Abstract
There is a renewed interest in better conceptualizing trajectories of attention-deficit/hyperactivity disorder (ADHD) from childhood to adulthood, driven by an increased recognition of long-term impairment and potential persistence beyond childhood and adolescence. This review addresses the following major issues relevant to the course of ADHD in light of current evidence from longitudinal studies: (1) conceptual and methodological issues related to measurement of persistence of ADHD, (2) estimates of persistence rate from childhood to adulthood and its predictors, (3) long-term negative outcomes of childhood ADHD and their early predictors, and (4) the recently proposed new adult-onset ADHD. Estimates of persistence vary widely in the literature, and diagnostic criteria, sample characteristics, and information source are the most important factors explaining variability among studies. Evidence indicates that ADHD severity, comorbid conduct disorder and major depressive disorder, and treatment for ADHD are the main predictors of ADHD persistence from childhood to adulthood. Comorbid conduct disorder and ADHD severity in childhood are the most important predictors of adverse outcomes in adulthood among children with ADHD. Three recent population studies suggested the existence of a significant proportion of individuals who report onset of ADHD symptoms and impairments after childhood. Finally, we highlight areas for improvement to increase our understanding of ADHD across the life span.
Keywords: ADHD, Persistence, Outcomes, Predictors, Course, Longitudinal investigations
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurobiological disorder characterized by a persistent and impairing pattern of inattentive, hyperactive, and/or impulsive symptoms [1]. Meta-analyses suggest prevalence rates around 5 to 7.1 % in childhood and 2.5 to 5 % in adulthood [2–4]. The disorder is associated with adverse outcomes for affected individuals, their families, and society in general [5].
There is recent interest in better conceptualized adult ADHD [6] and its trajectories from childhood to adulthood [5]. A previous meta-analysis found that only 15 % of diagnosed children continued presenting full ADHD diagnosis in adulthood, although 65 % presented with a subsyndromal phenotype [7]. This figure would suggest a much lower adult ADHD prevalence rate (15 % of 5–7 % = 0.8–1.1 %) than what has been detected both in meta-analyses (2.5–5 %) [3, 4] and in a multinational study on ADHD prevalence in adults (3.4 %) [8]. There is a current debate about reasons for this discrepancy. Some investigators suggest that the 15 % persistence rate is a clear underestimation due to change of informants between adult and child assessments and inadequacy of the ADHD diagnostic criteria for adults. Major aspects of both the ADHD phenotype and its impairments might be different in adults, and other approaches to define persistence, like cognitive and social dysfunction, are lacking in the literature [6]. In addition, controversies also exist surrounding new findings suggesting an unexpected ADHD trajectory. Three recent population studies found a substantial number of individuals with onset of clinically significant ADHD symptoms and impairments after childhood, challenging the established notion of ADHD as exclusively a childhood-onset neurodevelopmental disorder [9•, 10•, 11•].
This narrative review of the literature addresses the following topics that might increase our understanding about these discrepant findings: (a) conceptual and methodological issues inherent to the study of ADHD trajectories, (b) data on persistence rates from longitudinal ADHD studies, (c) predictors of ADHD persistence from childhood to adulthood, (d) child and adolescent predictors of adult ADHD negative outcome, and (e) new adult-onset cases and their predictors.
Conceptual and Methodological Issues Inherent to the Study of ADHD Trajectories
(a) ADHD diagnosis
In the last 50 years, the diagnostic criteria for ADHD from Diagnostic and Statistical Manual of Mental Disorders second edition (DSM-II) to DSM-5 have been modified [1, 12–14]. Previous work has demonstrated that the use of different diagnostic criteria is one of the major factors influencing variability in ADHD prevalence rates worldwide over the last three decades [2, 15]. ADHD persistence is the proportion affected by the diagnostic definitions in childhood (denominator) who also meet the definition in adult life (numerator) [6, 9•]. A birth cohort study with assessments of the disorder from childhood to adulthood (15) provided different ADHD persistence rates depending on the diagnostic system used on multiple follow-up waves [16]. An adult norm-based diagnostic approach yielded the highest persistence rate compared to any other approaches (29.3 % against 11.2 to 13.8 % for strict criteria definitions). While some studies assessed individuals at baseline in childhood for ADHD by using previous classifications (DSM-II, DSM-III) [17–22], others used more contemporary systems such as DSM-IV [11•, 23, 24]. Assessments at follow-up are likewise a source of heterogeneity in persistence estimates: Studies have used DSM-III [18, 25], DSM-IV [16, 21, 22, 26], and DSM-5 [9•, 11•] criteria to determine ADHD diagnosis in adulthood. Differences of criteria may occur even in a same study in longitudinal assessments.
One study evaluated how differences in case definition might impact persistence estimates in the 16-year clinical follow-up of the Multimodal Treatment Study of ADHD (MTA) [27•]. Persistence estimates varied widely from 1.9 % (requiring DSM-IV criteria, combining parent and self-report in the Diagnostic Interview Schedule for Children (DISC) with an item-level AND rule) to 61.4 % (requiring norm-based symptom count, combining parent and self-report in the DISC with an item-level OR rule). Based on findings from a Receiver Operating Curve (ROC) analysis of impairment, the authors concluded that the best combination of sensitivity and specificity was achieved by using a norm-based threshold of four symptoms from either list (more than two standard deviations above the mean of the local normative comparison group) assessed with rating scales and combining parental and self-report information with an item-level OR rule. This approach yielded a persistence rate of 60 % for symptoms and 41 % for symptoms with impairment.
Although the presence of impairment has been required by the successive revisions of diagnostic criteria for ADHD, the level of impairment required is not unanimous. The level of impairment substantially affects variability in ADHD prevalence rates worldwide and across the last three decades [2, 15]. Using full DSM-5 criteria, a recent population study assessing ADHD prevalence in adults [28] found a rate of 3.55 % (95 % CI 2.98–4.12 %) for at least moderate impairment, but only 1.4 % for severe clinical impairment. Thus, diagnostic rates vary substantially from one study to another depending on the level of impairment required for diagnosing the disorder at baseline and endpoint [29].
