Abstract
Background
Prosthetic joint infection (PJI) is among the most-severe complications of a total joint arthroplasty. Identification of the causal organism is of paramount importance for successful treatment, and sonication of implants may aid in this identification. Dithiothreitol (DTT) treatment has been proposed as an alternative to sonication to improve diagnosis, reduce costs, and improve reliability of the procedure, but its efficacy remains poorly characterized.
Questions/purposes
(1) Are DTT and sonication more sensitive and/or more specific than standard cultures of tissue samples for the diagnosis of PJI? (2) Which test (DTT or sonication) is more sensitive when the clinician does not suspect infection before surgery? (3) Which test (DTT or sonication) is more sensitive when the clinician suspects infection before surgery?
Methods
Two hundred thirty-two patients undergoing revision of a knee or hip arthroplasty were prospectively evaluated in this randomized study. Cultures were performed on five tissue samples from each patient and on fluid obtained by prosthesis treatment in patients randomly assigned to sonication (117 patients) or DTT (115 patients). The reference standard against which cultures (on tissue samples and on fluids from sonication or DTT) were compared was the Musculoskeletal Infection Society definition of PJI.
Results
Cultures on sonication and DTT fluids provided higher sensitivity (89% and 91%, respectively) than those on standard cultures of tissue samples (79%; p < 0.001). Among patients in whom infection was not suspected before surgery, the sensitivity of DTT was greater than that for sonication and cultures on tissue samples (100% versus 70% and 50%; p < 0.001). Among patients in whom infection was suspected before surgery, the sensitivity of DTT and sonication were not greater than that for standard cultures (89% and 94% versus 86%).
Conclusions
In this randomized study, we found no difference in sensitivity between DTT and sonication for the detection of PJI, and both of those tests were more sensitive than standard tissue cultures. Thus, cultures of sonication or DTT fluid should be considered important additional tools to standard cultures for definition of PJI and should be considered together with other criteria, especially in settings where infection is not suspected before revision surgery.
Level of Evidence Level I, diagnostic study.
Introduction
The prevalence of prosthetic joint infection (PJI) in knee and hip arthroplasties is increasing as the number of joint arthroplasties grows, leading to severe consequences for patients owing to long hospital stays, expensive treatments, and multiple operations [9, 11]. The diagnosis of PJI is challenging [3, 12], and although many different clinical parameters can suggest the presence of an infection, only identification of the infecting microorganism provides the diagnosis with the highest level of certainty. Microbiologic findings are included as a major criterion in the Musculoskeletal Infection Society (MSIS) and the Infectious Diseases Society of America diagnostic criteria for PJI [12, 13]. Isolation of pathogens by culture of tissue samples remains the gold standard [1, 19], although such samples may produce false-negative results in up to 30% of patients [14, 15]. To improve the diagnosis of PJI, several techniques for detection of biofilm-related infections have been developed. Culture of samples obtained by sonication of the removed prosthesis has been shown to be more sensitive than conventional tissue cultures, especially in patients treated with antibiotics before surgery [15, 16, 19]. However, some limitations of this method have been highlighted, such as the necessity for dedicated laboratory tools and the intrinsic risk of contamination owing to possible damage or inappropriate sealing of sample containers, the size of explanted prostheses, and bacteria proliferation in the water of the sonication bath [18].
To overcome the limitations of sonication, a novel treatment of the implant with dithiothreitol (DTT) solution has been proposed. Drago et al. [4, 5] showed the ability of DTT to detach bacteria from biofilm on orthopaedic devices with comparable or even higher yields than sonication and periprosthetic tissue culture. However, those findings are yet to be confirmed in a larger cohort of patients and in the specific setting of only prosthetic devices.
In this prospective randomized study, we compared the performance of sonication and DTT with standard cultures (without sonication or DTT), and we compared sonication and DTT treatment with one another, using the MSIS criteria as the reference standard for the diagnosis of PJI. We asked: (1) Are these tests (DTT and sonication) more sensitive and more specific to standard cultures of tissue samples for the diagnosis of PJI? (2) Which test (DTT or sonication) is more sensitive when the clinician does not suspect infection before surgery? (3) Which test (DTT or sonication) is more sensitive when the clinician suspects infection before surgery?
