Abstract
Background
Whole lung lavage is the current standard therapy for pulmonary alveolar proteinosis (PAP) that is characterized by the alveolar accumulation of surfactant. Rituximab showed promising results in auto-immune PAP (aPAP) related to anti-GM-CSF antibody.
Methods
We aimed to assess efficacy of rituximab in aPAP in real life and all patients with aPAP in France that received rituximab were retrospectively analyzed.
Results
Thirteen patients were included. No patients showed improvement 6 months after treatment, but, 4 patients (30%) presented a significant decrease of alveolar-arterial difference in oxygen after 1 year. One patient received lung transplantation and one patient was lost of follow-up within one year. Although a spontaneous improvement cannot be excluded in these 4 patients, improvement was more frequent in patients naïve to prior specific therapy and with higher level of anti-GM-CSF antibodies evaluated by ELISA. No serious adverse event was evidenced.
Conclusions
These data do not support rituximab as a second line therapy for patients with refractory aPAP.
Keywords: Whole lung lavage, GM-CSF, Surfactant, Interstitial lung disease, Therapy
Background
Pulmonary alveolar proteinosis (PAP) is a rare disease characterized by deposition of extracellular lipoproteinaceous material within pulmonary alveoli [1]. PAP usually results from failure of clearance of surfactant by alveolar macrophages [2–4]. The most common cause of PAP is autoimmune (in 90% of cases, aPAP) relevant to the presence of anti-GM-CSF (Granulocyte-macrophage colony-stimulating factor) autoantibodies. GM-CSF, a growth factor for granulocytes and monocytes, stimulates the differentiation, the proliferation and the survival of myeloid cells [5]. Anti-GM-CSF antibodies bind with high affinity to GM-CSF thus blocking receptor binding and its specific activity [6–8]. Alveolar macrophages are then no longer able to clear alveolar surfactant, and also have a poor local anti-infectious activity [9]. The pathogenicity of anti-GM-CSF antibodies has been proven by the development of PAP after administration of anti-GM-CSF antibodies to non human primates [10, 11].
Whole lung lavage is the current standard therapy for PAP and has been shown to improve the prognosis. Whole lung lavage is effective in almost 85% of patients [12]. However, whole lung lavage is associated with adverse effects such as infections, fever, convulsions, pneumothorax, pleural effusion, hypoxemia or even death. Moreover, 15% of patients do not improve and 10% of patients need repeated whole lung lavage [13–19]. Corticosteroids are not effective and may be associated with an increased risk of opportunistic infection [20]. Inhaled or sub-cutaneous GM-CSF is currently investigated as an alternative to therapeutic lung lavage (NCT02702180). Retrospective and prospective data suggest a persistent improvement in 48% to 100% of the cases [21–23]. Rituximab, a monoclonal antibody directed against the CD20 antigen, has been shown to be effective in several autoimmune diseases such as rheumatoid arthritis or granulomatosis with polyangiitis [24–26]. Rituximab has been reported to improve aPAP in 3 isolated cases and in 7/9 patients of a prospective series [27–30]. While, rituximab showed early promising results and appears to be an interesting alternative treatment, we aimed to evaluate its efficacy on aPAP in real life.
Methods
Study design
We retrospectively identified all French patients with aPAP who received rituximab between 2007 and 2014, from a mailing list to the French network of competence and reference centers for rare pulmonary diseases. In France, almost all of the patients with rare lung diseases, and particularly with aPAP, are referred to one of the competence/reference center.
All patients gave informed consent for data collection and the use of rituximab beyond marketing authorization. The Institutional Review Board of the French-learned society for respiratory medicine (Société de Pneumologie de Langue Française) approved this retrospective study (CEPRO 2012–016).
Inclusion criteria
The patients were included in the study if 1) they had evidence for PAP as assessed by bronchoalveolar lavage (BAL), transbronchial biopsy or open lung biopsy; and 2) an anti-GM-CSF antibody was detected; and 3) they had received at least one dose of rituximab.
