Abstract
Different root parts with or without increased iron-reducing activities have been studied in iron-deficient and iron-sufficient control sugar beet (Beta vulgaris L. Monohil hybrid). The distal root parts of iron-deficient plants, 0 to 5 mm from the root apex, were capable to reduce Fe(III)-chelates and contained concentrations of flavins near 700 μm, two characteristics absent in the 5 to 10 mm sections of iron-deficient plants and the whole root of iron-sufficient plants. Flavin-containing root tips had large pools of carboxylic acids and high activities of enzymes involved in organic acid metabolism. In iron-deficient yellow root tips there was a large increase in carbon fixation associated to an increase in phosphoenolpyruvate carboxylase activity. Part of this carbon was used, through an increase in mitochondrial activity, to increase the capacity to produce reducing power, whereas another part was exported via xylem. Root respiration was increased by iron deficiency. In sugar beet iron-deficient roots flavins would provide a suitable link between the increased capacity to produce reduced nucleotides and the plasma membrane associated ferric chelate reductase enzyme(s). Iron-deficient roots had a large oxygen consumption rate in the presence of cyanide and hydroxisalycilic acid, suggesting that the ferric chelate reductase enzyme is able to reduce oxygen in the absence of Fe(III)-chelates.
Iron deficiency is a widespread agricultural problem in many crops grown in alkaline, calcareous soils. Iron in these soils, although abundant, is often not soluble and therefore is unavailable for the roots (Lindsay and Schwab, 1982). Based on the mechanisms of iron uptake and on the physiological responses to iron deficiency, plants can be classified into two groups: (a) Strategy I plants, which include dicotyledonous and non-Graminaceae monocotyledonous species and (b) Strategy II plants, which include Graminaceae species (Marschner et al., 1986). When grown under a limited iron supply Strategy I and II plants increase their capacity for iron uptake. Strategy II plants respond by an increased synthesis and secretion of phytosiderophores to the rizosphere (Marschner and Römheld, 1994). Strategy I plants develop morphological changes, such as increased formation of lateral roots, root hairs, and transfer cells. All these changes increase the root surface available for iron uptake (Kramer et al., 1980; Landsberg, 1982). Strategy I also includes biochemical changes, such as a higher proton extrusion capacity (Brown, 1978), a release of reducing and/or chelating substances such as phenolics and flavins (Welkie and Miller, 1960; Susín et al., 1994), and the development of a two-step mechanism for iron uptake. In this mechanism iron is first reduced by a plasma membrane (PM)-bound ferric-chelate reductase (FC-R) enzyme (Moog and Brüggemann, 1994; Susín et al., 1996; Robinson et al., 1999; Schmidt, 1999) and subsequently absorbed as Fe(II) (Chaney et al., 1972) by a specialized transporter (Eide et al., 1996; Fox and Guerinot, 1998).
When grown under iron deficiency, Strategy I plants accumulate organic acids, mainly citrate and malate in leaves (Iljin, 1951; Landsberg, 1981) and roots (Brown, 1966; Alhendawi et al., 1997). The role of these organic acids in the iron deficiency responses is not well established (Schmidt, 1999), although it is commonly accepted that citrate could play an important role in the translocation of iron in roots (Tiffin, 1966; White et al., 1981) and in iron transport via xylem to the mesophyll cells (Brown, 1966; Brown et al., 1971).
Two hypotheses have been put forward so far to explain the accumulation of organic acids in iron-deficient roots. Landsberg (1986) reported that organic acid increases coincided with the enhanced proton extrusion found in iron-deficient roots. This may occur through cytoplasm alkalinization associated to proton efflux, which could activate phosphoenolpyruvate carboxylase (PEPC) activity (Rabotti et al., 1995, and refs. therein). The second hypothesis (de Vos et al., 1986) suggested that iron deficiency causes an alteration in the glycolytic pathway, as reported in fungi (Habison et al., 1979). Under iron deficiency, phosphofructokinase may lose its regulation by citrate and pyruvate kinase (PK) would be inhibited by citrate, causing an accumulation of PEP, that in turn, via PEPC activity, would cause increases in organic acid contents. The cytoplasm acidification produced by the increases in organic acids would be responsible for H+ extrusion. An increased PEPC activity may lead to organic acid accumulation to maintain the ionic balance of the root cell cytoplasm (pH-stat theory; Davies, 1973). Organic acid concentrations, however, also increased in iron-deficient plants in which proton extrusion was not increased (Landsberg, 1981). Miller et al. (1990) reported increases in CO2 fixation, organic acid contents, and PEPC activity in roots of iron-deficient plants, and suggested that the increased PEPC activity may feed the TCA cycle via malate, thus bypassing the key control point at PK (Lance and Rustin, 1984).
The aim of this work was to investigate the organic acid metabolism in iron-deficient plants to further understand the biochemical responses of plants to iron deficiency. We have measured organic acid concentrations and the enzymatic activities of PEPC, carbonic anhydrase (CA), glucose 6 phosphate dehydrogenase (G6PDH), several enzymes involved in organic acid metabolism, and lactate dehydrogenase (LDH) and pyruvate decarboxylase (PDC), two enzymes related to anaerobic metabolism, in root tips of sugar beet (Beta vulgaris L. Monohil hybrid) affected by iron deficiency. The redox poise of the pyridine nucleotide and mitochondrial quinone (Q) pools and the ATP levels were also determined. The possible relationships between the accumulation of organic acids and root tip O2 consumption were also investigated. Based on the experimental results found we propose a new metabolic model that provides a comprehensive scheme for the functioning of sugar beet root cells under iron deficiency.
RESULTS
Changes in Flavin Concentrations and FC-R Activity in Roots with Iron Deficiency
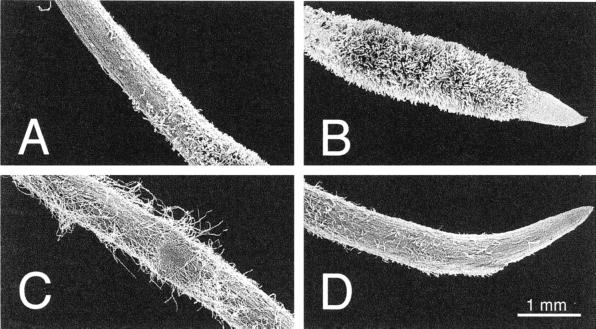
The distal roots from iron-deficient plants were divided in two parts, the yellow zone (YZ; approximately 0–5 mm from the apex), which has short root hairs (Fig. 1B), is enriched in flavins and has increased Fe(III)-reductase activity (Table I; Susín et al., 1993, 1996), and an adjacent white zone (WZ; approximately 5–10 mm from the apex), which does not have root hairs (Fig. 1A) and has much lower flavin concentrations and Fe(III)-reducing activities (Table I). In the control roots no differences were found between flavin concentrations and Fe(III)-reductase activities in the 0 to 5 mm and 5 to 10 mm fractions (data not shown). Because of this reason, only mean values corresponding to the root 0 to 10 mm section from the apex are given. In the control roots (Fig. 1, C and D) flavin concentrations and Fe(III)-reductase activities were low (Table I).
Figure 1.
Scanning electron micrographs showing the root distal segments of iron-deficient plants 5 to 10 mm from the apex (A), 0 to 5 mm from the apex (B), and iron-sufficient sugar beet plants 5 to 10 mm from the apex (C), and 0 to 5 mm from the apex (D).
Table I.
Ferric chelate reductase activities (nmol Fe reduced g−1 fresh wt min−1 and flavin concentrations (nmol g−1 fresh wt) in root tips from Fe-sufficient and -deficient sugar beet
| +Fe (0–10 mm) | −Fe
|
−Fe YZ/+Fe | −Fe WZ/+Fe | ||
|---|---|---|---|---|---|
| YZ | WZ | ||||
| FC-R activity | 13 ± 2 | 138 ± 5 | 39 ± 5 | 11 | 3 |
| Riboflavin 3′-sulfate | 4 ± 1 | 406 ± 79 | 91 ± 30 | 102 | 23 |
| Riboflavin 5′-sulfate | 2 ± 1 | 98 ± 12 | 30 ± 4 | 49 | 15 |
| FAD | 10 ± 2 | 67 ± 3 | 49 ± 9 | 7 | 5 |
| Riboflavin | n.d. | 13 ± 2 | 6 ± 1 | – | – |
Data are means ± se of five replicates. n.d., Not detected.