Patients and Methods
This prospective randomized study compares the performance of DTT and sonication with that of standard cultures performed on tissue samples for the diagnosis of PJI, and it also compares sonication and DTT treatment with one another.
The trial was approved by the Istituto Ortopedico Rizzoli ethics committee. Informed consent for study participation was obtained from all patients enrolled. Based on results previously reported by Drago et al. [4], calculation of the population sample power was made based on the hypothesis that DTT had 14 points higher sensitivity than sonication and cultures performed on tissue samples. If the α value was 0.05 and the statistical power was set to 80%, the sample size for each sample was 105 per group.
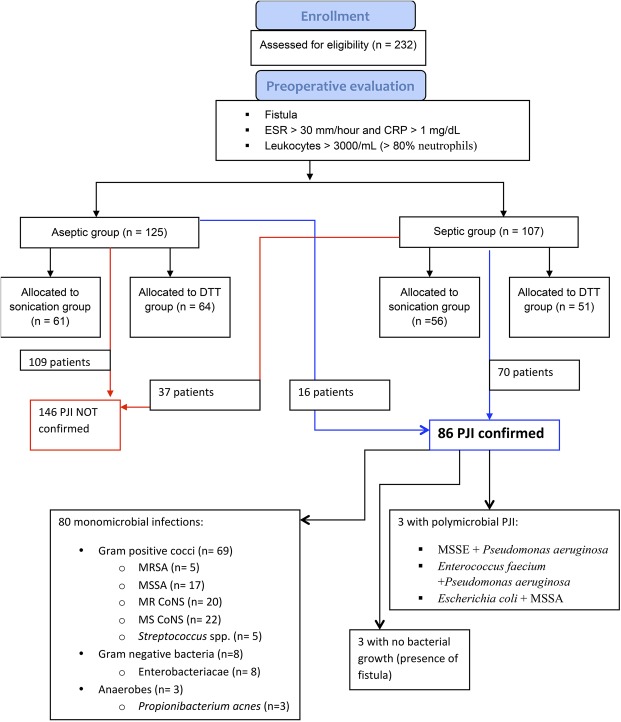
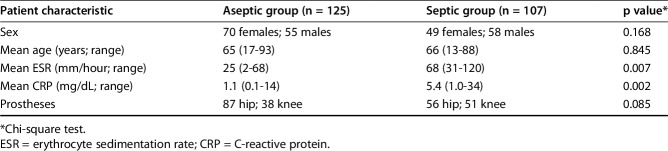
A total of 232 adult (older than 18 years) patients undergoing removal and revision of a total knee or hip prosthesis for aseptic indications or suspected infection at the Istituto Ortopedico Rizzoli, Bologna, Italy, were enrolled between April 2014 and July 2016. Exclusion criteria were mechanical failure of the prosthesis or periprosthetic fracture. Patients also were excluded if they had an acute PJI, if the prosthesis did not fit in the container provided for it, if they received antibiotic therapy during the previous 2 weeks, or if only one tissue sample was cultured (Fig. 1). One hundred seven patients met the criteria for suspected infection; 125 patients were considered as not having PJI preoperatively (Table 1).
Fig. 1.
The flowchart shows enrollment and microbiologic results for the patients. According to clinical and laboratory presentation, 232 patients were considered to have an infection (septic group, n = 107) or not have an infection (aseptic group, n = 125). All patients were randomly assigned by a sealed envelope technique to the sonication group (117 patients) or to the DTT group (115 patients). According to MSIS criteria, 146 patients (109 in the aseptic group and 37 in the septic group) were confirmed as not having an infection (red arrow and box). Eighty-six patients (16 in the aseptic group and 70 in the septic group) had PJIs confirmed based on MSIS criteria (blue arrow and box). MSIS = Musculoskeletal Infection Society; DTT = dithiothreitol; PJI = prosthetic joint infection; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-susceptible S aureus; MSSE = methicillin-susceptible Staphylococcus aureus; MR = methicillin-resistant; CoNS = coagulase-negative staphylococci; MS = methicillin-sensitive; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein.