Data collection
The clinical charts of the patients were reviewed and the following data were collected by use of a standardized and anonymous collection form: at the first rituximab infusion (day 0 or baseline), and at day 14, at months 3 (M3), M6, M9, M12. We collected clinical data, standard biological tests results, anti-GM-CSF antibodies titer, chest high-resolution computed tomography (CT), and results of lung function tests, and blood gases when available.
Anti-GM-CSF titer
GM-CSF neutralizing activity of serum was evaluated in a functional bioassay [4]. Briefly, the GM-CSF-dependent TF-1 cell line was incubated with serial serum dilutions in the presence of 1 ng/mL recombinant GM-CSF (Cellgenix, Germany). After 48 h incubation, 3H-thymidine was added for further 8 h. Thymidine uptake by TF-1 cells was assessed by quantification of the radioactivity with a scintillation counter. The GM-CSF antibody titer was calculated as the inverse of the serum dilution blocking 50% of the maximum TF-1 proliferation.
In addition, in some patients, the serum GM-CSF antibody concentration was assessed by ELISA as previously described [4, 31, 32], with some modifications. Briefly, 96-wells Maxisorp® plates (Corning, USA) were coated overnight with 1 μg/mL GM-CSF in phosphate buffer saline (PBS) then washed with PBS-0.05% Tween and blocked with PBS-1% bovine serum albumin for 2 h. After 3 washing steps, diluted sera were added to the wells and incubated for 40 min at room temperature. Then wells were washed 5 times with PBS-0.1% Tween and bound GM-CSF antibodies were detected by anti-human IgG biotinylated antibody (BD Pharmingen, USA) combined to streptavidin conjugated to horseradish peroxidase. Chromogenic substrate solution (TMB Substrate Reagent, BD Pharmingen) was added. Optical absorbance was measured at 450 nm. Anti-GM-CSF concentration was calculated using a standard calibration curve obtained with human polyclonal intravenous immunoglobulins.
All assays were done in one single expert center. Functional assays were done at time of blood taking, while ELISA measurements were done for all samples at the same time.
Chest CT analysis
Chest CT obtained before (baseline), or 6 months and 12 months after rituximab infusion, were anonymously analyzed by an experienced thoracic radiologist (MPD), patient by patient, blinded to the time from treatment. The extent of CT reticulations and ground-glass opacities was scored between 6 and 30 by summing the grades of 6 pulmonary zones (adapted from [33]). The more extended the CT lesions, the higher the score.
Assessments
Improvement after rituximab therapy was defined by a decrease ≥10 mmHg of the alveolar–arterial gradient at rest (D(A-a)O2) as compared to baseline. A deterioration was defined by an increase ≥10 mmHg of the D(A-a)O2.
Other endpoints considered as significantly improved were: ≥ 10 mmHg increase of arterial partial pressure of oxygen (PaO2), ≥ 10% increase in diffusing capacity of the lung for carbon monoxide (DLCO) or absolute value of vital capacity (VC), ≥ 50% decrease in GM-CSF antibody level, ≥ 3 points decrease in CT-grade. The disease severity score (DSS) was assessed as follow: DSS 1: no symptoms and PaO2 ≥ 70 mmHg, DSS 2: symptomatic and PaO2 ≥ 70 mmHg, DSS 3: PaO2 ≥ 60 mmHg and < 70 mmHg, DSS 4: PaO2 ≥ 50 mmHg and < 60 mmHg and DSS 5: PaO2 < 50 mmHg [22].
Statistical analysis
Continuous data are presented as mean (minimum-maximum) and compared by the Mann-Whitney’s test. Categorical data are presented as numbers (%) and compared by the Fisher’s test. All tests were bilateral with a p < 0.05 considered as significant. All analyses were performed with the GraphPAd prism software (La Jolla, USA).
Results
Patients
We identified a total of 13 patients (9 men) from 11 centers, including one patient previously reported [28] (Table 1). The mean age at aPAP diagnosis was 46 years and the mean time since diagnosis was 28.5 months. Seven patients (54%) were smokers, including 4 active smokers, and 8 patients (62%) had been exposed to inhaled toxics.
Table 1.