Another conceptual issue is the source of impairment, which has varied across studies. Some studies used general measures of impairment, such as the Clinical Global Assessment Scale [30], Clinical Global Impression Scale [31], or the Global Assessment of Functioning [32], while others used measures that specifically assess impairment derived from ADHD symptoms, such as questions included in ADHD modules of structured or semi-structured diagnostic instruments [11•, 21, 22]. Instruments created to assess impairment specifically related to ADHD, as the Barkley Functional Impairment Scale (BFIS) were also used [23]. Two paramount clinical follow-ups, the multimodal treatment study of children with ADHD (MTA) [27•] and the Pittsburgh ADHD Longitudinal Study (PALS) [33], used the Impairment Rating Scale (IRS) proposed by Fabiano et al. for children with ADHD [34]. Although it is questionable whether the source of impairment can be clearly specified when comorbidity is the rule, persistence rates ascertained by different instruments, even for the same level of impairment, may be substantially different.
(b) Sample characteristics
The origin of the sample (community or clinical) affects prevalence rates and clinical correlates of psychiatric disorders [35]. Clinical samples of individuals with ADHD in general include more severe cases than population samples and thus report increased comorbidity [36]. Part of this increased morbidity is expected according to the “Berkson’s bias,” a mathematical bias due to restricting the sample to those individuals seeking clinical treatment and showing greater levels of severity and comorbidity [35, 37]. Thus, it is not surprising that ADHD persistence rates appear to be higher in clinical samples [19, 23, 24, 29] than in population-based samples [9•, 10•, 11•]. Barriers to health services across countries also affect persistence rates. It is expected that clinical samples will select more severe and socially deprived cases in countries with accessible health care like the UK or Scandinavian countries [38].
Additionally, retention rate is related to selection bias [39] affecting the representativeness of the sample, especially in population-based samples. A population-based sample with a substantial amount of participants lost to follow-up might underestimate persistence rates, since severe cases may have a higher risk of persistence and a higher risk of not attending several longitudinal assessments [40].
(c) Demographic aspects
Longitudinal ADHD studies assess individuals at different ages both at baseline in childhood and endpoint during adulthood [11•, 41]. Considering the general trend that prevalence rates of ADHD decrease across the life cycle, regardless of the criteria used (see Faraone et al. [7]), age at assessment might be another factor influencing persistence rates. The literature shows that ADHD prevalence in adolescence is about half of that in childhood [2] and prevalence estimates continue to decrease in adulthood. This has been illustrated most clearly by a long ADHD follow-up study that assessed participants with childhood-onset ADHD at different time points in adulthood. The prevalence of ADHD declined to 43 % at 18 years of age and 22 % at 41 years (Mannuzza et al. [18]; Klein et al. [42]). In addition to attrition, these studies used different informants and diagnostic criteria classifications at different assessment points in adulthood, making it unclear whether the decrease in rate was mainly due to age or methodology. Regardless, age at entry into the study and age at endpoint clearly affect reported persistence rates.
The literature in general also suggests that females might have a higher persistence rate than males, as well as more negative outcomes in adulthood [43], although this could not be confirmed in the MTA sample. This sex difference might be responsible in part for the lower, in some studies absent, male/female preponderance during adulthood (see Matte et al. [28]; Vitola et al. [44]). Thus, the proportion of females in the study may affect the observed persistence rate. This might be even more important in studies reporting persistence rates based on samples composed exclusively of males or females [18, 21, 22]. However, these differences might also be due to higher severity, comorbidity, or adverse social background of girls diagnosed with ADHD compared to boys, instead of being only determined by gender.
(d) Informants
Who is reporting the information is a major factor explaining heterogeneity in worldwide ADHD prevalence in childhood and adolescence [2]. The agreement between parents and teachers on ADHD symptoms is low in childhood, and the literature indicates that children tend to underreport their ADHD symptoms [5]. Consequently, the choice of the informant in childhood impacts the estimate of prevalence, and changing sources may impact estimates of persistence. As a complication, this informant effect may differentially influence various aspects of ADHD diagnostic criteria (e.g., either symptoms or impairment).
Although some reports suggest good inter-rater reliability between adult self-report and parent reports of childhood and adulthood symptoms [45], others documented that neither are reliable for retrospectively reporting ADHD symptoms in childhood [9•, 46]. In adult clinical studies, parents or relatives that knew the individual during childhood tend to report retrospectively fewer childhood ADHD symptoms than adult retrospective self-reports [33, 47], the opposite of adult current report on symptoms and impairments [27•, 43].
Thus, persistence rates will depend heavily on which information source was selected during childhood and adulthood. This is especially important because some studies change information source from the parent source in childhood to affected individual (self) source in adulthood, potentially artificially deflating persistence rates [9•, 10•, 11•].
Data on Persistence Rates From Longitudinal ADHD Studies From Childhood to Adulthood
Based on the issues discussed above, it is not surprising that ADHD persistence rates from childhood to adulthood vary substantially among studies (Fig. 1). The figure shows estimates of full ADHD diagnostic persistence reported by longitudinal studies that followed children to a mean age of at least 18 years. A comparison of the extremes is informative. The lowest rate detected was 4 % in a clinical study in the USA [18]. This study followed referred boys diagnosed with DSM-II hyperkinetic disorder at ages 5 to 11 years and reassessed their ADHD status 17 years later with DSM-III-R criteria. Potential factors responsible for this low rate include the following: (1) The sample was composed exclusively of males (see below); (2) patients with comorbid conduct disorder at baseline were excluded; (3) change of diagnostic system and assessment approach: DSM-II with clinical interview at baseline and DSM-III-R with structured interview at follow-up; (4) requirement of endorsement of childhood ADHD symptoms and impairment at follow-up to diagnose adult ADHD; and (5) the strict use of a DSM threshold instead of a norm-based approach. The authors emphasize that recall bias might have constrained the persistence rate (see Klein et al. [42]). The highest ADHD persistence rate found was 76 % in the UK study by Cheung and colleagues [23]. Authors followed children and adolescents (mean age 11.8, 87 % males) with ADHD combined type (DSM-IV) criteria for 6.6 years. Factors that might be responsible for this very high rate include (1) a short follow-up time; (2) similarity of assessment in the two time points, using DSM-IV and a structured interview and no change of information source (parent report); (3) a clinical sample composed of only ADHD combined type (see below); and (4) relatively young age at follow-up.
