Abstract
Background
Previously published studies reported benefits of computer-assisted surgery (CAS) in terms of radiographic implant position in TKA, but whether these improvements result in clinically important survival differences or functional differences that a patient might perceive at a minimum 10-year followup remains largely unknown.
Questions/purposes
We performed a prospective randomized trial and asked whether CAS (1) improved survival free from aseptic loosening; and (2) demonstrated any clear difference in patient-reported outcomes at latest followup using validated outcome measures at minimum 10-year followup.
Methods
Between January 2004 and December 2005, 80 patients scheduled for TKA were randomly assigned either to the CAS group or to the conventional technique group by the Hospital Informatics Department. The patient inclusion criteria were age 20 to 80 years old, weight < 100 kg, and consent to receive a primary knee arthroplasty performed through a medial parapatellar approach by the senior author. The exclusion criteria were a history of prior knee surgery, TKA performed for a posttraumatic indication, or revision knee surgery. The first 80 patients meeting these criteria were included in the study. There were 21 women and 19 men and in each group; mean age was 66 years (range, 58-77 years), and mean body mass index was 27 ± 4 kg/m2. An initial published study using this patient group investigated only differences regarding implant positioning in the coronal and sagittal planes. This is a secondary analysis of patients from the earlier study protocol at a minimum of 10-year followup with different endpoints. Kaplan-Meier survivorship was compared between groups, and functional patient-reported outcome measures (PROMs) were evaluated using the SF-12, Knee Injury and Osteoarthritis Outcome Score (KOOS), Forgotten Joint Score, and the new Knee Society Score. Those PROMs were not available at the time of the original randomized controlled trial and we therefore do not have baseline preoperative values demonstrating that our two groups were comparable. However, our groups were created using strict randomization and were similar in terms of demographic parameters and knee deformities. Our secondary analysis was not powered for survival analysis but had 80% power to detect a difference > 6 points on the SF-12 components and > 6 points out of 100 on the KOOS subscores (published minimal clinically important difference: 8 points) at the p < 0.05 level.
Results
With the numbers available, there was no difference between the CAS group and the conventional TKA group in terms of survivorship free from aseptic loosening 10 years after TKA (97%, 95% confidence interval [CI], 95%-99% versus 97%, 95% CI, 95%-99%; p = 0.98). Investigation of the latest followup PROM scores showed no difference between SF-12 scores (respectively, for CAS and control patients, physical SF-12: 72 ± 12 versus 73 ± 13 mean difference 0, 95% CI -3 to 3, p = 0.9; mental SF-12: 75 ± 8 versus 73 ± 10, mean difference 2, 95% CI 0−4, p = 0.3) as well as for all KOOS subscores (all p > 0.1). Forgotten Joint Scores were similar in both groups with 83 ± 4 for CAS and 82 ± 5 for control patients (mean difference 1, 95% CI 0−2, p = 0.2). Finally, the new Knee Society Scores were not statistically different between groups with a mean objective score of 82 ± 13 for CAS patients versus 79 ± 12 for control patients (mean difference 2, 95% CI 0−5, p = 0.5) and a mean subjective score of 83 ± 11 versus 85 ± 12, respectively (mean difference 2, 95% CI 0−5, p = 0.5).
Conclusions
Our observations suggest that CAS used for TKA alignment with restoration of a neutral mechanical axis as the goal did not confer any substantial advantage in survivorship, function, or quality of life at 10 years after TKA. Larger studies with longitudinal collection of PROMs for functional assessment and greater numbers to assess survivorship are needed to confirm these findings.
Level of Evidence:
Level III, therapeutic study.
Introduction
Computer-assisted surgery (CAS) in TKA was developed to improve implant positioning and restore a neutral mechanical axis with the aim of improving patient function and implant survivorship [22]. However, the role that coronal alignment of TKA plays in these areas remains somewhat controversial [21]. Although several studies have indicated that coronal plane alignment beyond the range of 0° ± 3° is a risk factor for impaired results, much of the literature on alignment and survivorship is historical and surprisingly weak in terms of lower limb alignment definitions [1]. In 2008, Parratte et al. [21] reported that TKAs that are mechanically aligned (0° ± 3°) versus those that are outside that range have identical survivorship 15 years after surgery. This research opened the debate on alternatives to the mechanical axis as a target for contemporary TKAs [13].