Patients characteristics at inclusion
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (year) | 44 | 56 | 50 | 30 | 53 | 41 | 49 | 44 | 59 | 41 | 51 | 49 | 45 |
| Gender | M | M | M | F | M | F | M | F | F | M | M | M | M |
| Duration of PAP (months) | 38 | 12 | 12 | 29 | 64 | 50 | 6 | 32 | 9 | 15 | 45 | 20 | 51 |
| Smoking status | Never | Never | Current | Current | Never | Never | Never | Never | Ex | Current | Ex | Ex | Current |
| Inhaled toxic | No | No | Yes | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Yes | No |
| Previous therapy | No | WLL GM-CSF |
WLL | WLL | WLL GM-CSF |
WLL GM-CSF |
No | WLL GM-CSF |
WLL | WLL | WLL GM-CSF |
WLL | No |
| Initial Improvement with WLL | Np | No | No | Yes | Yes | Yes | Np | Yes | No | No | No | Yes | Np |
| Aggravation in the 3 month before RTX | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Disease severity score | 3 | 2 | 2 | 2 | 2 | 4 | 3 | 4 | 2 | 2 | 3 | 2 | 3 |
| Anti GM-CSF titer before RTX | 10,000 | 480 | 400 | 700 | 1000 | 200 | 400 | 400 | 40 | 300 | 300 | 800 | 4000 |
| D(a-A)O2 | |||||||||||||
| At RTX injection | 36 | 36 | 40 | 38 | 31 | 50 | 46 | 57 | 37 | 35 | 47 | 35 | 40 |
| At M12 | 20 | 32 | 60 | 51 | 30 | Na | 35 | 60 | 38 | Na | 15 | 26 | 27 |
PAP Pulmonary alveolar proteinosis, M Male, F Female, WLL Whole Lung lavage, RTX Rituximab, Na Not available, Np not performed
For patients without dosage at rituximab injection, the last available concentration of anti-GM-CSF is reported
Prior to rituximab, 10 patients (77%) were treated with whole lung lavage, with a mean of 2.8 lavages and a median of 2.0 lavages per patient. No patient received whole lung lavage within 3 months before inclusion. Five patients (38%) had received GM-CSF prior to rituximab, including one who was still treated by inhaled GM-CSF at first infusion of rituximab.
Rituximab therapy and efficacy
Twelve patients received two infusions of rituximab (1000 mg) (at day 0 and day 14) among whom three received a third infusion at month 6, 9 and 12 respectively. One patient received only one infusion of rituximab (1000 mg) and was lost to follow-up after month 3. One patient was treated with plasma exchange one month after enrollment, and then received a lung transplant. Eleven patients were still followed from M3 (Table 2).
Table 2.
Characteristics at inclusion and after rituximab
| Mean values | At Rituximab injection | After rituximab | p-value* | ||
|---|---|---|---|---|---|
| M3 | M6 | M12 | |||
| Number of patients | 13 | 11 | 11 | 11 | |
| D(A-a)O2 (mmHg) | 40 (31–57) | 43 (34–60) | 40 (31–56) | 35.9 (15–60) | 0.19 |
| PaO2 (mmHg) | 68 (57–82) | 63 (55–70) | 65 (52–78) | 70 (49–92) | 0.51 |
| Disease Severity Score | 2.7 (2–4) | 3.0 (2–4) | 2.8 (2–4) | 2.3 (1–5) | 0.44 |
| VC (%predicted value) | 79 (36–100) | 78 (61–87) | 79 (50–100) | 84 (62–102) | 0.81 |
| DLCO (% predicted value) | 54 (19–92) | 46 (30–86) | 50 (29–79) | 63 (35–97) | 0.38 |
| Serum anti-GM-CSF | |||||
| ELISA (μg/mL)/Available | 99 (18–220)/8 | Na | 85 (19–187)/7 | 7.7 (3.1–158)/6 | 0.19 |
| Functional (titer)/Available | 853 (200–4000)/9 | Na | 831 (30–4000)/11 | 436 (0–1400)/8 | 0.21 |
| CT-grade | 19.3 (12–28) | Na | 19.3 (13–28) | 17.3 (6–26) | 0.73 |
*between at rituximab injection and M12, VC Vital capacity, DLCO Diffusing capacity of the lung for carbon monoxide, CT Computed tomography, D(A-a)O2 alveolar–arterial gradient at rest, na not available
In the whole population, rituximab was not associated with significant improvement in any of the endpoints at M3, M6 or M12 (Table 2). However when assessed individually some patients did improve (Fig. 1).
