SUMMARY
Monoamine and acetylcholine neurotransmitters from the autonomic nervous system (ANS) regulate insulin secretion in pancreatic islets. The molecular mechanisms controlling neurotransmitter signaling in islet β cells and their impact on diabetes development are only partially understood. Using a glucose-intolerant, MafA-deficient mouse model, we demonstrate that MAFA controls ANS-mediated insulin secretion by activating the transcription of nicotinic (ChrnB2 and ChrnB4) and adrenergic (Adra2A) receptor genes, which are integral parts of acetylcholine-and monoamine-signaling pathways. We show that acetylcholine-mediated insulin secretion requires nicotinic signaling and that nicotinic receptor expression is positively correlated with insulin secretion and glycemic control in human donor islets. Moreover, polymorphisms spanning MAFA-binding regions within the human CHRNB4 gene are associated with type 2 diabetes. Our data show that MAFA transcriptional activity is required for establishing β cell sensitivity to neurotransmitter signaling and identify nicotinic signaling as a modulator of insulin secretion impaired in type 2 diabetes.
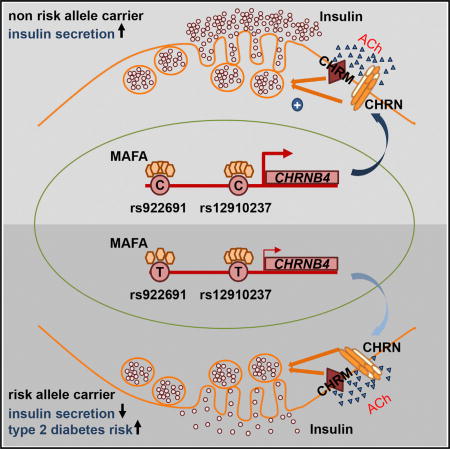
Graphical abstract
INTRODUCTION
Glucose homeostasis is maintained by hormone secretion from the islets of Langerhans in the pancreas. Insulin secreted by islet β cells promotes glucose uptake, whereas glucagon from α cells stimulates glucose release. Loss, dysfunction, and dedifferentiation of islet β cells results in a profound imbalance in blood glucose homeostasis (Halban et al., 2014), leading to the development of type 2 diabetes. Regulation of physiological insulin and glucagon secretion is achieved by direct sensing of glucose and other nutrients in α and β cells but also indirectly through communication between islet cells and the sympathetic and parasympathetic branches of the autonomic nervous system (ANS) (reviewed in Thorens, 2011). Glucose-sensing neurons stimulate sympathetic norepinephrine release to repress insulin secretion and promote glucagon release under physical and mental stress conditions (Porte and Williams, 1966). In contrast, parasympathetic acetylcholine signaling through cholinergic muscarinic receptors is critical for the pre-absorptive phase of insulin secretion, prior to the increase in blood glucose levels in response to food intake (Ahrén and Holst, 2001). Alleles that increase the risk of type 2 diabetes have been identified in the adrenoceptor ADRA2A (Rosengren et al., 2010) and the cholinergic muscarinic receptor CHRM3 genes (Guo et al., 2006), further highlighting the importance of neurotransmitter signaling in glucose homeostasis.
The transcription network regulating neurotransmitter signaling pathways in pancreatic β cells is unknown, making it difficult to assess how sensitivity to neurotransmitter signaling is maintained and adjusted in response to changing physiological conditions. The β cell-enriched MAFA transcription factor activates genes critical for glucose sensing, insulin production, and secretion (Artner et al., 2010; Hang et al., 2014), and it has been established that MAFA expression is lost in human type 2 diabetes islets most likely contributing to diabetic β cell dysfunction (Guo et al., 2013). Here, we show that β-cell-specific deletion of the MafA transcription factor in a mouse model, which develops glucose intolerance, leads to a complete loss of insulin secretion in response to stimulation of the ANS in vivo. We show that this defect is most likely caused by MAFA activating transcription of adrenergic and nicotinic neurotransmitter receptor expression including genes encoding CHRNB2 and B4 subunits and ADRA2A. Importantly, this transcriptional regulation by MAFA was conserved between mouse and human β cells. In addition, polymorphisms in nicotinic receptor genes correlated to insulin secretion and type 2 diabetes in a large cohort of patients. These findings establish MAFA as a critical regulator of neurotransmitter signaling in β cells and identify nicotinic signaling as a modulator of insulin secretion, suggesting that smoking-induced nicotine exposure may directly affect insulin secretion, thereby linking the increased risk of developing type 2 diabetes and smoking on a cellular level.
RESULTS
Islet β-Cell-Specific Deletion of MafA Results in Impaired ANS-Stimulated Insulin Secretion
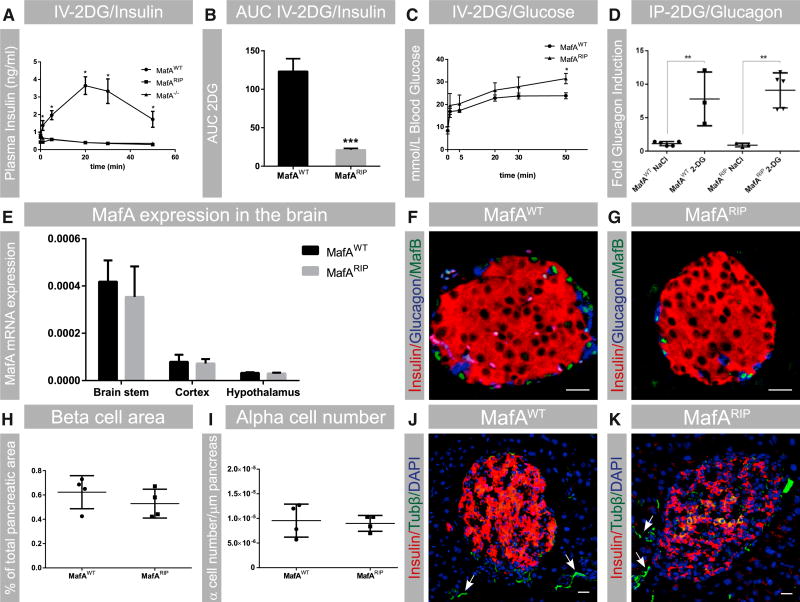
Loss of MafA results in adult β cell dysfunction, which leads to glucose intolerance (Zhang et al., 2005; Figures S1A and S1B). To test whether loss of MafA affects the responsiveness of β cells to neurotransmitter signaling, mice wild-type for MafA (MafAWT), completely lacking MafA (MafA−/−), and only lacking MafA in β cells (MafARIP) were treated with 2-deoxy-D-glucose (2DG), which stimulates the ANS to induce insulin and glucagon secretion (Karlsson et al., 1987). Both MafA−/− and MafARIP animals failed to increase insulin secretion in response to 2DG (Figures 1A and 1B), whereas insulin secretion increased in MafAWT mice, as expected (Figures 1A and 1B), and glucose was cleared (Figure 1C). By contrast, no difference in glucagon secretion levels was found between MafARIP and wild-type animals treated with 2DG (Figure 1D), suggesting that loss of MafA selectively affects ANS-driven insulin secretion.
Figure 1. β-Cell-Specific Deletion of MafA Results in Impaired Glucose Clearance and ANS-Stimulated Insulin Secretion.
(A and B) 2DG-stimulated insulin secretion in adult MafAWT, MafA−/−, and MafARIP mice is shown; n = 9 or 10.
(C) Glucose levels in 2DG-treated MafAWT and MafARIP animals; n = 9 or 10.
(D) Glucagon secretion induced by 2DG in MafAWT and MafARIP mice, with saline (NaCl) treatment as a control; n ≥ 3.
(E) MafA mRNA expression in the hypothalamic, cortex, and brainstem regions in MafARIP and MafAWT mice. Data were normalized to the geomean of HPRT and β-actin mRNA levels. n = 4 or 5.
(F and G) Immunohistochemistry staining for MafB (green), α cells (stained for glucagon; blue), and β cells (stained for insulin; red) of freshly isolated pancreatic sections from MafAWT and MafARIP mice.
(H) β cell area in MafARIP and MafAWT mice; n = 4.
(I) α cell area in MafARIP and MafAWT mice; n = 4.
(J and K) Islet innervation in MafAWT and MafARIP mice was assessed by β-tubulin immunostaining (Tubβ; green). Autonomic nerve fibers are denoted by white arrows. Insulin is visualized in red and nuclei in blue (DAPI staining).
