Abstract
The effects of methyl jasmonate (JA-Me) on early light-inducible protein (ELIP) expression in barley (Hordeum vulgare L. cv Apex) have been studied. Treatment of leaf segments with JA-Me induces the same symptoms as those exhibited by norflurazon bleaching, including a loss of pigments and enhanced light stress that results in increased ELIP expression under both high- and low-light conditions. The expression of both low- and high-molecular-mass ELIP families is considerably down-regulated by JA-Me at the transcript and protein levels. This repression occurs despite increased photoinhibition measurable as a massive degradation of D1 protein and a delayed recovery of photosystem II activity. In JA-Me-treated leaf segments, the decrease of the photochemical efficiency of photosystem II under high light is substantially more pronounced as compared to controls in water. The repression of ELIP expression by JA-Me is superimposed on the effect of the increased light stress that leads to enhanced ELIP expression. The fact that the reduction of ELIP transcript levels is less pronounced than those of light-harvesting complex II and small subunit of Rubisco transcripts indicates that light stress is still affecting gene expression in the presence of JA-Me. The jasmonate-induced protein transcript levels that are induced by JA-Me decline under light stress conditions.
Excess light can be harmful to the photosynthetic apparatus, as it leads to free-radical formation and photooxidation processes (Barber and Andersson, 1992; Prasil et al., 1992), which cause photoinhibition and result in the inactivation of photosystem II (PSII) reaction centers, loss of chlorophyll, and reduced photosynthetic activity (Andersson et al., 1992; Aro et al., 1993). Plants respond to changing light conditions by altered gene expression so that maintenance of high photosynthetic efficiency is achieved and formation of toxic oxygen radicals is minimized under high-light (HL) fluxes (Andersson and Styring, 1991; Chory 1994; Anderson et al., 1995; Chory et al., 1996; Fankhauser and Chory, 1997; Kloppstech, 1997; Mustilli and Bowler, 1997). To cope with excess light conditions, plants have developed several mechanisms for adaptation, repair, and protection, including PSII repair via replacement of damaged D1 protein by de novo synthesized D1 (Mattoo et al., 1984; Andersson et al., 1992; Prasil et al., 1992; Aro et al., 1993) and protection by quenching of excess excitation energy via the activation of the xanthophyll cycle (Demming-Adams, 1990, 1996) and light-harvesting complex II (LHC II) phosphorylation.
The early light-inducible proteins (ELIPs) are thought to protect plastids against light stress (Adamska et al., 1992a, 1992b, 1993; Pötter and Kloppstech, 1993; Adamska, 1997). In barley (Hordeum vulgare), two ELIP families are known that differ in their molecular masses (Grimm et al., 1989; Green et al., 1991). ELIPs are expressed under light stress in green plants as well as in the early phase of greening of etiolated plants (Grimm et al., 1989; Adamska et al., 1992a, 1992b; Pötter and Kloppstech, 1993). The light stress-induced expression of ELIPs in mature plants increases with the intensity and the duration of the stress and thus it correlates with the degree of photoinhibition. The extent of light stress-induced ELIP expression depends on the circadian time and declines with the differentiation state of the cells (Adamska et al., 1992a, 1992b, 1993; Pötter and Kloppstech, 1993; Humbeck et al., 1994). In most cases, light stress-induced ELIP accumulation is accompanied by a decrease in the steady-state level of LHC II mRNA, while that of small subunit of Rubisco (SSU) is less affected. Under these conditions, an increase in β-carotene and zeaxanthin synthesis as well as degradation of the D1 protein are observed (Adamska et al., 1992a, 1992b; 1993; Pötter and Kloppstech, 1993; Anderson et al., 1995; Adamska, 1997; Montané et al., 1998). ELIPs have been shown to bind chlorophyll a and lutein and have been proposed to function as transient pigment carriers or chlorophyll exchange proteins (Adamska et al., 1999).
Jasmonates act as stress hormones and play a role in plant growth and development (Parthier, 1990, 1991; Creelman and Mullet, 1997). In plant tissues treated with jasmonates, distinct effects are exerted on gene expression. Jasmonates induce the expression of jasmonate-induced protein (JIPs) (Weidhase et al., 1987a; Müller-Uri et al., 1988), most of which seem to function as stress proteins and may protect and defend plants under stress conditions. For instance, this expression has been shown for the leaf thionin JIP 6 (Becker and Apel, 1992). The rapid activation of JIP genes occurs at the level of transcription (Weidhase et al., 1987a; Müller-Uri et al., 1988; Parthier, 1990 and 1991; Reinbothe et al., 1992). In the presence of jasmonates, the synthesis of Rubisco as well as the synthesis of other chloroplast proteins that are involved in photosynthesis (light-harvesting chlorophyll-protein complexes, psaA, psaB) is immediately decreased by negative control of translation as the transcript levels remain constant. At later times, the transcript amounts also decline. The corresponding proteins are degraded (Weidhase et al., 1987a, 1987b; Müller-Uri et al., 1988; Parthier, 1990, 1991; Reinbothe et al., 1993a, 1993b, 1993c, 1994a, 1997). This selective influence on gene expression leads to the typical symptoms that are interpreted as leaf senescence: chlorophyll destruction, yellowing, and protein degradation (Weidhase et al., 1987a, 1987b; Reinbothe et al., 1993a, 1993b). Jasmonates exert almost no influence on constitutive housekeeping proteins. D1 and several other plastid DNA-encoded proteins are only slightly affected by jasmonates (Reinbothe et al., 1993a, 1993b, 1993c, 1994a). After 48 h of jasmonate treatment a general decrease in protein synthesis is caused by the ribosome-inactivating protein JIP 60 (Chaudry et al., 1994; Reinbothe et al., 1994a, 1994b).
Barley leaf segments treated with methyl jasmonate (JA-Me) show symptoms that are similar to those of primary leaves bleached by norflurazon (NF) treatment. In the presence of NF the levels of carotenoids decline. This situation leads to substantially higher ELIP expression at both mRNA and protein levels, even under low-light (LL) conditions (Pötter and Kloppstech, 1993). The striking similarities between JA-Me-treated and NF-bleached plants led us to ask whether ELIPs would also be overexpressed following JA-Me treatment. To examine the effects of JA-Me on the expression of ELIPs, the incubation with JA-Me in LL was followed by treatment with HL as ELIPs are inducible by light stress. The effects of HL on the JA-Me induced expression of JIPs, especially that of leaf thionin (JIP6), could be simultaneously analyzed.
RESULTS
Effects of JA-Me Treatment
Treatment of barley leaf segments with JA-Me leads to JIP induction and to effects that are interpreted as accelerated senescence, namely a loss of chlorophyll and the degradation of Rubisco (Weidhase et al., 1987a, 1987b; Müller-Uri et al., 1988). These effects were confirmed for the JA-Me-treated segments in our studies.
