Abstract
The neurotoxic effects of methamphetamine (MA) exposure in the developing and adult brain can lead to behavioral alterations and cognitive deficits in adults. Previous increases in the rates of adolescent MA use necessitate that we understand the behavioral and cognitive effects of MA exposure during adolescence on the adolescent brain. Adolescents using MA exhibit high rates of nicotine (NIC) use, but the effects of concurrent MA and NIC in the adolescent brain have not been examined, and it is unknown if NIC mediates any of the effects of MA in the adolescent. In this study, the long-term effects of a neurotoxic dose of MA with or without NIC exposure during early adolescence (postnatal day 30-31) were examined later in adolescence (postnatal day 41-50) in male C57BL/6J mice. Effects on behavioral performance in the open field, Porsolt forced swim test, and conditioned place preference test, and cognitive performance in the novel object recognition test and Morris water maze were assessed. Additionally, the effects of MA and/or NIC on levels of microtubule associated-2 (MAP-2) protein in the nucleus accumbens and plasma corticosterone were examined. MA and NIC exposure during early adolescence separately decreased anxiety-like behavior in the open field test, which was not seen following co-administration of MA/NIC. There was no significant effect of early adolescent MA and/or NIC exposure on the intensity of MAP-2 immunoreactivity in the nucleus accumbens or on plasma corticosterone levels. These results show that early adolescent MA and NIC exposure separately decrease anxiety-like behavior in the open field, and that concurrent MA and NIC exposure does not induce the same behavioral change as either drug alone.
Keywords: methamphetamine, nicotine, adolescence, anxiety-like behavior, nucleus accumbens, mice
1. Introduction
Methamphetamine (MA) is a psychomotor stimulant that impacts multiple neurotransmitter systems, including the dopamine (DA) system, and is associated with high abuse potential, DA neurotoxicity, and cognitive impairments in adults [1–3]. While rates of adolescent MA use are relatively low compared to other drugs of abuse, studies suggest the rates of adolescents seeking treatment for MA abuse increased in the early 2000s [4, 5]. The behavioral, cognitive, and neurobiological effects of MA may differ in adolescents compared to adults, as adolescence is a dynamic period of brain and behavioral maturation [6]. Co-abuse of MA and nicotine (NIC) is common [7, 8], and the average age of initiation for NIC use is 13 years of age [9]. Relatively little is known about the behavioral and cognitive effects of MA and MA/NIC co-administration in adolescence compared to adulthood. Therefore in the current study we examined the effects of MA and/or NIC on behavioral and cognitive performance in adolescent male mice.
Adolescent MA users are more likely to have had previous psychiatric treatment and a family history of drug abuse [10], previous treatments for drug abuse [5], and increased rates of depression and suicide ideation compared to non-users and adolescents using other drugs of abuse [11]. Adolescent MA users have poorer treatment responses, higher recidivism rates compared to non-MA using adolescent drug users [11], and increased rates of general risk-taking behaviors as well as risky sexual behaviors associated with adolescent pregnancy [12, 13]. Adolescent MA users also show increased depression and anxiety levels, increased plasma cortisol levels following a social stressor [14], and executive function impairments [15] relative to non-MA users following 4-11 months of abstinence.
Animal models have been used to examine the effects of adolescent MA exposure. MA exposure during adolescence impairs spatial learning in the Morris water maze and sequential learning in the Cincinnati water maze in rats, but these effects do not occur in pre-adolescence or adulthood, which suggests that adolescence is a period of increased susceptibility to MA-induced cognitive impairments [16]. Adolescent rats show enhanced rates of MA self-administration [17], enhanced MA-induced locomotor activation, and enhanced MA conditioned place preference (CPP) [18] compared to adult rats. MA exposure during adolescence induces long-term impairments in visual discrimination and reversal learning in rats [19]. In mice, MA exposure during early adolescence increases depression-like behavior and decreases vasopressin-immunoreactive neurons in the paraventricular nucleus of the hypothalamus [20].
NIC also exerts unique effects in adolescence compared to adulthood. Compared to adult NIC exposure, exposure to NIC in adolescence increases the density of DA transporters (DAT) in the nucleus accumbens (NAc), caudate, and putamen in rats [21], increases DA release in the NAc [22], and decreases anxiety-like behaviors [23] in rats. NIC also appears to have a protective effect against MA-induced DA neurotoxicity in adult rodents. For example, adult MA-exposed male mice exhibit reductions in striatal DA levels to 18% of controls, while mice exposed to NIC prior to MA show striatal DA levels measuring 57% of controls [24]. NIC prevents MA-induced striatal DA depletion [25] and MA-induced DA terminal neurodegeneration [26] in adult male mice and in adult rats, NIC pre-treatment reduces MA self-administration [27]. Furthermore, NIC exposure beginning in either adolescence or adulthood mitigates MA-induced deficits in novel object recognition in adult rats [28]. However, the effects of MA and NIC co-administration have not been examined in adolescent rodents.
Microtubule associated protein 2 (MAP-2) can be used as a dendritic marker to assess the effects of MA and NIC on the integrity of the striatum. MAP-2 is a dendritic protein responsible for the assembly of the microtubules in the dendrites and is important for dendritic plasticity. MAP-2 is required for dendrite elongation, protein kinase A (PKA) anchoring in dendrites, and proper PKA signal transduction [29]. Additionally, MAP-2 is a sensitive marker for age-related changes in rodents [30, 31] and nonhuman primates [32]. Previous work has shown that MAP-2 levels in the CA3 region of the hippocampus and the entorhinal cortex of 4-month-old mice were reduced following neonatal treatment with MA [33]. In adult rats, MA exposure decreases MAP-2 mRNA and protein levels in the cortex and hippocampus [34]. Thus, MAP-2 is useful for quantifying MA-induced brain changes. We were specifically interested in examining MAP-2 levels in the NAc, as NAc functioning shows long-term alterations following MA exposure in rodents [35–38] and humans [39]. Because little is known about the effects of MA in the adolescent brain, we investigated MAP-2 levels in the NAc to better understand the possible effects of MA on the NAc in the adolescent brain.
To the best of our knowledge, no previous studies have explored the effects of MA and NIC co-administration in adolescence. Therefore in the current study we examined the effects of early adolescent MA and/or NIC exposure on behavioral and cognitive performance later in adolescence in male mice. MAP-2 levels in the NAc core and shell and plasma corticosterone levels were also assessed in order to delineate the effects of MA and/or NIC on the integrity of the NAc and the stress response.
2. Methods
2.1. Mice
Seventy-two male C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME., USA). Mice arrived on postnatal day (PND) 27 and were weighed upon arrival and then daily from PND 30 to PND 39. The mice were housed in groups of four (one mouse per treatment group in each cage) and maintained on a 12-hour light/dark cycle (06:00/18:00). Food and water were provided ad libitum. All experimental procedures and testing paradigms were approved by the Institutional Animal Care and Use Committee at Sewanee: The University of the South.