Data are presented as mean ± SEM and were analyzed with multiple (A and C) and Student’s (B, D, E, H, and I) t test. *p < 0.05 and **p < 0.01. The scale bar represents 20 µm. See also Table S1 and Figure S1 for additional characterization of MafARIP animals.
We found that the reduction of insulin secretion was due to loss of MafA in MafARIP β cells, as no changes in the expression levels of MafA in the brainstem, hypothalamus, or cortex (Figure 1E) were found. In fact, in the CNS, MafA mRNA levels were 500 times lower than in pancreatic islets and no protein expression was detected (Figure 1E; data not shown), which suggests that MafA function in β cells, and not the CNS, is critical for ANS-driven insulin secretion. MafARIP mice also did not express the closely related transcription factor MafB in β cells (Figures 1F and 1G), which has been shown to partially compensate for the loss of MafA (Artner et al., 2010). In addition, there was no reduction in β cell area (Figure 1H), α cell number (Figure 1I), or alterations in islet morphology (Figures 1F, 1G, S1C, and S1D) in MafARIP mice, although such reduction has been previously reported for MafA−/− mice (Zhang et al., 2005). Moreover, islet innervation seemed to be unaffected in MafARIP mice, as shown by the presence of autonomic nerve fibers (Figures 1K, 1J, and S1E–S1H) and 2DG-induced glucagon secretion in MafARIP animals (Figure 1D). These findings suggest that the effects observed in MafARIP are specific to the loss of MAFA function in β cells and not due to reduced β cell area, altered islet morphology, or ectopic MAFB expression. Taken together, our data show that MAFA function in β cells is essential for ANS-driven insulin secretion.
Expression of Adrenergic and Cholinergic Nicotinic Receptor Genes Is Affected in MafARIP Islets
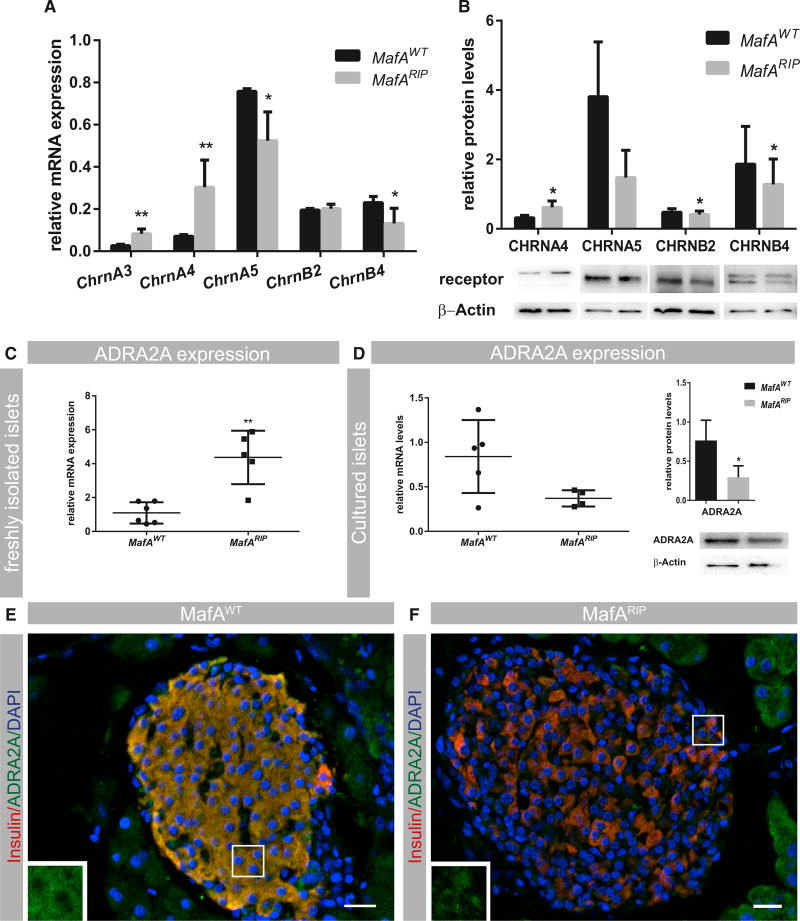
Sympathetic and parasympathetic nerve endings secret norepinephrine, acetylcholine, and neuropeptides to signal to target cells. Signal reception is mediated by adrenergic (sympathetic nervous system), muscarinic, and nicotinic acetylcholine and neuropeptide receptors (parasympathetic nervous system), whereas monoamine degradation is performed by several enzymes including monoamine oxidases (MAOs). As alterations in adrenergic and cholinergic receptors, as well as parasympathetic neuropeptide receptor expression, affect human and rodent β cell function (Guo et al., 2006; Rosengren et al., 2010; Winzell and Ahrén, 2007), we performed mRNA expression analysis to determine whether loss of ANS-mediated insulin secretion in MafARIP mice was due to deficient neurotransmitter signaling. Expression of the parasympathetic neuropeptide receptor genes VipR, PACAPR, and GrpR was unchanged in MafARIP mutant islets (Table 1), suggesting that neuropeptide receptor signaling is not affected in MafARIP. Expression of the muscarinic receptor genes ChrM3 and ChrM5 has been observed in β cells (Duttaroy et al., 2004; Molina et al., 2014), and in line with this, we found that ChrM3 transcript levels were detectable in MafARIP islets and similar to expression in MafA wild-type littermates (Table 1). In contrast, ChrM1, ChrM2, ChrM4, and ChrM5 transcript levels were low in both wild-type and MafARIP islets. Systematic expression analysis of nicotinic α and β receptor subunits (Chrn genes), which form pentameric ion channels consisting of two α and three β subunits, showed that several α (CHRNA3, CHRNA4, and CHRNA5) and β (CHRNB2 and CHRNB4) subunits were produced in islet cells (Figure 2A). mRNA levels of ChrnA3 and ChrnA4 genes were increased, whereas ChrnB2 expression was unchanged and ChrnA5 and ChrnB4 subunit transcript levels were reduced in MafARIP islets (Figure 2A). Western blot analysis demonstrated that these changes in expression levels were also present on protein level (Figure 2B). Expression of other CHRN subunits was low and unchanged in MafARIP islets compared with MafAWT islets (Table 1). Moreover, transcript levels of MaoB, the main MAO enzyme expressed in islets (Table 1), were reduced, whereas expression of the adrenoceptor Adra2A, which inhibits insulin secretion, was elevated in MafARIP islets (Table 1; Figure 2C). This expression analysis shows that loss of MafA in β cells selectively affects expression of nicotinic acetylcholine and adrenergic receptor, as well as MAO genes.
Table 1.
Relative Neurotransmitter-Signaling Gene Expression in Islets from MafA Wild-Type and MafARIP Mice
| Gene | Relative mRNA Levels MafAWT |
Relative mRNA Levels MafARIP |
p Value | Difference |
|---|---|---|---|---|
| Muscarinic receptors | ||||
| ChrM1 | 0.001 | 0.0009 | 0.02 | 0.0007 |
| ChrM2 | 0.029 | 0.039 | 0.02 | −0.009 |
| ChrM3 | 0.083 | 0.065 | 0.11 | 0.0187 |
| ChrM4 | 0.002 | 0.004 | 0.04 | −0.001 |
| ChrM5 | 0.001 | 0.002 | 0.33 | −0.0003 |
| Neuropeptide receptors | ||||
| PACAPR | 0.001 | 0.001 | 0.63 | 0.0004 |
| VipR | 1.613 | 1.267 | 0.13 | 0.346 |
| GrpR | 0.002 | 0.001 | 0.28 | 0.0009 |
| Monoamine oxidases | ||||
| MaoA | 0.006 | 0.007 | 0.93 | −0.0004 |
| MaoB | 0.338 | 0.120 | 0.008a | 0.219 |
| Nicotinic receptors | ||||
| ChmA1 | 0.003 | 0.001 | 0.0001a | 0.002 |
| ChrnA2 | 0.002 | 0.002 | 0.59 | 0.0004 |
| ChrnA6 | 9.85E-05 | 0.000136 | 0.44 | −3.75E-05 |
| ChrnA7 | 0.005 | 0.014 | 0.004a | −0.009 |
| ChrnA9 | 0.0005 | 0.0008 | 0.42 | −0.0003 |
| ChmA10 | 7.77E-05 | 0.00016 | 0.05 | −8.68E-05 |
| ChrnBI | 0.003 | 0.004 | 0.50 | −0.0007 |
| ChrnB3 | 0.0009 | 0.001 | 0.09 | −0.0004 |
| ChrnD | 0.002 | 0.004 | 0.13 | −0.002 |
| ChrnE | 0.0001 | 0.0001 | 0.90 | 6.00E-06 |
| ChrnG | 0.0002 | 0.0004 | 0.39 | −0.0002 |
Genes that were significantly downregulated in MafARIP compared to MafAWT islets. p ≤ 0.01 (n ≥ 4).