Fig. 1.
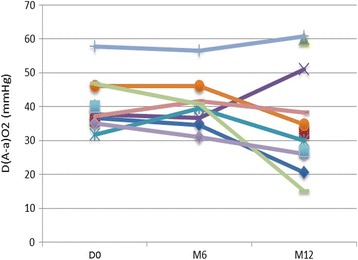
Changes in D(A-a)O2 before (D0) and 6 (M6) et 12 (M12) months after rituximab therapy
At M6 after rituximab infusion, 3 patients deteriorated their lung function and required whole lung lavage or GM-CSF therapy. D(A-a)O2, PaO2 or VC was not improved in any patient. DLCO improved in one patient and CT scan improved in two patients.
At M12 after rituximab infusion, a significant improvement of D(A-a)O2 was observed in 4/11 patients (36%, none of them had received any additional therapy after rituximab), while D(A-a)O2 was stable in 5 patients (45%, including 2 patients who received specific aPAP therapies because of an early deterioration) and decreased in 2 patients (18%). In the improved group, the mean D(A-a)O2 decreased from 42.4 mmHg before infusion to 24.3 mmHg at M12 and the mean PaO2 get from 64.3 mmHg before infusion to 82.8 mmHg at M12 (Table 3). In parallel, at M12, VC increased in 1/10 patients (10%), DLCO in 5/10 patients (50%), and CT score decreased in 4/9 patients (44%). Three patients had both increased DLCO and D(A-a)O2 and decreased CT score.
Table 3.
Factors associated with therapeutic response in patients evaluated at M12
| Improved (n = 4) | Non-improved (n = 7) | p-value | |
|---|---|---|---|
| Age | 47 (41–51) | 48 (30–59) | 0.70 |
| Gender male | 4 (100%) | 4 (57%) | 0.24 |
| Toxics inhalation or significant smoking | 3 (75%) | 5 (71%) | 1.0 |
| Prior treatments to rituximab | 1 (25%) | 7 (100%) | 0.02 |
| Severity | |||
| PaO2 (mmHg) | 64.3 (63–66) | 70.7 (57–82) | 0.07 |
| D(A-a)O2 (mmHg) | 42.4 (36.7–46) | 39.4 (31.7–57.7) | 0.32 |
| VC (%) | 94.3 (85–105) | 83.0 (54–95) | 0.92 |
| DLCO (%) | 49.0 (19–64) | 56.9 (38–92) | 0.30 |
| CT-grade | 17.8 (12–23) | 20.1 (14–28) | 0.50 |
| Serum anti-GM-CSF | |||
| ELISA (μg/mL) | 169.3 (78–220) | 58.2 (18–104) | 0.03 |
| Functional (titer) | 1566.7 (300–4000) | 496.7 (200–800) | 1.0 |
VC Vital capacity, DLCO Diffusing capacity of the lung for carbon monoxide, CT Computed tomography, D(A-a) O2 alveolar–arterial gradient at rest
Evolution of anti-GM-CSF titers
Data were both available at rituximab injection and at M12 for 6 patients evaluated by the functional assay and 4 patients evaluated by ELISA.
Globally, the mean level of anti-GM-CSF antibodies was not significantly modified before and after rituximab, evaluated either by ELISA or by functional assay (Table 2). However, 4 patients (67%) showed a significant decrease (≥ 50%) of anti-GM-CSF antibody titers at M12 as evaluated by the functional assay, among which 2 showed an improvement of D(A-a)O2, while 1 patient (25%) showed a significant decrease of anti-GM-CSF antibody titers as evaluated by ELISA, and this patient did not experience improvement of D(A-a)O2.
