Abstract
Background
Bladder cancer caused by exposure to aniline dyes, chronic cystitis, and smoking is detected in approximately 70 000 new cases annually. In the USA alone, it leads to 15 000 deaths every year. In the present study, we investigated the role of 3-((4′-amino-[1,1′-biphenyl]-4-yl)amino)-4-bromo-5-oxo-2,5-dihydrofuran-2-yl acetate (ABDHFA) in the inhibition of bladder cancer cell viability.
Material/Methods
Viability of cells was examined using MTT assay and distribution of cell cycle was assessed by flow cytometry. Expression of cyclin D1, androgen, prostate-specific antigen (PSA), and miR-449a was analyzed using Western blot and quantitative real-time polymerase chain reaction assays.
Results
The results demonstrated that ABDHFA treatment inhibited viability of UMUC3 and TCCSUP AR-positive bladder cancer cells. ABDHFA treatment led to break-down of AR in UMUC3 and TCCSUP cells after 48 h in a dose-dependent manner. Up-regulation of miR-449a by lentivirus transfection down-regulated the AR signalling pathway. In UMUC3 and TCCSUP cells, ABDHFA treatment led to inhibition of mRNA and protein expression corresponding to AR.
Conclusions
In summary, the present study demonstrates that proliferation of AR-positive bladder carcinoma cells is markedly reduced by ABDHFA treatment through arrest of cell cycle and degradation of AR protein. Thus, ABDHFA, a novel compound, can be used for the treatment of bladder cancer.
MeSH Keywords: Androgens, Prostate-Specific Antigen, Urinary Bladder Neoplasms
Background
Bladder cancer is detected in approximately 70 000 new cases and leads to 15 000 deaths annually in the USA alone. The disease is commonly caused by exposure to aniline dyes, chronic cystitis, and smoking [1,2]. Various studies have been performed to understand the mechanism of bladder cancer and to develop treatment strategies [3–5]. Treatment of bladder cancer involves cystoscopy and bladder tumor transurethral resection. However, a limitation of these techniques is the migration of floating cells to adjacent epithelium, which enhances the risk of disease recurrence. On the other hand, recurrence and progression of the cancer demands instillation of intravesical immunotherapeutic agents. This leads to up-regulation of cytokine expression and is accompanied by granulocytes and dendritic cell influx [6,7]. The side effects associated with instillation of intravesical immunotherapeutic agents are sometimes life-threatening [6]. The level of androgens determines the rate of cell proliferation and progression of cancer in the early stage. Although early-stage progression can be controlled by anti-androgen treatment, the therapy has no effect at the advanced stage [8]. The physiological effects of androgen are mediated by androgen receptor (AR), which promotes the proliferation rate of cancer cells [9,10]. There are drugs that block the binding of androgens with AR, leading to inhibition of cancer cell proliferation [11]. However, development of drug resistance demands the new treatment strategies for bladder cancer [12,13]. It is reported that miR-449a, an miRNA, has a significant role in the suppression of several types of tumors by targeting gene expression. Progression of cell cycle is arrested by miR-449a either through inhibition of Rb phosphorylation or by targeting expression of HDAC1 [14,15].
Natural products containing 2(5H)-furanone scaffold have been found to exhibit various biological activities, such as antiviral, anti-HIV, and anti-cancer effects [16]. These scaffolds, especially 4-arylamino-2(5H)-furanone moiety, serve as the building blocks for bioactive natural compound and drug candidates [17]. The presence of biphenyl group in various bioactive molecules has attracted the attention of synthetic organic chemists and highlighted the need for its incorporation into pharmaceutical molecules [18]. SAR studies have revealed that presence of aminobiphenyl or benzidine moiety in compounds with antitumor activity [19]. The present study investigated the effect of 3-((4′-amino-[1,1′-biphenyl]-4-yl)amino)-4-bromo-5-oxo-2,5-dihydrofuran-2-yl acetate (ABDHFA) on proliferation of AR-positive bladder carcinoma cells. ABDHFA inhibited AR-positive bladder carcinoma cell proliferation through cell cycle arrest through up-regulation of miR-449a expression.