Figure 2. MafARIP Islets Have Altered Neurotransmitter Receptor Expression.
(A) qPCR measurement of nicotinic receptor (Chrn) mRNA expression in freshly isolated MafAWT and MafARIP islets (n ≥ 4).
(B) Quantitative analysis of Chrn protein levels (n ≥ 4).
(C) qPCR measurement of Adra2A mRNA expression in freshly isolated MafAWT and MafARIP islets (n ≥ 4).
(D) Left panel: qPCR measurement of Adra2A mRNA expression in cultured MafAWT and MafARIP islets (n ≥ 4). Right panel: quantitative analysis of ADRA2A protein levels is shown (n ≥ 4). qPCR data were normalized to the geomean of HPRT and β-actin mRNA levels (n ≥4).
(E and F) Immunohistochemistry staining for ADRA2A (green) in MafAWT and MafARIP pancreatic islets, with insulin (red) and nuclear counterstaining with DAPI (blue). ADRA2A expression in β cells is further illustrated by insets presented in the left corner of respective images. The insets were derived from portions of the images marked by white rectangles.
Data are represented as mean ± SEM and were analyzed using the multiple t test (A and C) and ratio t tests (B and D). *p < 0.05 and **p < 0.01. The scale bar represents 20 µm. See Figure S2A for validation of the ADRA2A antibody.
The sympathetic nervous system inhibits insulin secretion through ADRA2A signaling in islet β cells. However, ADRA2A is also expressed in presynaptic nerve endings as part of a negative feedback loop terminating adrenergic signaling. Previous studies have established that localized accumulation of norepinephrine can lead to upregulation of Adra2A expression in nerve endings presynaptically (Gilsbach et al., 2006). MafARIP islets lack expression of the monoamine-oxidizing enzyme MAOB (Table 1), which mediates degradation of norepinephrine locally. Thus, we hypothesized that the increase in Adra2A expression observed in MafARIP islets may be due to an increased expression in islet-associated synapses and not in the islet cells themselves. To determine whether the increase in Adra2A expression occurred in islet cells or islet-associated nerves, islets were cultured to degrade severed nerve fibers and the effect on transcript and protein levels was measured. We found that Adra2A transcript and protein levels were decreased in cultured MafARIP islets, albeit changes were only significant on the protein level (Figure 2D), indicating that the enhanced Adra2A expression observed in freshly isolated MafARIP islets originated from nerve endings, whereas Adra2A transcription was reduced specifically in islet cells. In support of this, ADRA2A protein expression was also reduced in MafARIP islets (Figures 2E and 2F). Our data suggest that MAFA is required for Adra2A expression in islet β cells whereas elevation of norepinephrine levels in MafARIP mutant islets (due to the lack of MAOB) results in upregulation of Adra2A in presynaptic nerve fibers associated with pancreatic islets.
Chrn Gene Expression Depends on MAFA in Mouse Pancreatic β Cells
We have shown that MafARIP animals lack insulin secretion in response to autonomic stimulation but that transcription of muscarinic receptor genes, which mediate autonomic stimulation of insulin secretion, is not reduced in MafARIP islets (Table 1). These findings suggest that alternate cholinergic signaling pathways might be present in β cells. As we also observed that expression of some Chrn genes changed in MafARIP islets, we next investigated whether expression of these genes is regulated by MAFA.
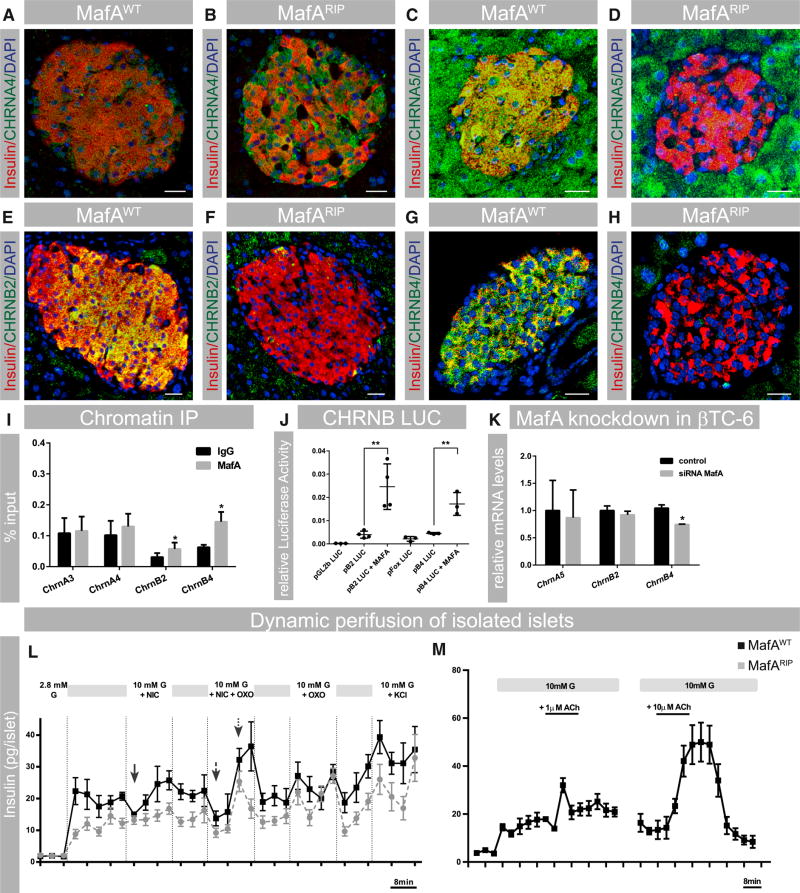
The CHRNA4 protein was detected in all islet cells by immunohistochemistry, with production enhanced in MafARIP islets (Figures 3A and 3B). By contrast, CHRNA5 (Figure 3C) and B4 (Figure 3G) were detected in all pancreatic cell types, whereas CHRNB2 was predominantly expressed in β cells (Figure 3E) of MafAWT islets. CHRNA3 protein was not detected by immunohistochemistry. Expression of CHRNA5, CHRNB2, and CHRNB4 was reduced in MafARIP islets (Figures 3D, 3F, and 3H), suggesting that a reduced amount of functional ion channels (A3/B4 and A4/B2) is present, which could affect nicotinic channel signaling in MafARIP β cells. Chromatin immunoprecipitation analysis demonstrated that MAFA selectively bound to upstream regulatory sequences in ChrnB2 and ChrnB4, but not ChrnA3 and ChrnA4, genes (Figure 3I) indicating that only ChrnB2 and ChrnB4 are directly regulated by MAFA. The ability of co-transfected MAFA to stimulate ChrnB2- or ChrnB4-driven reporter activity (Figure 3J) and reduction of ChrnB4 transcript levels upon siRNA-mediated knockdown of MAFA in βTC-6 cells (Figure 3K) provided additional strong support for direct activation of Chrn genes in mouse β cells. In summary, these data show that nicotinic subunits are expressed in mouse islets and that MAFA is directly activating expression of ChrnB2 and ChrnB4.
Figure 3. MafA-Dependent Nicotinic Receptor Expression Modulates Insulin Secretion.
(A–H) Immunohistochemistry of MafAWT and MafARIP islets to show expression of CHRNA4 (A and B; green), CHRNA5 (C and D; green), CHRNB2 (E and F; green), and CHRNB4 (G and H; green). β cells are stained for insulin (red) and nuclei (DAPI; blue); scale bar represents 20 µm.
(I) qPCR amplification of Chrn upstream sequences after immunoprecipitation of βTC-6 chromatin with a MAFA or rabbit IgG antibody are presented as % input. Differences in percent input for IgG reflect variations in primer efficiencies. n = 4.