Fig. 1.
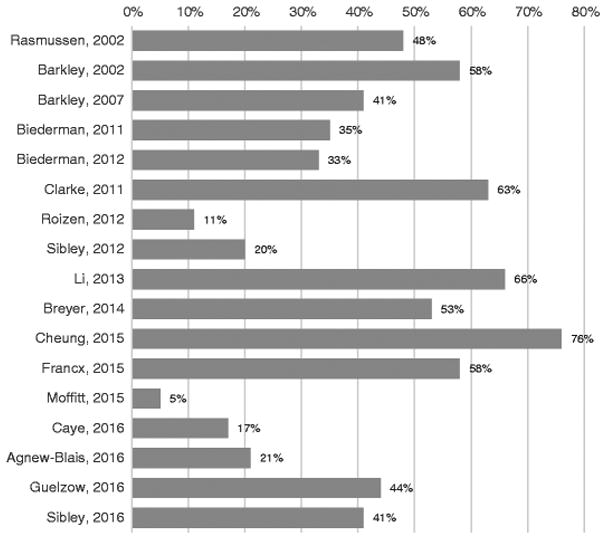
Estimates of ADHD persistence rates into adulthood in longitudinal studies. All reported studies are longitudinal studies with mean age at follow-up of at least 18 years old and a full diagnosis (syndromatic) definition of persistence
Predictors of ADHD Persistence From Childhood to Adulthood
The comprehensive review of persistence rates found few studies that report factors in childhood related to the course of symptoms into adulthood. A recent systematic review and meta-analysis summarizing the findings thus far concluded that available reports are heterogeneous and hard to combine [48••]. However, a meta-analysis of predictors assessed and reported by at least three studies is summarized in Table 1.
Table 1.
Summary of risk factors reported in the systematic review and meta-analysis by Caye and colleagues [48••]
| Factors meta-analyzed and significantly associated with persistence | |||
|---|---|---|---|
| Predictor | Odds ratio | 95 % Confidence interval | P value |
| Severe ADHD | 2.33 | 1.6–3.39 | <0.001 |
| Treatment for ADHD | 2.09 | 1.04–4.18 | 0.037 |
| Comorbid major depressive disorder | 1.80 | 1.1–2.95 | 0.019 |
| Comorbid Conduct Disorder | 1.85 | 1.06–3.24 | 0.03 |
| Factors meta-analyzed nonsignificantly associated with persistencea | |||
| Predictor | Odds ratio | 95 % Confidence interval | P value |
| Female gender | 1.23 | 0.84–1.81 | 0.295 |
| Comorbid ODD | 1.65 | 0.75–3.65 | 0.213 |
| Factors meta-analyzed and consistently not associated with persistence | |||
| Predictor | Odds ratio | 95 % Confidence interval | P value |
| Single parent family | 1.08 | 0.25–1.29 | 0.179 |
| Predictor | SMD | 95 % Confidence interval | P value |
| Intelligence quotient | 0.03 | −0.18–−0.23 | 0.8 |
| Factors not meta-analyzed but associated with persistence in individual studies | |||
| Combined ADHD subtype • comorbid bipolar disorder • parental ASPD | |||
SMD standardized mean difference, ASPD antisocial personality disorder
Authors note that sensitive analysis or the adoption of less conservative meta-analysis techniques (fixed-effects models) would result in a positive and significant association for comorbid ODD and female gender, whereas single parent family and intelligence quotient have consistent small and not significant effects on persistence across included studies
Characteristics of the clinical syndrome were the most consistent risk factors for persistence: comorbid conditions like conduct disorder (CD) and major depressive disorder (MDD), severe ADHD, and treatment for ADHD are associated with ADHD persistence. The finding that ADHD treatment is a risk factor for persistence is not surprising, since the most severe cases are selected for treatment. Barriers to health care may influence this finding; lack of access to treatment might be a marker of environmental or socioeconomic risk factors (e.g., ethnic minorities or living in an area with limited resources [49, 50]). Importantly, the two studies that found the effect of ADHD treatment adjusted their findings for disorder severity, but possibly, the treatment-severity relationship was not fully captured by the instruments used. A clinical study that followed individuals for 5 years after a 12-month randomized clinical trial found that medication adherence was related to greater improvement but higher endpoint symptoms, while symptom severity at baseline was the most important single predictor of persistent symptoms at follow-up [51]. Disentangling this bias adequately would require a randomized clinical trial with good adherence and retention for several years comparing outcome between allocated groups at baseline. However, maintaining adherence to assigned treatment over long periods may not be possible.
An analysis of the MTA evaluated childhood factors influencing persistence of ADHD into adulthood at a mean age of 24.7 years [52]. ADHD symptom severity, comorbidities, and parental mental health problems were the most important risk factors for persistence, while childhood IQ, socioeconomic status, parental education, and parent-child relationships showed no association with persistence. These findings are, in general, similar to what was reported in the meta-analysis (see Table 1). However, treatment assignment was not evaluated as a risk factor, having been found in previous reports to have lost significant association with symptom severity by 3 years of follow-up [53, 54].
Evaluation and Prediction of Trajectories of ADHD Symptoms
Another possible approach to evaluate persistence and remission is to investigate trajectories of symptoms rather than categorical diagnosis. Since few studies using this approach followed subjects from childhood to adulthood, we also included studies where the last assessment was in late adolescence in this section.