The treated segments exhibited a typical JA-Me symptom (Weidhase et al., 1987b): strong yellowing, caused by an intense loss of chlorophylls a and b. This yellowing was first visible after 24 h of incubation and progressed substantially during incubation. The total chlorophyll content decreased by 30% after 1 d, 50% after 2 d, and 70% after 3 d of JA-Me treatment (data not shown). In addition, a substantial loss of carotenoids occurred as the total content decreased 60% after 1 d and 65% after 3 d of JA-Me treatment (data not shown). The control segments that floated for 1 to 3 d on water did not exhibit a loss of pigment in comparison to freshly detached segments (data not shown).
JA-Me-treated segments were similar to leaves bleached in the presence of NF. NF blocks carotenoid biosynthesis (Chamovitz et al., 1990) and the lack of carotenoids leads in turn to photooxidation of chlorophylls. Besides their light-harvesting function as accessory pigments, carotenoids serve primarily as quenchers of excess excitation energy by promoting non-radiative dissipation and thus prevent the generation of reactive oxygen species (Demming-Adams, 1990, 1996). When carotenoid biosynthesis is prevented, a situation analogous to light stress can occur under light conditions which are optimal for growth (Oelmüller, 1989). Thus the damaging effect of light is strongly enhanced compared to the situation in untreated leaves. The striking similarities between JA-Me-treated and NF-bleached plants raised the question of whether light stress and the extent of photoinhibition are also strongly increased after JA-Me treatment.
Effects of JA-Me Treatment on Photoinhibition
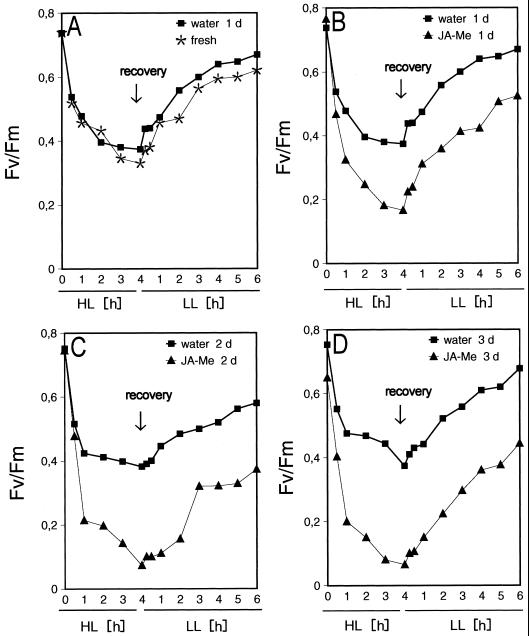
In freshly detached segments, the photochemical efficiency of PSII expressed as the maximum photochemical efficiency of PSII in the dark-adapted state (Fv/Fm) decreased by 55% during 4 h of HL (2,500 μmol m−2 s−1). During the following 6 h of recovery under LL (100 μmol m−2 s−1) Fv/Fm increases to 85% of the value at the beginning of HL treatment (Fig. 1). This increase is in accordance with previous data (Pötter, 1994) and leads to an equilibrium between D1 destruction and repair which depends on the light intensity and a restauration phase during which PSII activity is recovered due to the integration of the newly formed D1 protein. In control segments that were incubated for 1 to 3 d on water prior to the HL treatment, the Fv/Fm values at the beginning of HL and the Fv/Fm time course are almost unchanged.
Figure 1.
In vivo measurement of variable chlorophyll fluorescence. After 7 d of growth barley leaf segments were floated for 24 h (1 d; A and B), 48 h (2 d; C), or 72 h (3 d; D) on either tap water (water) or 45 μm of JA-Me (JA-Me) or sampled immediately (fresh; A). The segments were exposed to HL of 2,500 μmol m−2 s−1 floating on tap water for 4 h followed by 6 h recovery under LL conditions (100 μmol m−2 s−1). At the indicated times fluorescence induction kinetics were measured. To ensure that no diurnal changes in the chlorophyll fluorescence properties occurred during these 10 h (4 h HL + 6 h recovery) control segments were incubated for the same period of time under LL conditions floating on tap water or JA-Me, respectively.
In JA-Me-treated segments, Fv/Fm decreases faster, and after 4 h of HL, Fv/Fm is close to zero. The Fv/Fm values at the beginning of HL treatment are only slightly diminished even after 3 d of incubation. The faster decrease of Fv/Fm indicates that the same light intensity of 2,500 μmol m−2 s−1 causes substantially higher light stress in the JA-Me-treated segments. The increase of Fv/Fm during recovery occurs substantially more slowly as compared to the controls and finally reaches only about 65% of control values. This increase is in agreement with earlier findings showing that the recovery of photosynthesis is inversely related to the extent of the preceding light stress (Adamska et al., 1993).
HL exposure of NF-treated leaves leads to loss of variable fluorescence, chlorophyll bleaching, loss of turgor, and finally to total photodestruction of the leaf (Pötter, 1994). In JA-Me-treated leaf segments, similar phenomena are observed; however, the turgor remains almost constant during incubation under LL but decreases strongly under HL. The treatment with JA-Me and HL occasionally results in small necrotic lesions whose numbers increase with the time of incubation.
Effects of JA-Me Treatment on the Expression of ELIPs
In control as well as in JA-Me-pretreated segments exposed to LL, neither ELIP transcripts nor the correspondent proteins were detectable despite the fact that in JA-Me-pretreated segments light stress should have occurred under LL due to the loss of pigments. However, it was surprising that under LL conditions, Fv/Fm was only very slightly decreased.
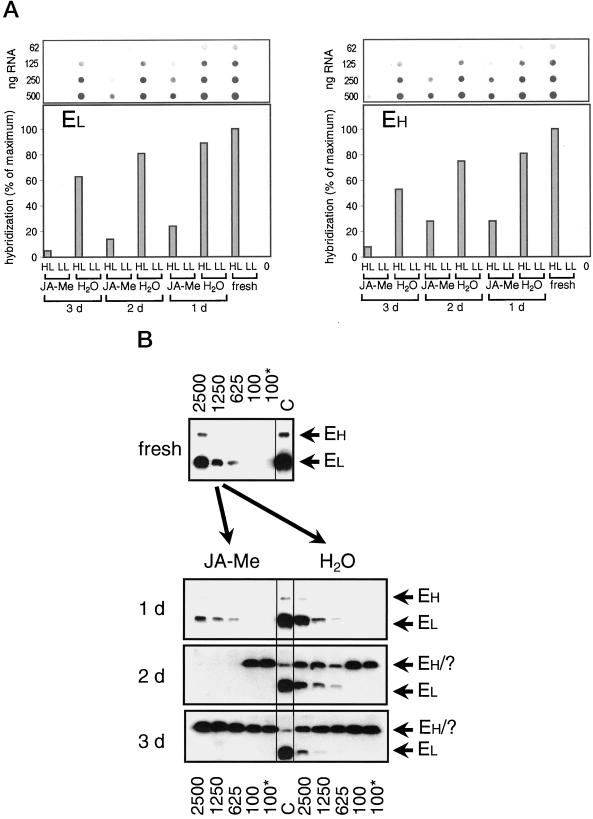
In freshly detached segments, transcripts of small and large ELIPs start to accumulate at light fluxes of 625 μmol m−2 s−1. The induced amounts increase considerably with light intensity (data not shown) as described previously for the entire leaf (Pötter and Kloppstech, 1993). The transcript amounts of small and large ELIPs which are induced by a 4 h HL-treatment at fluxes of 2,500 μmol m−2 s−1 are highest in freshly detached segments but decrease to about 60% with incubation time (Fig. 2A).