Material and Methods
Reagents
ABDHFA supplied by Sigma-Aldrich (St. Louis, MO, USA) was dissolved in dimethyl sulfoxide to prepare stock solution. Antibodies against AR (rabbit polyclonal) and glyceraldehyde-phosphate dehydrogenase (GAPDH; mouse monoclonal) were provided by Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal antibody against cyclin D1 was supplied by Proteintech (Chicago, IL, USA).
Cell culture
UMUC3 and TCCSUP AR-positive bladder carcinoma cell lines were supplied by the American Type Culture Collection (ATCC; Rockville, MD, USA). For culture of cells we used RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and 1% penicillin-streptomycin. Both cell lines were cultured at a temperature of 37°C in humidified air containing 5% carbon dioxide.
Transfection of lentivirus
The miR-449a was overexpressed in UMUC3 and TCCSUP cell lines by transfection of lentivirus. Briefly, 293T cells were transfected with lentivirus and incubated for 48 h. Following incubation, the supernatant containing viral infection was collected, mixed with polybrene (10 μg/ml), and then infected into UMUC3 and TCCSUP cells. The cells were cultured in this medium for 24 h, followed by replacement of fresh medium.
Analysis of cell viability
We used the MTT assay to assess the effect of ABDHFA on viability of UMUC3 and TCCSUP cells; 2×104 cells/well were distributed onto 96-well culture plates and incubated for 12 h. Then, medium was exchanged with fresh medium containing 10-, 20-, 30-, 40-, 50-, and 60-μM doses of ABDHFA. In this medium, culture of cells was performed at a temperature of 37°C for 48 h. Following this, MTT (0.5 mg/ml) solution was put into each well of the plate. Incubation was continued for 4 h at 37°C, after which medium was discarded and DMSO was put into plates for dissolving the formazan crystals. Absorbance at a wavelength of 490 nm for each well of the plate in triplicates was recorded by a microplate autoreader (Bio-Tek Instruments Inc., Winooski, VT, USA).
Flow cytometric analysis
Flow cytometry was used for the analysis of cell cycle distribution and apoptosis in UMUC3 and TCCSUP cells. Incubation of cells with 10-, 20-, 30-, 40-, 50-, and 60-μM doses of ABDHFA was carried out for 48 h at 37°C. After incubation, the cells were washed twice with PBS and subsequently fixed in 70% ethyl alcohol for 24 h at −20°C. Cell apoptosis induced by ABDHFA was determined by propidium iodide (PI) and Annexin V-FITC (BD Pharmingen, San Diego, CA, USA) staining as per the instructions by the manufacturer. For analysis of cell cycle distribution, PBS-washed cells were incubated at room temperature for 45 min with PI (50 μg/ml) and RNase A (50 μg/ml). The FACSCalibur system with CellQuest software version 3.3 (both from Becton Dickinson, San Jose, CA, USA) was used for flow cytometry. ModFit LT software (version 3.0; Verity Software House, Topsham, ME, USA) was used for calculation of cell percentage in different cell cycle phases.
Western blot analysis
UMUC3 and TCCSUP cells were harvested following 48 h of treatment with 10-, 20-, 30-, 40-, 50-, and 60-μM doses of ABDHFA at 37°C. The cells were treated with RIPA buffer [50 mM Tris (pH 8.0), 150 mM naCl, 0.1% SDS, 1% nP-40, and 0.5% sodium deoxycholate] containing 1% cocktail and 1 mM PMSF. The lysates were subjected to centrifugation at 25 000 rpm for 20 min at 4˚C. Then, separation of proteins was achieved by electrophoresis on sodium dodecyl sulfate polyacrylamide gel (10%; SDS-PAGE). We used the BCA Protein Assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) to quantify the proteins enhanced. The protein samples were incubated on nitrocellulose membranes at 4°C overnight with primary antibody (Wuhan Boster Biological Technology, Ltd., Wuhan, China; 1: 1000). The non-specific sites in the membranes were blocked by incubation with 5% non-fat dry milk in TBS. The antibodies used were rabbit polyclonal (N20) anti-AR, anti-glyceraldehyde-phosphate dehydrogenase (GAPDH) (from Santa Cruz, CA, USA), and mouse monoclonal anti-cyclin D1 (Proteintech, Chicago, IL, USA). The membranes were then washed with TBS Tween-20 and subsequently incubated at room temperature for 1 h with HRP-conjugated secondary antibodies. β-actin used as loading control was examined on the same membrane.