2.2. Treatments
(+)-MA hydrochloride (Sigma Aldrich, St. Louis, MO, USA) and (-)-NIC tartrate (Sigma Aldrich, St. Louis, MO, USA) were diluted in 0.9% injectable saline to appropriate concentrations. Intra-peritoneal (IP) injections (0.1 mL) were administered four times daily at two-hour intervals to all mice on PND 30 and PND 31 (early adolescence; [6]). Treatment groups consisted of MA (7.5 mg/kg × 4 injections), NIC (0.3 mg/kg × 4 injections), MA and NIC (7.5 mg/kg and 0.3 mg/kg, respectively × 4 injections), and saline (× 4 injections). This injection schedule and paradigm was chosen to replicate previous research examining the effects of early adolescent MA exposure in mice [20, 40] and all solutions were administered via IP injections for consistency between groups. All injections were counterbalanced within the housing cages.
2.3. Behavioral Testing
Behavioral testing began on PND 41 (later in adolescence). The mice were individually housed beginning three days before the start of behavioral testing. A total of 36 mice (9 per treatment group) underwent behavioral testing in the following order: open field test, novel object recognition test, Porsolt forced swim test, and the Morris water maze on the days indicated below. These specific tests were chosen to replicate previous research examining the effects of adolescent MA exposure [20]. A separate group of 36 mice (9 per treatment group) underwent the same exposure paradigm and were subsequently trained and tested in the CPP test starting on PND 41.
2.3.1. Open Field
Locomotor activity and anxiety-like behavior were assessed in the open field test on PND 41. Mice were tested in a brightly lit 40 × 40 cm clear, Plexiglas arena. The interior 20 × 20 cm section of the chamber was designated as the center of the arena [20, 41]. Anymaze Video Tracking Software (Stoelting, Wood Dale, IL, USA) was used to record distance traveled and percent time in the center of the arena during the single ten-minute trial. The percent of time spent in the center of the arena was calculated as a measure of anxiety-like behavior [42, 43].
2.3.2. Novel Object Recognition
Object memory was evaluated in the novel object recognition test. All testing was carried out under dim lighting in the same arenas used to conduct the open field test. All objects used were similar in size and texture, and novel object positioning was counterbalanced across treatment groups.
For two subsequent days (PND 42 and PND 43) prior to testing, all mice underwent single, ten-minute habituation trials with no objects present. For novel object recognition testing, all mice underwent two trials on PND 44. Each trial lasted for ten minutes and included a five-minute inter-trial interval. For trial one, two identical plastic toy fish (5.1 cm W × 7.6 cm L × 6.4 cm H) were placed in opposing corners of the arena. In trial two, a plastic basketball (novel object; 6.4 cm W × 6.4 cm L × 6.4 cm H) replaced one of the first objects. This retention interval of 5 minutes was used to assess short-term memory, and previous studies have shown that neonatal and adolescent rats are able to perform the novel object recognition test with an interval of this length [44].
The time spent exploring each object during each trial was recorded using Anymaze Video Tracking Software. The average distance moved in the trials was measured, and the preference delta score for the novel object (time spent exploring new object – time spent exploring old object) as well as the percent time exploring the novel object in trial 2 were calculated as measures of object recognition memory.
2.3.3. Porsolt Forced Swim Test
Depression-like behavior was analyzed in the Porsolt forced swim test on PND 45. 1,000 mL glass beakers were filled with 700 mL of room temperature water (20° C). All mice were placed in a beaker for one five-minute trial. The percent of time spent immobile versus swimming was quantified by Anymaze Video Tracking Software. Increased immobility served as an indicator of depression-like behavior and/or learned helplessness [45].
2.3.4. Morris Water Maze
Spatial learning and memory were assessed using the Morris water maze from PND 46 to PND 50. The procedure was performed as previously described [20, 46]. For analyses, the maze (circular tub, 122 cm diameter) was divided into four virtual quadrants using Anymaze Video Tracking Software. The maze was filled with water made opaque by the addition of chalk. All testing occurred under bright lighting. Mice were trained to locate a submerged platform hidden in one of the four quadrants. Each test day consisted of one morning and one afternoon session. Each session involved three 60-second trials with an approximately 10-minute inter-trial interval. Mice that were unable to locate the platform during training were led there by the experimenter and allowed to remain on the platform for 3 seconds prior to removal. Mice were placed in the maze facing the exterior wall at 9 discrete drop locations that were alternated between trials. Anymaze Video Tracking Software was used to record swimming patterns and to measure swim speed and escape latency.
For visible (non-spatial) training on PND 46 and 47, white curtains surrounded the maze and a beacon was used to identify the target platform. The target platform was moved to a different quadrant for each visible training session. The curtains and beacon were subsequently removed for hidden platform sessions (spatial training) that occurred from PND 48-50. The platform remained in the same quadrant for all hidden training sessions. Approximately one hour after each hidden platform spatial training session, spatial memory was assessed in a probe trial. The platform was removed from the pool and the mice were analyzed in a single 60-second probe trial. The cumulative distance from the previous platform location was quantified using Anymaze Video Tracking Software. Decreased cumulative distance from the hidden platform location served as an indicator of spatial memory.
2.3.5. Conditioned Place Preference
A separate group of 36 mice underwent the same MA treatment paradigm and were tested in the CPP test beginning on PND 41. Mice were exposed to an unbiased two-compartment place conditioning procedure based on a previous protocol [47] using an unbiased apparatus (Lafayette Instruments, Lafayette, IN., USA). Mice were randomly assigned to receive MA (2 mg/kg I.P.) paired with one of two floor types: grid (5 mice per treatment group) or hole (4 mice per treatment group). The grid floor was 21.6 × 21.6 cm with 4 mm rods and a 3.35 mm gap between the rods, and the hole floor was 21.6 × 21.6 cm with 6.4 mm holes with 9.5 mm staggered centers. In the Grid+ group, MA was injected immediately before placement onto the grid floor while saline was injected immediately before placement on the hole floor. In the Grid− group, saline was injected immediately before placement onto the grid floor whereas MA was injected before placement onto the hole floor. Mice received one 30 min trial per day for 8 days, with a total of four trials of each type alternating between days. Injection and floor pairings were counterbalanced across treatment groups. Distance moved in each trial was measured. On PND 49, a preference test was conducted in which all mice received an injection of saline (I.P.) and were placed in the center of the apparatus with access to both the grid and hole floor for 30 minutes. Preference was expressed as the time spent on the grid floor, calculated as seconds per minute spent on the grid floor for mice that had MA paired with the grid floor (Grid+) or the hole floor (Grid−). A difference between the groups in time spent on the grid floor would provide evidence of place conditioning and CPP expression would be indicated if the mice in the Grid+ group show increased sec/min spent on the grid floor compared to the mice in the Grid− group.