Table 1.
Demographic characteristics in the suspected aseptic and septic groups at baseline
Preoperatively, all information regarding the type of prosthesis, clinical presentation, and laboratory tests (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], white blood cell count and differential, and fibrinogen) were recorded for each patient. If the patient had a fistula communicating with the implant, patients were considered to have an infection. In the absence of a fistula but the presence of elevated ESR (> 30 mm/hour) and CRP (> 1 mg/dL), joint aspiration was performed and patients were considered to have a suspected infection if leukocytes were greater than 3000/mL with more than 80% neutrophils (Fig. 1). Patients not meeting those criteria were counted among the patients believed not to have an infection before surgery.
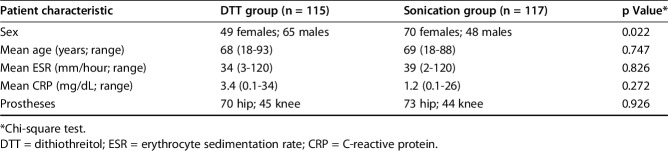
At the time of admission, patients were randomly assigned by a sealed envelope technique using a random permuted blocks protocol by one of the authors (MC), who was not involved directly in laboratory procedures, to the sonication group (117 patients) or to the DTT group (115 patients) (Table 1). The two groups were balanced considering preoperative suspicion (aseptic or septic) (Table 2).
Table 2.
Demographic characteristics in the DTT and sonication groups at baseline
During the surgery, five separate biopsy specimens were collected from different areas of periprosthetic tissues, as specified in the study protocol. All the prosthetic components were removed in the operating room under sterile conditions and transported to the microbiology laboratory for sonication or elution with DTT solution. In most of the patients, the whole prosthesis was removed; the femoral stem and the acetabulum in THAs and the femoral and tibial components in TKAs were sent for treatment separately. Both components removed from the same patients received identical treatment. The prosthetic components removed were placed in a sterile, wide-mouthed, airtight, polypropylene container and transported immediately to the microbiology laboratory. All the samples were collected intraoperatively before any empiric antibiotic therapy was started.
Homogenization of tissue samples was performed in the original container, vortexing the specimen in 3 mL tryptic soy broth [14].
Each tissue specimen homogenate was inoculated in sheep blood agar, thioglycollate broth medium, and tryptic soy broth. All the media were incubated at 36° ± 1° C for 7 days and examined daily for evidence of growth. For isolation of individual colonies, aliquots from enrichment broth tubes were spread using a sterile loop on Columbia CNA blood agar, mannitol salt agar, MacConkey agar, and chocolate agar and incubated at 36° ± 1° C under aerobic conditions for 24 hours. They also were subcultured on chocolate agar under anaerobic conditions for 72 hours at 36° ± 1° C. All the media were from Biolife Italiana (Milan, Italy). Negative thioglycollate broth medium and tryptic soy broth incubates were reincubated up to 14 days at 36° ± 1° C and examined daily for evidence of growth. Identification and antimicrobial susceptibility testing were performed with a MicroScan® WalkAway® system (Beckman Coulter, Sacramento, CA, USA).
Sonication was performed according to the technique of Trampuz et al. [19]. Briefly, the container was filled with sterile saline until the device was submersed, carefully sealed, vortexed, and sonicated in an ultrasound bath (VWR International Srl, Milan, Italy) for 5 minutes with a frequency of 40 kHz at room temperature.
DTT treatment was performed as previously described by Drago et al. [4]. Prostheses were immersed in a solution of 0.1% w/v DTT (Sigma-Aldrich S.R.L., Milan, Italy) in sterile saline and mechanically stirred for 15 minutes at room temperature.