Isolation of total RNA and reverse transcription
UMUC3 and TCCSUP cells at a density of 3×105 in 5 ml of DMEM supplemented with 10% FBS were incubated with 10-, 20-, 30-, 40-, 50-, and 60-μM doses of ABDHFA for 48 h and then total RNA was extracted from whole cells using a NucleoSpin® RNA II kit (Macherey-Nagel, Düren, Germany). Reverse transcription of the extracted RNA was performed using PrimerScript™ RT Master Mix (Takara Biotechnology Co., ltd., Dalian, China) as per the instructions for users. The mixture of 2 μl 5X PrimeScript RT Master Mix, 1 μl RNA and 7 μl RNase-free-dH2O were subjected to reactions for 15 min at 37°C and 10 s at 80°C.
Isolation and reverse transcription of miRNA
After 48 h of treatment with ABDHFA, UMUC3 and TCCSUP cells were collected to extract miRNA using TRIzol reagent. Quantification of the extracted miRNA was performed by recording absorbance at 265 nm. Reverse transcription of miRNA was done by PrimerScript™ RT Master Mix in accordance with the manual protocol.
Quantitative real-time polymerase chain reaction (qPCR)
For the qPCR, a mixture of 13.0 μl of SYBRR Premix Ex Taq II, 1 μl of 10 μM primer solution, 2 μl of cDNA, and 8.5 μl of RNase-free water was prepared. The mixture was denatured at 95°C for 45 s, then 1 cycle and PCR reaction for 510 s at 90°C, followed by 45 s at 55°C, 40 cycles, and the third stage of dissociation at 95°C for 15 s followed by 60°C for 30 s and at 90°C for 20 s. The internal loading control used was GAPDH. For amplification, the following primers were used: AR forward, 5′-CCAgggACCATgTTTTgCC-3′ and AR backward, 5′-Cg AAgACgACAAgATggACAA-3′; PSA forward, 5′-gTgTgT ggACCTCCATgTTATT-3′ and PSA backward, 5′-CCACTC ACCTTTCCCCTCAAg-3′.
Statistical analysis
The Statistical Package for Social Sciences (SPSS for Windows, version 17.0; SPSS, Inc., Chicago, IL, USA) was used for processing all data. Analysis of the data was done using mono-factorial analysis of variance. The data presented are the mean of ± standard deviation. The differences were considered to be statistically significant at a P-value of <0.05.
Results
UMUC3 and TCCSUP AR-positive cell proliferation was inhibited by ABDHFA
UMUC3 and TCCSUP cells were subjected to incubation in medium containing 10, 20, 30, 40, 50, and 60 μM of ABDHFA for 48 h. MTT assay results showed that increase in ABDHFA dosage promoted inhibition of UMUC3 and TCCSUP cell viability. ABDHFA at 50-μM concentration reduced viability of UMUC3 and TCCSUP cells to 31% and 27%, respectively (Figure 1).
Figure 1.
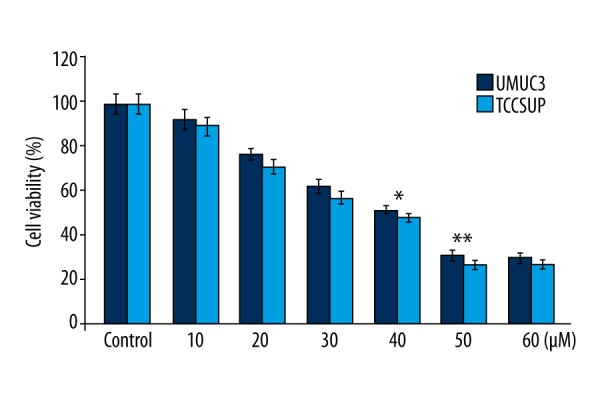
ABDHFA inhibits bladder cancer cell viability. For analysis of UMUC3 and TCCSUP cell viability after ABDHFA treatment, the MTT assay was used. GraphPad Prism software was used for constructing the bar graphs representing cell viability. Control cells were treated with dimethyl sulfoxide alone; * P<0.01 and ** P<0.02 vs. the cells treated with dimethyl sulfoxide alone.