2.4. MAP-2 Immunoreactivity
On PND 51 or 52, the mice that completed the battery of behavioral tests were transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (pH = 7.4) as previously described [33]. Brains were removed and post-fixed overnight in 4% paraformaldehyde at 4°C, transferred to 30% sucrose solution, and embedded in cryoprotectant. Serial coronal sections (50 μm) were collected onto Superfrost microscope slides through the NAc (Bregma +1.96 mm to +0.61 mm) with a 150 μm inter-section distance.
MAP-2 immunofluorescence was performed on the sections. Briefly, slides were rinsed in phosphate-buffered saline (PBS) 3 times for 5 minutes each then blocked in 5% normal goat serum made in 0.3% triton-X solution. Slides were then incubated overnight in primary antibody (MAP2, mouse monoclonal; Millipore, MAB3418) at a 1:1000 concentration. The following day, slides were rinsed in PBS 3 × 5 minutes then incubated for 2 hours in secondary antibody (goat anti-mouse AlexaFluor488; Life Technologies, A-11001) at a 1:250 concentration. Slides were rinsed in PBS 5 × 5 minutes. Once dry, slides were cover-slipped with VectaShield Mounting Media with DAPI. Images were subsequently taken at 20× magnification using an Olympus IX-81 microscope and analyzed using Slidebook 6.0 software. After all images had been acquired, the average intensity of MAP-2 immunoreactivity in the region of interest was analyzed for the NAc core (triangle, 410 μm × 310 μm × 510 μm) and shell (rectangle, 280 μm × 370 μm). The researcher performing the immunohistochemistry, microscopy, and quantification procedures was blind to the treatment of the subjects.
2.5. Corticosterone ELISA
On PND 50, the mice that completed the CPP test were euthanized by cervical dislocation and decapitation in order to collect plasma that was stored at −80°C until use. Plasma corticosterone levels were measured in 5-7 mice per treatment group using a specific ELISA kit according to the manufacturer’s instructions (Abcam, Cambridge, MA., USA).
2.6. Statistical Analysis
Analysis of variance (ANOVA) was used to test the effects of treatment on behavioral and cognitive performance, intensities of MAP-2 immunoreactivity in the NAc shell and core, and plasma corticosterone levels. Dunnett’s correction for multiple comparisons was used for the MAP-2 immunoreactivity analysis. Repeated measures ANOVA was used to analyze performance in the Morris water maze learning trials, distance moved in the CPP trials, and body weight data. Mauchly’s test of sphericity was used to test the assumption of sphericity for all repeated measure ANOVAS, and when violated a Greenhouse-Geisser correction was used. Shapiro-Wilk tests were used to assess normality of the data distributions and Levene’s test was used to assess the assumption of homogeneity of variance. In cases where these assumptions were violated, a Kruskal-Wallis non-parametric test was used to analyze the data. All statistical analyses were conducted using SPSS software (IBM, Armonk, N.Y., USA). A significance level of p < 0.05 was used for all statistical tests.
3. Results
3.1. Body Weights
The mice that underwent the battery of behavioral and cognitive tests were weighed upon arrival in our colony and from PND 30-39. The assumption of sphericity was violated and a Greenhouse-Geisser correction was used. There was a significant effect of day (F (3.8, 121.7) = 102.4, p < 0.01) and an interaction between day and treatment group (F (11.4, 121.7) = 3.2, p < 0.01). Further analysis of the effects of treatment on each day showed that there was a main effect of treatment on PND 31 (F (3, 32) = 7.8, p < 0.01). A Newman-Keuls post hoc test showed that the saline and NIC groups weighed significantly more than the MA and MA/NIC groups on PND 31 (Table 1). There were no effects of treatment group on weight for any other day, suggesting that any effect of MA on weight did not persist past PND 31, which was the second day of injections.
Table 1.
Mouse weights
| Treatment group | PND 27 | PND 30 | PND 31 | PND 32 | PND 33 | PND 34 | PND 35 | PND 36 | PND 37 | PND 38 | PND 39 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline | 15.0 ± 0.7 | 17.0 ± 0.5 | 17.2 ± 0.5 | 17.1 ± 0.5 | 17.4 ± 0.4 | 17.9 ± 0.3 | 17.8 ± 0.4 | 17.7 ± 0.4 | 17.9 ± 0.4 | 18.0 ± 0.4 | 18.4 ± 0.3 |
| MA | 15.0 ± 0.3 | 17.2 ± 0.2 | 15.9 ± 0.4 | 17.1 ± 0.3 | 17.6 ± 0.3 | 18.4 ± 0.2 | 18.6 ± 0.2 | 18.2 ± 0.2 | 18.4 ± 0.2 | 18.2 ± 0.2 | 18.4 ± 0.2 |
| NIC | 16.2 ± 0.5 | 18.0 ± 0.4 | 18.1 ± 0.3* | 17.9 ± 0.4 | 18.0 ± 0.3 | 18.6 ± 0.2 | 18.4 ± 0.2 | 18.8 ± 0.3 | 18.8 ± 0.2 | 18.7 ± 0.2 | 19.2 ± 0.2 |
| MA/NIC | 15.2 ± 0.4 | 17.6 ± 0.4 | 16.0 ± 0.4 | 17.3 ± 0.4 | 17.7 ± 0.3 | 18.1 ± 0.3 | 18.2 ± 0.4 | 18.2 ± 0.4 | 18.7 ± 0.4 | 18.6 ± 0.3 | 18.7 ± 0.4 |
Note: PND = postnatal day, MA = methamphetamine, NIC = nicotine.
All measures shown as group means ± SEM.
n = 9 mice per treatment group.
p < 0.05 higher compared to MA and MA/NIC groups.
3.2. Open Field
To determine the effects of treatment on locomotor activity and anxiety-like behavior, total distance moved and percent time spent in the center of the open field arena were assessed. There was no significant effect of treatment on total distance moved in the open field test (p = 0.07). There was a main effect of treatment on percent time spent in the center of the open field (F (3, 32) = 41.7, p < 0.01). A Newman-Keuls post hoc test revealed that the MA and NIC groups spent significantly greater percent time in the center of the open field compared to the saline and MA/NIC groups. The assumption of homogeneity of variance was violated for percent time in the center of the arena, and thus we also analyzed the data using a Kruskal-Wallis non-parametric test. The Kruskal-Wallis test also showed that there was a statistically significant difference in percent time in the center of the arena between the different treatment groups (χ2 (3) = 10.75, p = 0.01), with a mean rank percent time spent in center score of 11.61 for saline, 14.06 for MA/NIC, 23.44 for NIC, and 24.89 for MA (Figure 1).
Figure 1.