Characteristics of patients who improved after rituximab
We assessed the characteristics of patients who improved defined by a decrease ≥10 mmHg of the alveolar–arterial gradient at rest (D(A-a)O2) at M12 as compared to baseline. The patients who improved were four men aged 45 to 51 years. Three were current or ex-smokers and one of them was exposed to wool dusts and bird feathers. There was no significant difference between improved and non-improved patients on age, gender, smoking history or toxic inhalation, and disease severity at rituximab initiation. Patients who improved had higher serum titers of anti-GM-CSF antibodies evaluated by ELISA, received less specific aPAP therapies prior to rituximab and tended to have lower PaO2 at inclusion (Table 3). None of the patients who improved received adjuvant PAP therapy in the 12 months following rituximab infusion.
Adverse events
No serious adverse events related to rituximab were identified. A cutaneous rash or pruritus occurred after the first rituximab administration in 3 patients, and spontaneously resolved. One patient developed deep-vein thrombosis and one patient complained of sleep and concentration troubles. During follow-up, 2 patients developed viral upper respiratory tract infection and one patient was given a diagnosis of Mycobacterium avium airway colonization. No opportunistic infection was observed.
Discussion
This series is the largest series evaluating rituximab in aPAP. Rituximab was well tolerated and adverse effects were rare and minor. Rituximab was associated with an objective improvement in only 4 of the 13 patients treated (30%) after 12 months. Interestingly, improvement only occurred after 6 months of treatment and was more frequent in patients naive of specific aPAP therapy and with higher level of GM-CSF auto-antibodies evaluated by ELISA.
One prospective clinical trial by Kavuru et al., which included 9 aPAP patients, reported a 78% objective response at M12 [29]. In the current series, the absence of a significant improvement after rituximab in the whole population and the 30% objective response were disappointing. Numerous hypotheses may explain this discrepancy between both series. Firstly, the patients included in the current series were less hypoxemic (mean initial PaO2: 68 mmHg) than those studied by Kavuru et al. (PaO2: 54 mmHg) [29]. Interestingly, we observed that patients who improved had lower initial PaO2 than patients who did not improve (p = 0.07). Secondly, in the current series, patients who already received a specific therapy for aPAP were less prone to respond, although patients from the Kavuru et al. trial had received a mean of 5 whole lung lavage before inclusion [29]. Some of the patients from the present series may present irreversible interstitial lung disease or pulmonary fibrosis, although we were not able to identify them by CT scan [34].
Our study showed that the improvement of the D(A-a)O2 was delayed after rituximab infusion, as it was only evidenced at M12 while it was absent at M6. Again, our results are discordant with Kavuru et al. who showed an improvement of D(A-a)O2, PaO2, pulmonary function tests and CT-scan from M6 [29]. Such a delayed efficacy of rituximab is not reported in other autoimmune diseases such as autoimmune hemolytic anemia or ANCA associated vasculitis. This delayed effect could be due to 1) the time needed to restore a normal alveolar macrophagic activity, that may be delayed from circulating monocyte activity and 2) to the time needed to evidence a functional effect of an improved alveolar surfactant clearance [35].
We cannot exclude that rituximab was not responsible for the improvement observed in 4 patients at M12, since spontaneous improvement has been reported in up to 30% of cases in the literature. Spontaneous improvement is mostly observed in patients with PaO2 > 70 mmHg, D(A-a)O2 < 40 mmHg and with higher DLCO [13, 17, 19, 36]. In the current series, mean initial PaO2 in the responder group was 64 mmHg, D(A-a)O2 was 42 mmHg, and DLCO was 49%.
This study has obvious limits such as its uncontrolled and retrospective nature and the relatively small number of patients studied. However, all French patients that received rituximab for aPAP were included in this study and therefore the 30% in intention to treat rate of response is a real life evaluation of rituximab efficacy in this very rare disease.
Conclusions
This retrospective study does not support rituximab as a second line therapy for patients with refractory aPAP. Since whole lung lavage and inhaled GM-CSF are already very effective for this rare disease, the evaluation of rituximab as a first line therapy is unlikely due to its unconfirmed slowly developing effect.