Figure 2.
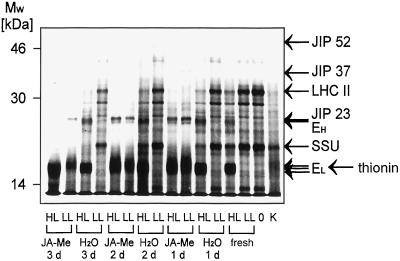
ELIPs: mRNA and protein accumulation. A, Dot-blot analysis of poly(A)-rich mRNA. After 7 d of growth barley leaf segments were floated for 24 h (1 d), 48 h (2 d), or 72 h (3 d) on either tap water (H2O) or 45 μm of JA-Me (JA-Me) or sampled immediately (fresh). Following these pre-incubations the leaves were either exposed for 4 h to HL of 2,500 μmol m−2 s−1 (HL) or further incubated for 4 h at LL conditions of 100 μmol m−2 s−1 (LL). In addition, segments of barley grown for 7 d were frozen in liquid nitrogen directly after detachment (0). Poly(A)-rich mRNA was dotted (in four different concentrations as indicated in the figure) and hybridized with homolog probes to EL = HV90 (low-molecular-mass ELIP) and EH = HV8F6 (high-molecular-mass ELIP). After checking for linearity the maximum signal is set as 100%. The other signals are shown as percent of the maximum signal. B, Western-blot analysis of total protein extracts. After 7 d of growth barley leaf segments were floated for 24 h (1 d), 48 h (2 d), or 72 h (3 d) on either 45 μm of JA-Me (JA-Me) or tap water (H2O) or sampled immediately (fresh). Thereafter leaves were either subjected for 4 h to HL of 2,500 μmol m−2 s−1 (2500), 1,250 μmol m−2 s−1 (1250), or 625 μmol m−2 s−1 (625), or further incubated for 4 h at LL conditions of 100 μmol m−2 s−1 (100) or directly frozen in liquid nitrogen (100*). Total protein extracts (15 μg of protein per lane) were analyzed by western blot probed with anti-ELIP following SDS-PAGE (15% [w/v]gels). One major band of 13.5 kD (EL) is recognized by the antibody. Large ELIPs (EH) show slight cross reactivity. Control for ELIP expression (C): 5 μL total protein extract from etiolated leaves after 10 h at 100 μmol m−2 s−1. The four western blots were performed in parallel and treated identically. EH/?, High-molecular-mass ELIP or unidentified band, respectively, which runs directly below the ELIPs (see text).
Treatment with JA-Me reduces the transcript levels considerably. After 1d of incubation the mRNA levels of small and large ELIPs are reduced to about one quarter of the amount found in freshly detached segments. The levels declined further to 5 to 8% at d 3 (Fig. 2A) so that the expression of both ELIP families remained inducible at the transcript level even after 3 d of JA-Me treatment. The amounts of HL-induced transcripts of small and large ELIPs were also substantially reduced when compared to the corresponding controls on water. The ratio of ELIP mRNA induced in the presence to that induced in the absence of JA-Me decreased with time.
The levels of both ELIP proteins increased with light intensity (Fig. 2B). In freshly removed leaves small ELIPs are detected at a light intensity of 625 μmol m−2 s−1 and large ELIPs at a light intensity of 1,250 μmol m−2 s−1 onwards. Segments incubated for 1 d in water did not show any difference in the levels of accumulated ELIP. With incubation on water, the amounts of small ELIPs induced decreased while the threshold at the 625-μmol m−2 s−1 intensity and the dependence on light fluxes remained unchanged. After 2 d of pre-incubation in either water or JA-Me in water, a strong ELIP cross-reactive band appeared that overlapped with the large ELIPs so that no further analysis of these ELIPs by quantification was possible (Fig. 2B). The pattern of appearance argues against a JIP, as the band is also abundant in untreated segments. The pattern also does not represent a typical ELIP, as it is abundant under LL conditions. The cross-reactive band could eventually represent a wound-inducible protein such as glutathione S-transferase (McConn et al., 1997).
In JA-Me-pretreated segments, ELIP protein accumulation declined dramatically (Fig. 2B). After 1 d of incubation, small ELIPs were expressed with the same dependency on the light intensity as in freshly detached segments; however, the induced amounts of small and large ELIPs are considerably smaller than those in the corresponding controls in water. After 2 and 3 d of preincubation in JA-Me, small ELIPs were hardly detected.
Based on the results above, we conclude that ELIP expression is dramatically repressed by JA-Me at the transcript level. An additional negative control by JA-Me was found at the protein level as the amounts of accumulated ELIP protein were still smaller than could be expected from the remaining amounts of ELIP transcripts (Fig. 2).
It follows that in NF-bleached leaves, ELIP expression was increased due to the strongly enhanced light stress, but under JA-Me treatment ELIP expression is strongly reduced despite the fact that the light stress is considerably enhanced. This finding could be explained by a severe intervention of JA-Me in gene regulation that is superimposed on the effect of the light stress.
Effects of JA-Me Treatment and HL on the Expression of LHC II and Rubisco
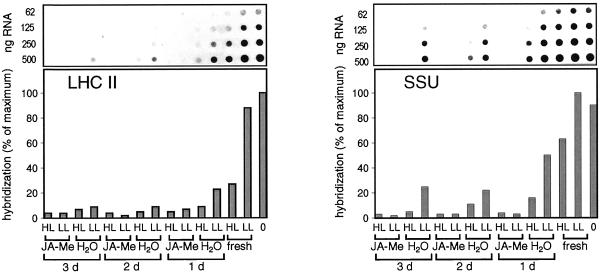
In JA-Me-treated segments, the transcripts for LHC II and SSU disappeared almost completely within 1 d of treatment (Fig. 3). The amounts of LHC II, SSU, and large subunit of Rubisco proteins decreased with time of incubation in the presence of JA-Me, but remained constant in the controls (data not shown). This finding was consistent with the repression of synthesis and destruction of photosynthesis proteins being typical effects of JA-Me (Weidhase et al., 1987a, 1987b; Müller-Uri et al., 1988; Reinbothe et al., 1993a, 1993b, 1997). The transcript levels of SSU and LHC II also declined more slowly during incubation in water. This decrease, which was more pronounced for LHC II transcripts, could be explained by an increase in the levels of JA-Me or abscisic acid (ABA) caused by the wounding (Reinbothe et al., 1992, 1993a; Pötter, 1994).