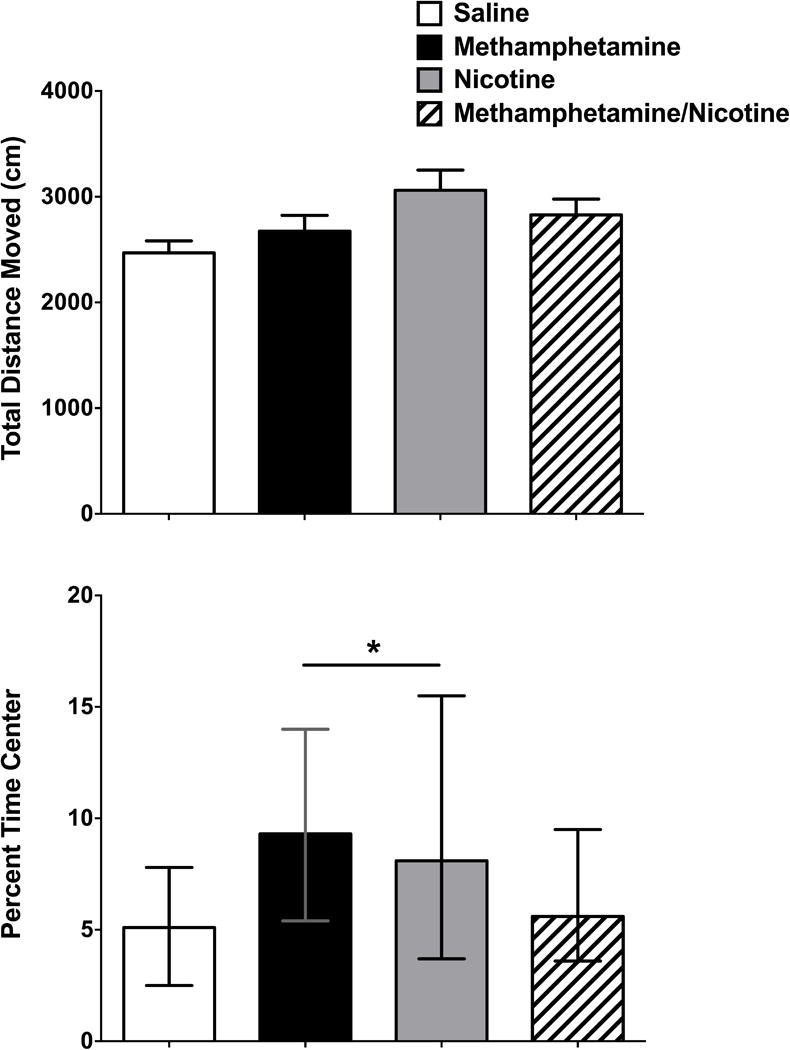
Total distance moved (A) and percent time in the center (B) of the open field test. A. There were no group differences in the total distance moved in the open field test. Data represented as means ± SEMs. B. MA-treated mice and NIC-treated mice spent a greater percent time in the center of the open field compared to saline-treated mice and MA/NIC-treated mice. Because a nonparametric Kruskal-Wallis test was used, data are represented as the medians and the upper and lower range of scores (χ2 (3) = 10.75, p = 0.01). n = 9 mice per group. *p < 0.01 versus saline-treated mice and MA/NIC-treated mice.
3.3. Novel Object Recognition
Novel object recognition was used to assess the potential effects of treatment on object memory. There were no group differences in the average total distance moved (p = 0.3) or total time exploring the objects (p = 0.09) in trial 1. For the novel object trial (trial 2), there was no effect of treatment on the average total distance moved (p = 0.3), on the delta score (p = 0.3), or percent time exploring the novel object (p = 0.3; Table 2).
Table 2.
Behavioral and cognitive performance of mice
| Test | Measure | Saline | MA | NIC | MA/NIC |
|---|---|---|---|---|---|
| Novel object recognition | Average total distance moved (cm) | 1970.2 ± 120.4 | 2250.9 ± 118.0 | 2073.8 ± 170.1 | 2164.6 ± 73.7 |
| Delta score trial 2 (sec) | 0.54 ±1.4 | 1.4 ± 1.2 | 3.1 ± 0.8 | 0.2 ± 1.1 | |
| Time exploring new object trial 2 (%) | 4.9 ± 1.4 | 6.5 ± 1.1 | 4.9 ± 1.0 | 7.1 ± 0.7 | |
| Porsolt forced swim test | Time immobile (%) | 53.6 ± 3.4 | 59.0 ± 5.0 | 52.9 ± 4.4 | 57.6 ± 3.5 |
| Morris water maze | Average latency to escape hidden sessions (sec) | 47.3 ± 2.9 | 44.2 ± 3.9 | 42.2 ± 4.6 | 46.4 ± 4.6 |
| Average cum. Distance probe trials (cm) | 3440.6 ± 180.2 | 3286.5 ± 246.3 | 2932.7 ± 318.9 | 3436.0 ± 312.0 |
Note: MA = methamphetamine, NIC = nicotine, Cum. = cumulative.
All measures shown as group means ± SEM.
n = 9 mice per treatment group.
3.4. Porsolt Forced Swim Test
To determine the effects of treatment on depression-like behavior, the percent time spent immobile in the Porsolt forced swim test was measured. There was no effect of treatment on the percent time spent immobile in the Porsolt forced swim test (p = 0.9; Table 2).
3.5. Morris Water Maze
Morris water maze performance was used to assess the effects of treatment on spatial learning and memory. For the visible training sessions, the assumption of sphericity was violated and a Greenhouse-Geisser correction was used. There was an effect of session on latency to find the platform (F (2.3, 73.65) = 18.29, p < 0.01). All mice improved their performance with training and showed a reduction in the latency to the platform over the visible training sessions (data not shown). There was no interaction between the visible training session and treatment during the visible platform training (p = 0.5). For the hidden training sessions, the assumption of sphericity was violated and a Greenhouse-Geisser correction was used. All mice improved their performance and there was an effect of session on latency to find the platform (F (3.4, 109.4) = 7.83, p < 0.01). Overall the latency to find the hidden platform decreased over training sessions. There was no effect of treatment (p = 0.8) or an interaction between hidden training session and treatment (p = 0.2) during the hidden platform training (Table 2). For the 3 probe trials, there was no effect of treatment on cumulative distance to the previous platform location in probe trial 1 (p = 0.9), probe trial 2 (p = 0.5), or probe trial 3 (p = 0.2; Table 2).
3.6. Conditioned Place Preference
The rewarding effects of MA were assessed in the CPP test. For the average distance moved over the 8 CPP training trials, there was no effect of trial (p = 0.2) or an interaction between trial and treatment (p = 0.9). However, there was an effect of treatment on average distance moved over the 8 CPP training trials (F (3, 32) = 10.33, p < 0.01). A Newman-Keuls post hoc test showed that MA/NIC mice moved a greater average distance than all the other groups, and the MA mice moved a greater average distance than the NIC mice during the CPP training trials (Table 3).
Table 3.