Previously published studies demonstrated benefits of CAS in terms of radiographic implant position, particularly with regard to outliers [10, 12], but whether these improvements result in clinically important differences that patients might perceive at a minimum 10-year followup remains largely debated [5, 18]. Although some series demonstrated clinical benefits of TKA performed using CAS [3-5], most of them are short- to midterm studies with followup < 60 months [4, 5]. Series with longer followup failed to demonstrate clinical benefits or improved implant survivorship when comparing CAS and conventionally instrumented TKA [11, 16].
We earlier designed a randomized controlled trial to compare implant positioning using CAS versus a conventional surgical technique [17]. Using this same study group, we performed a secondary analysis 10 years after that study and asked whether CAS (1) improved implant survival free from aseptic loosening; and (2) demonstrated any clear difference in patient-reported outcomes at latest followup using validated outcome measures at minimum 10-year followup.
Patients and Methods
Between January 2004 and December 2005, we performed 373 elective TKAs for primary or secondary arthritis. Of those, 86 (23%) were performed using the CAS technique [16]. Between April 2004 and April 2005, a randomized study was designed to assess implant positioning using CAS versus conventional instrumentation TKA (defined as control patients). The patient inclusion criteria were age 20 to 80 years old, weight < 100 kg, and consented for primary knee arthroplasty performed through a medial parapatellar approach by the senior author (J-NA). The exclusion criteria were a history of prior knee surgery, TKA performed for a posttraumatic indication, or revision knee surgery (Fig. 1). All patients provided informed consent to participate in the study.
Fig. 1.
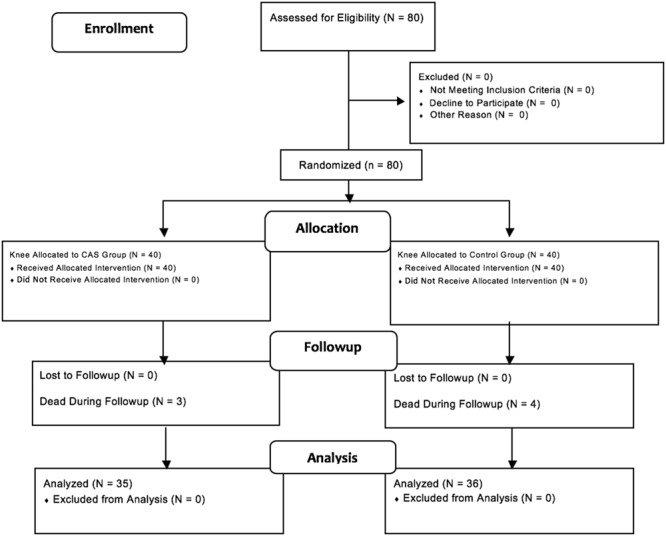
This is a flow diagram illustrating patients’ enrollment, allocation, followup, and analysis.
The first 80 patients meeting the inclusion criteria of this study were randomly assigned to the CAS or control group by the Hospital Informatics Department with use of a systematic sampling method. Patients were randomized after stratification on age and gender to try to ensure comparability of control and CAS patients (Fig. 1). The study protocol (including the use of navigation and postoperative evaluation) and consent forms were approved by the local ethical committee, and this study was subsequently published [17].
Ten years after initiation of this study, we performed a secondary analysis of this group. Of those who were treated with CAS, three patients (7.5%) had died, whereas 37 patients (37 knees) were available for followup at a minimum of 10 years (median, 11 years; range, 11-13 years); no patients were lost to followup. Of those treated with conventional instrumentation, four patients died (10%) leaving 36 patients (36 knees) for clinical and radiologic evaluation at a minimum of 10 years (median, 11 years; range, 11-13 years).
There were no differences in demographic data between the two study groups with the numbers available. There were 21 women and 19 men. The median age of the patients was 66 years (range, 40-80 years) in the CAS group and 64 years (range, 46-78 years) in the control conventional technique group (p = 0.31). The mean body mass index was 27 ± 4 kg/m2 (range, 19-41 kg/m2) in the CAS group and 26 ± 5 kg/m2 (range, 20-44 kg/m2) in the control group (p = 0.44). The etiologies were primary osteoarthritis in 32 knees and eight with secondary arthritis in the CAS group and primary osteoarthritis in 29 knees and 11 with secondary arthritis in the control group.