Figure 3.
LHC II and SSU: mRNA expression. Dot-blot analysis of poly(A)-rich mRNA: application of JA-Me, light treatment, and analysis of poly(A)-rich mRNA were performed as described in the legend of Figure 2A. Hybridization was performed with homologous probes to LHC II and SSU. The exposure times of the autoradiograms were different for each probe.
During HL treatment of freshly detached segments, the transcript levels declined considerably for LHC II and moderately for SSU (Fig. 3). These declines could be attributed to the higher light stress sensitivity of LHC II (Pötter and Kloppstech, 1993). With prolonged incubation of segments the extent of reduction of SSU transcript levels under HL increased in segments incubated on water. This increase in light stress sensitivity of SSU might be explained by a higher ABA concentration in wounded segments as the SSU transcript levels have been found to be reduced under HL in the presence of ABA (Pötter, 1994).
JA-Me repressed the expression of SSU and LHC II transcripts more efficiently and quickly than the light stress-induced ELIP expression (Figs. 2A and 3). The delayed repression of ELIPs in comparison to LHC II and SSU showed that the light stress-regulated ELIPs remained inducible for a longer time than LHC II and SSU that normally were continuously abundant in the cell.
Effects of JA-Me and HL Treatment on the Expression of D1
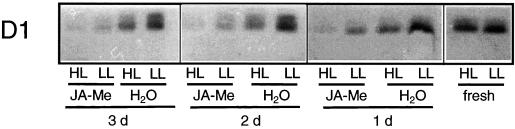
No change in the amount of D1 protein occurred during 1 to 3 d of incubation of segments with water (Fig. 4). During HL treatment (2,500 μmol m−2 s−1 for 4 h), the steady-state level of D1 protein remained almost unchanged in freshly detached segments although it was considerably reduced in segments preincubated for 1 to 3 d in water. In JA-Me-treated segments the amount of D1 was strongly reduced without HL treatment to values that were substantially lower than those of the corresponding water controls after 4 h of HL treatment (Fig. 4). It was surprising that the Fv/Fm values were only very slightly reduced under LL conditions. This reduction could be explained if one considers that a pool of D1 protein exists which is not assembled into PSII. Exposure of JA-Me-treated segments to HL of 2,500 μmol m−2 s−1 further reduced the level of D1 so that almost no D1 protein remained (Fig. 4). The amount of D1 protein was reduced by incubation with JA-Me and, in addition, by treatment with HL intensities.
Figure 4.
D1: protein accumulation. Application of JA-Me and light treatment were performed as described in the legend of Figure 2A. Total protein extracts (15 μg per lane) were analyzed by western-blot analysis probed with anti-D1 following SDS-PAGE (12.5% [w/v] gels). The figure is composed of two western blots that were performed in parallel and treated identically. The same total extracts for segments incubated for 1 d were loaded onto both western blots and gave equally strong signals ensuring that signals of both western blots are comparable.
In freshly detached segments, almost no change in the steady-state level of D1 was found during HL treatment (2,500 μmol m−2 s−1 for 4 h). This finding corresponds with the situation in freshly detached primary leaves of barley (Pötter, 1994). The strong additional reduction of the D1 steady-state level during HL treatment that was observed in segments pretreated with water or JA-Me during 1 to 3 d suggests an increase in light stress sensitivity of D1. This finding corresponds with the situation in pea (Pisum sativum) (Adamska et al., 1992a, 1992b, 1993).
Effects of HL on the Expression of JIPs
The application of JA-Me induces a strong JIP expression within 1 d (data not shown) (Reinbothe et al., 1992). The JIP amounts increased with incubation time while the amounts of their transcripts decreased moderately (Figs. 5 and 6). This finding is in accordance with earlier studies (Weidhase et al., 1987a; Müller-Uri et al., 1988; Reinbothe et al., 1994a, 1997). In the non-JA-Me-treated controls, a small amount of leaf thionin was present (Fig. 6A) in contrast to other JIPs, which were not detectable (Gausing, 1987; Reimann-Philipp et al., 1989).
Figure 5.
Changes in mRNA population under JA-Me and/or HL treatment: in vitro translation of poly(A+)-rich RNA. Application of JA-Me and light treatment were performed as described in the legend of Figure 2A. After in vitro translation of rate-limiting amounts of poly(A+)-rich RNA, equal amounts of incorporated radioactivity (50,000 cpm) of each probe were separated by SDS-PAGE and analyzed by fluorography. Poly(A+)-rich RNA isolated from segments pre-incubated with JA-Me for 3 d and then subjected to HL (3 d JA-Me, HL) could be translated less effectively than the RNA isolated from all other segments, so that a higher volume of this probe had to be used to load equal counts. Lane C represents poly(A+)-rich RNA from etiolated, 2-h-illuminated barley leaves as a positive control. In freshly detached segments and water controls at least 2 or 3 different precursors of small or large ELIPs were detectable. For segments pretreated with JA-Me and then subjected to HL, no clear differentiation between precursor proteins of small ELIPs and the precursor protein of thionin was possible as their bands overlap. Poly(A+)-rich RNA from all segments could be translated in vitro and the analysis of the in vitro translation products gave the same results as obtained in the dot-blot analysis (see Figs. 2A, 3, and 6A).
Figure 6.
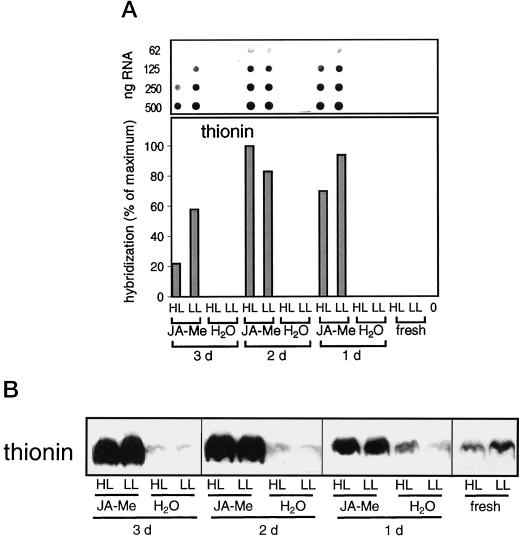
Leaf thionin: mRNA and protein accumulation. A, Dot-blot analysis of poly(A)-rich mRNA. Application of JA-Me, light treatment, and analysis of poly(A)-rich mRNA were performed as described in the legend of Figure 2A, except that hybridization was done with a homologous probe to leaf thionin. B, Western-blot analysis of total protein extracts. Total protein extracts (15 μg of protein per lane) were analyzed by western blot probed with anti-leaf thionin following SDS-PAGE (20% [w/v] gels). The figure is composed of two western blots that were performed in parallel and treated identically (see also legend to Fig. 4).