Conditioned place preference and plasma corticosterone levels
| Measure | Saline | MA | NIC | MA/NIC |
|---|---|---|---|---|
| Average distance moved in training trials (cm) | 4758.2 ± 244.0 | 5200.6 ± 236.7# | 4236.1 ± 207.4 | 5929.8 ± 201.2* |
| Average distance moved in test trial (cm) | 6535 ± 477.8 | 6318.2 ± 251.7 | 5694.9 ± 341.5 | 6749.1 ± 271.7 |
| Average time on grid floor for Grid+ group (sec/min) | 36.17 ± 2.38** | 37.01 ± 2.71** | 37.35 ± 2.91** | 36.51 ± 1.53** |
| Average time on grid floor for Grid− group (sec/min) | 27.96 ± 4.22 | 25.26 ± 1.58 | 26.80 ± 1.63 | 28.63 ± 2.50 |
| Plasma corticosterone (ng/mL) | 22.96 ± 2.22 | 10.97 ± 3.30 | 19.54 ± 4.73 | 14.21 ± 3.30 |
Note: MA = methamphetamine, NIC = nicotine.
All measures shown as group means ± SEM.
n = 9 mice per treatment group for conditioned place preference testing.
n = 5-7 mice per treatment group for corticosterone ELISA.
p < 0.05 higher compared to all other treatment groups.
p < 0.05 higher compared to NIC treatment group.
p < 0.05 higher compared to Grid− group.
There was no difference between the treatment groups on average distance moved during the CPP test (p = 0.8). The average time (measured as sec/min) spent on the grid floor during the CPP test between the treatment groups and the conditioning subgroup (Grid+ or Grid-) was assessed. There was an effect of conditioning subgroup on the time (sec/min) spent on the grid floor (F (1, 28) = 27.69, p < 0.01). The Grid+ group spent significant more time on the grid floor compared to the Grid− group. However, this finding was not modulated by treatment group, as indicated by a lack of an effect of treatment group (p = 0.9) or a significant interaction between treatment group and conditioning subgroup (p = 0.9) on the time spent on the grid floor (Table 3).
3.7. MAP-2 Immunoreactivity
The effect of treatment on MAP-2 immunoreactivity in the NAc was assessed. There was no significant effect of treatment on the intensity of MAP-2 immunoreactivity in the NAc core (p = 0.1; Figure 2B) or the NAc shell (p = 0.2; Figure 2C).
Figure 2.
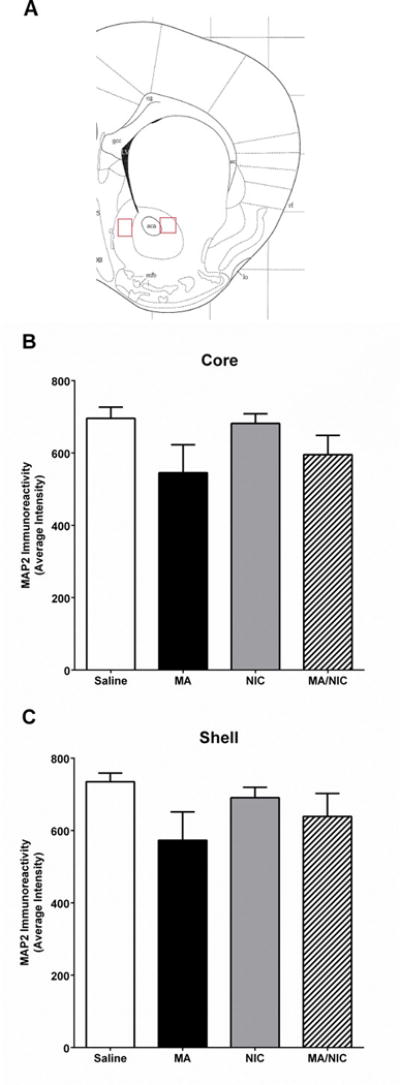
Intensity of MAP-2 immunoreactivity in the core (B) and shell (C) of the nucleus accumbens (NAc). A. Representative image of area of interest. B. No effects of treatment on intensity of MAP-2 immunoreactivity in the NAc core were found. C. No effects of treatment on intensity of MAP-2 immunoreactivity in the shell were found. Data represented as means ± SEMs. Saline: n = 5 mice; MA: n = 4 mice; NIC: n = 5 mice; MA/NIC: n = 4 mice. MAP-2 = microtubule associated protein 2, MA = methamphetamine, NIC = nicotine.
3.8. Plasma Corticosterone Levels
The effect of treatment on the stress response was assessed by measuring plasma corticosterone levels. There was no effect of treatment group on plasma corticosterone levels (p = 0.1; Table 3).
4. Discussion
The purpose of this study was to examine the effects of early adolescent MA and/or NIC exposure on behavior, cognition, MAP-2 levels in the NAc, and plasma corticosterone levels later in adolescence in mice. We found that neurotoxic doses of MA and NIC in early adolescence each reduced anxiety-like behavior in the open field test compared to saline controls, while co-exposure to MA and NIC did not cause this reduction in anxiety-like behavior.
Our study found that early adolescent MA exposure decreased anxiety-like behavior in the open field. This reduction in anxiety-like behavior following adolescent MA exposure is in contrast to what is observed in abstinent adolescent human MA users, who show increased anxiety levels following a social stressor [14]. However, studies examining the effects of MA on anxiety in rodents have generated conflicting findings. For example, acute adult MA exposure decreases anxiety-like behavior in the open field [48, 49] and elevated plus maze [49–51] in rats while acute adolescent MA exposure increases anxiety-like behavior in the open field [52] in mice. In mice, a neurotoxic dose of MA in adulthood increases anxiety-like behavior in the light-dark test one week after MA exposure [53] while a neurotoxic dose of MA in adolescence does not alter anxiety-like behavior in the open field later in adolescence [20]. Considering our finding that adolescent MA exposure decreased anxiety-like behavior later in adolescence, these data suggest that the effect of MA on anxiety-like behavior depends on the age of exposure as well as the age at which the behavior is measured. MA has direct effects on the DA, norepinephrine, and serotonin neurotransmitter systems [54], and indirect effects on the acetylcholine (ACh) system [55, 56], and alterations in anxiety-like behavior may arise from MA’s acute and chronic effects on these neurotransmitter systems. More research is needed to elucidate how MA alters anxiety-like behavior in an age-dependent manner and the neurobiological mechanisms underlying these changes.
Our study found that early adolescent NIC exposure decreased anxiety-like behavior in the open field test. Consistent with our findings, previous research has shown that adolescent NIC exposure decreases anxiety-like behavior in adolescent rats in the elevated plus maze [23]. However there is also conflicting research on the effects of NIC on anxiety-like behavior among different age groups. For example, adolescent exposure to NIC has been shown to increase anxiety-like behavior [57] or have no effect on anxiety-like behavior [58] in adulthood in rats in the elevated plus maze. Adult NIC exposure does not alter anxiety-like behavior in the elevated plus maze [57]. Prenatal NIC exposure increases anxiety-like behavior in adulthood but not in adolescence in rats in the elevated plus maze [59]. NIC alters the levels of ACh, DA, norepinephrine, and serotonin, and changes in these neurotransmitter systems following NIC exposure differ between adolescents and adults [22]. Similar to MA, NIC exerts developmental age-specific effects on anxiety-like behavior that depend on the age of exposure as well as the age at which the behavior is measured.