(J) Induction of luciferase reporter activity of ChrnB2 (pB2LUC) and ChrnB4 (pB4LUC) luciferase reporter constructs upon co-transfection with MAFA. Empty vector control (pGl2b and pFOX) is set to one; n = 3 or 4.
(K) qPCR measurements of Chrn expression levels in MafA siRNA-treated βTC6 cells; n ≥ 3. Data were normalized to the geomean of HPRT and β-actin mRNA levels.
(L) Dynamic insulin secretion of MafAWT and MafARIP islets stimulated with 10 mM glucose (G) and 100 µM nicotine (NIC), 100 µM nicotine + 100 µM oxotremorine (NIC+OXO), and 100 µM oxotremorine (OXO). The transient decrease in insulin secretion upon NIC treatment in MafAWT islets is marked by a solid arrow. The biphasic insulin secretion induced by NIC+OXO treatment is indicated by a dashed arrow and a dotted arrow; n = 8.
(M) Dynamic insulin secretion of wild-type islets with 1 or 10 µM acetylcholine (n ≥ 5). Acetylcholine treatment is illustrated by black lines.
Data are mean ± SEM and were analyzed using one-way ANOVA and Tukey multiple comparison tests (J) or paired t test (I and K). *p < 0.05 and **p < 0.01. See Figure S2B for validation of the nicotinic receptor antibodies.
CHRN Activity Modulates Acetylcholine-Mediated Insulin Secretion
Acetylcholine promotes insulin secretion through activation of muscarinic receptors (reviewed in Ahrén, 2000), whereas the role of nicotinic signaling in this process has been considered to be minor (Ohtani et al., 2006; Rodriguez-Diaz et al., 2011b; Yoshikawa et al., 2005). We wanted to investigate whether the MAFA-regulated Chrn activity contributes to acetylcholine-stimulated insulin release. Thus, dynamic insulin secretion studies on islets from MafAWT and MafARIP mice were conducted. Nicotine exposure (which selectively stimulates CHRN) resulted in a transient decrease in insulin secretion in wild-type, but not MafARIP, islets (Figure 3L). Next, a combined treatment with nicotine and the muscarinic agonist oxotremorine was performed to determine the effect of a simultaneous stimulation of nicotinic and muscarinic receptor activity, which resembles the physiological response to acetylcholine. This treatment caused a biphasic insulin response consisting of an initial reduction in insulin release that is then followed by a phase of enhanced insulin secretion in wild-type and MafARIP islets (Figure 3L). However, nicotine and oxotremorine stimulation of insulin release from MafARIP islets was muted (Figure 3L), which is most likely caused by the selective reduction in nicotinic receptor gene expression. In contrast, stimulation of muscarinic receptors was comparable between wild-type and MafARIP islets (Figure 3L), suggesting that CHRN expression in β cells is promoting acetylcholine-mediated insulin secretion. Stimulation of wild-type islets with acetylcholine resulted in a similar biphasic insulin secretion (Figure 3M), as observed for nicotine and oxotremorine treatment, suggesting that activation of nicotinic and muscarinic receptors is required for physiological insulin secretion. The presence of a biphasic synergistic response to stimulation of nicotinic and muscarinic receptors supports the idea that activation of fast-acting nicotinic ion channels is temporarily inhibiting insulin secretion to prime β cells for muscarinic-receptor-induced insulin secretion.
Genetic Variants in the CHRNB4 Gene Correlate with Islet Expression and Type 2 Diabetes Susceptibility in Humans
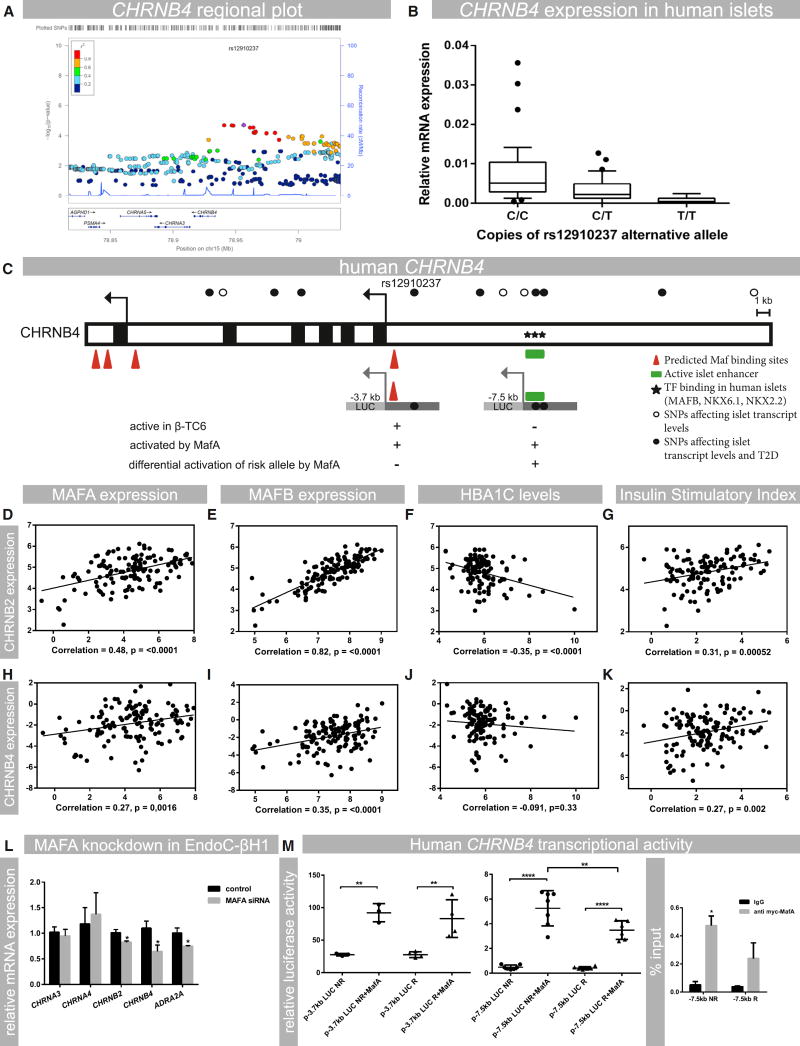
The expression and functional analysis of MafARIP mice suggest that Chrn genes are critical for β cell function in rodent islets. To evaluate whether expression of Chrn genes in islets is also critical for human β cell function, an expression quantitative trait loci (eQTL) analysis of 89 human islet samples (Fadista et al., 2014) was performed to see whether there were any SNPs in CHRN genes that are associated with islet expression, glucose clearance, and type 2 diabetes. We identified a cluster of SNPs that was associated with low expression of CHRNB4 (Figure 4A). Specifically, two copies of the alternate allele (T/T) for the CHRNB4 SNP rs12910237 resulted in a significant reduction of CHRNB4 transcript levels (Figure 4B). Moreover, this SNP was also associated with patients having high fasted blood glucose levels and low insulin secretion after an oral glucose challenge in the MAGIC and DIAGRAM databases (Table 2). The SNP was not associated with change in BMI, indicating that this polymorphism does not affect overall food intake and energy expenditure. These results identify SNPs in the CHRNB4 gene that are associated with type 2 diabetes and indicate that reduced expression in pancreatic islets, which may impair nicotinic signaling in β cells, is correlated with glucose clearance in humans.
Figure 4. Human Nicotinic Receptor Expression in Islets Is Associated with Type 2 Diabetes and Controlled by MAF Transcription Factors.
(A) Regional plot of the CHRNB4 gene showing the presence of a cluster of SNPs that infer low expression of CHRNB4 in human islets. The leading SNP (rs12910237) is indicated in purple.
(B) Effect of alternate rs12910237 allele copy numbers on CHRNB4 transcript levels in islets from human donors; n = 89 (p = 1.98E–05).
(C) Overview of the CHRNB4 upstream region containing SNPs affecting islet gene transcription and the risk for developing type 2 diabetes. MAF and other transcription-factor-binding sites and active islet enhancer regions are shown (Pasquali et al., 2014). Transcriptional activity in the β cell line β-TC6 and activation by MafA is indicated.
(D–G) Analysis of RNA-seq data from human donor islets to show the correlation between CHRNB2 expression and MAFA and MAFB transcript levels, insulin secretion (stimulatory index), and HbA1c levels.