One study evaluated baseline differences between trajectories of ADHD symptoms (persistently high compared to declining) through grades 3 to 12, when participants are expected to be 17 or 18 years old [55]. Participants with a more chronic trajectory were more aggressive and more hyperactive at school and more emotionally dysregulated at home than their peers with a declining trajectory of ADHD symptoms. The investigators also reported a more stable pattern of inattentive symptoms compared to hyperactive symptoms, a finding that was reported in previous studies [56]. In a different study, 8395 twin pairs were assessed for ADHD at ages 8, 12, 14, and 16 with a DSM-IV ADHD symptom subscale [57]. Consistent with population-based and clinical studies, there was a general decline of symptoms across ages, and inattentive symptoms persisted more than hyperactive/impulsive symptoms. Authors reported important inter-individual differences in the developmental course of symptoms, mostly explained by genetic influences independently of baseline severity. Another study showed protective effects related to parenting and attendance in college that were manifested in the transition from adolescence to adulthood [58]. In the adult assessment of the MTA, a dimensional outcome based on symptom severity showed a large difference between the overall ADHD group and comparison group. However, neither initial random allocation to treatment with medication nor self-selected, extended use of medication significantly predicted adult outcomes on this variable within the ADHD group (Swanson et al., personal communication).
The Avon Longitudinal Study of Parents and Children (ALSPAC), a large birth cohort in the UK, analyzed factors associated with latent-class trajectories of ADHD symptoms age 4 to 17 years. The persistent class (3.9 % of the sample and 40.2 % of participants with high childhood scores) had mostly males (72.9 %) and higher conduct problems, language impairments, and social communication problems and lower IQs. Also, the persistent group had higher ADHD genetic liability as indexed by ADHD polygenic risk scores, whereas other psychiatric genetic risk scores (schizophrenia, bipolar disorder, and depression) were not associated with trajectories [59].
Predictors of Adult ADHD Deleterious Outcomes That Can Be Detected in Childhood and Adolescence
There is substantial evidence documenting adverse outcomes for those affected by ADHD compared to those without the disorder [5, 6]. ADHD affects a wide range of functional domains including academic, social, and occupational contexts. Studies have documented lower academic achievement [60, 61], higher unemployment, and lower income for probands with ADHD followed into adulthood [29, 62, 63]. The risk of substance use disorders (SUD) and antisocial personality disorder is higher in patients with ADHD than in nonaffected individuals [64–68]. Individuals with ADHD are more likely to have traffic accidents than the general population [69–71]. Other documented outcomes include obesity [72, 73], dysfunctional family relationships [29, 74], and emotional dysregulation [75]. These functional impairments may result in reduced perception of well-being [76] and be related to adverse outcomes like higher overall mortality rates in individuals with current or past ADHD diagnosis [77••]. A comprehensive meta-analysis has confirmed a longitudinal association of childhood ADHD with adverse outcomes, the most relevant being mental disorders and substance abuse, academic and professional underachievement, criminality, and risky driving behaviors [78••]. The 16-year follow-up of the MTA showed that adverse outcomes in education, work, and risky sexual behavior were associated with ADHD and symptom persistence; the risk increases in a progressive fashion: The local normative comparison group (LNCG) had the lowest risk, symptom-persistent ADHD the highest, with symptom-desistent ADHD in between. For emotional outcomes, like anxiety and depression, their difference was not significant between those whose symptoms remitted and the LNCG, while both were doing better than ADHD persisters. Alcohol use and jail time did not differ significantly across any of the groups assessed, probably because alcohol use was so common and jail time so rare [79••].
Although these adverse adult outcomes associated with ADHD are unquestionable, much less clear are their childhood predictors. Several factors have been suggested as influencing the outcome in ADHD subjects, like the clinical profile (ADHD severity and comorbidities), prenatal factors [80], genetic and family loading, gene-environment interactions, and protective factors like exercise and cognitive ability [5, 81].
A recent systematic review and meta-analysis on ADHD and criminality consistently identified these risk factors for arrests, convictions, and incarcerations [82]: male sex, low intelligence quotient, severe ADHD, and comorbid conduct disorder. Low socioeconomic status was associated in univariate analysis, but the effect faded in multivariate analysis. A study of unimodal (medication only) and multimodal treatments initiated in the 1970s evaluated long-term effects and showed an initial protective effect in the multimodal treatment group that dissipated in the adult follow-up [83, 84].
ADHD severity, comorbid conduct disorder and oppositional defiant disorder, sexual abuse, school suspension, family history of SUD or ADHD, and male gender were associated with SUD development in ADHD, whereas ADHD inattentive subtype and a fearful temperament were inversely associated [85]. One study found that the development of SUD in adulthood was predicted by age of treatment initiation in childhood (the later, the higher the risk for SUD) and that the relation was moderated by antisocial personality disorder [86]. The MTA found no residual effect of initial assignment to 14-month treatment with medication and no effect of current treatment with medication in the development of SUD in adolescence [87]. The PALS, a clinical follow-up, found medication to be a risk factor that lost significance when controlling for other factors at baseline [88].
A cross-sectional analysis of data from nationwide registers found the overall mortality rate higher among ADHD patients than in the general population, and the risk was especially higher in females and with comorbid oppositional defiant disorder, conduct disorder, and SUD. The mortality rate ratio was 4.25 (95 % confidence interval 3.05–5.78) for individuals diagnosed with ADHD at ages 18 or older, compared to 1.86 for 5 or younger and 1.58 for those diagnosed between 6 and 17 years of age [77••].
In the Milkauwee follow-up study, higher ADHD scores in childhood predicted a wide range of worse outcomes like educational, occupational, financial, and driving problems, whereas lower IQ was associated only with worse educational and occupational outcomes [29].