Riboflavin 3′-sulfate (SI) and riboflavin 5′-sulfate (SII) concentrations increased in the YZs of iron-deficient roots 102- and 49-fold, respectively, when compared with the controls (Table I). The concentration of flavin adenine dinucleotide (FAD) was also increased 7-fold in the YZs when compared with the controls (Table I). In the WZs of the iron-deficient roots the SI, SII, and FAD concentrations were 23-, 15-, and 5-fold higher than the control values (Table I).
Changes in Root Organic Anion Concentrations with Iron Deficiency
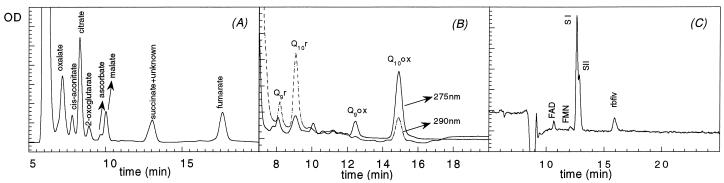
A typical chromatographic separation of the organic anions present in roots is shown in Figure 2A. Retention times for oxalate, cis-aconitate, citrate, 2-oxoglutarate, ascorbate, malate, and fumarate were 6.5, 7.5, 8.2, 8.6, 10.0, 10.2, and 17.4 min, respectively. The other peak with a retention time of 13.0 min contained succinate and an unidentified component with absorption maxima at 210 and 261 nm. In all root zones the major organic anions (more than 95% of the total organic anion contents) were oxalate, citrate, malate, and ascorbate. In the control roots no differences were found between the organic anion concentrations in the 0 to 5 mm and 5 to 10 mm fractions (data not shown). Because of this reason only mean values corresponding to the root 0 to 10 mm section from the apex are given (Table II).
Figure 2.
Separation of organic acids (A), quinones (B), and flavins (C) by HPLC. Organic acids (detected at 210 nm) were oxalate, cis-aconitate, citrate, 2-oxoglutarate, ascorbate, malate, succinate, and fumarate. Flavins (detected at 445 nm) were FAD, FMN, SI, SII, and riboflavin. Reduced Q9 and Q10 were detected at 290 nm and oxidized Q9 and Q10 at 275 nm.
Table II.
Concentrations of organic anions (μmol g−1 fresh wt) in root tips from Fe-sufficient and Fe-deficient sugar beet
| Organic Anion | +Fe (0–10 mm) | −Fe
|
−Fe YZ/+Fe | −Fe WZ/+Fe | |
|---|---|---|---|---|---|
| YZ | WZ | ||||
| Malate | 0.37 ± 0.05 | 5.95 ± 0.78 | 4.32 ± 0.80 | 16.1 | 11.7 |
| Oxalacetate | <0.001 | <0.001 | <0.001 | – | – |
| Citrate | 0.10 ± 0.02 | 2.60 ± 0.54 | 2.70 ± 0.65 | 26.0 | 27.0 |
| cis-Aconitate | 0.030 ± 0.002 | 0.403 ± 0.140 | 0.040 ± 0.001 | 13.4 | 1.3 |
| Isocitrate | <0.001 | <0.001 | <0.001 | – | – |
| 2-Oxoglutarate | 0.020 ± 0.006 | 0.065 ± 0.016 | 0.020 ± 0.006 | 3.2 | 1.0 |
| Succinatea | 0.047 | 0.123 | 0.048 | 2.6 | 1.0 |
| Fumarate | 0.006 ± 0.001 | 0.800 ± 0.300 | 0.298 ± 0.040 | 133.3 | 49.7 |
| Oxalate | 7.71 ± 1.54 | 18.40 ± 4.50 | 19.60 ± 8.20 | 2.4 | 2.5 |
| Ascorbate | 0.23 ± 0.07 | 2.40 ± 0.37 | 2.55 ± 0.50 | 10.4 | 11.1 |
| Pyruvate | <0.001 | <0.001 | <0.001 | – | – |
| Total | 8.47 | 30.62 | 29.53 | 3.6 | 3.5 |
Data are the mean ± se of 10 replicates.
Succinate co-eluted with an unidentified compound.
In the YZs of iron-deficient sugar beet root tips there was a 3.6-fold increase in total organic anion concentration when compared with iron-sufficient control roots. This was associated to 26.0-fold increases in citrate, 16.1-fold increases in malate, and 10.4-fold increases in ascorbate (Table II). Oxalate, however, was increased only by 2.4-fold with iron deficiency. The minor organic anions cis-aconitate, 2-oxoglutarate, succinate, and fumarate (less than 5% of the total organic anion concentration in all cases) increased 13.4-, 3.2-, 2.6-, and 133-fold in the yellow parts of the iron-deficient root tips when compared with the iron-sufficient controls (Table II).
The concentrations of the major organic anions oxalate, citrate, malate, and ascorbate were similar in the YZs and WZs of the iron-deficient sugar beet root tips (Table II). However, the concentrations of cis-aconitate, succinate, and 2-oxoglutarate in the white parts of the iron-deficient roots were much lower than those present in the yellow, flavin-enriched areas, and similar to those found in iron-sufficient root tips (Table II). The fumarate concentration in the white parts of the iron-deficient roots was intermediate between those found in iron-sufficient roots and in the yellow areas of the iron-deficient roots (Table II).
Changes in Root Extract Enzymatic Activities with Iron Deficiency
We measured five enzymatic activities involved in organic acid metabolism. All these enzymatic activities were markedly increased in the extracts from the yellow parts of iron-deficient roots when compared with those obtained from iron-sufficient roots (Table III). Increases were 16.4-fold for malate dehydrogenase (MDH), 8.7-fold for fumarase, 14.2-fold for isocitrate dehydrogenase (ICDH), 5.5-fold for aconitase, and 44.8-fold for citrate synthase (CS).
Table III.
Enzymatic activities (in μmol g−1 fresh wt min−1 for MDH, ICDH, PEPC, GGPDM, LDH, and PDC, and in nmol g-1 fresh wt min−1 for CS, aconitase, fumarase, and CA) in root tip homogenates from Fe-sufficient and Fe-deficient sugar beet
| Enzyme | +Fe (0–10 mm) | −Fe
|
−Fe YZ/+Fe | −Fe WZ/+Fe | |
|---|---|---|---|---|---|
| YZ | WZ | ||||
| MDH | 6.84 ± 0.48 | 112.10 ± 24.60 | 25.30 ± 2.01 | 16.4 | 3.7 |
| CS | 0.036 ± 0.021 | 1.611 ± 0.360 | 0.133 ± 0.010 | 44.8 | 3.7 |
| Aconitase | 282 ± 65 | 1560 ± 133 | 380 ± 140 | 5.5 | 1.3 |
| ICDH | 0.168 ± 0.048 | 2.391 ± 0.342 | 0.356 ± 0.030 | 14.2 | 2.1 |
| Fumarase | 381 ± 99 | 3340 ± 1100 | 594 ± 112 | 8.7 | 1.6 |
| PEPC | 0.222 ± 0.066 | 13.320 ± 2.520 | 0.542 ± 0.041 | 60.0 | 2.4 |
| CA | 13.921 ± 3.220 | 11.690 ± 2.301 | 21.883 ± 5.410 | 0.8 | 1.6 |
| G6PDH | 0.589 ± 0.070 | 2.270 ± 0.573 | 0.785 ± 0.127 | 3.8 | 1.3 |
| LDH | 0.016 ± 0.006 | 0.216 ± 0.059 | 0.087 ± 0.024 | 13.5 | 5.4 |
| PDC | 0.048 ± 0.024 | 0.288 ± 0.120 | 0.097 ± 0.026 | 6.0 | 2.0 |
Data are the mean ± se of five replicates. In the control roots no differences were found between enzymatic activities in the 0 to 5 mm and 5–10 mm fractions (data not shown). Because of this reason, only mean values corresponding to the root 0 to 10 mm section from the apex are given.