During a 4-h exposure of leaf segments to 2,500 μmol m−2 s−1, the amount of thionin transcripts was reduced by 26% of the initial level within 1 d and to 62% in segments incubated for 3 d in JA-Me (Figs. 5 and 6A). This finding indicates that the sensitivity of thionin transcripts to light stress increased with duration of JA-Me pretreatment. In vitro translation of poly(A)-rich mRNA showed that during HL treatment, the transcript amounts of JIP 23, JIP 37, and JIP 52 also decreased in segments incubated for 1 to 3 d with JA-Me (Fig. 5). Independent of the preincubation, no effect of HL was noted on the thionin level (Fig. 6B). Therefore it appears that the accumulated thionin protein was stable during the 4-h HL treatment while the JIP transcript levels declined under light stress.
Effects of Abscisic Acid and Cytokinin on the Expression of ELIPs
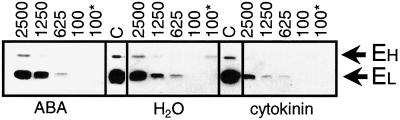
ABA often acts synergistically to JA-Me while cytokinin is considered an antagonist to JA-Me (Weidhase et al., 1987a; Parthier, 1990; Sembdner and Parthier, 1993). Thus it was of interest to examine the influence of ABA and cytokinin on ELIP levels. Under all light intensities cytokinin exerted a negative effect on ELIP accumulation and reduced the induced amounts of small and large ELIPs by about more than 50% (Fig. 7). Thus the light stress-induced ELIP expression is reduced in presence of cytokinin, JA-Me, or both (Figs. 2B and 7). Consistent with previous studies (Pötter and Kloppstech, 1993), ABA at concentrations of 1 mm had no clear effect on ELIP accumulation under light stress in barley.
Figure 7.
Effects of ABA and cytokinin on ELIP accumulation. After 7 d of growth barley leaf segments were floated for 24 h on either tap water (H2O) or 2 mm of cytokinin (cytokinin) or 1 mm of ABA (ABA). Following these pre-incubations the segments were either subjected for 4 h to HL of 2,500 μmol m−2 s−1 (2500), 1,250 μmol m−2 s−1 (1250), or 625 μmol m−2 s−1 (625) or further incubated for 4 h at LL conditions of 100 μmol m−2 s−1 (100) or directly frozen in liquid nitrogen (100*). Fifteen μg of protein per lane were analyzed by western blot probed with anti-ELIP after SDS-PAGE (15% [w/v] gels). See also legend to Figure 2 B.
DISCUSSION
The stress symptoms induced by JA-Me treatment are reminiscent to those observed in NF-treated leaves in which the lack of carotenoids leads to an increased light stress under HL and to a situation analogous to light stress already under normal light conditions (Oelmüller, 1989). In NF-bleached leaves, ELIP expression and translation product accumulation is strongly enhanced under HL treatment and a significant increase in ELIP occurs already under light conditions of normal growth light (70 μmol m−2 s−1) (Pötter and Kloppstech, 1993). In JA-Me-treated segments, light stress is also considerably enhanced, an effect that may be caused by the loss of carotenoids and chlorophylls. This enhanced stress becomes manifest in a substantially stronger reduction of the photochemical efficiency of PSII under HL treatment that is due to a massive degradation of D1 and a delayed recovery of PSII activity. Thus in JA-Me-treated segments, ELIP expression is also expected to increase as a result of the considerably enhanced light stress. However, it is surprising that ELIP expression under HL treatment is substantially reduced at both the mRNA and protein levels. Thus JA-Me abolishes the correlation established so far between the extent of ELIP expression and the degree of light stress.
These unexpected results could be explained if one considers that the regulation of the ELIP level in plants exposed to light stress in the presence of JA-Me is the result of a competition between ELIP induction as a result of the light stress and inhibition of its expression by JA-Me.
The extent of repression by JA-Me increases with the incubation time and consequently the HL-induced transcript and protein amounts decrease during incubation despite an increase in light stress. However, in the presence of JA-Me, the HL-induced ELIP transcript level is considerably less reduced than those of LHC II and SSU, which are continuously abundant in the cell. Thus an increasing influence of the enhanced light stress on ELIP expression can be deduced indirectly. The remaining induction of ELIPs under light stress in the presence of JA-Me represents a positive transcription control showing that the control of gene expression by HL is still active.
JA-Me-treated leaves exposed to LL show symptoms which normally occur only under conditions of light stress, namely substantially reduced LHC II and SSU transcript and D1 protein levels. The reduction in D1 levels in JA-Me-treated leaf-segments might be due in part to the diminished carotenoid content as β-carotene is required for the assembly of D1 protein into functional PSII reaction centers. It was observed that in NF-bleached algal cells, the D1 protein was completely degraded after 1 h of HL (Trebst and Depka, 1997).
The correlation between Fv and the levels of D1 appears very peculiar. If one compares the situation in JA-Me-treated segments with the situation in the corresponding controls, one will observe that the reduction in D1 levels in the presence of JA-Me correlates well with a decrease in the Fv/Fm levels under HL but not under LL conditions (Figs. 1 and 4). When the water controls are compared with freshly detached segments, one will observe that despite a pronounced reduction in the D1 protein level under HL, the Fv/Fm levels under HL remain unchanged in the water controls.
A loss of chlorophylls, the repression of synthesis, and degradation of proteins of the photosynthetic apparatus are typical symptoms for JA-Me treatment in mature leaves. These symptoms resemble those of senescence and were interpreted accordingly. More recently, these effects have also been discussed as signs of dedifferentiation of mature leaves. As jasmonate levels were found to be high in young leaves as well as in dividing tissues, it was suggested that jasmonate in developing leaves might play a role in prevention of premature accumulation of photosynthetic structures and in support of accumulation of nutritive storage proteins for further leaf development. Application of jasmonates to mature leaves would then lead to dedifferentiation toward an earlier stage of leaf development (Creelman and Mullet, 1997). The juvenilization of plastids might include the degradation of proteins that function in photosynthesis.
Previous studies suggested that synthesis or accumulation of the nuclear-encoded JIPs might require the presence of functional chloroplasts (Weidhase et al., 1987a; Parthier, 1991). Plastidic factors (Surpin and Chory, 1997; Goldschmidt-Clermont, 1998) have been postulated to be degraded under light stress, so that in turn LHC II expression is repressed at the transcript level (Oelmüller, 1989; Taylor, 1989). The steady-state levels of JIP transcripts are reduced under HL treatment; for example, those of leaf thionin, JIP 23, JIP 37, and JIP 52 were examined. Thus it is conceivable that the presence of plastidic factors might also be required for JIP expression.