Interestingly, the co-administration of MA/NIC did not produce the same anxiolytic effect of either drug alone in the open field, demonstrating an effect of co-administration that is distinct from that of either drug alone. While MA and NIC decreased anxiety-like behavior, the co-administration of MA/NIC resulted in anxiety-like behavior that was comparable to saline control levels. One possible neurobiological explanation for this finding could involve changes in the ACh and DA systems induced by NIC and MA. Elevated ACh and low DA levels in the NAc are associated with increased anxiety and stress responses [60]. It is therefore possible that adolescent exposure to MA or NIC in our study altered NAc DA and ACh levels, respectively, while co-administration of MA/NIC preserved the DA and ACh balance in the NAc, thus attenuating the behavioral changes associated with each drug alone. As we did not analyze ACh and DA changes in the NAc following adolescent MA and/or NIC exposure in the current study, future research is warranted to examine the age-specific effects of adolescent MA and/or NIC exposure on NAc neurotransmitter levels and how this might relate to changes in anxiety-like behaviors.
Adolescent MA and/or NIC treatment had no significant effect on the intensity of MAP-2 immunoreactivity in the NAc. This result is in contrast to findings in other age groups. For example, MAP-2 mRNA and protein levels in the cortex and hippocampus are decreased in adult rats following MA exposure [34]. Neonatal exposure to MA decreases MAP-2 levels in the hippocampus in adulthood in mice, although consistent with our findings, similar changes were not shown in the NAc [33]. There was also no effect of adolescent MA and/or NIC exposure on plasma corticosterone levels. Previous research has shown that MA increases plasma corticosterone several weeks after exposure in adult mice [61] and that acute MA exposure immediately increases corticosterone levels in neonatal rodents [33, 62–64] and adult rodents [51, 65–67], but not in adolescent rodents [52]. Consistent with these findings, our data suggest that adolescent MA exposure does not exert long-term effects on corticosterone levels in adolescent mice.
We found no differences in MA-induced CPP among the treatment groups. Previous studies show that prenatal exposure to MA does not alter MA-induced CPP in adult rats [68] and NIC pre-treatment has no effect on MA CPP in adult rats [69]. Similarly, our findings suggest that pre-exposure to MA and/or NIC during early adolescence does not alter subsequent MA CPP. Adolescent MA exposure also did not affect memory in either the novel object recognition test or the Morris water maze. Adult MA exposure induces long-term impairments in object recognition memory in rats [70–72] and adult and neonatal MA exposure impairs novel object recognition memory and water maze memory performance later in life in rodents [16, 46, 73–75]. However, consistent with our findings, adolescent MA exposure does not impair novel object recognition or memory in the Morris water maze [20]. This suggests that adolescent rodents are potentially protected from the effects of MA exposure on memory compared to other age groups. However, it should be noted that we used a short retention interval for the novel object recognition test (5 minutes), and a more challenging, longer interval may reveal effects of MA on novel object recognition in adolescent mice. Thus further research is required to assess the effects of MA on memory performance in adolescence. Finally we also found no effects of MA or NIC exposure on depression-like behavior in the Porsolt forced swim test. In contrast, previous research has shown increased depression-like behavior in male mice following adolescent MA exposure [20]. The lack of any significant effect of MA on depression-like behavior in our study could have been due to differences in behavioral testing order. Future research is warranted to further elucidate the effects of early adolescent MA exposure on adolescent depression-like behavior.
Future studies might increase the dose of MA exposure in adolescence. The dose used in the current study was based on previous research using similar dosing paradigms [20, 40, 76] and we did not experience any MA-induced lethality in our study. We based our dose of 7.5 mg/kg MA (4 injections per day for 2 days) on previous studies in rodents showing that 10 mg/kg of MA (4 injections for only one day) is neurotoxic and decreases DA levels in adult mice [61] and 5 mg/kg of MA (4 injections for 2 days) during adolescence decreases the DA system in adult mice [76]. While the treatment paradigm used in this study was neurotoxic to the DA system, it is possible that higher doses of MA are needed to produce more significant effects in certain tests used in our study and to further assess the potentially mitigating effects of concurrent NIC exposure on MA-induced brain and behavioral changes.
In summary, the findings from this study suggest that MA and NIC exposure during early adolescence each decrease anxiety-like behavior in the open field test, and that this effect is not produced by the co-administration of MA and NIC together. Future studies examining the effects of early adolescent MA and/or NIC exposure are necessary to better understand the effects of early adolescent MA exposure and how NIC modulates these effects.
Acknowledgments
This work was supported by a James D. Kennedy III Faculty Fellowship from Sewanee: The University of the South, and by the development account of Dr. Raber. All authors contributed to the data analysis and writing of the manuscript.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to report.