One surgeon with subspecialty training and interest in TKA (J-NA) performed all the surgeries. Traditional mechanical guides were used in the control group. Before the beginning of the study, the senior author had performed > 2000 TKAs, which included > 50 computer-assisted procedures.
NexGen® LPS Flex (Zimmer, Warsaw, IN, USA) posterior-stabilized femoral components and NexGen all-polyethylene patella components were cemented in all patients. All TKAs were done without a tourniquet using a standard paramedian incision and a medial parapatellar approach. The tibial resection was performed with an extramedullary guide and the distal femoral resection with an intramedullary guide. Femoral rotation was set according to a previously described technique [24]. In the CAS group, an imageless femoral and tibial implant positioning, computer-based navigation system was used following a previously described protocol [17]. Two additional skin incisions were made to position the rigid bodies required for navigation in the CAS group. Once the standard approach was done and the knee exposed, the tibial and femoral rigid bodies were implanted through separate incisions. After calibration of the instruments, the first step of the CAS procedure was the acquisition of predetermined landmarks and surfaces on the distal femur and the proximal tibia using a bone morphing technique. The mechanical axis of the limb and of the femur and tibia was then determined with the registration of the center of the femoral head (circumduction of the entire limb), the center of the trochlear notch, the center of the tibial spines, and palpation of the medial and lateral malleoli to determine the center of the ankle. After this registration process, the tibial cut was then made using the specific navigated tibial jig following the indications of frontal alignment and slope given by the software. The femoral cuts were then done following the same principles. Both cuts were then checked using the dedicated navigated probe. Size of the femoral implant was given by the computer, but standard instruments were used to determine femoral rotation. At the time of the trial, ROM, frontal alignment, and varus/valgus laxity were assessed using the software. Using this information, complementary releases were performed and a thicker liner was tried when needed. At the time of inclusion, the instrumented ligament tensor was not available. A pure measured resection technique was used in both groups. The flexion and extension gaps were tested after the cuts and appropriate ligament release was performed when needed [24]. Varus and valgus stresses were performed to assess the stability of the knee at the time of the trials. The goal of the surgeon was to obtain a neutral postoperative mechanical axis as defined by the center of the hip, center of the knee, and center of the ankle. Femoral and tibial rotations were set according to a previously described technique mainly based on a measured resection technique. The same combination of classic bony landmarks was used in both groups. CAS was only used to determine the frontal and sagittal alignment of the implants as a result of the limited accuracy of CAS to determine femoral and tibial rotation. The same postoperative rehabilitation protocol was used for all patients.
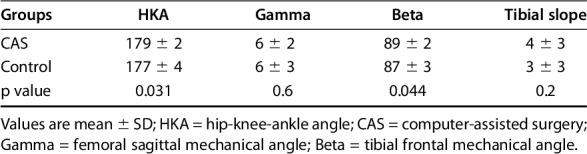
As part of our earlier study on alignment with these same groups, we observed a difference in implant positioning referenced by the hip-knee-angle (evaluated on long-axis radiographs (CAS: 179° ± 2° versus control: 177° ± 4°, mean difference 2° ± 3°, 95% confidence interval [CI] -2° to 5°, p = 0.031) and tibial implant position in the coronal plane (CAS: 89° ± 2° versus control: 87° ± 3°, mean difference 2° ± 4°, 95% CI -1° to 6°, p = 0.044; Table 1).
Table 1.
Summary of postoperative implant position analysis
Survivorship was compared in the two groups using the Kaplan-Meier method [15] defining the endpoint as revision of one or more of the components resulting from aseptic loosening or mechanical failure (fracture excepted) defined as substantial migration of one or more component(s), substantial wear, or osteolysis at last followup, potentially calling for a revision [8]. Septic revision or revision resulting from periprosthetic fracture was not included in our survivorship analysis.
We also elected to compare the subjective functional outcome and quality of life of the two groups, because they were judged to be equivalent based on the earlier randomization process. However, because the original randomization had been performed for an alignment study, preoperative patient-reported outcome measures (PROMs) had not been obtained. Furthermore, the PROMs in question were not common, available, and/or validated in French at the time of the original randomization. Nevertheless, PROMs of the following scores (SF-12, Knee Injury and Osteoarthritis Outcome Score [KOOS], Forgotten Joint Score [FJS], new Knee Society Score [KSS]) were assessed at an individual clinical examination to offer a general basis of comparison between the groups, accepting the obvious limitation of not having preoperative and serial scores for comparison.
