubMed] [Google Scholar]
- 52.Riley JL 3rd, Gibson E, Zsembik BA, Duncan RP, Gilbert GH, Heft MW. Acculturation and orofacial pain among Hispanic adults. J Pain. 2008; 9: 750–758. doi: 10.1016/j.jpain.2008.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flores G, Tomany-Korman SC. The language spoken at home and disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008; 121: e1703–14. doi: 10.1542/peds.2007-2906 [DOI] [PubMed] [Google Scholar]
- 54.Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, Griffen AL. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One. 2012; 7: e47722 doi: 10.1371/journal.pone.0047722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Corby PM, Lyons-Weiler J, Bretz WA, Hart TC, Aas JA, Boumenna T, et al. Microbial risk indicators of early childhood caries. J Clin Microbiol. 2005; 43: 5753–5759. doi: 10.1128/JCM.43.11.5753-5759.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanasi E, Dewhirst FE, Chalmers NI, Kent R Jr, Moore A, Hughes CV, et al. Clonal analysis of the microbiota of severe early childhood caries. Caries Res. 2010; 44: 485–497. doi: 10.1159/000320158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 2015; 86: 611–622. doi: 10.1902/jop.2015.140520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mason MR, Nagaraja HN, Camerlengo T, Joshi V, Kumar PS. Deep sequencing identifies ethnicity-specific bacterial signatures in the oral microbiome. PLoS One. 2013; 8: e77287 doi: 10.1371/journal.pone.0077287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miranda AO and Matheny KB. Socio-psychological predictors of acculturative stress among Latino adults. J Ment Health Couns. 2000; 22: 306–317. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial richness as measured by observed OTU (A) and Chao index (C) is inversely associated with age in women who have lived >50% of their life in the US (observed OTU: r = -0.28, P<0.01; Chao: r = -0.32, P<0.01) versus Mexico (observed OTU: r = -0.07, P = 0.31; Chao: r = -0.05, P = 0.46). Among women who resided longer in the US, country of birth did not affect this relationship (US vs Mexico, P = 0.56 for observed OTU and P = 0.87 for Chao) (B & D). (E) Shannon diversity did not vary with age, irrespective of country of longest residence (US, P = 0.14; Mexico, P = 0.12). Among women residing longer in the US, the relationship between Shannon diversity and age was not modified by country of birth (US vs Mexico, P = 0.22) (F). OTU, operational taxonomic unit.
(PDF)
OTU co-occurrence relationships among Mexican American women identified by SparCC correlation analysis. Analysis was restricted to OTUs detected at ≥0.1% relative abundance in at least one-third of samples. Positive correlations (co-occurrence) are shown in shades of blue and negative correlations (co-exclusion) in red. Correlation strength is indicated by circle size, with larger circles depicting stronger associations. Only significant relationships are shown (FDR Q = 0.1). Black rectangles demarcate results of hierarchical clustering.
(PDF)
Relative abundance of “core” genera in Mexican American women by DMM cluster. (A) Stacked bar graphs indicate the proportion of each sample represented by each core genus. (B) Box-plots of core taxa by DMM cluster. Post-multiple comparison adjustment, pairwise P<0.05: a “Streptococcus” cluster vs “Fusobacterium” cluster; b “Streptococcus” cluster vs “Prevotella” cluster; c “Fusobacterium” cluster vs “Prevotella” cluster.
(PDF)
(PDF)
(PDF)
(PDF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.