(H–K) Analysis of RNA-seq data from human donor islets to show the correlation between CHRNB4, MAFA, and MAFB; stimulatory index; and HbA1c levels. Experiments were analyzed with linear regression and Pearson correlation analysis; p values are indicated in the respective graphs; n = 131.
(L) siRNA-mediated knockdown of MAFA in EndoC-βH1 cells, showing the effect on mRNA levels of CHRNB2 (p = 0.04), CHRNB4 (p = 0.05), and ADRA2A (p = 0.055); n = 3 or 4.
(M) Effect of pCMVMafA (MafA) expression on luciferase (Luc) activities of human CHRNB4 upstream reporter constructs (p-3.7kbLUC and p-7.5kbLUC) spanning sequences that contain identified risk(R) and corresponding non-risk alleles (NR) for rs12910237 or rs922691 and islet enhancer regions. Activity was assessed in HEK293 cells, which do not have endogenous MAFA activity. n = 4.
Data are mean ± SEM and were analyzed using one-way ANOVA and Tukey multiple comparison tests (M) and Student’s t test (L). *p < 0.05; **p < 0.01; ****p < 0.001. Abbreviations: T2D, type 2 diabetes; TF, transcription factor. See also Figures S3 and S4 and Table S2 for additional correlation and transcriptional data.
Table 2.
SNPs Upstream of the CHRNB4 Gene Are Associated with CHRNB4 Gene Expression in Islets from Human Donors
| SNP | chr (hg19) |
Position (hg19) |
p Value | FDR | GWAS Hit for Type 2 Diabetes and/or Glycemic Traits (p Value < 0.05)? |
Islet Regulome TF-Binding Sites |
|---|---|---|---|---|---|---|
| Chr15:78942385D | chr15 | 78 942 385 | 1.87E–05 | 0.00861 | ||
| rs12910237 | chr15 | 78 956 338 | 1.98E–05 | 0.009019 | MAGIC_2hrGlucose_AdjustedForBMI + DIAGRAM_T2D | |
| rs12915539 | chr15 | 78 940 795 | 2.05E–05 | 0.009252 | ||
| rs1021070 | chr15 | 78 946 863 | 2.07E–05 | 0.009323 | DIAGRAM_T2D | |
| rs7181405 | chr15 | 78 948 152 | 2.15E–05 | 0.009608 | DIAGRAM_T2D | |
| rs922691 | chr15 | 78 963 994 | 2.85E–05 | 0.011931 | DIAGRAM_T2D | NKX6.1,NKX2.2 |
| rs58946838 | chr15 | 78 961 449 | 2.87E–05 | 0.012026 | ||
| rs61630816 | chr15 | 78 965 866 | 5.44E–05 | 0.019905 | ||
| rs34694149 | chr15 | 78 965 917 | 5.44E–05 | 0.019905 | ||
| rs12905641 | chr15 | 78 964 362 | 6.16E–05 | 0.021855 | MAGIC_2hrGlucose_AdjustedForBMI + DIAGRAM_T2D | |
| rs34275594 | chr15 | 78 981 282 | 6.44E–05 | 0.022621 | ||
| rs7178007 | chr15 | 78 965 254 | 6.46E–05 | 0.022671 | ||
| rs8038920 | chr15 | 78 974 545 | 6.88E–05 | 0.023829 | DIAGRAM_T2D |
Transcription factor (TF)-binding sites were obtained from the human islet regulome database (http://gattaca.imppc.org/isletregulome/regulome/cgi-bin/regulome.cgi). FDR, false discovery rate; GWAS, genome-wide association study.
The sequences spanning the CHRNB4 SNPs are located upstream of the CHRNB4 transcription start site. Analysis of potential β-cell-transcription-factor-binding sites and published data on human pancreatic islet enhancers (Pasquali et al., 2014) showed that the CHRNB4 SNPs are contained within an enhancer region associated with β cell transcription factor binding in human islets (Table 2; Figure 4C; Pasquali et al., 2014). The CHRNB4 upstream region is transcriptionally active in β cell lines as assessed by reporter gene activity (Figures 4C and S4A). Collectively, these results demonstrate that SNPs that affect susceptibility to type 2 diabetes are located in a transcriptional control region of the CHRNB4 gene.
MAFA Is Critical for Expression of Nicotinic and Adrenergic Receptors, Including CHRNB2, CHRNB4, and ADRA2A, in Human β Cells
Our biochemical and reporter gene data suggest that MAFA directly regulates the nicotinic receptor CHRNB4 gene in mouse and human islet β cells. However, human β cells produce both MAFA and MAFB, whereas mice only express MafA (Dai et al., 2012). Thus, we performed an analysis of the correlation between expression of nicotinic and adrenergic receptor genes and expression of MAFA and MAFB. Analysis of islet RNA-seq data collected from human donors (n = 131) showed that MAFA and MAFB expression were strongly correlated with each other (Figure S3A) and moderately correlated with low HbA1c levels (Figures S3B and S3C), a measure of long-term blood glucose levels. Both factors were significantly correlated with ADRA2A (Figures S3D and S3E; strong correlation of MAFA with ADRA2A), CHRNB2 (Figures 4D and 4E; strong correlation), and CHRNB4 (Figures 4H and 4I; weak to moderate correlation) transcript levels, but not with other nicotinic receptor subunits (Figures S3G–S3O). Importantly, CHRNB2 and CHRNB4 were also positively correlated with insulin secretion as assessed by insulin stimulatory index (SI) (Figures 4G and 4K; moderate correlation) and low levels of glycated hemoglobin (HbA1c; Figures 4F and 4J; moderate correlation). By contrast, CHRNA3, CHRNA4, and CHRNA5 transcript levels were not linked to glycemic control or insulin secretion (Figure S3I, S3L, S3O, and S4E–S4G). These correlation studies suggest that high expression levels of CHRNB4 and CHRNB2 genes are critical to insulin secretion and long-term blood glucose control not only in mice but also humans.
To determine whether MAFA directly regulates nicotinic and adrenergic receptor gene expression in human cells, MAFA siRNA knockdown was performed in human EndoC-βH1 cells. This human β cell line expresses unique markers associated with human β cell function and secretes insulin in a glucose-responsive manner (Ravassard et al., 2011). Following 50% knockdown of MAFA, ADRA2A, CHRNB2, and CHRNB4 transcript levels were reduced (20%–30% reduction; p ≤ 0.05; Figure 4L), demonstrating that regulation of these genes by MAFA is conserved in human β cells. Additional support for this conclusion was obtained by the finding that human CHRNB4 control sequences spanning the identified type 2 diabetes SNPs (Figure 4C) were bound and activated by MAFA in transfection assays in non-β cell lines (Figure 4M). Mutations of predicted MAFA-binding sites (Figure S4B) within these sequences resulted in reduced activation by MafA, and reporter constructs containing the alternate allele of the diabetes-associated SNP rs922691 had reduced transcriptional activity upon co-transfection with MAFA (Figure 4M). These findings suggest that SNPs in the upstream region of CHRNB4, which are associated with type 2 diabetes and reduced gene expression, are located within a gene region controlling expression and that MAFA is critical for islet-specific expression of CHRNB4.
DISCUSSION
Animal studies have established that MafA promotes the glucose-sensing and proliferative properties of islet β cells (Artner et al., 2010; Hang et al., 2014; Zhang et al., 2005). Here, we demonstrate that MAFA is essential for the transcription of acetylcholine- and monoamine-signaling genes in mouse and human β cells. These results show that MAFA is key in regulating β cell function by connecting the physiological insulin secretion response to nutrients with neurotransmitter signaling. When these processes are impaired in islets lacking MAFA, glucose homeostasis is disrupted and type 2 diabetes develops (Figure 1; Guo et al., 2013).
The finding that stimulation of the parasympathetic nervous system with 2DG results in insulin secretion in wild-type, but not MafA−/− and MafARIP, animals indicates that this transcription factor is essential for initiating ANS-driven insulin secretion. Other possible explanations are unlikely, as the RIP-Cre mouse line used here (Herrera, 2000) does not contain the human growth hormone minigene, which has been associated with numerous β cell defects (Brouwers et al., 2014). Moreover, RIP-Cre activity in the CNS does not alter MafA transcript levels, and changes in islet innervation and ANS-induced glucagon secretion were not observed. So, we conclude that the defects in autonomic signal reception observed in the MafA mutant animals are caused by loss of MAFA function in islet β cells.