References
- 1.NIDA. Research Report Series : Methamphetamine Abuse and Addiction. 2006:1–8. [Google Scholar]
- 2.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–49. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Rawson R, Gonzales R, McCann M, Ling W. Use of methamphetamine by young people: is there reason for concern? Addiction. 2007;102:1021–1022. doi: 10.1111/j.1360-0443.2007.01899.x. [DOI] [PubMed] [Google Scholar]
- 5.Gonzales R, Ang A, McCann MJ, Rawson RA. An emerging problem: methamphetamine abuse among treatment seeking youth. Subst Abus. 2008;29:71–80. doi: 10.1080/08897070802093312. [DOI] [PubMed] [Google Scholar]
- 6.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 7.Yen CF, Chong MY. Comorbid psychiatric disorders, sex, and methamphetamine use in adolescents: a case-control study. Compr Psychiatry. 2006;47:215–20. doi: 10.1016/j.comppsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Brecht ML, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 9.Brecht ML, Greenwell L, Anglin MD. Substance use pathways to methamphetamine use among treated users. Addict Behav. 2007;32:24–38. doi: 10.1016/j.addbeh.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Miura H, Fujiki M, Shibata A, Ishikawa K. Prevalence and profile of methamphetamine users in adolescents at a juvenile classification home. Psychiatry Clin Neurosci. 2006;60:352–7. doi: 10.1111/j.1440-1819.2006.01513.x. [DOI] [PubMed] [Google Scholar]
- 11.Rawson RA, Gonzales R, Obert JL, McCann MJ, Brethen P. Methamphetamine use among treatment-seeking adolescents in Southern California: participant characteristics and treatment response. J Subst Abuse Treat. 2005;29:67–74. doi: 10.1016/j.jsat.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Zapata LB, Hillis SD, Marchbanks PA, Curtis KM, Lowry R. Methamphetamine use is independently associated with recent risky sexual behaviors and adolescent pregnancy. J Sch Health. 2008;78:641–8. doi: 10.1111/j.1746-1561.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- 13.Embry D, Hankins M, Biglan A, Boles S. Behavioral and social correlates of methamphetamine use in a population-based sample of early and later adolescents. Addict Behav. 2009;34:343–51. doi: 10.1016/j.addbeh.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King G, Alicata D, Cloak C, Chang L. Psychiatric symptoms and HPA axis function in adolescent methamphetamine users. J Neuroimmune Pharmacol. 2010;5:582–91. doi: 10.1007/s11481-010-9206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King G, Alicata D, Cloak C, Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl) 2010;212:243–9. doi: 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41-50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21-30 or P31-40) or adult rats (P51-60) Neurotoxicol Teratol. 2005;27:117–34. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Anker JJ, Baron TR, Zlebnik NE, Carroll ME. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye T, Pozos H, Phillips TJ, Izquierdo A. Long-term effects of exposure to methamphetamine in adolescent rats. Drug Alcohol Depend. 2014;138:17–23. doi: 10.1016/j.drugalcdep.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joca L, Zuloaga DG, Raber J, Siegel JA. Long-Term Effects of Early Adolescent Methamphetamine Exposure on Depression-Like Behavior and the Hypothalamic Vasopressin System in Mice. Dev Neurosci. 2014;36:108–118. doi: 10.1159/000360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. Eur J Pharmacol. 2004;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res Bull. 2008;76:626–39. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Elliott BM, Faraday MM, Phillips JM, Grunberg NE. Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav. 2004;77:21–8. doi: 10.1016/j.pbb.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Bondy SC, Ali SF, Kleinman MT. Exposure of mice to tobacco smoke attenuates the toxic effect of methamphetamine on dopamine systems. Toxicol Lett. 2000;118:43–6. doi: 10.1016/s0378-4274(00)00267-8. [DOI] [PubMed] [Google Scholar]
- 25.Maggio R, Riva M, Vaglini F, Fornai F, Molteni R, Armogida M, Racagni G, Corsini GU. Nicotine prevents experimental parkinsonism in rodents and induces striatal increase of neurotrophic factors. J Neurochem. 1998;71:2439–46. doi: 10.1046/j.1471-4159.1998.71062439.x. [DOI] [PubMed] [Google Scholar]
- 26.Ryan RE, Ross SA, Drago J, Loiacono RE. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–6. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neugebauer NM, Harrod SB, Bardo MT. Nicotine elicits methamphetamine-seeking in rats previously administered nicotine. Drug Alcohol Depend. 2010;106:72–8. doi: 10.1016/j.drugalcdep.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieira-Brock PL, McFadden LM, Nielsen SM, Smith MD, Hanson GR, Fleckenstein AE. Nicotine Administration Attenuates Methamphetamine-Induced Novel Object Recognition Deficits. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada A, Teng J, Takei Y, Oguchi K, Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J Cell Biol. 2002;158:541–9. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 31.Peister A, Zeitouni S, Pfankuch T, Reger RL, Prockop DJ, Raber J. Novel object recognition in Apoe(−/−) mice improved by neonatal implantation of wild-type multipotential stromal cells. Exp Neurol. 2006;201:266–9. doi: 10.1016/j.expneurol.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Haley GE, Kohama SG, Urbanski HF, Raber J. Age-related decreases in SYN levels associated with increases in MAP-2, apoE, and GFAP levels in the rhesus macaque prefrontal cortex and hippocampus. Age (Dordr) 2010;32:283–96. doi: 10.1007/s11357-010-9137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acevedo SF, Pfankuch T, van Meer P, Raber J. Role of histamine in short- and long-term effects of methamphetamine on the developing mouse brain. J Neurochem. 2008;107:976–86. doi: 10.1111/j.1471-4159.2008.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putzke J, Spina MG, Buchler J, Kovar KA, Wolf G, Smalla KH. The effects of p-chloroamphetamine, methamphetamine and 3,4-methylenedioxymethamphetamine (ecstasy) on the gene expression of cytoskeletal proteins in the rat brain. Addict Biol. 2007;12:69–80. doi: 10.1111/j.1369-1600.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- 35.Loewinger GC, Beckert MV, Tejeda HA, Cheer JF. Methamphetamine-induced dopamine terminal deficits in the nucleus accumbens are exacerbated by reward-associated cues and attenuated by CB1 receptor antagonism. Neuropharmacology. 2012;62:2192–201. doi: 10.1016/j.neuropharm.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse. 1997;27:153–60. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 37.Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol. 1999;371:123–35. doi: 10.1016/s0014-2999(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 38.Trulson ME, Cannon MS, Faegg TS, Raese JD. Tyrosine hydroxylase immunochemistry and quantitative light microscopic studies of the mesolimbic dopamine system in rat brain: effects of chronic methamphetamine administration. Brain Res Bull. 1987;18:269–77. doi: 10.1016/0361-9230(87)90201-2. [DOI] [PubMed] [Google Scholar]
- 39.Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, Furukawa Y, Ang L, Adams V, Reiber G, Anthony RA, Wickham D, Kish SJ. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry. 2000;5:664–72. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- 40.Good RL, Radcliffe RA. Methamphetamine-induced locomotor changes are dependent on age, dose and genotype. Pharmacol Biochem Behav. 2011;98:101–11. doi: 10.1016/j.pbb.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Q, Long Y, Hang A, Zan GY, Shu XH, Wang YJ, Liu JG. The anxiolytic- and antidepressant-like effects of ATPM-ET, a novel kappa agonist and mu partial agonist, in mice. Psychopharmacology (Berl) 2016;233:2411–8. doi: 10.1007/s00213-016-4292-z. [DOI] [PubMed] [Google Scholar]