New Adult-Onset Cases and Their Predictors
Historically, ADHD has been conceptualized as a child-onset neurodevelopmental disorder [89]. The last DSM version (DSM-5) launched in 2013 [1] included the disorder in the neurodevelopmental disorder section. Three recent population studies from diverse cultures challenged the notion that ADHD has its onset only in childhood by suggesting the existence of a significant large proportion of individuals who report onset of ADHD symptoms and impairments after childhood [9•, 10•, 11•]. The most surprising finding among the three studies is the similarity in the rates of these new adult-onset cases in the three studies: 87 % of the adults with ADHD presented new adult-onset in the New Zealand study [9•], 87.4 % in the Brazilian study [10•], and 67.5 % in the UK investigation [11•]. However, issues have been raised about the meaning of these findings. One hypothesis is that the new onset cases are the result of the false positive paradox. Another explanation is that in all three samples, there was a shift from parent report or teacher report in childhood to self-report in adulthood. However, the British study has controlled for this potential bias in secondary analyses [11•]. A recent analysis in the ALSPAC cohort relying on the same source information for assessments, and using a screening instrument for ADHD (hyperactive Strengths and Difficulties Questionnaire scale) at ages 7 and 17 years old (parental assessment), found that 54 % of the adult cases were new-onset cases. In addition, the persistence rate was only 22 % [59].
In an analysis of predictors in childhood for the adult-onset ADHD cases, the British study [11•] found that higher IQ and lower externalizing and internalizing scores differentiated the adult-onset individuals from the ADHD-persistent group. One possible explanation for this would be that high intelligence and lack of comorbidity allow the disorder to go undetected during childhood and adolescence. In the Dunedin study, the following childhood factors differentiate the adult ADHD group from non-ADHD adult group: higher ADHD scores by teachers’ report, conduct disorder, and lower reading ability scores [9•]. Future investigations need to clarify which factors predict adult-onset cases compared to individuals without ADHD. An international effort comparing data sets from different cultures on this question is ongoing.
Conclusions
Several methodological factors intrinsically related to the ADHD diagnosis (e.g., diagnostic criteria), demographic and sample characteristics (e.g., clinical or population origin and age), and information source (self or other) seem to be responsible for different persistence rates from childhood to adulthood among studies. Since evidence from longitudinal studies on ADHD is scarce and extremely heterogeneous in methodology, it is difficult to disentangle with statistical methods the role of each of these factors in explaining the heterogeneity of ADHD persistence rate. This scenario results in a wide range of observed persistence rates among studies, from as low as 4 % [18] to as high as 76 % [23].
The available literature indicates that ADHD severity, co-morbid conduct disorder and major depressive disorder, and treatment for ADHD are the main predictors of ADHD persistence from childhood to adulthood [48••]. Comorbid conduct disorder in childhood is ubiquitous as a predictor of multiple adverse outcomes like premature mortality, SUD, and criminality, whereas other factors have controversial effects depending on the study. Male sex is a risk factor for SUD and criminality but is protective in terms of the overall mortality rate. Stimulant medication use may protect against the development of SUD (although the MTA, the largest prospective study, failed to find such an effect). Severity of ADHD appears to be positively associated with criminality and SUD, but its relationship with mortality could not be assessed due to lack of data.
Finally, innovative investigations like those suggesting the possibility of an adult-onset ADHD trajectory predicted by higher cognitive reserve and lower symptomatology in childhood are important to expand our knowledge about ADHD trajectories across the life cycle.
Biographies
James Swanson reported receiving research support, advisory board membership, speaker’s bureau membership, and/or consulting for Alza, Richwood, Shire, Celgene, Novartis, Celltech, Gliatech, Cephalon, Watson, CIBA, UCB, Janssen-Cilag, McNeil, and Eli-Lilly.
Lily Hechtman has been on advisory boards and a speaker for Shire, Jannsen, Ironshore, and Purdue Pharma. She has also received research funds from Purdue.
L. Eugene Arnold has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, Supernus, and YoungLiving (as well as NIH and Autism Speaks); has consulted with Gowlings, Neuropharm, Organon, Pfizer, Sigma Tau, Shire, Tris Pharma, and Waypoint; been on advisory boards for Arbor, Ironshore, Novartis, Noven, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, and Shire; and received travel support from Noven.
Luis Augusto Rohde was on the speakers’ bureau/advisory board and/or acted as consultant for Eli-Lilly, Janssen-Cilag, Novartis, and Shire in the last 3 years. He receives authorship royalties from Oxford Press and ArtMed. He also received travel awards for taking part in the 2014 APA meeting and 2015 WFADHD meeting from Shire. The ADHD and Juvenile Bipolar Disorder Outpatient Programs chaired by him received unrestricted educational and research support from the following pharmaceutical companies in the last 3 years: Eli-Lilly, Janssen-Cilag, Novartis, and Shire.
Footnotes
This article is part of the Topical Collection on Attention-Deficit Disorder
Compliance with Ethical Standards
Conflict of Interest Arthur Caye, Anita Thapar, Margaret Sibley, Louise Arseneault, Janni Niclasen, and Terrie Moffitt declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.APA. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 3.Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurother: J Am Soc Exp NeuroTher. 2012;9(3):490–9. doi: 10.1007/s13311-012-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon V, Czobor P, Balint S, Meszaros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry: J Ment Sci. 2009;194(3):204–11. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- 5.Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat rRev Dis Primers. 2015;1:15020. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- 6.Asherson P, Buitelaar J, Faraone SV, Rohde LA. Adult attention-deficit hyperactivity disorder: key conceptual issues. Lancet Psychiatry. 2016;3(6):568–78. doi: 10.1016/S2215-0366(16)30032-3. [DOI] [PubMed] [Google Scholar]