In the iron-deficient root extracts the activities of the five measured enzymes were markedly lower in the WZ than in the YZ (Table III). Activities in the WZ of iron-deficient roots were generally intermediate between the activities found in the YZ and those found in the iron-sufficient controls. The increases over the control values were approximately 4-, 2-, 2-, and 4-fold for MDH, fumarase, ICDH, and CS, respectively. The activity of aconitase was similar to the control values.
The PEPC activity in extracts of the YZ of iron-deficient roots was 60 times higher than in iron-sufficient root tips at pH 8.5 (Table III) and 40 times at pH 7.3 (data not shown). In the WZ of the iron-deficient roots, however, the PEPC activity was only 2.4-fold of that found in the iron-sufficient controls (Table III). The inhibition of the PEPC activity (measured at pH 7.3) by 500 μm malate was approximately 59% and 41% in iron-sufficient and iron-deficient yellow root tip extracts (data not shown).
The CA activity in the extracts of the YZ of iron-deficient roots was similar to that found in the controls, whereas in the WZ of the same roots CA activity was slightly increased (Table III). G6PDH activity increased 3.8-fold in the YZs of iron-deficient roots respect to the controls, whereas the WZs had similar values than the controls. LDH and PDC activities were also 13.5- and 6-fold higher in the yellow parts of the iron-deficient roots when compared with the controls. The activities of LDH and PDC in the WZ of the iron-deficient root tips were between the activities found in the YZ and those found in the iron-sufficient controls.
Changes in Root Nucleotide Concentrations with Iron Deficiency
The pool of pyridine nucleotides increased 3.3-fold in the yellow root tips of iron-deficient plants when compared with the controls (Table IV). Iron deficiency increased the concentrations of both reduced and oxidized nucleotide forms. The largest increase was 8-fold for NAD+, followed by 4.5-fold for NADP+, 2.2-fold for NADPH, and 1.5-fold for NADH. As a result of these changes, the NADH/NAD+ and NADPH/NADP+ ratios decreased by 82% and 50%, respectively, in the yellow tips of iron-deficient roots when compared with the controls.
Table IV.
Concentrations of pyridine nucleotides and ATP (nmol g−1 fresh wt) in root tips from Fe-sufficient and Fe-deficient sugar beet
| +Fe (0–10 mm) | −Fe
|
−Fe YZ/+Fe | −Fe WZ/+Fe | ||
|---|---|---|---|---|---|
| YZ | WZ | ||||
| NAD+ | 0.80 ± 0.47 | 6.42 ± 0.17 | 1.91 ± 0.01 | 8.0 | 2.4 |
| NADP+ | 1.41 ± 0.92 | 6.34 ± 0.34 | 2.13 ± 0.28 | 4.5 | 1.5 |
| NADH | 1.87 ± 0.27 | 2.75 ± 0.51 | 3.23 ± 0.31 | 1.5 | 1.7 |
| NADPH | 1.70 ± 0.09 | 3.73 ± 1.18 | 2.58 ± 0.44 | 2.2 | 1.5 |
| Total pool | 5.78 | 19.24 | 9.85 | 3.3 | 1.7 |
| NADH/NAD+ | 2.3 | 0.4 | 1.7 | 0.2 | 0.7 |
| NADPH/NADP+ | 1.2 | 0.6 | 1.2 | 0.5 | 1.0 |
| ATP (×10−3) | 0.25 ± 0.03 | 1.26 ± 0.37 | 0.64 ± 0.13 | 5.0 | 2.6 |
Data are the mean ± se of seven replications. In the control roots no differences were found between pyridine nucleotide concentrations in the 0 to 5 mm and 5 to 10 mm fractions (data not shown). Because of this reason, only mean values corresponding to the root 0 to 10 mm section from the apex are given.
The WZ of iron-deficient root tips had pyridine nucleotide concentrations only 1.7-fold higher than those found in iron-sufficient root tips. The concentrations of reduced forms in these white parts were not significantly different to those found in the yellow parts (Table IV). However, the concentrations of the oxidized forms were intermediate between those found in the yellow parts of the same root and the control, iron-sufficient values. In these white parts the NADH/NAD+ and NADPH/NADP+ ratios were similar to those found in iron-sufficient root tips (Table IV).
The ATP concentration in the yellow part of the iron-deficient root tips was 5-fold higher than that found in iron-sufficient root tips (Table IV). The white parts of iron-deficient roots had ATP concentrations still 2.6-fold higher than those found in the controls.
Changes in Root Tip Oxygen Consumption Rates Induced by Iron Deficiency
The yellow parts of the roots from iron-deficient plants had increased O2 consumption rates when compared with the control roots. The yellow and white parts of the iron-deficient roots consumed approximately 627 (Table V) and 137 nmol O2 min−1 g−1 fresh weight (not shown), whereas control roots consumed 164 nmol O2 min−1 g−1 fresh weight (Table V).
Table V.
O2 consumption rates (nmol O2 g−1 fresh wt min−1) in root tips from Fe-sufficient and Fe-deficient sugar beet
| Oxygen Consumption | +Fe | −Fe (YZ) |
|---|---|---|
| Initial rate | 164 ± 23 | 627 ± 69 |
| 2 mm KCN | 83.4 ± 6 (49%) | 326 ± 23 (48%) |
| 2 mm SHAM | 113 ± 26 (31%) | 499 ± 23 (20%) |
| 2 mm KCN + 2 mm SHAM | 0 (100%) | 108 ± 31 (82%) |
| 2 mm KCN + 2 mm SHAM + 2 mm Fe(III)-EDTA | – | 0 (100%) |
| 2 mm KCN + 2 mm Fe(III)-EDTA | – | 263 ± 11 (58%) |
| 2 mm SHAM + 2 mm Fe(III)-EDTA | – | 422 ± 20 (33%) |
| 2 mm Fe(III)-EDTA | 164 ± 23 (0%) | 514 ± 7 (18%) |
Data are the mean ± sd of 10 replications. Data in brackets are the percentage of inhibition of O2 consumption respect to the initial rate.
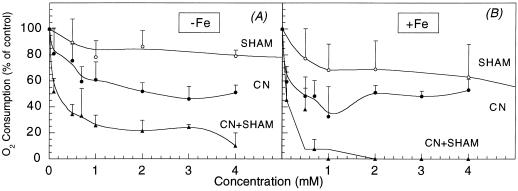
Cyanide-resistant O2 consumption was approximately 50% of the total consumption in both iron-sufficient and -deficient roots (Table V; Fig. 3). In the YZ of iron-deficient roots the presence of hydroxisalycilic acid (SHAM) decreased root O2 consumption by 20% at 4 mm (Fig. 3A) and by 80% at 20 mm (data not shown). In the controls 4 mm SHAM decreased O2 consumption by 40% (Fig. 3B), whereas 20 mm SHAM inhibited completely O2 consumption (data not shown). Residual O2 consumption, the fraction of oxygen uptake that is resistant to the combination of KCN and SHAM (Ribas-Carbó et al., 1997), was approximately 20% of the maximal rates in iron-deficient roots and practically zero in the controls.
Figure 3.
Changes in O2 consumption rates in yellow, iron-deficient (A) and iron-sufficient root tips (B) with different concentrations of SHAM (○), CN− (●), and CN− + SHAM (▴). Data are means ± se of three different replications. Actual O2 consumption rates are shown in Table V.
The addition of 2 mm Fe(III)-EDTA decreased the O2 consumption rate in iron-deficient root tips by approximately 20%, whereas in iron-sufficient roots the rate was unaffected (Table V). The inhibition of root O2 consumption rates by 2 mm Fe(III)-EDTA reached values of 58%, 33%, and 100% in iron-deficient roots treated with KCN, SHAM, or KCN plus SHAM, respectively (Table V).
Redox Poise of the Quinones Pool
The Q10 homolog was the predominant Q form present in sugar beet roots. The total pool of Q10 increased in the yellow and white parts of iron-deficient roots by 3- and 2.5-fold, respectively, when compared with the controls (Table VI). The Q10 pools were 42% reduced in iron-sufficient roots and 52% and 27% reduced in the yellow and WZs of iron-deficient roots, respectively (Table VI). The total amount of Q9 was always less than 5% of that of Q10 (not shown).
Table VI.