At the end of each treatment (either sonication or DTT), the obtained fluids were collected in sterile tubes and centrifuged at 3000 rpm for 10 minutes at room temperature. The pellet was suspended in a volume of 2 mL of the same solution. A total of 100 μL of each sample was plated on chocolate agar, sheep blood agar, and inoculated in tryptic soy broth and thioglycollate broth medium. Sheep blood agar was incubated aerobically, whereas chocolate agar plates were incubated in 5% CO2 atmosphere at 36° ± 1° C for 7 days. Broths were incubated for 7 and 14 days at 36° ± 1° C and, if negative, the incubation was extended to 14 days and examined daily for evidence of growth; terminal subcultures were performed. Subcultures and identification were performed as previously described for the cultures performed on biopsy specimen. Sonicated or DTT-treated device fluids were considered positive if at least five colonies grew on agar plates after 24 hours and up to 7 days or if growth was observed during broth enrichment.
The gold standard for the presence or absence of infection in this study was the MSIS criteria [13] , which was evaluated at the time of data analysis. In this study, the intention-to-treat and per-protocol analyses were identical, since all the patients received the treatment to which they had been assigned.
In the definition of PJI according to MSIS criteria, cultures performed on sonication and DTT fluids were considered together with cultures performed on tissue samples, because we considered these as additional tools to reach the diagnosis of PJI. Cultures (from tissue samples and fluids from sonication or DTT) were positive when at least one showed the growth of a strict pathogen (Staphylococcus aureus, Pseudomonas aeruginosa, and Enterobacteriaceae) or when two yielded a skin commensal organism (coagulase-negative Staphylococci or Propionibacterium acnes) [12, 17]. When only one of two samples from sonication or DTT was positive, sonication (or DTT) was considered positive if there was growth of a strict pathogen. The performance of cultures on tissue samples and on DTT and sonication fluids was compared, using the definition of infection according to the PJI criteria of the MSIS [13]. In addition, an indirect comparison between DTT and sonication was made, because a direct comparison is not possible since each prosthetic component can undergo only a single treatment.
After analysis of clinical, bacteriologic, and histologic criteria, 86 of 232 patients met the MSIS definition for PJI [13]. PJI was identified in 16 subjects who were included in the aseptic group in the preoperative stage; conversely, 37 patients who were provisionally included in the septic group had a totally negative set of results so PJI was excluded (Fig. 1).
Statistical Analysis
The baseline characteristics of the PJI group and the aseptic failure group were compared using the chi-square test. Differences between the results obtained by culture-based techniques were assessed by the t test. Probability values less than 0.05 were considered significant. All analyses were completed using the Statistical Package for Social Science (SPSS Statistics for Windows, Version 22.0; IBM Corporation, Armonk, NY, USA).
Results
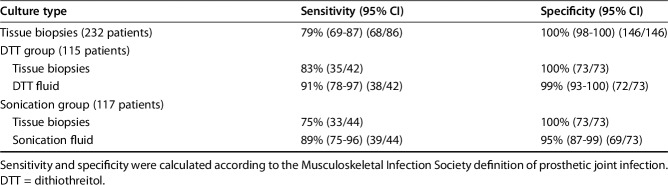
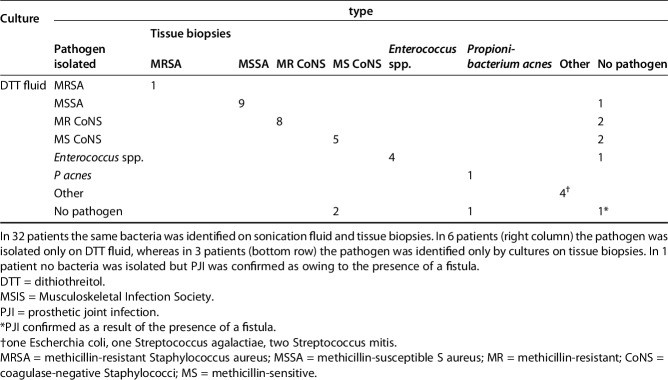