- 7.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006;36(2):159–65. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 8.Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry: J Ment Sci. 2007;190:402–9. doi: 10.1192/bjp.bp.106.034389. [DOI] [PubMed] [Google Scholar]
- 9•.Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, et al. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am J Psychiatry. 2015;172(10):967–77. doi: 10.1176/appi.ajp.2015.14101266. The first time that the late-onset ADHD was reported in an analysis of a four-decade longitudinal cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Caye A, Rocha TB, Anselmi L, Murray J, Menezes AM, Barros FC, et al. Attention-deficit/hyperactivity disorder trajectories from childhood to young adulthood: evidence from a birth cohort supporting a late-onset syndrome. JAMA Psychiatry. 2016;73(7):705–12. doi: 10.1001/jamapsychiatry.2016.0383. A Brazilian longitudinal cohort found similar results in regard to the late-onset ADHD and tested for multiple confounding factors in secondary analyses. [DOI] [PubMed] [Google Scholar]
- 11•.Agnew-Blais JC, Polanczyk GV, Danese A, Wertz J, Moffitt TE, Arseneault L. Evaluation of the persistence, remission, and emergence of attention-deficit/hyperactivity disorder in young adulthood. JAMA Psychiatry. 2016;73(7):713–20. doi: 10.1001/jamapsychiatry.2016.0465. An UK longitudinal cohort found similar results in regard to the late-onset ADHD and reported factors from childhood related to this trajectory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.APA American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. 1980. [Google Scholar]
- 13.APA. Diagnostic and statistical manual of mental disorders. 4. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 2. Washington, DC: American Psychiatric Association; 1968. [PubMed] [Google Scholar]
- 15.Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–42. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbaresi WJ, Weaver AL, Voigt RG, Killian JM, Katusic SK. Comparing methods to determine persistence of childhood adhd into adulthood: a prospective, population-based study. J Atten Disord. 2015 doi: 10.1177/1087054715618791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss G, Hechtman L, Milroy T, Perlman T. Psychiatric status of hyperactives as adults: a controlled prospective 15-year follow-up of 63 hyperactive children. J Am Acad Child Psychiatry. 1985;24(2):211–20. doi: 10.1016/s0002-7138(09)60450-7. [DOI] [PubMed] [Google Scholar]
- 18.Mannuzza S, Klein RG, Bessler A, Malloy P, LaPadula M. Adult psychiatric status of hyperactive boys grown up. Am J Psychiatry. 1998;155(4):493–8. doi: 10.1176/ajp.155.4.493. [DOI] [PubMed] [Google Scholar]
- 19.Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111(2):279–89. [PubMed] [Google Scholar]
- 20.Yan W. An investigation of adult outcome of hyperactive children in Shanghai. Chin Med J. 1996;109(11):877–80. [PubMed] [Google Scholar]
- 21.Biederman J, Petty CR, Clarke A, Lomedico A, Faraone SV. Predictors of persistent ADHD: an 11-year follow-up study. J Psychiatr Res. 2011;45(2):150–5. doi: 10.1016/j.jpsychires.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biederman J, Petty CR, O’Connor KB, Hyder LL, Faraone SV. Predictors of persistence in girls with attention deficit hyperactivity disorder: results from an 11-year controlled follow-up study. Acta Psychiatr Scand. 2012;125(2):147–56. doi: 10.1111/j.1600-0447.2011.01797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung CH, Rijdijk F, McLoughlin G, Faraone SV, Asherson P, Kuntsi J. Childhood predictors of adolescent and young adult outcome in ADHD. J Psychiatr Res. 2015;62:92–100. doi: 10.1016/j.jpsychires.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francx W, Zwiers MP, Mennes M, Oosterlaan J, Heslenfeld D, Hoekstra PJ, et al. White matter microstructure and developmental improvement of hyperactive/impulsive symptoms in attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry, Allied Disciplines. 2015 doi: 10.1111/jcpp.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gittelman R, Mannuzza S, Shenker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Arch Gen Psychiatry. 1985;42(10):937–47. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- 26.Chang WLY, Qian Q, Tang H, Wang Y. Related factors of early adulthood attention deficit hyperactivity disorder. Chin Ment Health J. 2011;25(12):5. [Google Scholar]
- 27•.Sibley MH, Swanson JM, Arnold LE, Hechtman LT, Owens LE, Stehli A, et al. Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. Journal of Child Psychology and Psychiatry. 2016 doi: 10.1111/jcpp.12620. In press. This was the first study to analyze a wide range of ADHD persistence definitions and test for the accuracy of those definitions within one clinical sample. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matte B, Anselmi L, Salum GA, Kieling C, Goncalves H, Menezes A, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361–73. doi: 10.1017/S0033291714001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russel A, Barkley MF. ADHD in adults: what the science says. New York: Guilford Press; 2007. [Google Scholar]
- 30.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–71. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 31.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. [Google Scholar]
- 32.Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–75. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 33.Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waxmonsky JG, Waschbusch DA, et al. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. J Consult Clin Psychol. 2012;80(6):1052– 61. doi: 10.1037/a0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabiano GA, Pelham WE, Jr, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, et al. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. J Clin Child Adolesc Psychol: Off J Soc Clin Child Adolesc Psychol, Am Psychol Assoc, Div 53. 2006;35(3):369–85. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- 35.Cohen P, Cohen J. The clinician’s illusion. Arch Gen Psychiatry. 1984;41(12):1178–82. doi: 10.1001/archpsyc.1984.01790230064010. [DOI] [PubMed] [Google Scholar]
- 36.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry, Allied Disciplines. 1999;40(1):57–87. [PubMed] [Google Scholar]
- 37.Du Fort GG, Newman SC, Bland RC. Psychiatry comorbidity and treatment seeking: sources of selection bias in the study of clinical populations. J Nerv Ment Dis. 1993;181(8):467–74. [PubMed] [Google Scholar]