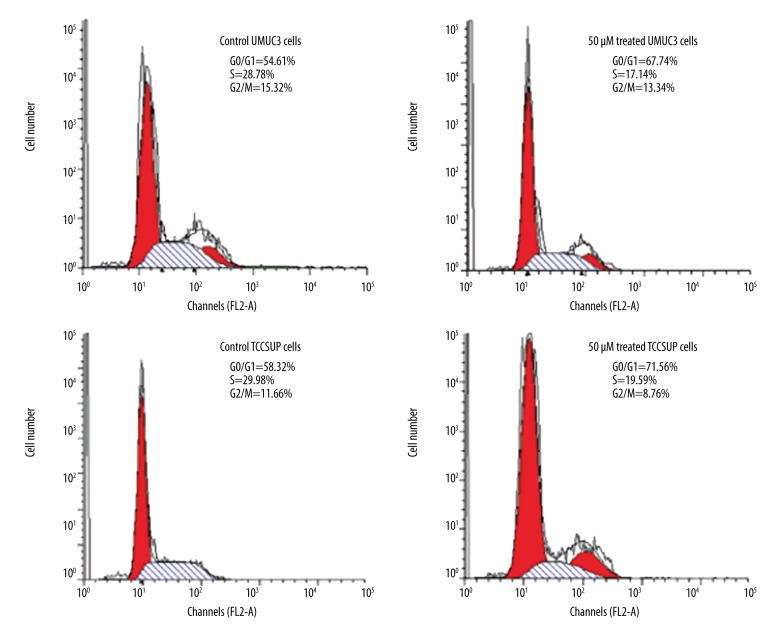
In UMUC3 and TCCSUP AR-positive cells, ABDHFA treatment led to cell cycle arrest
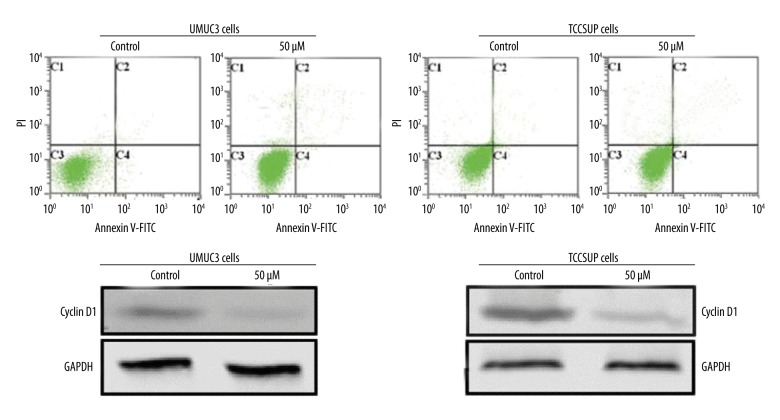
UMUC3 and TCCSUP cells treated with ABDHFA showed significant cell cycle arrest (p<0.002) in G0/G1 phase (Figure 2). Subsequently, the percentage of UMUC3 and TCCSUP cells in G2/M and S phases was markedly reduced. Western blot analysis revealed that ABDHFA treatment led to a marked decrease in the level of cyclin D1 in both UMUC3 and TCCSUP cells (Figure 2).
Figure 2.
In AR-positive bladder cancer cells, ABDHFA treatment caused cell cycle arrest. Distribution of UMUC3 and TCCSUP bladder cancer cells in cell cycle was determined by flow cytometry. Western blot assay was used for analysis of expression of protein (cyclin D1) involved in regulation of cell cycle. Control cells were treated with dimethyl sulfoxide alone and in the treatment group with 50 μM concentration of ABDHFA for 48 h.
In UMUC3 and TCCSUP AR-positive cells, ABDHFA had no role in inducing apoptosis
In logarithmic growth phase, UMUC3 and TCCSUP cells were incubated with various doses of ABDHFA for 48 h. Flow cytometry revealed that a 50-μM concentration of ABDHFA induced apoptosis in 4.29% of UMUC3 cells compared to 3.67% in the untreated control cells. The percentage of apoptotic TCCSUP cells was 5.49% compared to 4.93% in the untreated control cells (Figure 3).
Figure 3.
ABDHFA failed to induce apoptosis in UMUC3 and TCCSUP bladder cancer cells. Apoptosis of the bladder cancer cells was examined using flow cytometry.