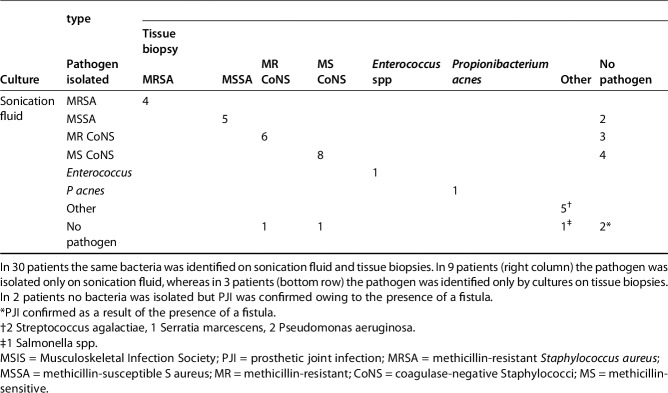
Sensitivity of DTT and sonication both were greater than that observed from tissue culture (91% [95% CI, 78%-97%] and 89% [95% CI, 75%–96%] versus 79% [95% CI, 69%–87%], p < 0.001). The specificity of DTT and sonication was not greater than that from tissue culture (99% [95% CI, 93%-100%] and 95% [95% CI, 87%–99%] versus 100% [95% CI, 98%–100%]). Positive predictive value of DTT and sonication did not differ from that observed from tissue culture (97% [95% CI, 87%-100%] and 91% [95% CI, 78%–97%] versus 99% [95% CI, 9%–100%]). Negative predictive value of DTT and sonication showed no difference to that observed from tissue culture (95% [95% CI, 87%-99%] and 93% [95% CI, 85%–98%] versus 89% [95% CI, 83%–93%]) (Table 3). Forty-two patients in the DTT group (Table 4) and 44 in the sonication group (Table 5) had MSIS-confirmed PJIs. All the microorganisms with the exception of one P acnes, which was isolated after 14 days of incubation from all tissue cultures but not from the prosthetic eluate (DTT), were isolated within 7 days of incubation as per standard procedures.
Table 3.
Performance of the diagnostic techniques
Table 4.
Microbiologic culture results for patients meeting the MSIS definition of having a PJI
Table 5.
Microbiologic culture results for patients meeting the MSIS definition of PJI
Among patients in whom infection was not suspected before surgery, the sensitivity of DTT was greater than that for sonication and cultures on tissue samples (100% versus 70% and 50%; p < 0.001) (Table 6). The specificity of DTT and sonication were not greater than that from tissue culture (98% and 96% versus 100%. Positive predictive value of DTT and sonication did not differ from that observed from tissue culture (87.5% and 77.8% versus 100%) and negative predictive value of DTT and sonication were not greater than that observed from tissue culture (98% and 96% versus 93%).
Table 6.
Performance of the diagnostic techniques in the suspected groups
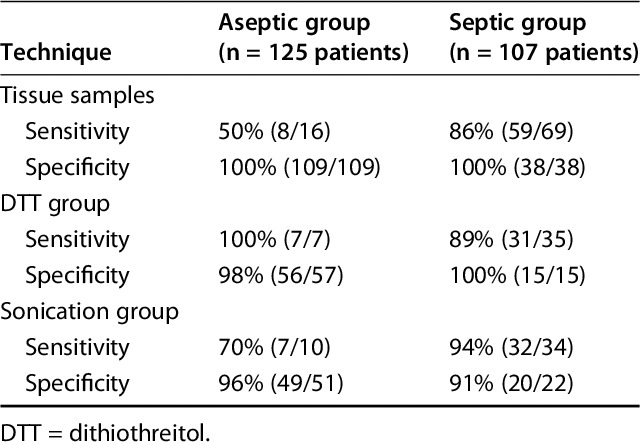
Among patients in whom infection was suspected before surgery, the sensitivity and specificity of DTT and sonication were not greater than those for standard cultures (89% and 94% versus 86%; 100% and 91% versus 100%) (Table 6). The specificity of DTT and sonication were not greater than that from tissue culture, and the positive predictive value of DTT and sonication did not differ from that observed from tissue culture (100% and 94% versus 100%). Negative predictive value of DTT and sonication were not greater than that observed from tissue culture (79% and 91% versus 79%). Of the patients in this group, 103 had the whole prosthesis removed (58 stems and cups, 45 femur and tibia components). Both components removed from the same patients received identical treatment. In the majority of these patients, there was concordance between results obtained by culturing the two prosthetic components. Forty of the 49 patients with confirmed PJIs (41 in the septic group, eight in the aseptic group) showed growth of the same bacteria on both eluate fluids; nevertheless, in nine patients, only one eluate showed growth of bacteria. Interestingly, in all these patients, the culture test was positive only in tissue samples from the same joint site (eg, cup, stem, femur, tibia).