We found that MAFA regulated specific neurotransmitter signaling genes in the sympathetic branch of the ANS, including the adrenergic receptor Adra2A as well as the monoamine metabolizing enzyme MaoB. Islet cells express receptor and transporter proteins for norepinephrine, a monoamine neurotransmitter reported to have a negative effect on insulin secretion (reviewed in Ahrén, 2000). Our results suggest a direct effect of MAFA activity on Adra2A transcription, because ADRA2A expression was reduced following knockdown of MAFA in a human β cell line. By contrast, MAOB expression, which was reduced in MafARIP islets, was not associated with human MAFA activation in EndoC-βH1 (Figure S4G), suggesting that MAFA only regulates MaoB expression in the mouse (Ganic et al., 2015). The regulation of Adra2A expression indicates that MAFA controls autonomic input of the sympathetic, as well as the parasympathetic, branch of the ANS.
Gene expression analysis showed that MafA regulates the expression of several genes that are critical for autonomic signaling. The main group of neurotransmitter receptor genes that are transcriptionally regulated by MAFA encode subunits of the cholinergic nicotinic receptors. Acetylcholine from islet a cells (human) and parasympathetic nerves (mouse and human) has been shown to stimulate the pre-absorptive phase of insulin secretion (Ahrén and Holst, 2001; Rodriguez-Diaz et al., 2011a, 2011b). The importance of muscarinic acetylcholine receptors in this process has been established in both human and animals studies. However, nicotinic acetylcholine receptor activity was previously thought to not be significantly involved in this process (Gautam et al., 2006; Rodriguez-Diaz et al., 2011b). This is in contrast to previous findings that pre-absorptive insulin secretion is inhibited by the nicotinic ganglionic blocker trimethaphan but only partially impaired by muscarinic antagonists (Ahrén and Holst, 2001), which argues for a function of nicotinic receptor activity in human islet cells. Our insulin secretion studies extended these previous observations by showing that nicotine has a dynamic impact on insulin release with transient inhibition of insulin secretion. In addition, co-stimulation with nicotine and oxotremorine, which activates muscarinic acetylcholine receptor signaling, resulted in synergistic insulin secretion similar to that observed upon stimulation with acetylcholine (Figure 3L). This suggests that the physiological acetylcholine response in β cells consists of an initial opening of nicotinic channels that primes β cells for subsequent muscarinic receptor activity. In support of this conclusion, MafA mutant islets that had reduced expression of nicotinic, but not muscarinic, acetylcholine receptor expression showed neither transient inhibition of insulin secretion upon nicotine exposure nor synergistic insulin secretion in response to treatment with nicotine and oxotremorine.
To establish whether nicotinic receptors are also required for human β cell function and whether MAFA transcription factor activity is regulating CHRN transcription, we analyzed RNA-seq and genotyping data from human donor islets. We established a positive correlation between CHRNB2, CHRNB4, MAFA, and MAFB transcript levels, insulin secretion, and glucose clearance, further supporting a critical role for nicotinic receptors in β cell function. Importantly, MAFA controlled nicotinic receptor CHRNB2 and CHRNB4 gene transcription in human EndoC-βH1 cells, suggesting that the transcriptional control of mouse and human nicotinic receptor genes is conserved. The CHRNB4 upstream enhancer region contained several SNPs critical for islet CHRNB4 transcript levels, insulin secretion after an oral glucose challenge, and type 2 diabetes, further linking the transcriptional control sequences of nicotinic receptors with the genetics of type 2 diabetes. This islet-specific enhancer contained binding sites for several islet-enriched transcriptional regulators, NKX6.1, NKX2.2, and MAFB (Pasquali et al., 2014). Mutations in this MAF-binding site, as well as in the adjacent type 2 diabetes-associated SNP rs922691 showed a reduction in MAFA transactivation potential, demonstrating that MAFA contributes to the transcriptional regulation of CHRNB4 and suggests that loss of MAFA and/or MAFB in type 2 diabetic islets may result in compromised CHRNB4 expression and subsequently CHRN activity, thus contributing to the β cell dysfunction observed (Guo et al., 2013).
We have identified a role for CHRN genes in β cell function, insulin secretion, and human type 2 diabetes. Previous studies have shown that nicotine addiction, specifically smoking, elevates the risk to develop type 2 diabetes by 40% (Willi et al., 2007). This has been partially attributed to nicotine’s effect on insulin sensitivity in peripheral tissues but also to the induction of β cell apoptosis (reviewed in Xie et al., 2009). Here, we show that nicotinic signaling also directly modulates acetylcholine-mediated insulin secretion. Thus, nicotine exposure may directly affect the β cell’s ability to respond to endogenous acetylcholine signaling, as chronic stimulation of CHRN proteins leads to receptor desensitization (Alkondon et al., 2000). However, targeted activation of nicotinic signaling in specific CHRNB4 risk allele carriers could also provide beneficial cues to improve β cell function.
EXPERIMENTAL PROCEDURES
Animal Models and Cell Lines
The MafAfl/fl, MafA−/−, and RIP (rat insulin promoter)-cre mouse lines have been previously described (Artner et al., 2010; Herrera, 2000). The RIP-Cre mouse line does not contain the human growth hormone minigene, which is in contrast to other commonly used pancreas-specific Cre deleter lines, which exhibit defects associated with the ectopic expression of human growth hormone in islets (Brouwers et al., 2014). Islet-β-cell-specific deletion of MafA (MafARIP) resulted in complete loss of MAFA expression (Figures S1C and S1D), impaired glucose clearance after an intraperitoneal glucose challenge (Figures S1A and S1B), and reduced expression of MAFA target genes Slc2a2, Slc30a8, Pdx1, and Insulin2 (Table S1). Mice were maintained on a mixed C57BL/6 and Sv129 background. HEK293 and the mouse β (β-TC6) cell lines were used for luciferase reporter and chromatin immunoprecipitation assays as indicated.
Western Blot Analysis
Western blot analysis with MafAWT and MafARIP islets was performed as previously described (Rosengren et al., 2010). Primary antibodies were α-CHRNB2 antibody (1:200; no. ANC-012; Alomone Labs), α-ADRA2A antibody (1:500; no. A271; Sigma-Aldrich), α-CHRNA5 antibody (1:400; no. NBP1–69122; Novus Biologicals), α-CHRNA4 antibody (1:500; no. ANC-004; Alomone Labs), α-CHRNB4 antibody (1:500; no. ANC-014; Alomone Labs), and monoclonal mouse α-pactin antibody (1:2,000; Sigma). Secondary antibodies were goat α-rabbit IgG (1:1,000; Cell Signaling Technology) and goat anti-mouse IgG (1:2,000; Dako). Signals were visualized using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific) and Chemi-Doc MP system (Bio-Rad).
Immunohistochemistry
Pancreata from 2- to 3-month-old wild-type and mutant mice were processed for paraffin and frozen sections. Immunohistochemical analysis was performed as described previously (Matsuoka et al., 2003). The primary antibodies used were guinea pig α-insulin (1:2,000; Dako), mouse α-glucagon (1:2,000; Sigma), rabbit α-MAFA (1:5,000; Bethyl Laboratories), rabbit α-MAFB (1:1,000; Bethyl Laboratories), rabbit α-CHRN-A4, A5, B2, and B4 (1:100; Alomone Labs), goat α-ADRA2A (1:100; Sigma), rabbit α-ADRA2A, rabbit α-TH (1:1,000; Sigma), goat α-VAChT (1:1,000; Millipore), and rabbit α-β-tubulin (1:5,000; Covance). The specificity of primary nicotinic receptor antibodies was assessed using absorption assays (Figure S2). Secondary antibodies used were Cy2-, Cy3-, and Cy5- conjugated α-guinea pig, α-mouse, α-goat, and α-rabbit (1:500; Jackson ImmunoResearch). Nuclear counter-staining was performed using DAPI (1:6,000; Invitrogen).
Image Analysis and Quantification
Immunofluorescence images were obtained using Zeiss 780 confocal microscope and Zen software or Zeiss Axioplan 2 imaging and AxioVision Rel 4.9 software (Zeiss); the latter was used for quantification of β cell area and α cell number. Immunohistochemistry and quantifications are based on four wild-type and MafA mutant pancreata. β cell area and α cell number were assessed by quantifying the pancreatic-, glucagon-, and insulin-stained area in sections at a 720-µm interval throughout the entire pancreatic organ. Mean differences were tested using the Student’s t test.