- 38.Eklund H, Cadman T, Findon J, Hayward H, Howley D, Beecham J, et al. Clinical service use as people with attention deficit hyper-activity disorder transition into adolescence and adulthood: a prospective longitudinal study. BMC Health Serv Res. 2016;16:248. doi: 10.1186/s12913-016-1509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JAD, Sterne JAC. Chapter 8: assessing risk of bias in included studies. In: Higgins JPTGS, editor. Cochrane handbook for systematic reviews of interventions. 5.1.0 ed. The Cochrane Collaboration; 2011. [Google Scholar]
- 40.Szklo M. Population-based cohort studies. Epidemiol Rev. 1998;20(1):81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
- 41.Breyer JL, Lee S, Winters KC, August GJ, Realmuto GM. A longitudinal study of childhood ADHD and substance dependence disorders in early adulthood. Psychol Addict Behav: J Soc Psychol Addict Behav. 2014;28(1):238–46. doi: 10.1037/a0035664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klein RG, Mannuzza S, Olazagasti MA, Roizen E, Hutchison JA, Lashua EC, et al. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiatry. 2012;69(12):1295–303. doi: 10.1001/archgenpsychiatry.2012.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guelzow BT, Loya F, Hinshaw SP. How persistent is ADHD into adulthood? Informant report and diagnostic thresholds in a female sample. J Abnorm Child Psychol. 2016 doi: 10.1007/s10802-016-0174-4. [DOI] [PubMed] [Google Scholar]
- 44.Vitola ES, Bau CHD, Salum GA, Horta BL, Quevedo L, Barros FC, et al. Exploring DSM-5 ADHD criteria beyond young adulthood: phenomenology, psychometric properties and prevalence in a large three-decade birth cohort. Pyshcological Medicine. 2016 doi: 10.1017/S0033291716002853. (in press) [DOI] [PubMed] [Google Scholar]
- 45.Murphy P, Schachar R. Use of self-ratings in the assessment of symptoms of attention deficit hyperactivity disorder in adults. Am J Psychiatry. 2000;157(7):1156–9. doi: 10.1176/appi.ajp.157.7.1156. [DOI] [PubMed] [Google Scholar]
- 46.Miller CJ, Newcorn JH, Halperin JM. Fading memories: retrospective recall inaccuracies in ADHD. J Atten Disord. 2010;14(1):7–14. doi: 10.1177/1087054709347189. [DOI] [PubMed] [Google Scholar]
- 47.Breda V, Rovaris DL, Vitola ES, Mota NR, Blaya-Rocha P, Salgado CA, et al. Does collateral retrospective information about childhood attention-deficit/hyperactivity disorder symptoms assist in the diagnosis of attention-deficit/hyperactivity disorder in adults? Findings from a large clinical sample. Aust N Z J Psychiatry. 2016;50(6):557–65. doi: 10.1177/0004867415609421. [DOI] [PubMed] [Google Scholar]
- 48••.Caye A, Spadini AV, Karam RG, Grevet EH, Rovaris DL, Bau CH, et al. Predictors of persistence of ADHD into adulthood: a systematic review of the literature and meta-analysis. Eur Child Adolesc Psychiatry. 2016 doi: 10.1007/s00787-016-0831-8. First systematic review of childhood predictors of ADHD persistence. Provides summarized estimates of risk with meta-analytic techniques. [DOI] [PubMed] [Google Scholar]
- 49.Alegria M, Lin JY, Green JG, Sampson NA, Gruber MJ, Kessler RC. Role of referrals in mental health service disparities for racial and ethnic minority youth. J Am Acad Child Adolesc Psychiatry. 2012;51(7):703–11e2. doi: 10.1016/j.jaac.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madsen KB, Ersboll AK, Olsen J, Parner E, Obel C. Geographic analysis of the variation in the incidence of ADHD in a country with free access to healthcare: a Danish cohort study. Int J Health Geogr. 2015;14:24. doi: 10.1186/s12942-015-0018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559–67. doi: 10.1097/00004583-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Roy A, Hechtman L, Arnold LE, Sibley MH, Molina BS, Swanson JM, et al. Childhood factors affecting persistence and desistence of attention-deficit/hyperactivity disorder symptoms in adulthood: results from the MTA. Journal of the American Academy of Child & Adolescent Psychiatry. 2016 doi: 10.1016/j.jaac.2016.05.027. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen PS, Arnold LE, Swanson JM, Vitiello B, Abikoff HB, Greenhill LL, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- 54.Molina BS, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasser TR, Kalvin CB, Bierman KL. Developmental trajectories of clinically significant attention-deficit/hyperactivity disorder (ADHD) symptoms from grade 3 through 12 in a high-risk sample: predictors and outcomes. J Abnorm Psychol. 2016;125(2):207–19. doi: 10.1037/abn0000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsson H, Dilshad R, Lichtenstein P, Barker ED. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: genetic effects, family risk and associated psychopathology. J Child Psychol Psychiatry, Allied Disciplines. 2011;52(9):954–63. doi: 10.1111/j.1469-7610.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 57.Pingault JB, Viding E, Galera C, Greven CU, Zheng Y, Plomin R, et al. Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry. 2015;72(7):651–8. doi: 10.1001/jamapsychiatry.2015.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howard AL, Strickland NJ, Murray DW, Tamm L, Swanson JM, Hinshaw SP, et al. Progression of impairment in adolescents with attention-deficit/hyperactivity disorder through the transition out of high school: contributions of parent involvement and college attendance. J Abnorm Psychol. 2016;125(2):233–47. doi: 10.1037/abn0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riglin L, Collishaw S, Thapar AK, Dalsgaard S, Langley K, Smith GD, et al. Association of Genetic Risk Variants With Attention-Deficit/Hyperactivity Disorder Trajectories in the General Population. JAMA Psychiat. 2016 doi: 10.1001/jamapsychiatry.2016.2817. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol. 2007;32(6):643–54. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- 61.Kuriyan AB, Pelham WE, Jr, Molina BS, Waschbusch DA, Gnagy EM, Sibley MH, et al. Young adult educational and vocational outcomes of children diagnosed with ADHD. J Abnorm Child Psychol. 2013;41(1):27–41. doi: 10.1007/s10802-012-9658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. MedGenMed: Medscape Gen Med. 2006;8(3):12. [PMC free article] [PubMed] [Google Scholar]