Subjective functional outcome and quality of life were assessed during individual clinical evaluation, performed by two authors not involved in the surgical interventions (MO, SP), using validated questionnaires (SF-12 [26], KOOS [23], FJS [25], and the new KSS [7]).
Statistical Analysis
A comparison of the functional outcomes at last followup between the two groups was performed using either a parametric or nonparametric test (depending on parameter distribution).
Our initial protocol was designed to detect differences in terms of lower limb alignment between two groups of patients undergoing CAS or conventional TKA. The present research protocol aimed to evaluate clinical and radiologic outcomes of those two groups 10 years after surgery. A post hoc analysis confirmed that regarding our primary outcome with our available sample size and postoperative score SD, this study had 80% power to detect a difference > 6 points out of 100 on the KOOS subscores (published minimally clinically important difference [MCID]: 8 points) and > 6 points on the SF-12 components (published MCID: 5 points) at the p < 0.05 level [4, 6]. Our study was not adequately powered for survival analysis.
Analysis was performed using SPSS software (Version 12; SPSS Inc, Chicago, IL, USA). All calculations assumed two-tailed tests.
Results
With the numbers available, there was no difference between the CAS group and the conventional TKA group in terms of survivorship free from aseptic loosening 10 years after TKA (97%, 95% CI 95%-99% versus 97%, 95% CI 95%-99%; p = 0.98; Fig. 2).
Fig. 2.
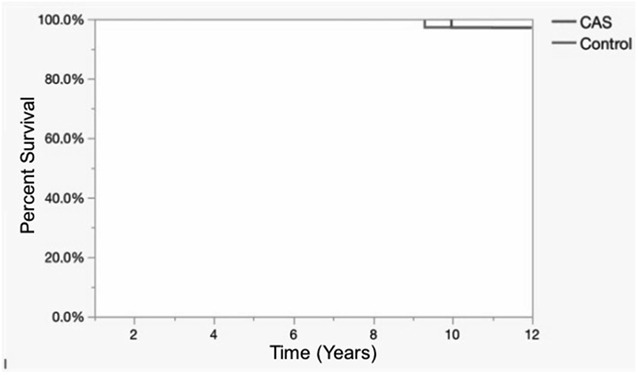
Kaplan-Meier analysis shows survivorship using revision for mechanical failure as the endpoint.
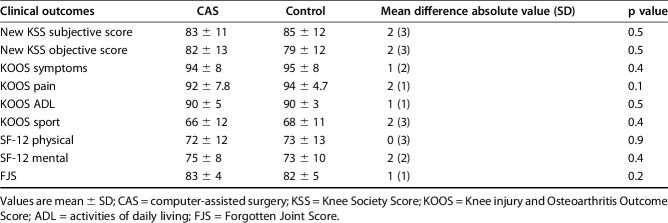
When investigating latest followup scores (improvement of those scores was not assessed because they were not available preoperatively), we found no difference between groups regarding SF-12 score (respectively, for CAS and control patients, physical SF-12: 72 ± 12 versus 73 ± 13 mean difference 0, 95% CI -3 to 3, p = 0.9; mental SF-12: 75 ± 8 versus 73 ± 10, mean difference 2, 95% CI 0−4, p = 0.3) as well as for all KOOS subscores (all p > 0.1; Fig. 3). FJS scores were similar in both groups with 83 ± 4 for CAS and 82 ± 5 for control patients (mean difference 1, 95% CI 0−2, p = 0.2) at last followup. Finally, the new KSS scores were not statistically different between groups with a mean objective score of 82 ± 13 for CAS patients versus 79 ± 12 for control patients (mean difference 2, 95% CI 0−5, p = 0.5) and a mean subjective score of 83 ± 11 versus 85 ± 12, respectively (mean difference 2, 95% CI 0−5, p = 0.5; Table 2).
Fig. 3.