IPGTT
Intraperitoneal glucose tolerance test (IPGTT) was performed on 2-month-old MafAWT and MafARIP mice after a 12-hr fasting period. Fasted blood glucose levels were measured with a handheld glucometer (OneTouch; Lifescan) prior to an intraperitoneal injection of 2 g glucose/kg body weight. Blood glucose was measured at 0, 5, 15, 30, 60, and 120 min after glucose injection. Samples were analyzed using a multiple t test.
Intravenous 2DG Insulin Secretion Measurement
2DG-stimulated insulin secretion assays were performed on random fed 2-month-old MafAWT and MafARIP animals using an injection of 0.5 g 2DG/kg body weight. Blood was collected from an incision in the distal part of the tail. Measurements were taken at 0, 1, 5, 20, 30, and 50 min after 2DG administration. Blood was collected into heparin-coated tubes (Sarstedt), and the serum fractions were analyzed with a mouse insulin ELISA (Mercodia) according to the manufacturer’s instructions. Samples were analyzed using a multiple t test.
Glucagon Secretion Measurements
Two-month-old wild-type and MafARIP mice were intraperitoneally injected with saline solution or 0.5 g 2DG/kg body weight. Blood samples were collected 0 and 30 min after injection into heparin-coated tubes. Samples were chilled immediately and centrifuged at 4°C. Plasma aliquots were stored at −20°C. Glucagon concentrations were determined using glucagon ELISA (Mercodia). Statistical analysis was conducted using Student’s t test.
Islet Isolation and RNA Extraction
Two-month wild-type and mutant pancreatic islets were isolated as previously described (Johansson et al., 2010), and brainstem, cortex, and hypothalamus samples were dissected from 2-month-old wild-type and mutant brains. For removal of nerve fibers, islets were cultured overnight (indicated in respective figure). Islet and brain RNA was extracted using an RNeasy mini kit (QIAGEN), and RNA quality was analyzed using a Bioanalyzer (Agilent). Samples with a RNA integrity greater than seven were further analyzed, and RNA concentrations were measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop).
Reverse Transcription and Real-Time qPCR
Reverse transcription was carried out with SuperScript III (Invitrogen) according to the manufacturer’s instructions using 100 ng RNA/sample. For real-time qPCR, cDNA samples were diluted 1:10 and all assays were performed with Fast SYBR Green Master Mix on a StepOnePlus Real-Time PCR instrument (Applied Biosystems). Primer sequences are available upon request. Formation of expected PCR products was confirmed by agarose melting curve analysis, and brain stem RNA samples were used as a positive control for neurotransmitter receptor expression. Gene expression data were normalized against expression of the internal control gene hypoxanthine-guanine phosphoribosyl transferase (HPRT) and β actin. Experiments were repeated for at least four wild-type and mutant mice, each in duplicate. The data are shown as mean expression with SEM and were analyzed with a multiple t test.
Dual Luciferase Reporter Assay
Sequences of interest were cloned from mouse genomic DNA and human DNA derived from risk and non-risk allele carriers into the pGL2basic (Promega) or pFOX (Raum et al., 2010) luciferase vector as indicated (primer sequences are presented in Table S2 and the location of the control sequences is indicated in Figures 4C, S4I, and S4J). Luciferase reporter assays were performed in HEK293 or β-TC6 cells as described (Mazur et al., 2013). Exogenous MafA protein was overexpressed using a pCMV MafA construct. Transient transfection of all plasmids was performed using Metafectene Pro (Biontex Laboratories). Empty vector transfections were used as controls where indicated. The data are mean expression with SEM and were analyzed with ANOVA and Tukey multiple comparison tests.
Chromatin Immunoprecipitation Assay
The β-TC6 cell line was maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Sigma) and 1% penicillin/streptomycin (PEST) (Invitrogen). Upon reaching 90% confluence, cells were harvested and chromatin was prepared (Mazur et al., 2013). For assessment of MAFA binding to sequences containing rs922691 risk and non-risk alleles, β-TC6 cells were transfected with specific human CHRNB4 reporter constructs and myc-tagged MAFA, as indicated, and chromatin was prepared 3 days after transfection. Protein/DNA chromatin fragments were immunoprecipitated with mouse anti-MAFA antibody (Bethyl Labs) or mouse IgG (Jackson Immuno Research) as previously described (Mazur et al., 2013). Enrichment was assessed by qPCR and is presented as percent input. Primer sequences are presented in Table S2; location of ChIP amplicons is shown in Figures 4C, S4I, and S4J.
In Vitro Islet Perifusion
Islets from MafAWT and MafARIP mice were isolated by collagenase digestion and handpicked under a stereomicroscope. Islets were incubated overnight in DMEM containing 4.5 g/l D-glucose supplemented with 10% FBS and 1% PEST. The next day, islets were loaded into perifusion chambers (50 islets/ chamber; Brandel). Islets were perifused in a secretion buffer (10% SAB-10X, 2.2 mM CaCl2, 1 mM MgCl2, 20 mM HEPES, and 2% w/v albumin [pH 7.4]) containing low (2.8 mM) or stimulatory (10 mM) concentrations of glucose with 1 or 10 µM acetylcholine, 100 µM nicotine, 100 µM oxotremorine, a combination of both, or 25 mM KCl. Fractions of perifusate were collected every 4th minute, and insulin was measured in each fraction using insulin ELISA (Mercodia).
RNA-Seq, eQTL, and Gene Co-expression Analysis of Human Islets
Islets from 131 cadaver donors of European ancestry were provided by the Nordic Islet Transplantation Program and processed for RNA-seq (see Fadista et al., 2014 and Taneera et al., 2012 for GEO accession codes). Raw counts were normalized using trimmed mean of M-values and log2-transformed correlation coefficients, and p values were calculated using Graphpad Prism. The eQTL analysis was done as previously described (Fadista et al., 2014) using islets from 89 cadaver donors. Cis-eQTLs were calculated between gene expression levels and all SNPs within 250 kb of each gene. A linear model adjusting for age and sex, as implemented in the R Matrix eQTL package (Shabalin, 2012), was used. Significance was assessed by filtering out eQTLs that did not pass false discovery rate <1% threshold and 10,000 permutations.
Insulin SI
Human islet insulin secretion was measured on a column during the dynamic glucose perifusion as described previously (Goto et al., 2004). Insulin content in the effluent was collected in 6-min intervals and quantified using human insulin ELISA (Mercodia). SI was defined as the ratio between the areas under the curves calculated for low (1.67 mM) and high (16.7 mM) glucose concentrations.
siRNA-Mediated MAFA Knockdown in the EndoC-βH1 Cell Line and βTC-6 Cells
Knockdown of MAFA in EndoC-βH1 (Ravassard et al., 2011) cells was achieved using the TARGETplus siRNA pool against human MAFA (no. L-027343-01). Briefly, 2 × 106 cells were transfected with siRNA (50 pmol) or a non-targeting control (no. D001810) using the Dharmafect no. 1 reagent (GE Dharmacon no. T-2001) following the manufacturer’s protocol. RNA was harvested 72 hr post-transfection and isolated using Trizol reagent (Life Technologies) and the DNA-Free RNA Kit (Zymo Research). The iScript cDNA synthesis kit (Bio-Rad) was used for cDNA synthesis, and qPCR reactions were performed on a LightCycler 480 II (Roche) and analyzed by the ΔΔCT method.
Knockdown of MafA or control sequences in βTC-6 cells was performed using 30 nM MAFA RNAi oligonucleotides (no. s196287; Thermo Scientific) or negative control no. 1 (Thermo Scientific) with transfection reagent (Dharmafect; Thermo Scientific). Total RNA was extracted after 72 hr transfection, and qPCR was performed as described above.
Knockdown of MAFA resulted in a 50% reduction of the MAFA transcript and protein levels (data not shown).
Study Approval
All animal work was approved by a local ethics committee for animal research, and the use of human tissue was approved by a Swedish government ethics committee.
Supplementary Material
In Brief.