- 42.Clement Y, Calatayud F, Belzung C. Genetic basis of anxiety-like behaviour: a critical review. Brain Res Bull. 2002;57:57–71. doi: 10.1016/s0361-9230(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 43.Demuyser T, Deneyer L, Bentea E, Albertini G, Van Liefferinge J, Merckx E, De Prins A, De Bundel D, Massie A, Smolders I. In-depth behavioral characterization of the corticosterone mouse model and the critical involvement of housing conditions. Physiol Behav. 2016;156:199–207. doi: 10.1016/j.physbeh.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Westbrook SR, Brennan LE, Stanton ME. Ontogeny of object versus location recognition in the rat: acquisition and retention effects. Dev Psychobiol. 2014;56:1492–506. doi: 10.1002/dev.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci. 2010;4:29. doi: 10.3389/fnbeh.2010.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acevedo SF, de Esch IJ, Raber J. Sex- and histamine-dependent long-term cognitive effects of methamphetamine exposure. Neuropsychopharmacology. 2007;32:665–72. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- 47.Dobbs LK, Cunningham CL. The role of the laterodorsal tegmental nucleus in methamphetamine conditioned place preference and locomotor activity. Behav Brain Res. 2014;265:198–202. doi: 10.1016/j.bbr.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herbert CE, Hughes RN. A comparison of 1-benzylpiperazine and methamphetamine in their acute effects on anxiety-related behavior of hooded rats. Pharmacol Biochem Behav. 2009;92:243–50. doi: 10.1016/j.pbb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Schutova B, Hruba L, Pometlova M, Slamberova R. Impact of prenatal and acute methamphetamine exposure on behaviour of adult male rats. Prague Med Rep. 2009;110:67–78. [PubMed] [Google Scholar]
- 50.Tamaki R, Yoshikawa M, Shinomiya T, Hashimoto A, Kawaguchi M, Byrne DW, Kobayashi H. Acute administration of methamphetamine decreases the mRNA expression of diazepam binding inhibitor in rat brain. Tokai J Exp Clin Med. 2008;33:51–6. [PubMed] [Google Scholar]
- 51.Szumlinski KK, Haskew RE, Balogun MY, Maisonneuve IM, Glick SD. Iboga compounds reverse the behavioural disinhibiting and corticosterone effects of acute methamphetamine: Implications for their antiaddictive properties. Pharmacol Biochem Behav. 2001;69:485–91. doi: 10.1016/s0091-3057(01)00564-0. [DOI] [PubMed] [Google Scholar]
- 52.Rud MA, Do TN, Siegel JA. Effects of early adolescent methamphetamine exposure on anxiety-like behavior and corticosterone levels in mice. Neurosci Lett. 2016;633:257–261. doi: 10.1016/j.neulet.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 53.Wu CF, Liu YL, Song M, Liu W, Wang JH, Li X, Yang JY. Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol Biochem Behav. 2003;76:103–9. doi: 10.1016/s0091-3057(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 54.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104:1085–99. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 55.Siegal D, Erickson J, Varoqui H, Ang L, Kalasinsky KS, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Brain vesicular acetylcholine transporter in human users of drugs of abuse. Synapse. 2004;52:223–32. doi: 10.1002/syn.20020. [DOI] [PubMed] [Google Scholar]
- 56.Dobbs LK, Mark GP. Comparison of systemic and local methamphetamine treatment on acetylcholine and dopamine levels in the ventral tegmental area in the mouse. Neuroscience. 2008;156:700–11. doi: 10.1016/j.neuroscience.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iniguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolanos-Guzman CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–24. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodwin AK, Lantz-McPeak SM, Robinson BL, Law CD, Ali SF, Ferguson SA. Effects of adolescent treatment with nicotine, harmane, or norharmane in male Sprague-Dawley rats. Neurotoxicol Teratol. 2015;47:25–35. doi: 10.1016/j.ntt.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Eppolito AK, Bachus SE, McDonald CG, Meador-Woodruff JH, Smith RF. Late emerging effects of prenatal and early postnatal nicotine exposure on the cholinergic system and anxiety-like behavior. Neurotoxicol Teratol. 2010;32:336–45. doi: 10.1016/j.ntt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Hoebel BG, Avena NM, Rada P. Accumbens dopamine-acetylcholine balance in approach and avoidance. Curr Opin Pharmacol. 2007;7:617–27. doi: 10.1016/j.coph.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grace CE, Schaefer TL, Herring NR, Graham DL, Skelton MR, Gudelsky GA, Williams MT, Vorhees CV. Effect of a neurotoxic dose regimen of (+)-methamphetamine on behavior, plasma corticosterone, and brain monoamines in adult C57BL/6 mice. Neurotoxicol Teratol. 2010;32:346–55. doi: 10.1016/j.ntt.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grace CE, Schaefer TL, Herring NR, Skelton MR, McCrea AE, Vorhees CV, Williams MT. (+)-Methamphetamine increases corticosterone in plasma and BDNF in brain more than forced swim or isolation in neonatal rats. Synapse. 2008;62:110–21. doi: 10.1002/syn.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol. 2000;22:751–9. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 64.Zuloaga DG, Siegel JA, Acevedo SF, Agam M, Raber J. Developmental methamphetamine exposure results in short- and long-term alterations in hypothalamic-pituitary-adrenal-axis-associated proteins. Dev Neurosci. 2013;35:338–46. doi: 10.1159/000351278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Braun AA, Herring NR, Schaefer TL, Hemmerle AM, Dickerson JW, Seroogy KB, Vorhees CV, Williams MT. Neurotoxic (+)-methamphetamine treatment in rats increases brain-derived neurotrophic factor and tropomyosin receptor kinase B expression in multiple brain regions. Neuroscience. 2011;184:164–71. doi: 10.1016/j.neuroscience.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herring NR, Schaefer TL, Tang PH, Skelton MR, Lucot JP, Gudelsky GA, Vorhees CV, Williams MT. Comparison of time-dependent effects of (+)-methamphetamine or forced swim on monoamines, corticosterone, glucose, creatine, and creatinine in rats. BMC Neurosci. 2008;9:49. doi: 10.1186/1471-2202-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuloaga DG, Johnson LA, Agam M, Raber J. Sex Differences in Activation of the Hypothalamic-Pituitary-Adrenal Axis by Methamphetamine. J Neurochem. 2014;129:495–508. doi: 10.1111/jnc.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slamberova R, Schutova B, Hruba L, Pometlova M. Does prenatal methamphetamine exposure affect the drug-seeking behavior of adult male rats? Behav Brain Res. 2011;224:80–6. doi: 10.1016/j.bbr.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 69.Berry JN, Neugebauer NM, Bardo MT. Reinstatement of methamphetamine conditioned place preference in nicotine-sensitized rats. Behav Brain Res. 2012;235:158–65. doi: 10.1016/j.bbr.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marshall JF, Belcher AM, Feinstein EM, O’Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007;102(Suppl 1):61–9. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- 71.Belcher AM, O’Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–34. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- 72.Belcher AM, Feinstein EM, O’Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;33:1453–63. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- 73.Siegel JA, Craytor MJ, Raber J. Long-term effects of methamphetamine exposure on cognitive function and muscarinic acetylcholine receptor levels in mice. Behav Pharmacol. 2010;21:602–14. doi: 10.1097/FBP.0b013e32833e7e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siegel JA, Park BS, Raber J. Long-term effects of neonatal methamphetamine exposure on cognitive function in adolescent mice. Behav Brain Res. 2011;219:159–164. doi: 10.1016/j.bbr.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003;168:329–38. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- 76.Good RL, Liang LP, Patel M, Radcliffe RA. Mouse strain- and age-dependent effects of binge methamphetamine on dopaminergic signaling. Neurotoxicology. 2011;32:751–9. doi: 10.1016/j.neuro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