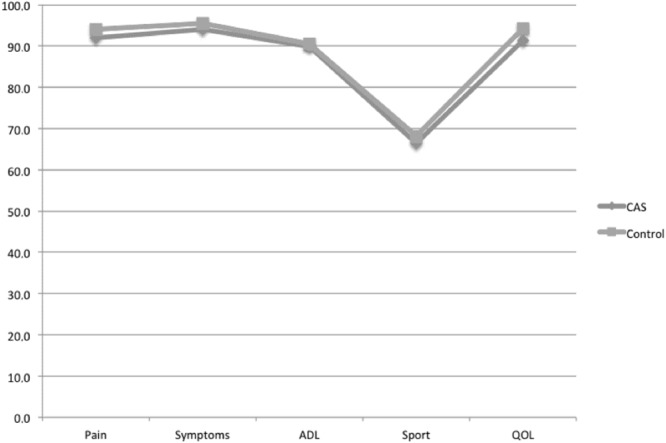
These results show a comparison of the KOOS score between CAS and control patients. *All p > 0.1. ADL = activities of daily living; QOL = quality of life.
Table 2.
Clinical score evaluation at last followup
Discussion
The premise of CAS in TKA was to improve implant positioning and restore a neutral mechanical axis with the goal of improving function and implant survivorship [22]. The benefits of CAS to improve implant positioning around a defined target goal and to decrease outliers have been demonstrated, but whether improved positioning will impact these other goals remains uncertain [10, 12]. The results of our earlier randomized controlled trial focusing on postoperative implant positioning in these two groups of patients randomly assigned to CAS or conventional TKA showed improved lower limb alignment and tibial implant position in the coronal plane for the CAS group [17]. To evaluate any potential benefits of this improved alignment, in this secondary analysis, we compared clinical outcomes and implant survivorship between the same groups with a minimum followup of 10 years [4, 17]. Specifically, we asked whether CAS would improve 10-year survivorship and certain PROMs as compared with conventional instrumentation. Those PROMs were not included in our initial study protocol and thus the functional scores we assessed in the present study were only available at the latest followup period. The results of our study suggest that CAS used for TKA placement with restoration of a neutral mechanical axis as the target goal did not confer any substantial advantage in survivorship, function, or quality of life 10 years after TKA.
Several limitations can be outlined in our study. First, the study is underpowered to detect all but very large differences in survivorship. When the study protocol was developed, the groups were powered for radiographic analysis of alignment; functional and survivorship data were not available to calculate sample size because all PROMs used in this secondary analysis were either not available or not translated into French in the preoperative/postoperative period. Although our groups were initially comparable in terms of demographics and preoperative knee deformities, we can only suspect that they were also comparable in terms of pain and function at baseline. However, with the numbers available and a power of 80%, we could demonstrate a clinically important difference for the SF-12 and the KOOS (minimal clinically important difference > 6 points) if one indeed were present [4, 6].
Second, the target used in this study was based on the mechanical alignment philosophy. Controversy surrounds the ideal alignment goal in TKA. Historically “restoring” mechanically neutral alignment through bone cuts aligned to the limb’s mechanical axis has been suggested to offer patients the best chance for long-term prosthetic knee function and survival [1, 2]. More recently, questions have been raised as to whether these targets should shift to achieve more patient-specific alignment goals, restoring the individual’s premorbid alignment and thus restoring knee kinematics [13, 21]. We might have chosen the wrong target in our series; however, to date no scientific evidence of a better “systematic option” has been demonstrated [27].
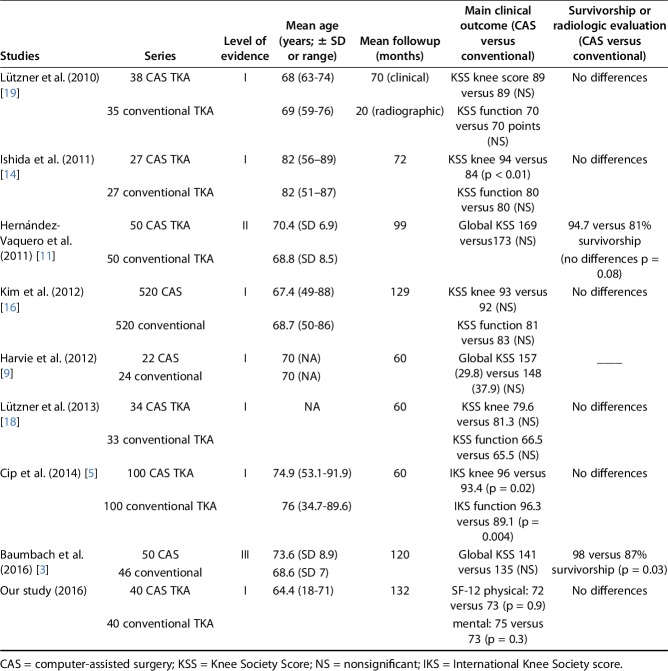
With the numbers available, we found no survivorship advantage in patients treated with CAS TKA 10 years after surgery. Although our results are consistent with the results from others authors [3, 9, 11, 14, 16, 19, 20] (Table 3), our series was not adequately powered for survival analysis. Because we found the same implant survivorship between the groups (if aseptic loosening was defined as the endpoint), a post hoc analysis evaluated that 1500 patients would be needed in each group to be able to detect a 2% survivorship difference 10 years after surgery. Those numbers demonstrate the difficulty in planning a controlled trial powered to distinguish such survivorship improvement.