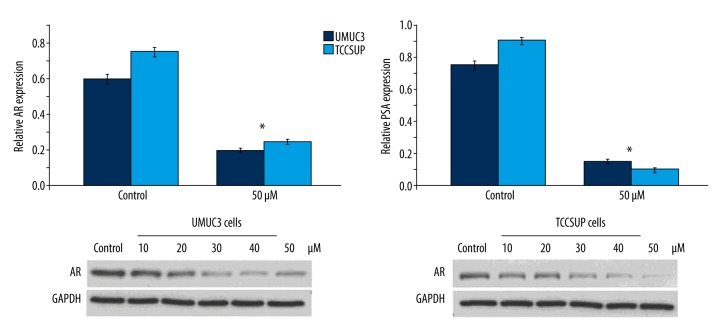
ABDHFA inhibited AR activation and expression of AR mRNA in UMUC3 and TCCSUP cells
In both UMUC3 and TCCSUP cells, expression of AR mRNA was reduced significantly (p<0.002) by ABDHFA treatment in comparison to the control group (Figure 4). PSA expression in both cell lines treated with ABDHFA was markedly inhibited after 48 h compared to cells treated with DMSO alone. The expression of AR protein was also reduced significantly (p<0.001) in UMUC3 and TCCSUP cells after treatment with different concentrations of ABDHFA for 48 h (Figure 4).
Figure 4.
Activation of AR in UMUC3 and TCCSUP cells was inhibited by ABDHFA. AR mRNA expression in bladder cancer cells was inhibited by treatment with ABDHFA for 48 h compared to control cells. The level of mRNA corresponding to PSA was also markedly reduced by ABDHFA treatment, as revealed by real-time PCR. Western blot assay confirmed that ABDHFA treatment markedly reduced expression of AR protein. * P<0.002 and ** P<0.001 vs. cells treated with DMSO alone.
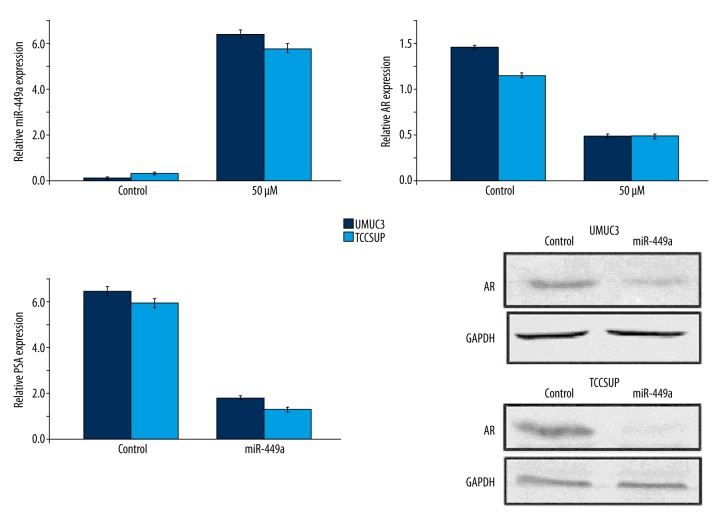
ABDHFA targeted AR expression by up-regulation of miR-449a level
In UMUC3 and TCCSUP cells, ABDHFA treatment increased the expression of miR-449a after 48 h (Figure 5). Up-regulation of miR-449a by lentivirus in UMUC3 and TCCSUP cells caused down-regulation of AR in both cell lines (Figure 5). The expression of PSA mRNA and AR in both cell lines was reduced by up-regulation of miR-449a due to lentivirus transfection (Figure 5).
Figure 5.
Up-regulation of miR-449a expression by ABDHFA treatment targeted AR expression. ABDHFA promoted miR-449a expression in TCCSUP and TCCSUP bladder cancer cells and inhibited AR level. Transfection of lentivirus markedly increased miR-449a expression in bladder cancer cells, which subsequently reduced PSA level. The miR-449a up-regulation by lentivirus reduced expression of AR protein.
Viability of bladder cancer cells was inhibited by miR-449a up-regulation
MTT assay showed that growth of bladder cancer cells was significantly inhibited by miR-449a over-expression caused by transfected lenti-miR449a (Figure 6). UMUC3 and TCCSUP cell viability was reduced to 29.45% and 25.67%, respectively, after 48 h of lenti-miR449a transfection (Figure 6).
Figure 6.