Acknowledgments
Funding
This work was supported by a grant from La Fondation du souffle. The funding institution had no role in (1) conception, design, or conduct of the study, (2) collection, management, analysis, interpretation, or presentation of the data, or (3) preparation, review, or approval of the manuscript.
Availability of data and materials
All data are available on request.
Authors’ contributions
RB and BC study concept and design. DBS, RB, CM, JC, LC, VC, EG, SMA, CHM, JMN, HN, MRG, LS, AT, LWS, MPD and BC acquisition of the data. BS, RB, CM and BC data analysis and interpretation. BS, RB, CM and BC manuscript preparation and drafting. BS and RB statistical methods, statistical data analysis. All authors made manuscript critique, review and approved the manuscript submitted. BS, RB and BC take responsibility for the integrity of the work as a whole.
Ethics approval and consent to participate
The Institutional Review Board of the French-learned society for respiratory medicine (Société de Pneumologie de Langue Française) approved this retrospective study (CEPRO 2012–016).
Consent for publication
All patients consent to publication.
Competing interests
RB reports personal fees and grants from Savarapharma, Roche and Boerhinger Ingelheim. JC reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from Roche, grants and personal fees from Astra Zeneca, grants and personal fees from Pfizer, personal fees from Lilly, personal fees from BMS, outside the submitted work;. VC reports personal fees from Actelion, personal fees from Boehringer Ingelheim, personal fees from Bayer, personal fees from Biogen Idec, personal fees from Gilead, personal fees from GSK, personal fees from MSD, personal fees from Novartis, personal fees from Roche, personal fees from Sanofi, grants from Boehringer Ingelheim, grants from Roche, personal fees from Promedior, personal fees from Celgene, personal fees from Galapagos, outside the submitted work. EG reports non financial support from Actélion, Roche, Boehringer Ingelheim. LS reports grants, personal fees and non-financial support from Actelion, grants, personal fees and non-financial support from Bayer, grants, personal fees and non-financial support from GSK, grants, personal fees and non-financial support from MSD, outside the submitted work;. MPD reports personal fees from Roche and Boehringer-Ingelheim, non financial support from Astrazeneca, General Electric and Guerbet. BC reports grants and personal fees from Boehringer Ingelheim, personal fees from Roche, personal fees from Sanofi, personal fees from Apellis, personal fees from Astra-Zeneca, grants from MedImmune, outside the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Berenice Soyez, Email: Berenice.soyez@aphp.fr.
Raphael Borie, Phone: +33 1 4025 6800, Email: Raphael.borie@aphp.fr.
Cedric Menard, Email: cedric.menard@chu-rennes.fr.
Jacques Cadranel, Email: Jacques.cadranel@aphp.fr.
Leonidas Chavez, Email: lchavez@wanadoo.fr.
Vincent Cottin, Email: vincent.cottin@chu-lyon.fr.
Emmanuel Gomez, Email: e.gomez@chu-nancy.fr.
Sylvain Marchand-Adam, Email: sylvain.marchand-adam@univ-tours.fr.
Sylvie Leroy, Email: leroy.s2@chu-nice.fr.
Jean-Marc Naccache, Email: Jean-marc.naccache@aphp.fr.
Hilario Nunes, Email: hilario.nunes@aphp.fr.
Martine Reynaud-Gaubert, Email: MartineLouise.REYNAUD@ap-hm.fr.
Laurent Savale, Email: laurent.savale@aphp.fr.
Abdellatif Tazi, Email: Abdellatif.tazi@aphp.fr.
Lidwine Wemeau-Stervinou, Email: Lidwine.WEMEAU@CHRU-LILLE.FR.
Marie-Pierre Debray, Email: marie-pierre.debray@aphp.fr.
Bruno Crestani, Email: bruno.crestani@aphp.fr.