Table 3.
Level I and II studies reporting clinical and radiologic outcomes for CAS versus conventional TKA
Parratte et al. also showed that the restoration of a neutral mechanical axis is perhaps not the best predictor of survivorship [21]. More specific targets of alignment may be required and several new concepts have emerged recently such as kinematic alignment or constitutional alignment [1, 2]. These techniques are too recent to have long-term survivorship; and if some clinical improvements have been shown at midterm followup [13], a recent randomized controlled study failed to demonstrate any clinical benefit of the kinematic alignment concept 2 years after TKA [27].
The results of the clinical evaluation showed no benefit for navigated patients. This is in agreement with other publications with at least 5 years of followup [9, 11, 16, 18, 19]. Harvie et al. [9] found no differences in patient satisfaction 5 years postoperatively and Kim et al. [16] did not find any differences in KSS or Hospital for Special Surgery score evaluation at 10 years. Similar KSS scores were reported by Lützner et al. [18, 19] and Hernández-Vaquero et al. [11] at, respectively, 5 years and 7 years of followup. We did not find any long-term report demonstrating a clinically important difference between CAS and conventional TKA. However, Ishida et al. [14] found superior KSS scores at 60 months (94 versus 84), but they found no differences in KSS function scores between CAS and conventional instrumented TKA. Cip et al. [5] demonstrated higher KSS pain and function scores 60 months after navigated TKAs, but the observed differences were below the MCID, suggesting that patients are unlikely to perceive the differences as clinically important. In our series, we aimed to evaluate patients based on self-reported and validated questionnaires [7, 23, 25, 26]. It is difficult to compare our results with some of those earlier reported because many were based on surgeon-reported scoring systems (eg, prior version of the KSS).
In conclusion, our series failed to demonstrate either long-term survival or clinical benefits of CAS-performed TKA over conventional implantation techniques designed to restore the neutral mechanical axis. Because CAS is associated with added costs and surgical time, future studies need to identify what clinically relevant advantages it offers to justify its continued use in TKA.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his institution approved for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Institute for Locomotion, Aix-Marseille University, Marseille, France.
References
- 1.Abdel MP, Oussedik S, Parratte S, Lustig S, Haddad FS. Coronal alignment in total knee replacement: historical review, contemporary analysis, and future direction. Bone Joint J. 2014;96:857–862. [DOI] [PubMed] [Google Scholar]
- 2.Allen MM, Pagnano MW. Neutral mechanical alignment: is it necessary? Bone Joint J. 2016;98:81–83. [DOI] [PubMed] [Google Scholar]
- 3.Baumbach JA, Willburger R, Haaker R, Dittrich M, Kohler S. 10-year survival of navigated versus conventional TKAs: a retrospective study. Orthopedics. 2016;39:S72–76. [DOI] [PubMed] [Google Scholar]
- 4.Berliner JL, Brodke DJ, Chan V, SooHoo NF, Bozic KJ. Can preoperative patient-reported outcome measures be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res. 2017;475:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cip J, Widemschek M, Luegmair M, Sheinkop MB, Benesch T, Martin A. Conventional versus computer-assisted technique for total knee arthroplasty: a minimum of 5-year follow-up of 200 patients in a prospective randomized comparative trial. J Arthroplasty. 2014;29:1795–1802. [DOI] [PubMed] [Google Scholar]
- 6.Clement ND, MacDonald D, Simpson AH. The minimal clinically important difference in the Oxford knee score and Short Form 12 score after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22:1933–1939. [DOI] [PubMed] [Google Scholar]
- 7.Debette C, Parratte S, Maucort-Boulch D, Blanc G, Pauly V, Lustig S, Servien E, Neyret P, Argenson JN. French adaptation of the new Knee Society Scoring System for total knee arthroplasty. Orthop Traumatol Surg Res. 2014;100:531–534. [DOI] [PubMed] [Google Scholar]