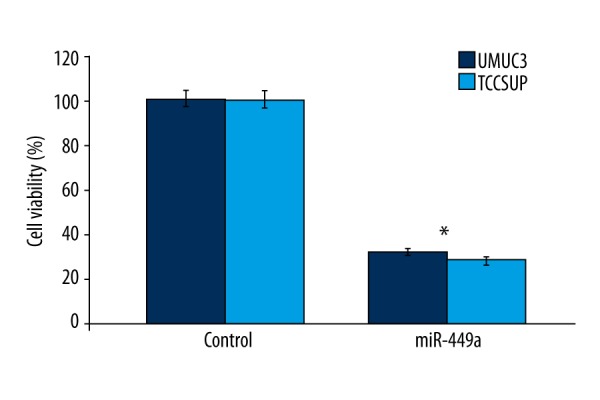
Transfection of lenti-miR449a reduced viability of bladder cancer cells. MTT assay was used to analyze the effect of lenti-miR449a on UMUC3 and TCCSUP cell viability. * P<0.05 vs. cells treated with DMSO alone (control cells).
Discussion
The present study demonstrates the anti-proliferative effect of ABDHFA on bladder cancer cells and showed the mechanism involved. Natural products bearing 2(5H)-furanone scaffold have been found to act as potent antitumor, antiviral, and anti-HIV agents [14]. Results from the present study showed that ABDHFA, a biphenyl 2(5H)-furanone-containing compound, inhibits viability of bladder cancer cells in a concentration-dependent manner. Progression of cell cycle through various phases is regulated by various checkpoints, as determined by assessing the synthesis of desired proteins and integrity of DNA [20]. Disorder in the cell cycle leads to uncontrolled proliferation of cells. The present study showed that treatment of bladder cancer cells with ABDHFA led to arrest of cell cycle in G0/G1 phase without inducing apoptosis. At present, AR is considered to be one of the most important targets for treatment of different cancers. Disintegration of AR has been found to be an efficient treatment for cancer treatment. In pancreatic cancer cells, treatment with ASC-J9 inhibited cell growth through break-down of AR [21–23]. The present study demonstrated that ABDHFA treatment caused break-down of AR protein in bladder carcinoma cells, and also inhibited proliferation. This suggests that ABDHFA suppresses bladder cell proliferation by down-regulation of AR level.
Studies have revealed that activity and expression of AR in cancer cells is inhibited by miR-let-7c through targeting c-Myc activity [24]. It has also been reported that AR signalling is down-regulated by miR-331-3p through affecting expression of ERBB-2 and Akt [25]. In addition, up-regulation of miR-448 in cancer cells leads to suppression of the AR signalling pathway and inhibition of proliferation [26]. In prostate cancer cells, over-expression of miR-449a suppresses cell growth induced by AR [27]. Results from the present study show that ABDHFA treatment promoted the expression of miR-449a, which in turn led to down-regulation of AR and PSA. These finding suggest that promotion of miR-449a by ABDHFA in bladder cancer cells degrades AR protein and inhibits its activity. Over-expression of miR-449a further suppressed bladder cancer proliferation through cell cycle arrest.