Autonomic signaling regulates insulin secretion in response to food intake, a process disturbed in diabetes. Ganic et al. show that the MAFA transcription factor controls autonomic-nervous-system-mediated insulin secretion by regulating nicotinic signaling and identify diabetes risk alleles in human nicotinic receptor genes, suggesting a potential link between smoking and diabetes.
Highlights.
MAFA controls autonomic-nervous-system-mediated insulin secretion
MAFA activates nicotinic receptor expression in mouse and human β cells
Nicotinic receptor signaling is required for acetylcholine-mediated insulin secretion
Polymorphisms in human nicotinic receptor genes affect islet expression and diabetes
Acknowledgments
This work was supported by the Swedish Research Council (grant no. 521-2011-3750 and 2015-02330), the Juvenile Diabetes Research Foundation (2-2007-716), Diabetesfonden (DIA2014-027), Segerfalk foundation, Påhlsson foundation, Diabetes Wellness Foundation, and the European Foundation for the Study of Diabetes. The authors thank B. Ahrén (Department of Clinical Sciences, Lund University), C. Wohlheim (University of Geneva), and J.C. Raum (University of Pennsylvania) for discussing the data; M. Magnusson for mouse strain maintenance; and Drs. Scharfmann and Ravassard for providing EndoC-βH1 cells.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2016.02.002.
AUTHOR CONTRIBUTIONS
E.G. researched data and wrote the manuscript. T.S., J.K.J., C.L., H.A., H.A.C., J.F., P.S., H.B., and G.P. researched data. R.S. and M.F. researched data, reviewed the manuscript, and contributed to discussion. E.R. and L.G. reviewed the manuscript and contributed to discussion. I.A. designed research, researched data, and wrote the manuscript.
References
- Ahré n B. Autonomic regulation of islet hormone secretion-implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–2739. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, Stein R. MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes. 2010;59:2530–2539. doi: 10.2337/db10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers B, de Faudeur G, Osipovich AB, Goyvaerts L, Lemaire K, Boesmans L, Cauwelier EJ, Granvik M, Pruniau VP, Van Lommel L, et al. Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 2014;20:979–990. doi: 10.1016/j.cmet.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Brissova M, Hang Y, Thompson C, Poffenberger G, Shostak A, Chen Z, Stein R, Powers AC. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55:707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duttaroy A, Zimliki CL, Gautam D, Cui Y, Mears D, Wess J. Muscarinic stimulation of pancreatic insulin and glucagon release is abolished in m3 muscarinic acetylcholine receptor-deficient mice. Diabetes. 2004;53:1714–1720. doi: 10.2337/diabetes.53.7.1714. [DOI] [PubMed] [Google Scholar]
- Fadista J, Vikman P, Laakso EO, Mollet IG, Esguerra JL, Taneera J, Storm P, Osmark P, Ladenvall C, Prasad RB, et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl. Acad. Sci. USA. 2014;111:13924–13929. doi: 10.1073/pnas.1402665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganic E, Johansson JK, Bennet H, Fex M, Artner I. Islet-specific monoamine oxidase A and B expression depends on MafA transcriptional activity and is compromised in type 2 diabetes. Biochem. Biophys. Res. Commun. 2015;468:629–635. doi: 10.1016/j.bbrc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Gautam D, Han SJ, Hamdan FF, Jeon J, Li B, Li JH, Cui Y, Mears D, Lu H, Deng C, et al. A critical role for beta cell M3 muscarinic acetylcholine receptors in regulating insulin release and blood glucose homeostasis in vivo. Cell Metab. 2006;3:449–461. doi: 10.1016/j.cmet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Gilsbach R, Faron-Górecka A, Rogóz Z, Brüss M, Caron MG, Dziedzicka-Wasylewska M, Bönisch H. Norepinephrine transporter knockout-induced up-regulation of brain alpha2A/C-adrenergic receptors. J. Neurochem. 2006;96:1111–1120. doi: 10.1111/j.1471-4159.2005.03598.x. [DOI] [PubMed] [Google Scholar]
- Goto M, Eich TM, Felldin M, Foss A, Källen R, Salmela K, Tibell A, Tufveson G, Fujimori K, Engkvist M, Korsgren O. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004;78:1367–1375. doi: 10.1097/01.tp.0000140882.53773.dc. [DOI] [PubMed] [Google Scholar]
- Guo Y, Traurig M, Ma L, Kobes S, Harper I, Infante AM, Bogardus C, Baier LJ, Prochazka M. CHRM3 gene variation is associated with decreased acute insulin secretion and increased risk for early-onset type 2 diabetes in Pima Indians. Diabetes. 2006;55:3625–3629. doi: 10.2337/db06-0379. [DOI] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab. 2014;99:1983–1992. doi: 10.1210/jc.2014-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang Y, Yamamoto T, Benninger RK, Brissova M, Guo M, Bush W, Piston DW, Powers AC, Magnuson M, Thurmond DC, Stein R. The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes. 2014;63:1994–2005. doi: 10.2337/db13-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Johansson JK, Voss U, Kesavan G, Kostetskii I, Wierup N, Radice GL, Semb H. N-cadherin is dispensable for pancreas development but required for beta-cell granule turnover. Genesis. 2010;48:374–381. doi: 10.1002/dvg.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S, Bood M, Ahrén B. The mechanism of 2-deoxy-glucose-induced insulin secretion in the mouse. J. Auton. Pharmacol. 1987;7:135–144. doi: 10.1111/j.1474-8673.1987.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol. Cell. Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur MA, Winkler M, Ganic E, Colberg JK, Johansson JK, Bennet H, Fex M, Nuber UA, Artner I. Microphthalmia transcription factor regulates pancreatic β-cell function. Diabetes. 2013;62:2834–2842. doi: 10.2337/db12-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J, Rodriguez-Diaz R, Fachado A, Jacques-Silva MC, Berggren PO, Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63:2714–2726. doi: 10.2337/db13-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani M, Oka T, Badyuk M, Xiao Y, Kellar KJ, Daly JW. Mouse beta-TC6 insulinoma cells: high expression of functional alpha3beta4 nicotinic receptors mediating membrane potential, intracellular calcium, and insulin release. Mol. Pharmacol. 2006;69:899–907. doi: 10.1124/mol.105.014902. [DOI] [PubMed] [Google Scholar]
- Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, Mularoni L, Miguel-Esca-lada I, Akerman I, Tena JJ, Morán I, Gómez-Marín C, van de Bunt M, et al. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 2014;46:136–143. doi: 10.1038/ng.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porte D, Jr, Williams RH. Inhibition of insulin release by norepinephrine in man. Science. 1966;152:1248–1250. doi: 10.1126/science.152.3726.1248. [DOI] [PubMed] [Google Scholar]
- Raum JC, Hunter CS, Artner I, Henderson E, Guo M, Elghazi L, Sosa-Pineda B, Ogihara T, Mirmira RG, Sussel L, Stein R. Islet beta-cell-specific MafA transcription requires the 5’-flanking conserved region 3 control domain. Mol. Cell. Biol. 2010;30:4234–4244. doi: 10.1128/MCB.01396-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravassard P, Hazhouz Y, Pechberty S, Bricout-Neveu E, Armanet M, Czernichow P, Scharfmann R. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J. Clin. Invest. 2011;121:3589–3597. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011a;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011b;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, Nagaraj V, Reinbothe TM, Tuncel J, Eliasson L, et al. Overexpression of alpha2A–adrenergic receptors contributes to type 2 diabetes. Science. 2010;327:217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–1358. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneera J, Lang S, Sharma A, Fadista J, Zhou Y, Ahlqvist E, Jonsson A, Lyssenko V, Vikman P, Hansson O, et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 2012;16:122–134. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Thorens B. Brain glucose sensing and neural regulation of insulin and glucagon secretion. Diabetes Obes. Metab. 2011;13(Suppl 1):82–88. doi: 10.1111/j.1463-1326.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-anaysis. JAMA. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- Winzell MS, Ahrén B. Role of VIP and PACAP in islet function. Peptides. 2007;28:1805–1813. doi: 10.1016/j.peptides.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Xie XT, Liu Q, Wu J, Wakui M. Impact of cigarette smoking in type 2 diabetes development. Acta Pharmacol. Sin. 2009;30:784–787. doi: 10.1038/aps.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Hellström-Lindahl E, Grill V. Evidence for functional nicotinic receptors on pancreatic beta cells. Metabolism. 2005;54:247–254. doi: 10.1016/j.metabol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et al. MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.