Concentrations of mitochondrial quinones (in nmol g−1 fresh wt) in root tips from Fe-sufficient and Fe-deficient sugar beet
| +Fe (0–10 mm) | −Fe
|
−Fe YZ/+Fe | −Fe WZ/+Fe | ||
|---|---|---|---|---|---|
| YZ | WZ | ||||
| Qr (Q10) | 1.73 ± 0.74 | 6.48 ± 2.80 | 2.79 ± 0.48 | 3.7 | 1.6 |
| Qo (Q10) | 2.34 ± 0.62 | 6.01 ± 3.07 | 7.54 ± 2.80 | 2.6 | 3.2 |
| Qt (Q10) | 4.07 | 12.49 | 10.3 | 3.0 | 2.5 |
| Qr/Qt (Q10) | 42 ± 2% | 52 ± 4% | 27 ± 4% | 1.23 | 0.64 |
Data are the mean ± se of five replications. In the control roots no differences were found between Q10 concentrations in the 0 to 5 mm and 5 to 10 mm fractions (data not shown). Because of this reason, only mean values corresponding to the root 0 to 10 mm section from the apex are given.
Protein Quantification
The amount of soluble protein in root tips increased with iron deficiency, being approximately 2.8 ng protein μg−1 fresh weight in the yellow tip and 1.2 ng protein μg−1 fresh weight in the white adjacent zone. These values were approximately 4.2- and 2.4-fold higher than the protein concentration found in the control roots, which was approximately 0.7 ng protein μg−1 fresh weight.
DISCUSSION
Most studies on iron-deficient roots have focused on very specific aspects of their physiology or biochemistry, resulting in very fragmentary information (Welkie and Miller, 1993; Schmidt, 1999). In the present work we have made a comprehensive study of two zones of the distal part of the root of iron-deficient sugar beet, having increased or not increased iron-reducing activities. Measurements included Fe(III)-reducing activities, flavin concentrations, O2 consumption rates, concentrations of organic anions, enzymatic activities and redox poises of the nucleotide, and mitochondrial Q pools. The results found provide support for the view that iron deficiency leads to a large increase in carbon fixation (approximately 50-fold) together with a significant, but a smaller increase in O2 utilization by roots (approximately 5-fold).
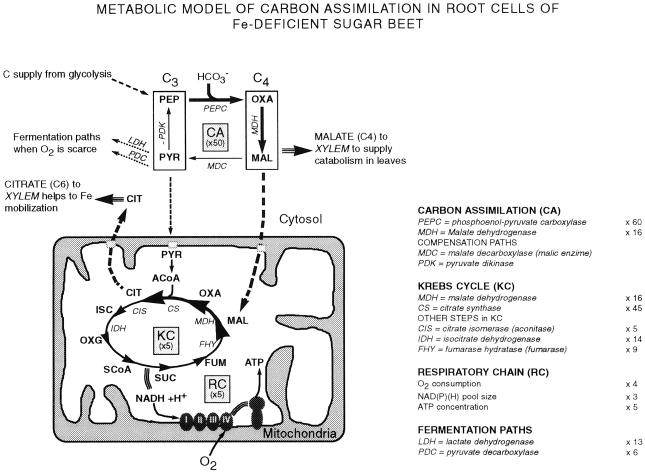
Iron-deficient root tips had a markedly enhanced capacity to fix carbon from bicarbonate. The main features of the metabolic pathway have been included in Figure 4. Carbon fixation was associated to large (40- to 60-fold on a fresh weight basis) increases in PEPC activity over the control values. PEPC catalyzes the carboxylation of PEP to oxalacetate, which could be subsequently reduced to malate via cytosolic MDH. Malate could then be transported to the mitochondria via the malate-oxalacetate shuttle and converted to citrate by CS. The increase in PEPC activity in the yellow iron-deficient roots was also accompanied by large increases in MDH and CS enzymatic activities, supporting the significance of carbon fixation by PEPC. In a thermodynamic manner, malate production from PEP, via PEPC and MDH is more favorable energetically than from pyruvate via PK (Lance and Rustin, 1984). CO2 fixation (Rhoads et al., 1959; Bedri et al., 1960; Rhoads and Wallace, 1960; Landsberg, 1986; Bienfait, 1988, 1989; Miller et al., 1990; Rabotti et al., 1995) and PEPC activity (Huffaker et al., 1959; Landsberg, 1986; Rabotti et al., 1995; Rombolà, 1998) have been reported to be stimulated by iron deficiency. Miller et al. (1990) suggested that PEPC activity may feed the tricarboxylic acid (TCA) cycle (Krebs Cycle; see also Welkie and Miller, 1993). PEP needed to maintain PEPC activity could possibly come from sugars, via glycolysis. Sugar concentrations have been reported recently to increase in iron-deficient roots, probably associated to an enhanced expression of several genes related to carbohydrate biosynthesis (Thoiron and Briat, 1999). Moreover, the activity of G3PDH, an enzyme involved in the glycolytic pathway, also increases in iron-deficient roots (Rabotti et al., 1995).
Figure 4.
Metabolic model for carbon assimilation in sugar beet roots under iron deficiency. Iron deficiency would cause an approximately 50-fold enhancement in carbon assimilation (C.A.) in the cytosol through the increase in PEPC activity, and approximately 5-fold increases in the Krebs cycle (K.C.) and respiratory chain (R.C.) in the mitochondria. Part of the malate and the citrate would be exported via xylem, thus providing respiratory substrates to the shoot. ACoA, Acetyl coenzyme A; CIT, citrate; FUM, fumarate; ISC, isocitrate; MAL, malate; OXA, oxalacetate; OXG, oxoglutarate; PYR, pyruvate; SCoA, succinyl coenzyme A; SUC, succinate.
The large increase in PEPC activity in iron-deficient roots is likely to be regulated at several levels. First, iron deficiency was associated to an increase in the amount of PEPC (results not shown). For instance, a 6-fold increase in the amount of a 56-kD fragment of PEPC was measured in two-dimensional gels with antibodies against PEPC (González-Vallejo, 1999). Second, increases of PEPC activity could be also mediated by post-transcriptional regulation through phosphorylation, as it occurs in the leaves of C4 and CAM species and in proteid roots of phosphorus-stressed plants (for review, see Chollet et al., 1996). This is supported by the fact that the PEPC sensitivity to malate was lower in extracts of iron-deficient roots than in those of controls, with 59% and 41% of the initial activity (at pH 7.3) remaining in the presence of 500 μm malate. Root tips of plants grown without iron in the absence of CaCO3 also had PEPC activities 40-fold higher (at pH 8.5) than the controls (data not shown). Also, CA activities did not increase with iron deficiency.
The yellow, Fe(III)-reducing tips of the iron-deficient roots (Fig. 1B) had signs of enhanced mitochondrial activity. These root parts had 4-fold increases in total O2 consumption rates and total protein, 3-fold increases in total nucleotide pools, 2-fold increases in total mitochondrial Q pools, and 5-fold increases in ATP concentrations over the controls. An enhanced mitochondrial activity is in agreement with the presence in these root tips of a large number of transfer cells containing mitochondria (Landsberg, 1994). Furthermore, both the pools of organic anions and enzymatic activities involved in organic acid metabolism were increased in the yellow, iron-deficient root tips when compared with the controls. For instance, the root concentrations of citrate and malate increased 26- and 16-fold, and the activities of CS, MDH, and fumarase increased 45-, 16-, and 9-fold when compared with the controls.
In these conditions the oxygen supply for the root could be limiting. Such a limitation is supported by the fact that the mitochondrial Q pool was more reduced in the yellow root tips of the iron-deficient plants than in the controls. The possible shortcoming of O2 for respiration could be enhanced by the substantial rate of residual O2 uptake, insensitive to the presence of KCN and SHAM, in the YZs of the iron-deficient sugar beet roots. This residual O2 uptake rate was approximately 130 nmol O2 g−1 fresh weight min−1, of the same order than the rates of reduction of Fe(III)-chelates in the same roots (Table I; see also Susín et al., 1996). When Fe(III)-EDTA was added to KCN plus SHAM-treated roots they ceased to consume O2. Since Fe(III)-EDTA cannot cross the PM, these data support that a FC-R enzyme induced by iron deficiency in the sugar beet root PM could use O2 when ferric chelates are not present. That O2 could be an acceptor for the PM FC-R enzyme had been suggested previously from the increases caused by anaerobiosis on the Fe(III)-reducing activity of purified PMs (González-Vallejo et al., 1998, 1999). The activities of two enzymes typical of anaerobic metabolism, PDC and LDH, increase markedly in activity (6- and 13-fold) in yellow root tips of iron-deficient plants when compared with the controls. These enzymes use pyruvate as substrate and form lactate and acetaldehyde, respectively. This again suggests that iron-deficient roots may suffer a certain degree of hypoxia as a consequence of the markedly increased O2 consumption rates found in these roots. Another enzyme of the anaerobic metabolism, FDH, has been shown to increase in activity with iron deficiency in roots of barley (Suzuki et al., 1998).