- 8.Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 9.Harvie P, Sloan K, Beaver RJ. Computer navigation vs conventional total knee arthroplasty: five-year functional results of a prospective randomized trial. J Arthroplasty. 2012;27:667–672.e1. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Vaquero D, Noriega-Fernandez A, Fernandez-Carreira JM, Fernandez-Simon JM, Llorens de los Rios J. Computer-assisted surgery improves rotational positioning of the femoral component but not the tibial component in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22:3127–3134. [DOI] [PubMed] [Google Scholar]
- 11.Hernández-Vaquero D, Suarez-Vazquez A, Iglesias-Fernandez S. Can computer assistance improve the clinical and functional scores in total knee arthroplasty? Clin Orthop Relat Res. 2011;469:3436–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández-Vaquero D, Suarez-Vazquez A, Sandoval-Garcia MA, Noriega-Fernandez A. Computer assistance increases precision of component placement in total knee arthroplasty with articular deformity. Clin Orthop Relat Res. 2010;468:1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell SM, Howell SJ, Kuznik KT, Cohen J, Hull ML. Does a kinematically aligned total knee arthroplasty restore function without failure regardless of alignment category? Clin Orthop Relat Res. 2013;471:1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishida K, Matsumoto T, Tsumura N, Kubo S, Kitagawa A, Chin T, Iguchi T, Kurosaka M, Kuroda R. Mid-term outcomes of computer-assisted total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19:1107–1112. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Kim Y-H, Park J-W, Kim J-S. Computer-navigated versus conventional total knee arthroplasty: a prospective randomized trial. J Bone Joint Surg Am. 2012;94:2017–2024. [DOI] [PubMed] [Google Scholar]
- 17.Lino L, Flecher X, Aubaniac J-M, Argenson J-N. Computer-assisted total knee arthroplasty with and without preoperative imaging. Orthop Proc. 2008;90:285–286. [Google Scholar]
- 18.Lützner J, Dexel J, Kirschner S. No difference between computer-assisted and conventional total knee arthroplasty: five-year results of a prospective randomised study. Knee Surg Sports Traumatol Arthrosc. 2013;21:2241–2247. [DOI] [PubMed] [Google Scholar]
- 19.Lützner J, Günther K-P, Kirschner S. Functional outcome after computer-assisted versus conventional total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc. 2010;18:1339–1344. [DOI] [PubMed] [Google Scholar]
- 20.Marques CJ, Daniel S, Sufi-Siavach A, Lampe F. No differences in clinical outcomes between fixed- and mobile-bearing computer-assisted total knee arthroplasties and no correlations between navigation data and clinical scores. Knee Surg Sports Traumatol Arthrosc. 2015;23:1660–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parratte S, Pagnano MW, Trousdale RT, Berry DJ. Effect of postoperative mechanical axis alignment on the fifteen-year survival of modern, cemented total knee replacements. J Bone Joint Surg Am. 2010;92:2143–2149. [DOI] [PubMed] [Google Scholar]
- 22.Pitto RP, Graydon AJ, Bradley L, Malak SF, Walker CG, Anderson IA. Accuracy of a computer-assisted navigation system for total knee replacement. J Bone Joint Surg Br. 2006;88:601–605. [DOI] [PubMed] [Google Scholar]
- 23.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28:88–96. [DOI] [PubMed] [Google Scholar]
- 24.Springer BD, Parratte S, Abdel MP. Measured resection versus gap balancing for total knee arthroplasty. Clin Orthop Relat Res. 2014;472:2016–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thienpont E, Opsomer G, Koninckx A, Houssiau F. Joint awareness in different types of knee arthroplasty evaluated with the Forgotten Joint score. J Arthroplasty. 2014;29:48–51. [DOI] [PubMed] [Google Scholar]
- 26.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 27.Young SW, Walker ML, Bayan A, Briant-Evans T, Pavlou P, Farrington B. The Chitranjan S. Ranawat Award: No difference in 2-year functional outcomes using kinematic versus mechanical alignment in TKA: a randomized controlled clinical trial. Clin Orthop Relat Res. 2017;475:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]