The combined effect of HL and JA-Me on the transcription machinery leads to a new gene expression pattern which is represented by the clear changes in the pattern of in vitro translation products of poly(A+)-rich RNA (Fig. 5). In untreated segments the predominant transcripts under LL conditions are those for SSU and LHC II. Under light stress conditions ELIP transcripts, especially those for small ELIPs, are predominant as ELIPs are induced while LHC II is strongly repressed and the light stress sensitivity of SSU increases with pre-incubation time. In JA-Me-treated segments, JIP transcripts, especially those of thionins, are predominant as they are induced while the expression of SSU and LHC II is repressed. Under HL treatment ELIP transcripts become next abundant to JIP transcripts but with a big difference as the HL-induced ELIP expression is strongly repressed in the presence of JA-Me. JIP transcripts are reduced by HL.
In conclusion, four different types of gene regulation by light stress and JA-Me can be distinguished: (a) negative regulation of photosynthesis-associated proteins by HL and JA-Me (LHC II, SSU, and D1), (b) positive regulation by JA-Me and light stress (chalcone synthase) (Feinbaum et al., 1991; Creelman et al., 1992), (c) positive regulation (induction) by HL but negative regulation by JA-Me (ELIPs), (d) positive regulation (induction) by JA-Me but negative regulation by HL (leaf thionin, JIP 23, JIP 37, and JIP 52). Thus it follows that the expression of ELIPs and leaf thionin is regulated antagonistically by light stress and JA-Me. The same holds also true for JIP 23, JIP 37, and JIP 52.
Therefore, ELIPs and leaf thionin are antagonistically regulated during greening of etiolated seedlings (Reimann-Philipp et al., 1989; Pötter and Kloppstech, 1993), during the day in green plants (Adamska et al., 1991; Beator and Kloppstech, 1996), and under light stress and in the presence of JA-Me, as is shown in this publication.
MATERIALS AND METHODS
Plant Growth, Application of JA-Me, and Light Treatment
Barley (Hordeum vulgare L. cv Apex) was grown on vermiculite for 7 d at 25°C under a light intensity of 100 μmol m−2 s−1 using a 12-h-light/12-h-dark cycle, starting the light at 8 am. Primary leaves of the same length were cut into 5-cm-long segments comprising the upper half of the leaf except the tip (1cm). Seventy-five segments were floated in Petri dishes on 200 mL of tap water or 45 μm of JA-Me (Firmenich Company, Geneva) dissolved in tap water and kept under the same growth conditions as before for 24, 36, or 72 h. Thereafter the segments were subjected to light treatments. Light-stress treatments with segments were performed at the indicated irradiances of white light as previously described (Pötter and Kloppstech, 1993) beginning at 8.30 am. At the end of the experiments the plant material was blotted on filter paper, frozen in liquid nitrogen, and stored at −70°C. Additional control segments were frozen after 7 d of growth and after 24 to 72 h of incubation in the presence or absence of 45 μm of JA-Me. The experiments presented in this study were repeated at least twice with the same outcome.
Plant Growth, Application of ABA and Cytokinin, and Light Treatment
The applications of the phytohormones ABA and cytokinin and light treatment were similar to those used in the studies with JA-Me but with the following modifications. ABA and cytokinin were dissolved in ethanol and added to tap water in concentrations of 1 mm ABA (cis/trans-isomer) or 2 mm cytokinin (6-benzylaminopurin). Controls received the same amount of ethanol (1% [v/v]). Thereafter segments were subjected to light treatment.
Chlorophyll Fluorescence Measurements
Fluorescence induction kinetics were measured using a pulse amplitude modulation fluorometer (Walz, Effeltrich, Germany). The leaves were placed between two glass plates and exposed to light through an aperture of 5 mm in diameter at a position 1 cm below the upper end of the segment (2 cm below the former leaf tip). The yield of minimal fluorescence (F0) was measured with pulse amplitude modulated light of low intensity that is not sufficient to produce any photosynthetic activity or significant variable fluorescence (Fv). The maximal fluorescence (Fm) was determined by application of a saturating white light flash (10,000 μmol m−2 sec−1) of 1-s duration. The photochemical efficiency of PSII (Adams et al., 1990) was calculated as the ratio Fv/Fm = (Fm − F0)/Fm for each segment. The presented data are arithmetic mean values of Fv/Fm based on the average of 10 measurements of independent segments per time point.
Analysis of RNA
Isolation of RNA
Poly(A)-rich mRNA from barley leaf segments was isolated using SDS/proteinase K extraction and oligo(dT)-cellulose chromatography (Pemberton et al., 1975) as described by Pötter and Kloppstech (1993).
Dot-Blot Analysis
Dot blotting of poly(A)-rich mRNA on replica filters and hybridization using 32P-labeled random-primed homologous cDNA inserts were performed as described by Kloppstech (1985). Quantification of signals on autoradiographs obtained from hybridized filters was carried out by densitometry using a scanner (Scanjet II cx; Hewlett-Packard, Palo Alto, CA) and the Scanpack-Software version 1.0, 1999 (Biometra, Goettingen, Germany). Autoradiography signals that were linear in intensity with the time of exposure and the amounts of RNA were scanned and the results calculated as “% of maximum” of the strongest signal of each experimental series. Signals were corrected for background noise. The following 32P-labeled cDNA probes were used: ELIP of low (EL = pHV90; Grimm et al., 1989) and high (EH = pHV8F6; Kruse and Kloppstech, 1992) molecular mass, SSU (pKG4626; Barkadottir et al., 1987), LHC II (pKG1490; Barkadottir et al., 1987), and thionin (pKG1348; Gausing, 1987).
Translation in Vitro
Poly(A)-rich mRNA was translated in a wheat-germ system in the presence of [35S]-Met according to Roberts and Paterson (1973). The translation products were separated on 12.5% (v/v) SDS/polyacrylamide gels (Neville, 1971) and analyzed by fluorography (Bonner and Laskey, 1974).
Protein Analysis
Protein Extraction and Gel Electrophoresis
For total protein extractions, leaf segments (0.5 g each) were homogenized in 5 mL of sample buffer (56 mm Na2CO3, 56 mm dithiothreitol, 2% [w/v] SDS, 12% [w/v] Suc, and 2 mm EDTA) in an all-glass potter homogenizer. After heating the suspensions to 70°C for 30 min, cellular debris was removed by centrifugation and equal amounts of supernatant protein were analyzed using SDS-PAGE (Neville, 1971). The protein concentration was determined by the method of Lowry et al. (1951). After electrophoresis the gels were stained with Coomassie Blue or blotted.