References
- 1.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 2.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 3.Gurel O, Ikegami M, Chroneos ZC, Jobe AH. Macrophage and type II cell catabolism of SP-A and saturated phosphatidylcholine in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1266–L1272. doi: 10.1152/ajplung.2001.280.6.L1266. [DOI] [PubMed] [Google Scholar]
- 4.Piccoli L, Campo I, Fregni CS, Rodriguez BM, Minola A, Sallusto F, Luisetti M, Corti D, Lanzavecchia A. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat Commun. 2015;6:7375. doi: 10.1038/ncomms8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966;44:287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T, Uchida K, Tanaka N, Tsuchiya T, Watanabe J, Yamada Y, Hanaoka K, Seymour JF, Schoch OD, Doyle I, et al. Serological diagnosis of idiopathic pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;162:658–662. doi: 10.1164/ajrccm.162.2.9910032. [DOI] [PubMed] [Google Scholar]
- 8.Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–1098. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 9.Greenhill SR, Kotton DN. Pulmonary alveolar proteinosis: a bench-to-bedside story of granulocyte-macrophage colony-stimulating factor dysfunction. Chest. 2009;136:571–577. doi: 10.1378/chest.08-2943. [DOI] [PubMed] [Google Scholar]
- 10.Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, Keller G, Wood RE, Wert SE, Ikegami M, et al. Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. Am J Respir Crit Care Med. 2010;182:49–61. doi: 10.1164/rccm.201001-0008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–235. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 13.Briens E, Delaval P, Mairesse MP, Valeyre D, Wallaert B, Lazor R, Cordier JF. Pulmonary alveolar proteinosis. Rev Mal Respir. 2002;19:166–182. [PubMed] [Google Scholar]
- 14.Shah PL, Hansell D, Lawson PR, Reid KB, Morgan C. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax. 2000;55:67–77. doi: 10.1136/thorax.55.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers RM, Levin DC, Gray BA, Moseley LW., Jr Physiologic effects of bronchopulmonary lavage in alveolar proteinosis. Am Rev Respir Dis. 1978;118:255–264. doi: 10.1164/arrd.1978.118.2.255. [DOI] [PubMed] [Google Scholar]
- 16.Prakash UB, Barham SS, Carpenter HA, Dines DE, Marsh HM. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc. 1987;62:499–518. doi: 10.1016/S0025-6196(12)65477-9. [DOI] [PubMed] [Google Scholar]
- 17.Selecky PA, Wasserman K, Benfield JR, Lippmann M. The clinical and physiological effect of whole-lung lavage in pulmonary alveolar proteinosis: a ten-year experience. Ann Thorac Surg. 1977;24:451–461. doi: 10.1016/S0003-4975(10)63440-6. [DOI] [PubMed] [Google Scholar]
- 18.Campo I, Luisetti M, Griese M, Trapnell BC, Bonella F, Grutters J, Nakata K, Van Moorsel CH, Costabel U, Cottin V, et al. Whole lung lavage therapy for pulmonary alveolar proteinosis: a global survey of current practices and procedures. Orphanet J Rare Dis. 2016;11:115. doi: 10.1186/s13023-016-0497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariman K, Kylstra JA, Spock A. Pulmonary alveolar proteinosis: prospective clinical experience in 23 patients for 15 years. Lung. 1984;162:223–231. doi: 10.1007/BF02715650. [DOI] [PubMed] [Google Scholar]
- 20.Akasaka K, Tanaka T, Kitamura N, Ohkouchi S, Tazawa R, Takada T, Ichiwata T, Yamaguchi E, Hirose M, Arai T, et al. Outcome of corticosteroid administration in autoimmune pulmonary alveolar proteinosis: a retrospective cohort study. BMC Pulm Med. 2015;15:88. doi: 10.1186/s12890-015-0085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan A, Agarwal R, Aggarwal AN. Effectiveness of granulocyte-macrophage colony-stimulating factor therapy in autoimmune pulmonary alveolar proteinosis: a meta-analysis of observational studies. Chest. 2012;141:1273–1283. doi: 10.1378/chest.11-0951. [DOI] [PubMed] [Google Scholar]