The pyridine nucleotide pool was more oxidized in the yellow parts of the iron-deficient sugar beet roots than in the controls, conversely to what has been reported previously in other species (Sijmons et al., 1984; Schmidt and Schuck, 1996). This total pool increased 3-fold in the yellow parts of the iron-deficient roots when compared with the controls, similarly to what has been reported previously for bean and Plantago lanceolata roots (Sijmons et al., 1984; Schmidt and Schuck, 1996). The shift toward oxidation occurred in spite of the increased potential for the production of reducing power associated to the large pools of organic acids and increased activities of several NADH and NADPH producing enzymes. This could be explained by the high concentrations in the cytosol of iron-deficient sugar beet roots of oxidized riboflavin sulfates (approximately 700 μm; see also Susín et al., 1993), since NADH and NADPH can reduce easily both flavin sulfates and riboflavin (González-Vallejo et al., 1998). These flavin compounds are not present in roots where the nucleotide pool is more reduced with iron deficiency. Also, the white parts of iron-deficient sugar beet roots do not have significant amounts of flavins and had NADPH/NADP+ ratios similar to the control roots. The increased activities of some NADH consuming enzymes, such as cytosolic LDH and PDC, could also contribute to oxidize the nucleotide pool. It should be mentioned that the shift to a more oxidized state with iron deficiency observed in the pyridine nucleotide pool does not reflect a generalized oxidation, since the pool of mitochondrial quinones was more reduced than in the controls.
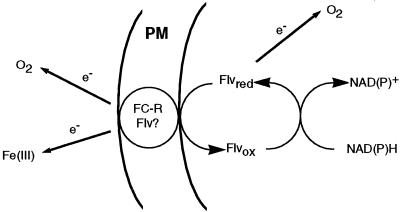
Our data support that flavins could be crucial in a metabolic link in iron-deficient sugar beet roots, involving a redox chain between organic acids and FC-R activity (Fig. 5). A link between organic acids and FC-R was suggested previously by Bienfait (1996). The major increase in PEPC activity in yellow iron-deficient roots would lead to carbon fixation, accumulation of organic acids, and in turn to increased activities of several NADPH-producing enzymes such as cytosolic ICDH, MDH, and G6PDH. Reduced pyridine nucleotides would then act as electron donors for flavins, including riboflavin sulfates and riboflavin, which are mainly oxidized and could reach root concentrations 35-fold higher than that of the nucleotide pool (700 and 20 μm, respectively). Electrons can pass from one flavin molecule to the next forming a “redox bridge” (Rawn, 1989), to reach different final acceptors at the level of the cell PM, including membrane flavoproteins or directly oxygen. Indeed the marked increases in FAD concentrations in the YZs of iron-deficient roots (7-fold over the control values) may suggest that the increases in FC-R activities (11-fold increases over the control values) could be associated to increases in a FC-R enzyme similar to the FAD-containing enzyme recently characterized in Arabidopsis (Robinson et al., 1999).
Figure 5.
Proposed electron transport pathways in iron-deficient sugar beet roots. Reduced pyridine nucleotides would reduce flavins (Flv), which are oxidized and in large amounts in the cytosol (up to 700 μm). Flavins would finally provide electrons to the FC-R enzyme of the PM. This enzyme would be able to reduce not only Fe(III)-chelates, but also oxygen when Fe(III)-chelates are absent.
The white parts of the iron-deficient roots, which do not contain flavin sulfates and do not have increased Fe(III)-reducing activities, had large pools of organic anions, similar to those of the yellow distal root parts. However, the respiration rates of these root zones were similar to the controls and increases of the TCA cycle enzymatic activities, although still significant, were less marked than in the yellow tips. Also, the increase in PEPC activity over the controls was much smaller than that found in the yellow tips. These data clearly show that accumulation of organic acids per se is not sufficient to increase Fe(III)-reducing activities.
Two of the major findings documented in this work, the utilization of carbon and O2 by the root zones expressing the biochemical responses typical of Strategy I plant species, may both constitute a metabolic protective mechanism, which implies ecologically significant advantages. The increased PEPC activity of iron-deficient roots and other enzyme and metabolite changes indicate the existence of a nonautotrophic, anaplerotic carbon fixation by roots, similar to that reported previously in phosphorus-stressed Lupinus albus (Johnson et al., 1994). It should be mentioned that iron deficiency occurs mostly in nature in soils with high carbonate content, where bicarbonate is in large supply. In general, CO2 uptake by roots is considered negligible (Farmer and Adams, 1991), although it may be of great importance for the carbon balance of roots (Vuorinen et al., 1992) and nitrogen-fixing root nodules (Vance et al., 1994). Carbon fixed anaplerotically in roots of iron-deficient plants can be exported via xylem (Bialzyk and Lechowski, 1992; López-Millán et al., 2000) and then used for basic maintenance processes in leaves with drastically reduced photosynthetic rates (Terry, 1980). The possibility that the PM FC-R enzyme may donate electrons to O2 under physiological conditions in the absence of Fe(III)-chelates would also provide a significant advantage for the iron-deficient roots, since, in the absence of ferric chelates, O2 usually available in the rhizosphere of aerated soils would permit the dissipation of reducing power that would otherwise accumulate in the cell and lead to adverse consequences.
MATERIALS AND METHODS
Plant Material
Sugar beet (Beta vulgaris L. Monohil hybrid from Hilleshög [Landskröna, Sweden]) was grown in a growth chamber with a photosynthetic photon flux density of 350 μmol m−2 s−1 photosynthetically active radiation at a temperature of 25°C, 80% relative humidity, and a photoperiod of 16 h of light/8 h of darkness. Seeds were germinated and grown in vermiculite for 2 weeks. Seedlings were grown for an additional 2 weeks in one-half-strength Hoagland nutrient solution (Terry, 1980) with 45 μm Fe(III)-EDTA and then transplanted to 20-L plastic buckets (four plants per bucket) containing one-half-strength Hoagland nutrient solution with either 0 or 45 μm iron. The pH of the iron-free nutrient solutions was buffered at approximately 7.7 by adding 1 mm NaOH and 1 g L−1 of CaCO3. Root tips from plants grown for 10 d in the presence or absence of iron were used in all experiments.
Root sections were taken approximately 0 to 5 and 5 to 10 mm from the root apex with a surgical blade. The 0- to 5-mm section from the zero-iron treatment had short root hairs (Fig. 1B) and was yellow due to the presence of flavins (Susín et al., 1993), whereas the 5- to 10-mm section in the zero-iron treatment (Fig. 1A) and the 0- to 5- (Fig. 1D) and 5- to 10-mm (Fig. 1C) sections in the control treatments had practically no root hairs and were white. Sampling was made after 3 to 4 h of the start of the light period.
Organic Anion Analysis
Root material (100 mg fresh weight) was frozen in liquid N2 and ground in a mortar with 8 mm sulfuric acid. Homogenates were boiled for 30 min, filtered with a 0.2 μm polyvinyl fluoride filter (LIDA, Kenosha, WI), taken to a final volume of 2 mL with 8 mm sulfuric acid, and kept at −80°C until analysis.