- 63.Altszuler AR, Page TF, Gnagy EM, Coxe S, Arrieta A, Molina BS, et al. Financial dependence of young adults with childhood ADHD. J Abnorm Child Psychol. 2016;44(6):1217–29. doi: 10.1007/s10802-015-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groenman AP, Oosterlaan J, Rommelse N, Franke B, Roeyers H, Oades RD, et al. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108(8):1503–11. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- 65.Lichtenstein P, Halldner L, Zetterqvist J, Sjolander A, Serlachius E, Fazel S, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006–14. doi: 10.1056/NEJMoa1203241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinhausen HC, Bisgaard C. Substance use disorders in association with attention-deficit/hyperactivity disorder, comorbid mental disorders, and medication in a nationwide sample. Eur Neuropsychopharmacol:J Eur Coll Neuropsychopharmacol. 2014;24(2):232–41. doi: 10.1016/j.euroneuro.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Levy S, Katusic SK, Colligan RC, Weaver AL, Killian JM, Voigt RG, et al. Childhood ADHD and risk for substance dependence in adulthood: a longitudinal, population-based study. PLoS ONE. 2014;9(8):e105640. doi: 10.1371/journal.pone.0105640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waschbusch DA, Biswas A, et al. The delinquency outcomes of boys with ADHD with and without comorbidity. J Abnorm Child Psychol. 2011;39(1):21–32. doi: 10.1007/s10802-010-9443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kieling RR, Szobot CM, Matte B, Coelho RS, Kieling C, Pechansky F, et al. Mental disorders and delivery motorcycle drivers (motoboys): a dangerous association. Eur Psychiatry: J Assoc Eur Psychiatrists. 2011;26(1):23–7. doi: 10.1016/j.eurpsy.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Chang Z, Lichtenstein P, D’Onofrio BM, Sjolander A, Larsson H. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319–25. doi: 10.1001/jamapsychiatry.2013.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson AL, Molina BS, Pelham W, Jr, Gnagy EM. Risky driving in adolescents and young adults with childhood ADHD. J Pediatr Psychol. 2007;32(7):745–59. doi: 10.1093/jpepsy/jsm002. [DOI] [PubMed] [Google Scholar]
- 72.Cortese S, Faraone SV, Bernardi S, Wang S, Blanco C. Adult attention-deficit hyperactivity disorder and obesity: epidemiological study. Br J Psychiatry: J Ment Sci. 2013;203(1):24–34. doi: 10.1192/bjp.bp.112.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortese S, Ramos Olazagasti MA, Klein RG, Castellanos FX, Proal E, Mannuzza S. Obesity in men with childhood ADHD: a 33-year controlled, prospective, follow-up study. Pediatrics. 2013;131(6):e1731–8. doi: 10.1542/peds.2012-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192–202. doi: 10.1097/01.chi.0000189134.97436.e2. [DOI] [PubMed] [Google Scholar]
- 75.Biederman J, Spencer T, Lomedico A, Day H, Petty CR, Faraone SV. Deficient emotional self-regulation and pediatric attention deficit hyperactivity disorder: a family risk analysis. Psychol Med. 2012;42(3):639–46. doi: 10.1017/S0033291711001644. [DOI] [PubMed] [Google Scholar]
- 76.Danckaerts M, Sonuga-Barke EJ, Banaschewski T, Buitelaar J, Dopfner M, Hollis C, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83– 105. doi: 10.1007/s00787-009-0046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77••.Dalsgaard S, Ostergaard SD, Leckman JF, Mortensen PB, Pedersen MG. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015 doi: 10.1016/s0140-6736(14)61684-6. An analysis of health records found a significant association between ADHD and overall mortality. [DOI] [PubMed] [Google Scholar]
- 78••.Erskine HE, Norman RE, Ferrari AJ, Chan GK, Copeland WE, Whiteford HA, Scott JG. Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: a systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry. 2016 doi: 10.1016/j.jaac.2016.06.016. In press. A comprehensive systematic review of long-term outcomes of ADHD and conduct disorder. Provides summarized estimates of risk with meta-analytic techniques. [DOI] [PubMed] [Google Scholar]
- 79••.Hechtman L, Swanson JM, Sibley MH, Stehli A, Owens BO, Mitchell JT, et al. Functional adult outcomes 16 years after childhood diagnosis of attention-deficit/hyperactivity disorder: MTA results. J Am Acad Child Adolesc Psychiatry. 2016 doi: 10.1016/j.jaac.2016.07.774. A report on long-term outcomes of ADHD children and controls within the larger clinical trial on the field and its relationship with symptom persistence and desistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu JL, Olsen J, Liew Z, Li J, Niclasen J, Obel C. Parental smoking during pregnancy and ADHD in children: the Danish national birth cohort. Pediatrics. 2014;134(2):e382–8. doi: 10.1542/peds.2014-0213. [DOI] [PubMed] [Google Scholar]
- 81.Rommel AS, Halperin JM, Mill J, Asherson P, Kuntsi J. Protection from genetic diathesis in attention-deficit/hyperactivity disorder: possible complementary roles of exercise. J Am Acad Child Adolesc Psychiatry. 2013;52(9):900–10. doi: 10.1016/j.jaac.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohr-Jensen C, Steinhausen HC. A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations. Clin Psychol Rev. 2016;48:32–42. doi: 10.1016/j.cpr.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Satterfield JH, Satterfield BT, Schell AM. Therapeutic interventions to prevent delinquency in hyperactive boys. J Am Acad Child Adolesc Psychiatry. 1987;26(1):56–64. doi: 10.1097/00004583-198701000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Satterfield JH, Schell A. A prospective study of hyperactive boys with conduct problems and normal boys: adolescent and adult criminality. J Am Acad Child Adolesc Psychiatry. 1997;36(12):1726–35. doi: 10.1097/00004583-199712000-00021. [DOI] [PubMed] [Google Scholar]
- 85.Nogueira M, Bosch R, Valero S, Gomez-Barros N, Palomar G, Richarte V, et al. Early-age clinical and developmental features associated to substance use disorders in attention-deficit/hyperactivity disorder in adults. Compr Psychiatry. 2014;55(3):639–49. doi: 10.1016/j.comppsych.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 86.Mannuzza S, Klein RG, Truong NL, Moulton JL, 3rd, Roizen ER, Howell KH, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008;165(5):604–9. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Molina BS, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. J Am Acad Child Adolesc Psychiatry. 2013;52(3):250–63. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sibley MH, Pelham WE, Molina BS, Coxe S, Kipp H, Gnagy EM, et al. The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. J Abnorm Psychol. 2014;123(2):362–74. doi: 10.1037/a0036585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doernberg E, Hollander E. Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS spectrums. 2016:1–5. doi: 10.1017/S1092852916000262. [DOI] [PubMed] [Google Scholar]