- 22.Tazawa R, Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, Nasuhara Y, Hizawa N, Kasahara Y, Tatsumi K, et al. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2010;181:1345–1354. doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papiris SA, Tsirigotis P, Kolilekas L, Papadaki G, Papaioannou AI, Triantafillidou C, Papaporfyriou A, Karakatsani A, Kagouridis K, Griese M, et al. Long-term inhaled granulocyte macrophage-colony-stimulating factor in autoimmune pulmonary alveolar proteinosis: effectiveness, safety, and lowest effective dose. Clin Drug Investig. 2014;34:553–564. doi: 10.1007/s40261-014-0208-z. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Olivo MA, Amezaga Urruela M, McGahan L, Pollono EN, Suarez-Almazor ME. Rituximab for rheumatoid arthritis. Cochrane Database Syst Rev. 2015;1:CD007356. doi: 10.1002/14651858.CD007356.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagnoux C, Guillevin L, French Vasculitis Study G, investigators M. Rituximab or azathioprine maintenance in ANCA-associated vasculitis. N Engl J Med. 2015;372:386–387. doi: 10.1056/NEJMc1414728. [DOI] [PubMed] [Google Scholar]
- 26.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amital A, Dux S, Shitrit D, Shpilberg O, Kramer MR. Therapeutic effectiveness of rituximab in a patient with unresponsive autoimmune pulmonary alveolar proteinosis. Thorax. 2010;65:1025–1026. doi: 10.1136/thx.2010.140673. [DOI] [PubMed] [Google Scholar]
- 28.Borie R, Debray MP, Laine C, Aubier M, Crestani B. Rituximab therapy in autoimmune pulmonary alveolar proteinosis. Eur Respir J. 2009;33:1503–1506. doi: 10.1183/09031936.00160908. [DOI] [PubMed] [Google Scholar]
- 29.Kavuru MS, Malur A, Marshall I, Barna BP, Meziane M, Huizar I, Dalrymple H, Karnekar R, Thomassen MJ. An open-label trial of rituximab therapy in pulmonary alveolar proteinosis. Eur Respir J. 2011;38:1361–1367. doi: 10.1183/09031936.00197710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagasawa J, Kurasawa K, Hanaoka R. Rituximab improved systemic lupus erythematosus-associated pulmonary alveolar proteinosis without decreasing anti-GM-CSF antibody levels. Lupus. 2016;25:783–784. doi: 10.1177/0961203315627204. [DOI] [PubMed] [Google Scholar]
- 31.Uchida K, Nakata K, Carey B, Chalk C, Suzuki T, Sakagami T, Koch DE, Stevens C, Inoue Y, Yamada Y, Trapnell BC. Standardized serum GM-CSF autoantibody testing for the routine clinical diagnosis of autoimmune pulmonary alveolar proteinosis. J Immunol Methods. 2014;402:57–70. doi: 10.1016/j.jim.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seymour JF, Doyle IR, Nakata K, Presneill JJ, Schoch OD, Hamano E, Uchida K, Fisher R, Dunn AR. Relationship of anti-GM-CSF antibody concentration, surfactant protein a and B levels, and serum LDH to pulmonary parameters and response to GM-CSF therapy in patients with idiopathic alveolar proteinosis. Thorax. 2003;58:252–257. doi: 10.1136/thorax.58.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Debray MP, Borie R, Revel MP, Naccache JM, Khalil A, Toper C, Israel-Biet D, Estellat C, Brillet PY. Interstitial lung disease in anti-synthetase syndrome: initial and follow-up CT findings. Eur J Radiol. 2015;84:516–523. doi: 10.1016/j.ejrad.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Akira M, Inoue Y, Arai T, Sugimoto C, Tokura S, Nakata K, Kitaichi M, Osaka Respiratory Diseases Symposia G Pulmonary fibrosis on high-resolution CT of patients with pulmonary alveolar Proteinosis. AJR Am J Roentgenol. 2016;207:544–551. doi: 10.2214/AJR.15.14982. [DOI] [PubMed] [Google Scholar]
- 35.Eguiluz-Gracia I, Schultz HH, Sikkeland LI, Danilova E, Holm AM, Pronk CJ, Agace WW, Iversen M, Andersen C, Jahnsen FL, Baekkevold ES. Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax. 2016;71:1006–1011. doi: 10.1136/thoraxjnl-2016-208292. [DOI] [PubMed] [Google Scholar]
- 36.Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177:752–762. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available on request.