Immunoblotting
For western blots, proteins were transferred onto polyvinylidene difluoride membranes according to Towbin et al. (1979). After incubation with primary antibody, immunoreactive bands were visualized using peroxidase coupled with anti-rabbit serum with chemiluminescence detection (enhanced chemiluminescent; Amersham, Buckinghamshire, UK).
ACKNOWLEDGMENTS
We thank Professor I. Ohad (Hebrew University, Jerusalem) for critical comments and discussion of the manuscript. We thank Dr. Kirsten Gausing (University of Aarhus, Denmark) for providing homologous cDNA clones of SSU, LHC II, and thionin of barley. We are also grateful to Dr. Klaus Apel (Eidgenössische Technische Hochschule, Zürich) and Dr. Joseph Hirschberg (Department of Genetics, Hebrew University) for the gift of the antibodies against leaf thionin and D1 protein, respectively.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft, Bonn.
LITERATURE CITED
- Adams WW, Demming-Adams B, Winter K, Schreiber U. The ratio of variable to maximum chlorophyll fluorescence from photosystem II, measured in leaves at ambient temperature and at 77K, as an indicator of the photon yield of photosynthesis. Planta. 1990;180:166–174. doi: 10.1007/BF00193991. [DOI] [PubMed] [Google Scholar]
- Adamska I. ELIPs: light-induced stress proteins. Physiol Plant. 1997;100:794–805. [Google Scholar]
- Adamska I, Kloppstech K, Ohad I. UV light stress induces the synthesis of the early light-inducible protein and prevents its degradation. J Biol Chem. 1992b;267:24732–24737. [PubMed] [Google Scholar]
- Adamska I, Kloppstech K, Ohad I. Early light-inducible protein in pea is stable during light stress but is degraded during recovery at low light intensity. J Biol Chem. 1993;268:5438–5444. [PubMed] [Google Scholar]
- Adamska I, Ohad I, Kloppstech K. Synthesis of the early light-inducible protein is controlled by blue light and related to light stress. Proc Natl Acad Sci USA. 1992a;89:2610–2613. doi: 10.1073/pnas.89.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamska I, Roobol-Boza M, Lindahl M, Andersson B. Isolation of pigment-binding early light inducible proteins from pea. Eur J Biochem. 1999;260:453–460. doi: 10.1046/j.1432-1327.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- Adamska I, Scheel B, Kloppstech K. Circadian oscillations of nuclear-encoded chloroplast proteins in pea (Pisum sativum) Plant Mol Biol. 1991;17:1055–1065. doi: 10.1007/BF00037144. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Chow WS, Pork YI. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- Andersson B, Salter AH, Virgin DJ, Vass I, Styring S. Photodamage to photosystem II-primary and secondary events. J Photochem Photobiol. 1992;15:15–31. [Google Scholar]
- Andersson B, Styring S. Photosystem II. Molecular organization function and acclimation. Curr Topics Bioenerg. 1991;16:1–81. [Google Scholar]
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II. Inactivation protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. doi: 10.1016/0005-2728(93)90134-2. [DOI] [PubMed] [Google Scholar]
- Barber J, Andersson B. Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci. 1992;17:61–66. doi: 10.1016/0968-0004(92)90503-2. [DOI] [PubMed] [Google Scholar]
- Barkadottir RB, Jensen BF, Kreiberg JD, Nielsen PS, Gausing K. Expression of selected nuclear genes during leaf development in barley. Dev Genet. 1987;8:495–511. [Google Scholar]
- Beator J, Kloppstech K. Significance of circadian gene expression in higher plants. Chronobiol Int. 1996;13:319–339. doi: 10.3109/07420529609012657. [DOI] [PubMed] [Google Scholar]
- Becker W, Apel K. Isolation and characterization of a cDNA encoding a novel jasmonate-induced protein of barley (Hordeum vulgare L) Plant Mol Biol. 1992;19:1065–1067. doi: 10.1007/BF00040538. [DOI] [PubMed] [Google Scholar]
- Bonner WM, Laskey RA. A film detection method for tritium labeled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974;46:83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chamovitz D, Pecker I, Sandmann G, Böger P, Hirschberg J. Cloning a gene for norflurazon resistance in cyanobacteria. Z Naturforsch. 1990;45:482–486. doi: 10.1515/znc-1990-0531. [DOI] [PubMed] [Google Scholar]
- Chaudry B, Müller-Uri F, Cameron-Mills V, Gough S, Simpson D, Skriver K, Mundy J. The barley 60 kDa jasmonate-induced protein (JIP60) is a novel ribosome inactivating protein. Plant J. 1994;6:815–824. doi: 10.1046/j.1365-313x.1994.6060815.x. [DOI] [PubMed] [Google Scholar]
- Chory J. Plant phototransduction: phytochrome signal transduction. Curr Biol. 1994;4:844–846. doi: 10.1016/s0960-9822(00)00189-5. [DOI] [PubMed] [Google Scholar]
- Chory J, Chatterjee M, Cook RK, Elich T, Fankhauser C, Li J, Nagpal P, Neff M, Pepper A, Poole D, Reed J, Vitart A. From seed germination to flowering: light controls development via the pigment phytochrome. Proc Natl Acad Sci USA. 1996;93:12066–12071. doi: 10.1073/pnas.93.22.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet J. Biosynthesis and action of Jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demming-Adams B. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta. 1990;1020:1–24. [Google Scholar]
- Demming-Adams B. Carotenoids 3: in vivo function of carotenoids in higher plants. FASEB J. 1996;10:403–412. doi: 10.1096/fasebj.10.4.8647339. [DOI] [PubMed] [Google Scholar]
- Fankhauser C, Chory J. Light control of plant development. Annu Rev Cell Dev Biol. 1997;13:203–229. doi: 10.1146/annurev.cellbio.13.1.203. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinbaum RL, Storz G, Ausubel FM. High intensity and blue light regulated expression of chimeric chalcone synthase genes in transgenic Arabidopsis thaliana plants. Mol Gen Genet. 1991;226:449–456. doi: 10.1007/BF00260658. [DOI] [PubMed] [Google Scholar]
- Gausing K. Thionin genes specifically expressed in barley leaves. Planta. 1987;171:241–246. doi: 10.1007/BF00391100. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. Coordination of nuclear and chloroplast gene expression. Int Rev Cytol. 1998;177:115–180. doi: 10.1016/s0074-7696(08)62232-9. [DOI] [PubMed] [Google Scholar]
- Green B, Pichersky E, Kloppstech K. Chlorophyll a/b-binding proteins: an extended family. Trends Biochem Sci. 1991;16:181–186. doi: 10.1016/0968-0004(91)90072-4. [DOI] [PubMed] [Google Scholar]
- Grimm B, Kruse E, Kloppstech K. Transiently expressed early light-inducible proteins share transmembrane domains with light-harvesting chlorophyll binding protein. Plant Mol Biol. 1989;13:583–593. doi: 10.1007/BF00027318. [DOI] [PubMed] [Google Scholar]