Conclusions
In summary, the present study demonstrates that ABDHFA treatment inhibits AR-positive bladder carcinoma cell proliferation through arrest of cell cycle and degradation of AR protein. ABDHFA treatment also up-regulated the expression of miR-449a, leading to down-regulation of the AR signalling pathway. Thus, ABDHFA, a novel compound, can be used for treatment of bladder cancer.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Natural Science Foundation of Jiangxi, China (No: 20151BAB205017), and the Foundation of Science and Technology of Education Bureau of Jiangxi, China (No: 150236)
References
- 1.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011;107:1059–64. doi: 10.1111/j.1464-410X.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 2.Madeb R, Golijanin D, Knopf J, Messing EM. Current state of screening for bladder cancer. Expert Rev Anticancer Ther. 2007;7:981–87. doi: 10.1586/14737140.7.7.981. [DOI] [PubMed] [Google Scholar]
- 3.Zhi-Feng Z, Kai W, Feng-Fu G, Hua L. Inhibition of T24 and RT4 human bladder cancer cell lines by heterocyclic molecules. Med Sci Monit. 2018;24:1156–64. doi: 10.12659/MSM.898265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohamad SFMN, Ahmad FAA, Khairul AMG, et al. Giant intradiverticular bladder tumor. Am J Case Rep. 2017;18:212–16. doi: 10.12659/AJCR.902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nariman AN, Jamie O, Tomislav MJ, Chun H. Primary urinary bladder angiosarcoma with osteoclast-like multinucleated giant cells: A case report and literature review. Am J Case Rep. 2016;17:143–49. doi: 10.12659/AJCR.896266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Z, Shen T, Wientjes MG, et al. Intravesical treatments of bladder cancer: Review. Pharm Res. 2008;25:1500–10. doi: 10.1007/s11095-008-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan CW, Shen ZJ, Ding GQ. The effect of intravesical instillation of antifibrinolytic agents on bacillus Calmette-Guerin treatment of superficial bladder cancer: A pilot study. J Urol. 2008;179:1307–11. doi: 10.1016/j.juro.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 8.Scher HI, Beer TM, Higano CS, et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1–2 study. Lancet. 2010;375:1437–46. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culig Z, klocker H, Bartsch G, Hobisch A. Androgen receptors in prostate cancer. Endocr Relat Cancer. 2002;9:155–70. doi: 10.1677/erc.0.0090155. [DOI] [PubMed] [Google Scholar]
- 10.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgendepleting therapies in prostate cancer: New agents for an established target. Lancet Oncol. 2009;10:981–91. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson PA, Chen YF, Balbas MD, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci USA. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noonan EJ, Place RF, Basak S, et al. miR-449a causes Rb-dependent cell cycle arrest and senescence in prostate cancer cells. Oncotarget. 2010;1:349–58. doi: 10.18632/oncotarget.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noonan EJ, Place RF, Pookot D, et al. miR-449a targets HDAC-1 and induces growth arrest in prostate cancer. Oncogene. 2009;28:1714–24. doi: 10.1038/onc.2009.19. [DOI] [PubMed] [Google Scholar]
- 16.Bihelovic F, Saicic RN. Total Synthesis of (−)-atrop-Abyssomicin C. Angew Chem Int Ed Engl. 2012;51:5687–91. doi: 10.1002/anie.201108223. [DOI] [PubMed] [Google Scholar]
- 17.Lattmann E, Dunn S, Niamsanit S, Sattayasai N. Synthesis and antibacterial activities of 5-hydroxy-4-amino-2(5H)-furanones. Bioorg Med Chem Lett. 2005;15:919–21. doi: 10.1016/j.bmcl.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Asselah T. NS5A inhibitors: A new breakthrough for the treatment of chronic hepatitis C. J Hepatol. 2011;54:1069–72. doi: 10.1016/j.jhep.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 19.Lai M-J, Lee H-Y, Chuang H-Y, et al. N-Sulfonyl-aminobiaryls as antitubulin agents and inhibitors of signal transducers and activators of transcription 3 (STAT3) signaling. J Med Chem. 2015;58:6549–58. doi: 10.1021/acs.jmedchem.5b00659. [DOI] [PubMed] [Google Scholar]
- 20.Park M-T, Lee S-J. Cell cycle and cancer. J Biochem Mol Biol. 2003;36:60–65. doi: 10.5483/bmbrep.2003.36.1.060. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita S, Lai KP, Chuang KL, et al. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14:74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin TH, Lee SO, Niu Y, et al. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) lead to promotion versus suppression of prostate cancer metastasis. J Biol Chem. 2013;288:19359–69. doi: 10.1074/jbc.M113.477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin TH, Izumi K, Lee SO, et al. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (Enzalutamide) or Casodex (Bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis. 2013;4:e764. doi: 10.1038/cddis.2013.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadiminty N, Tummala R, Lou W, et al. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. J Biol Chem. 2012;287:1527–37. doi: 10.1074/jbc.M111.278705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epis MR, Giles KM, Barker A, et al. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem. 2009;284:24696–704. doi: 10.1074/jbc.M109.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikand K, Slaibi JE, Singh R, et al. miR 488* inhibits androgen receptor expression in prostate carcinoma cells. Int J Cancer. 2011;129:810–19. doi: 10.1002/ijc.25753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Östling P, Leivonen SK, Aakula A, et al. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71:1956–67. doi: 10.1158/0008-5472.CAN-10-2421. [DOI] [PubMed] [Google Scholar]