Organic anions were analyzed by HPLC with an Aminex ion-exchange column (300 × 7.8 mm, HPX-87H, Bio-Rad, Hercules, CA) with an HPLC system (Waters, Milford, MA), including a 600E multisolvent delivery system, a 996 photodiode array detector, and Millenium 2010 software. Samples were injected with a Rheodyne injector (20-μL loop). Mobile phase (8 mm sulfuric acid) was pumped with a 0.6 mL min−1 flow rate. Organic anions were detected at 210 nm. Peaks corresponding to oxalate, cis-aconitate, citrate, 2-oxoglutarate, ascorbate, malate, succinate, and fumarate were identified by comparison of their retention times with those of known standards from Bio-Rad and Sigma (St. Louis; Fig. 2A). Identification was confirmed by the UV peak spectra and/or HPLC-MS (Waters; in this latter case formic acid was used as mobile phase instead of sulfuric acid). Quantification was made with known amounts of each organic anion using peak areas.
Enzyme Assays
Extracts for measuring enzyme activities were made by grinding 100 mg fresh weight of root material in a mortar with 1 mL of extraction buffer containing 30 mm sorbitol, 1% (w/v) bovine serum albumin, and 1% (w/v) polyvinylpyrrolidone in 100 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-KOH, pH 8.0. The slurry was centrifuged for 15 min at 10,000g and 4°C, and the supernatant was collected and analyzed immediately. The activities of all enzymes were analyzed in 1 mL (final volume) of the media indicated below.
MDH (EC 1.1.1.37) was determined with oxalacetate as substrate (Dannel et al., 1995) by measuring the decrease in A340 due to the enzymatic oxidation of NADH. The reaction was carried out with 5 μL of extract in 0.1 mm NADH, 0.4 mm oxalacetate, and 46.5 mm Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 9.5. CS (EC 4.1.3.7) was assayed spectrophotometrically according to Srere (1967) by monitoring the reduction of acetyl coenzyme (CoA) to CoA with 5–5′-dithio-bis-2-nitrobenzoic acid at 412 nm. The reaction was carried out with 50 μL of extract in 0.1 mm 5–5′-dithio-bis-2-nitrobenzoic, 0.36 mm acetyl CoA, 0.5 mm oxalacetate, and 100 mm Tris-HCl, pH 8.1. Aconitase (EC 4.2.1.3) activity was measured from the formation of cis-aconitate, monitored at 240 nm (Bacon et al., 1961) with 60 μL of extract in 500 mm Suc, 50 mm isocitrate, and 100 mm Tris-HCl, pH 8.5. ICDH (EC 1.1.1.42) was determined with 50 μL of extract by monitoring the reduction of NADP+ at 340 nm in a reaction mixture containing 3.5 mm MgCl2, 0.41 mm NADP+, 0.55 mm isocitrate, and 88 mm imidazole, pH 8.0 (Bergmeyer et al., 1974). Fumarase (EC 4.2.1.2) was assayed with 50 μL of extract following the increase in A240 due to the formation of fumarate (Bergmeyer et al., 1974). The reaction buffer was 50 mm malate and 100 mm phosphate, pH 7.4.
PEPC (EC 4.1.1.31) activity was measured in a coupled enzymatic assay with MDH according to Vance et al. (1983) with 75 μL of extract in 2 mm PEP, 10 mm NaHCO3, 5 mm MgCl2, 0.16 mm NADH, and 100 mm Bicine [N,N′-bis(2-hydroxyethylglycine)]-HCl, pH 8.5. The effect of malate on the PEPC activity in root extracts was assayed in 1 mL of a reaction mixture containing 75 μL of extract in 2 mm PEP, 1 mm NaHCO3, 5 mm MgCl2, 0.16 mm NADH, 50 mm HEPES, pH 7.3, and concentrations of malate from 50 μm to 5 mm. G6PDH (EC 1.1.1.49) was determined with d-Glc-6-P as substrate (Bergmeyer et al., 1974) by measuring the increase in A340 due to the enzymatic reduction of NADP+. The reaction was carried out with 50 μL of extract in 138 mm MgCl2, 20 mm Glc-6-P, 7.8 mm NADP+, and 100 mm HEPES, pH 7.6. For the determination of LDH (EC 1.1.1.27) and PDC (EC 4.1.1.1) the oxidation of NADH was monitored at 340 nm with 50 μL of extract. LDH was assayed in a reaction buffer containing 94.5 mm phosphate buffer (pH 9.5), 0.77 mm pyruvate, and 0.2 mm NADH. PDC was determined in 190 mm citrate-KOH buffer (pH 6.0), 30 mm pyruvate, 0.32 mm NADH, and 33 μg mL−1 alcohol dehydrogenase.
Extracts for measuring CA (EC 4.2.11) were made by grinding 200 mg fresh weight of root material in 1.5 mL of 100 mm Tris, 10 mm mercaptoethanol, and 1 mm EDTA, pH 8.3. The extract was stirred for 15 min at room temperature, centrifuged at 1,000g for 5 min, and the supernatant was stored on ice until assayed. The Wilbur-Anderson electrometric method was used to assay CA activity (Wilbur and Anderson, 1948). One milliliter of extract was added to 3 mL of 25 mm veronal (barbitone, 5–5-diethyl barbituric acid), pH 8.2. Four milliliters of CO2-saturated water were added and the time taken for the pH to change from 8.2 to 7.0 was measured. Blanks were run using 1 mL of extract buffer.
Nucleotide Analysis
Pyridine nucleotides were extracted from liquid N2-frozen root material (approximately 30 mg fresh weight) in 1 mL of 100 mm NaOH [for NAD(P)H] or 5% (w/v) TCA [for NAD(P)+]. The extracts were boiled for 6 min, cooled on ice, and centrifuged at 12,000g for 6 min. Samples were adjusted to pH 8.0 with HCl or NaOH and 100 mm bicine (pH 8.0). Nucleotides were quantified by the enzyme-cycling method of Matsumura and Miyachi (1980).
ATP was extracted by grinding 100 mg fresh weight of root material in a mortar with 1 mL of 2 mm EDTA and 100 mm Tris-acetate buffer, pH 7.75. The extract was centrifuged at 10,000g for 15 min at 4°C. The supernatant was mixed with dimethyl sulfoxide (1:9, v/v), and ATP was measured with a luminometer (Labsystems Luminoskan, Life Sciences International, Finland) using an ATP monitoring kit (Bio Orbit Oy, Finland).
Analysis of Q-Pool Redox Poise
The extraction of Q from root tips was conducted according to Millar et al. (1998). Root material (approximately 1 g fresh weight) was immersed in liquid N2 and crushed to a fine powder with mortar and pestle. The powder was freeze-dried to remove the root aqueous phase and decrease the possibility of Q oxidation during organic extraction. Dried samples were vortexed for 3 min in a mixture of 1.5 mL of methanol (containing 200 mm perchloric acid) and 1.5 mL of petroleum ether (35°C–50°C boiling point, d 0.64). After centrifugation for 3 min at 1,000g to separate the two phases, the upper phase was collected. Additional petroleum ether was added to the lower phase, and the procedure was repeated. The two upper phases were combined, dried under a stream of N2, and dissolved in 100 μL of methanol purged with N2 and containing 1 mm HCl.
The total pool and redox poise of mitochondrial Q in sugar beet roots were determined after Q organic extraction by reverse-phase HPLC (Fig. 2B). A Novapak C18 radial compression column (100 × 8 mm, Waters) was used, with an isocratic mobile phase (ethanol:methanol, 7:3, v/v; purged with N2), and a flow rate of 1 mL min−1. The oxidized and reduced forms of Q9 and Q10 were identified by their retention times and extinction coefficients at 275 and 290 nm (Millar et al., 1998; Millenaar et al., 1998). Retention times for reduced Q9, reduced Q10, oxidized Q9, and oxidized Q10 were 8.2, 10.0, 12.5, and 16.3 min, respectively. Both Q9 and Q10 were reduced with dithionite to their respective QH2 (Rich, 1978). Quantification of the Q-type compounds was made from the peak areas obtained with known amounts of Q9 and Q10 standards from Sigma.
O2 Consumption
Roots were excised under water at room temperature from plants illuminated for several hours. Root O2 consumption rates were measured from the decrease in O2 concentration in an aqueous phase with a Clark-type O2 electrode (Hansatech, Kings Lynn, UK). Calibration was made from the difference in signal between air and N2-saturated water (Walker, 1987). The effects of the respiration inhibitors KCN and SHAM were studied at different concentrations. Sequential additions of KCN were made directly to roots in the measurement cuvette. Roots were pre-incubated with different concentrations of SHAM for 30 min prior to measurement. A new batch of root material was used for each SHAM concentration. O2 consumption rates were also determined in the presence of 2 mm Fe(III)-EDTA alone or in combination with KCN, SHAM, or KCN plus SHAM.