- Humbeck K, Kloppstech K, Krupinska K. Expression of early light-inducible proteins in flag leaves of field grown barley. Plant Physiol. 1994;105:1217–1222. doi: 10.1104/pp.105.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppstech K. Diurnal and circadian rhythmicity in the expression of light-induced plant nuclear messenger RNAs. Planta. 1985;165:502–506. doi: 10.1007/BF00398095. [DOI] [PubMed] [Google Scholar]
- Kloppstech K. Light regulation of photosynthetic genes (Minireview) Physiol Plant. 1997;100:739–747. [Google Scholar]
- Kruse E, Kloppstech K. Integration of early light-inducible proteins into isolated thylakoid membranes. Eur J Biochem. 1992;208:195–202. doi: 10.1111/j.1432-1033.1992.tb17174.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr Al Randall RJ. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mattoo AK, Hoffman-Falk H, Marder JB, Edelman M. Regulation of protein metabolism: coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32 kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci USA. 1984;81:1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Creelman RA, Bell E, Mullet JE, Browse J. Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci USA. 1997;94:5473–5477. doi: 10.1073/pnas.94.10.5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montané MH, Tardy F, Kloppstech K, Havaux M. Differential control of xanthophylls and light-induced stress proteins as opposed to light-harvesting chlorophyll a/b protein during photosynthetic acclimation of barley leaves to light irradiance. Plant Physiol. 1998;118:227–235. doi: 10.1104/pp.118.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Uri F, Parthier B, Nover L. Jasmonate-induced alteration of gene expression in barley leaf segments analyzed by in vivo and in vitro protein synthesis. Planta. 1988;176:241–247. doi: 10.1007/BF00392451. [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Bowler C. Tuning in to the signals controlling photoregulated gene expression in plants. EMBO J. 1997;16:5801–5806. doi: 10.1038/sj.emboj.7590554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville DM. Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971;246:6328–6334. [PubMed] [Google Scholar]
- Oelmüller R. Photooxidative destruction of chloroplasts and its effect on nuclear gene excpression and extraplastidic enzyme level. Photochem Photobiol. 1989;49:229–239. [Google Scholar]
- Parthier B. Jasmonates: hormonal regulators or stress factors in leaf senescence? J Plant Growth Regul. 1990;9:1–7. [Google Scholar]
- Parthier B. Jasmonates new regulators of plant growth and development: many facts and few hypotheses on their actions. Bot Acta. 1991;104:446–456. [Google Scholar]
- Pemberton RE, Liberty PA, Baglioni C. Isolation of messenger RNA from polysomes by chromatography on oligo(dT)-cellulose. Anal Biochem. 1975;66:18–26. doi: 10.1016/0003-2697(75)90720-4. [DOI] [PubMed] [Google Scholar]
- Pötter E. Untersuchungen zur Genexpression der frühen lichtinduzierbaren Proteine der Gerste (Hordeum vulgare). PhD thesis. Hannover, Germany: University of Hannover; 1994. [Google Scholar]
- Pötter E, Kloppstech K. Effects of light stress on the expression of early light-inducible proteins in barley. Eur J Biochem. 1993;214:779–786. doi: 10.1111/j.1432-1033.1993.tb17980.x. [DOI] [PubMed] [Google Scholar]
- Prasil O, Adir N, Ohad I. Dynamics of photosystem II. Mechanism of photoinhibition and recovery processes. In: Barber J, editor. Topics in Photosynthesis. Vol. 43. Amsterdam: Elsevier Science Publishers; 1992. pp. 295–348. [Google Scholar]
- Reimann-Philipp U, Behnke S, Batschauer A, Schäfer E, Apel K. The effect of light on biosynthesis of leaf-specific thionins in barley (Hordeum vulgare) Eur J Biochem. 1989;182:283–289. doi: 10.1111/j.1432-1033.1989.tb14828.x. [DOI] [PubMed] [Google Scholar]
- Reinbothe C, Parthier B, Reinbothe S. Temporal pattern of jasmonate-induced alterations in gene expression of barley leaves. Planta. 1997;201:281–287. doi: 10.1007/s004250050067. [DOI] [PubMed] [Google Scholar]
- Reinbothe S, Mollenhauer B, Reinbothe C. JIPs and RIPs: The regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell. 1994a;6:1197–1209. doi: 10.1105/tpc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Heintzen C, Seidenbrecher C, Parthier B. A methyl jasmonate-induced shift in the length of the 5′ untranslated region impairs translation of the plastid rbcL transcript in barley. EMBO J. 1993b;12:1505–1512. doi: 10.1002/j.1460-2075.1993.tb05794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Lehmann J, Becker W, Apel K, Parthier B. JIP 60 a methyl jasmonate-induced ribosome-inactivating protein involved in plant stress reactions. Proc Natl Acad Sci USA. 1994b;91:7012–7016. doi: 10.1073/pnas.91.15.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Lehmann J, Parthier B. Differential accumulation of methyl jasmonate-induced mRNAs in response to abscisic acid and desiccation in barley (Hordeum vulgare) Physiol Plant. 1992;86:49–56. [Google Scholar]
- Reinbothe S, Reinbothe C, Parthier B. Methyl jasmonate-regulated translation of nuclear-encoded choroplast proteins in barley. J Biol Chem. 1993a;268:10606–10611. [PubMed] [Google Scholar]
- Reinbothe S, Reinbothe C, Parthier B. Methyl jasmonate represses translation initiation of a specific set of mRNAs in barley. Plant J. 1993c;4:459–467. [Google Scholar]
- Roberts BE, Paterson BM. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci USA. 1973;70:2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:569–589. [Google Scholar]
- Surpin M, Chory J. The co-ordination of nuclear and organellar genome expression in eukaryotic cells. Essays Biochem. 1997;32:113–125. [PubMed] [Google Scholar]
- Taylor WC. Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:211–233. [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamid gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trebst A, Depka B. Role of carotene in the rapid turnover and assembly of photosystem II in Chlamydomonas reinhardtii. FEBS Lett. 1997;424:267–270. doi: 10.1016/s0014-5793(96)01419-6. [DOI] [PubMed] [Google Scholar]
- Weidhase RA, Kramell HM, Lehmann J, Liebisch HW, Lerbs W, Parthier B. Methyl jasmonate-induced changes in the polypeptide pattern of senescing barley leaf segments. Plant Sci. 1987a;51:177–186. [Google Scholar]
- Weidhase RA, Lehmann J, Kramell H, Sembdner G, Parthier B. Degradation of ribulose-15-bisphosphate-carboxylase and chlorophyll in senescing barley leaf segments triggered by jasmonic acid methyl ester and counteraction by cytokinin. Physiol Plant. 1987b;69:161–166. [Google Scholar]