Iron-Reducing Activity
The FC-R activity of root tips was followed by measuring the formation of the Fe(II)- bathophenanthroline disulfonate complex from Fe(III)-EDTA at 535 nm (Bienfait et al., 1983). Three root tips were added to 1 mL of nutrient solution without microelements, 5 mm MES, pH 6.0, supplemented with 400 μm bathophenanthroline disulfonate and 500 μm Fe(III)-EDTA. The reaction was performed in the dark for 7 min. An 0.8-mL aliquot was taken, centrifuged at 10,000g for 3 min, and absorbance measured at 535 nm.
Flavin Determination
FAD, FMN, rivoflavin, SI, and SII were extracted from root tips and separated by reverse-phase HPLC according to Susín et al. (1993; Fig. 2C).
Protein Quantification
Extracts for protein quantification were made according to Granier (1988) with the solubilization buffer of Herbik et al. (1996). Samples were washed twice with TCA to avoid interferences by SDS and 2-mercaptoethanol and resuspended in ultrapure water. Proteins were quantified with the DC Protein System Assay (Bio-Rad) based on Lowry et al. (1951) using bovine serum albumin as standard.
Electron Microscopy
Electron microscopy was performed with root tips fixed in 2.5% (v/v) glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.4) for 2 h at room temperature, 20 h at 4°C, and then washed in the same buffer. After dehydration in acetone series, samples were critical-point dried, gold-palladium coated, and viewed at 10 kV in a LEO 430 scanning electron microscope (LEO Ltd., Cambridge, UK).
ACKNOWLEDGMENTS
The authors gratefully acknowledge the skillful technical assistance of Aurora Poc in growing the plants and Pilar Zanuy with the electron microscope. We also thank Drs. Beatriz Amorena and Juan Marín for use of equipment.
Footnotes
This work was supported by the Comisión Interministerial de Ciencia y Tecnología (grant no. AGR97–1177 to A.A.), the Dirección General de Investigación Científica y Técnica (grant no. PB97–1176 to J.A.), and the Commission of European Communities (grant nos. AIR3–CT94–1973 and PL971176 to J.A.). A.F.L.-M. was supported by a fellowship from the Spanish Ministry of Science and Education. F.M. and Y.G. were scientists on contracts from the Spanish Ministry of Education and Culture and the Spanish Council of Scientific Research, respectively.
LITERATURE CITED
- Alhendawi RA, Römheld V, Kirby EA, Marschner H. Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum and maize. J Plant Nutr. 1997;20:1731–1753. [Google Scholar]
- Bacon JSD, Palmer MJ, De Kock PC. The measurements of aconitase activity in the leaves of various normal and variegated plants. Biochem J. 1961;78:198–204. doi: 10.1042/bj0780198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedri AA, Wallace A, Rhoads WA. Assimilation of bicarbonate by roots of different plant species. Soil Sci. 1960;89:257–263. [Google Scholar]
- Bergmeyer HU, Gawwehn K, Grassl M. Enzymes as biochemical reagents. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 425–556. [Google Scholar]
- Bialzyk J, Lechowski L. Absorption of HCO3− by roots and its effect on carbon metabolism of tomato. J Plant Nutr. 1992;15:293–312. [Google Scholar]
- Bienfait HF. Mechanisms in iron-efficiency reactions of higher plants. J Plant Nutr. 1988;11:605–629. [Google Scholar]
- Bienfait HF. Prevention of stress in iron metabolism of plants. Acta Bot Neerl. 1989;38:105–129. [Google Scholar]
- Bienfait HF. Is there a metabolic link between H+ excretion and ferric reduction by roots of iron-deficient plants: a viewpoint. J Plant Nutr. 1996;19:1211–1222. [Google Scholar]
- Bienfait HF, Bino RJ, van der Bliek AM, Duivenvoorden JF, Fontaine JM. Characterization of ferric reducing activity in roots of iron-deficient Phaseolus vulgaris. Physiol Plant. 1983;59:196–202. [Google Scholar]
- Brown JC. Iron and Ca uptake as related to root-sap and stem-exudate citrate in soybeans. Physiol Plant. 1966;19:968–976. [Google Scholar]
- Brown JC. Mechanism of iron uptake by plants. Plant Cell Environ. 1978;1:249–257. [Google Scholar]
- Brown JC, Chaney RL, Ambler JE. A new tomato mutant inefficient in the transport of iron. Physiol Plant. 1971;25:48–53. [Google Scholar]
- Chaney RL, Brown JC, Tiffin LO. Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol. 1972;50:208–213. doi: 10.1104/pp.50.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O'Leary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Dannel F, Pfeffer H, Marschner H. Isolation of apoplasmic fluid from sunflower leaves and its use for studies on influence of nitrogen supply on apoplasmic pH. J Plant Physiol. 1995;50:208–213. [Google Scholar]
- Davies DD. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- de Vos CR, Lubberding HJ, Bienfait HF. Rhizosphere acidification as a response to iron deficiency in bean plants. Plant Physiol. 1986;81:842–846. doi: 10.1104/pp.81.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide D, Brodenius M, Fett J, Guerinot ML. A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci USA. 1996;93:5624–5628. doi: 10.1073/pnas.93.11.5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer AM, Adams MS. Carbon uptake by roots. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant Roots: The Hidden Half. New York: Marcel-Dekker; 1991. pp. 627–637. [Google Scholar]
- Fox TC, Guerinot ML. Molecular biology of cation transport in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:669–676. doi: 10.1146/annurev.arplant.49.1.669. [DOI] [PubMed] [Google Scholar]
- González-Vallejo EB. Caracterización de mecanismos de adquisición de Fe en plantas superiores. PhD thesis. Zaragoza, Spain: Univérsidad de Zaragozo; 1999. [Google Scholar]
- González-Vallejo EB, González-Reyes JA, Abadía A, López-Millán AF, Yunta F, Lucena JJ, Abadía J. Reduction of ferric chelates by leaf plasma membrane preparations from iron-deficient and iron-sufficient sugar beet. Aust J Plant Physiol. 1999;26:601–611. [Google Scholar]
- González-Vallejo EB, Susín S, Abadía A, Abadía J. Changes in sugar beet plasma membrane Fe(III)-chelate reductase activities mediated by iron-deficiency, assay buffer composition, anaerobiosis and the presence of flavins. Protoplasma. 1998;205:163–168. [Google Scholar]
- Granier F. Extraction of plant proteins for two dimensional electrophoresis. Electrophoresis. 1988;9:712–718. doi: 10.1002/elps.1150091106. [DOI] [PubMed] [Google Scholar]
- Habison A, Kubicek CP, Röhr M. Phosphofructokinase as a regulatory enzyme in citric acid producing Aspergillus niger. FEMS Microbiol Lett. 1979;5:39–42. [Google Scholar]
- Herbik A, Giritch A, Horstmann C, Becker R, Balzer HJ, Bäumlein H, Sthephan UW. Iron and copper nutrition-dependent changes in protein expression in a tomato wild type and the nicotianamine-free mutant chloronerva. Plant Physiol. 1996;111:533–540. doi: 10.1104/pp.111.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker RC, Hall DO, Shannon LM, Wallace A, Rhoads WA. Effects of iron and chelating agents on dark carboxylation reactions in plant homogenates. Plant Physiol. 1959;34:446–449. doi: 10.1104/pp.34.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iljin WS. Metabolism of plants affected with lime induced chlorosis: II. Organic acids and carbohydrates. Plant Soil. 1951;3:339–351. [Google Scholar]
- Johnson J, Allan DL, Vance CP. Phosphorous stress-induced proteoid roots show altered metabolism in Lupinus albus. Plant Physiol. 1994;104:657–665. doi: 10.1104/pp.104.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer D, Römheld V, Landsberg EC, Marschner H. Induction of transfer-cell formation by iron deficiency in the root epidermis of Helianthus annuus L. Planta. 1980;147:335–339. doi: 10.1007/BF00379842. [DOI] [PubMed] [Google Scholar]
- Lance C, Rustin P. The central role of malate in plant metabolism. Physiol Veg. 1984;22:625–641. [Google Scholar]
- Landsberg EC. Organic acid synthesis and release of hydrogen ions in response to iron deficiency stress of mono and dicotyledoneous plant species. J Plant Nutr. 1981;3:579–591. [Google Scholar]
- Landsberg EC. Transfer cell formation in the root epidermis: a prerequisite for iron-efficiency? J Plant Nutr. 1982;5:415–432. [Google Scholar]
- Landsberg EC. Function of rhizodermal transfer cells in the iron stress response mechanisms of Capsicum annum L. Plant Physiol. 1986;82:511–517. doi: 10.1104/pp.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg EC. Transfer cell formation in sugar beet roots induced by latent iron deficiency. Plant Soil. 1994;165:197–205. [Google Scholar]
- Lindsay WL, Schwab AP. The chemistry of iron soils and its availability to plants. J Plant Nutr. 1982;5:821–840. [Google Scholar]
- López-Millán AF, Morales F, Abadía A, Abadía J. Effects of iron deficiency on the composition of the leaf apoplastic fluid and xylem sap in sugar beet: implications for iron and carbon transport. Plant Physiol. 2000;124:873–884. doi: 10.1104/pp.124.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrougn NJ, Lewis-Farr A, Randall RJ. Protein measurement with the folin fenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marschner H, Römheld V. Strategies of plants for acquisition of iron. Plant Soil. 1994;165:261–274. [Google Scholar]
- Marschner H, Römheld V, Kissel M. Different strategies in higher plants in mobilization and uptake of iron. J Plant Nutr. 1986;9:695–713. [Google Scholar]
- Matsumura H, Miyachi S. Cycling assay for nicotinamide adenine dinucleotides. Methods Enzymol. 1980;69:465–470. [Google Scholar]
- Millar AH, Atkin OK, Menz RI, Henry B, Farquhar G, Day DA. Analysis of respiratory chain regulation in roots of soybean seedlings. Plant Physiol. 1998;117:1083–1093. doi: 10.1104/pp.117.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millenaar FF, Benschop JJ, Wagner AM, Lambers H. The role of the alternative oxidase in stabilizing the in vivo reduction state of the ubiquinone pool and the activation state of the alternative oxidase. Plant Physiol. 1998;118:599–607. doi: 10.1104/pp.118.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GW, Shigematsu A, Welkie GW, Motoji N, Szlek M. Potassium effect on iron stress in tomato: II. The effects on root CO2− fixation and organic acid formation. J Plant Nutr. 1990;13:1355–1370. [Google Scholar]
- Moog PR, Brüggemann W. Iron reductase systems on the plant plasma membrane: a review. Plant Soil. 1994;165:241–260. [Google Scholar]
- Rabotti G, de Nisi P, Zocchi G. Metabolic implications in the biochemical responses to iron deficiency in cucumber (Cucumis sativus L.) roots. Plant Physiol. 1995;107:1195–1199. doi: 10.1104/pp.107.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawn JD. Bioquimica. Madrid: Interamericana McGraw-Hill; 1989. [Google Scholar]
- Rhoads WA, Wallace A. Possible involvement of dark fixation of CO2 lime-induced chlorosis. Soil Sci. 1960;89:248–256. [Google Scholar]
- Rhoads WA, Wallace A, Romney EM. Lime-induced chlorosis studied physiology of disorder investigated to learn role of malonic acid and possibility of a block in organic acid metabolism. Calif Agric. 1959;6:15–16. [Google Scholar]
- Ribas-Carbó M, Lennon A, Robinson S, Giles L, Berry J, Siedow J. The regulation of electron partioning between the cytochrome and alternative pathways in soybean cotyledon and root mitochondria. Plant Physiol. 1997;113:903–911. doi: 10.1104/pp.113.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PR. Quinol oxidation in Arum maculatum mitochondria and its application to the assay, solubilisation and partial purification of the alternative oxidase. FEBS Lett. 1978;96:252–256. [Google Scholar]
- Robinson N, Procter C, Connolly E, Guerinot M. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- Rombolà AD. Aspetti fisiologici e biochimici della nutrizione ferrica in actinidia (A. deliciosa). PhD thesis. Bologna, Italy: Dipartimento di Colture Arboree, Universitá degli Studi di Bologna; 1998. [Google Scholar]
- Schmidt W. Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol. 1999;141:1–26. [Google Scholar]
- Schmidt W, Schuck C. Pyridine nucleotide pool size changes in iron-deficient Plantago lanceolata roots during reduction of external oxidants. Physiol Plant. 1996;98:215–221. [Google Scholar]
- Sijmons PC, van den Briel W, Bienfait HF. Cytosolic NADPH is the electron donor for extracellular Fe(III) reduction in iron-deficient bean roots. Plant Physiol. 1984;75:219–221. doi: 10.1104/pp.75.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Methods Enzymol. 1967;13:3–11. [Google Scholar]
- Susín S, Abadía A, González-Reyes JA, Lucena JJ, Abadía J. The pH requirement for in vivo activity of the iron-deficiency-induced “turbo” ferric chelate reductase: a comparison of the iron-deficiency-induced iron reductase activities of intact plants and isolated plasma membrane fractions in sugar beet. Plant Physiol. 1996;110:111–123. doi: 10.1104/pp.110.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susín S, Abián J, Peleato ML, Sánchez-Baeza J, Abadía A, Gelpí E, Abadía J. Flavin excretion from iron deficient sugar beet (Beta vulgaris L.) Planta. 1994;193:514–519. [PubMed] [Google Scholar]
- Susín S, Abián J, Sánchez-Baeza F, Peleato ML, Abadía A, Gelpí E, Abadía J. Riboflavin 3′- and 5′-sulfate, two novel flavins accumulating in the roots of iron-deficient Sugar Beet (Beta vulgaris) J Biol Chem. 1993;268:20958–20956. [PubMed] [Google Scholar]
- Suzuki K, Itai R, Suzuki K, Nakanishi H, Nishizawa NK, Yoshimura E, Mori S. Formate dehydrogenase, an enzyme of anaerobic metabolism, is induced by iron deficiency in barley roots. Plant Physiol. 1998;116:725–732. doi: 10.1104/pp.116.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting factors in photosynthesis: I. Use of iron stress to control photochemical capacity in vivo. Plant Physiol. 1980;65:114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoiron S, Briat J-F. Differential expression of maize sugar responsive genes in response to iron deficiency. Plant Physiol Biochem. 1999;37:759–766. [Google Scholar]
- Tiffin LO. Iron translocation: II Citrate/iron ratios in plant stem exudates. Plant Physiol. 1966;41:515–518. doi: 10.1104/pp.41.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Gregerson RG, Robinson DL, Miller SS, Gantt JS. Primary assimilation of nitrogen in alfalfa nodules: molecular features of the enzymes involved. Plant Sci. 1994;101:51–64. [Google Scholar]
- Vance CP, Stade S, Maxwell CA. Alfalfa root nodule carbon dioxide fixation: I. Association with nitrogen fixation and incorporation into amino acids. Plant Physiol. 1983;72:469–473. doi: 10.1104/pp.72.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorinen AH, Vapaavuori EM, Raatikainen O, Lapinjok SP. Metabolism of inorganic carbon taken up by roots in Salix plants. J Exp Bot. 1992;43:789–795. [Google Scholar]
- Walker D. The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis. Sheffield, UK: Oxygraphics Limited; 1987. [Google Scholar]
- Welkie GW, Miller GW. Iron nutrition of Nicotiana tabacum L. in relation to riboflavin, riboflavin-5′-phosphate, and flavin adenine dinucleotide content. Plant Physiol. 1960;35:516–520. doi: 10.1104/pp.35.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkie GW, Miller GW. Plant iron uptake physiology by nonsiderophore systems. In: Barton LL, Hemming BC, editors. Iron Chelation in Plants and Soil Microorganisms. San Diego: Academic Press; 1993. pp. 345–369. [Google Scholar]
- White MC, Baker FD, Chaney RL, Decker AM. Metal complexation in xylem fluid: II. Theoretical equilibrium model and computational computer program. Plant Physiol. 1981;67:301–310. doi: 10.1104/pp.67.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem. 1948;176:147–154. [PubMed] [Google Scholar]