Discussion
The diagnosis of PJI remains a laboratory challenge because a clinically assessed and analytically valid technique that can serve as a reference standard for this diagnosis is not available [6, 11]. Periprosthetic tissue culture has been considered the reference standard for identification of pathogens involved in PJI, although such cultures lack sufficient sensitivity (described as ranging from 70% to 90%) and specificity (ranging from 67% to 91%) [1, 2, 10]. Treating the prosthesis with DTT to affect the biofilm may be superior to sonication, but, to the best of our knowledge, it has yet to be investigated on a large cohort of patients [5]. Moreover, direct comparison between sonication and DTT is impossible because each prosthetic component can undergo only a single treatment. Separate treatment of different components with different techniques (such as treating an acetabular component with sonication and the same patient’s stem with DTT) in the same patient assumes that any infection is localized in both components with the same bacteria, and therefore important information might be missed. To the best of our knowledge, this is the first series in which DTT and sonication treatment were compared in a large cohort of patients undergoing prosthesis revision in a clinical setting.
The limitations of this study must be addressed. First, cultures from sonication or DTT treatment were included in the MSIS criteria for infection, which in this study was used as the gold standard for the presence or absence of infection. Nevertheless, in our opinion, sonication and DTT have to be considered additional tools in the diagnosis of PJI, so they should be included when analyzing MSIS criteria to reach a PJI diagnosis. Furthermore, the lack of followup and longer-term clinical data did not allow us to confirm whether any patient in whom PJI was excluded according to MSIS criteria later had a clinical infection develop. However, we do not think that long-term followup would be helpful in confirming patients with true asepis since a late infection could have been absent at the time of this analysis, but developed later.
The main finding of our study is that sonication (Table 5) and DTT treatment (Table 4) improved microbial detection compared with cultures performed on tissue samples, which was particularly evident in coagulase-negative Staphylococcus. Our results show that cultures from DTT-treated devices were not different from those for sonication fluids, with the numbers available. Furthermore, we report sensitivity data for sonication and DTT which are comparable to data from previous studies [4, 14, 19]. We also report higher sensitivity of cultures performed on tissue samples than reported by others [1, 2, 10]. This discrepancy could be because in our study, all samples were collected before antibiotic therapy, which is widely known to reduce sensitivity of culture procedures [7]. The evaluation of multiple fluid specimens (either by sonication or DTT treatment of different components of the same prosthesis) also increases sensitivity and specificity, as previously reported [8]. This might explain why we found higher specificity for culture tests than previous studies [4, 5] in which cultures were performed on a single specimen.
In 16 patients, we found a discrepancy between preoperative suspicion of aseptic loosening of the prosthesis and the definitive diagnosis after surgery considering the MSIS criteria. Specifically, in our study, treatment with DTT seemed to be more effective than sonication in the aseptic group. In this respect, pretreatment by DTT of the prosthetic component is recommended particularly in patients with delayed orthopaedic implant failure with no clear clinical signs of infection [16]. However, the small sample number when analyzing different subgroups does not allow us to draw any definitive conclusions regarding this point.
Analysis of the subgroup of patients with suspected infection preoperatively did not reveal any differences in sensitivity between sonication and DTT. Again, however, the sample size is not large enough to draw any definitive conclusion. In nine patients with confirmed PJIs, only one of the two fluids obtained by treating the two prosthetic components individually was positive by culture assay. In addition, in these patients cultures performed on tissue samples were positive only on tissue specimens derived from the same side of the prosthetic joint, perhaps suggesting that not all PJIs affect both implant components in the same joint. Nevertheless, because of the small number of patients in this subanalysis, this finding needs to be further investigated for confirmation. This fact clearly underlines that it is mandatory to perform identical microbe biofilm removal techniques on all the prosthetic components in such cases to obtain the most relevant microbial data. The results of previous studies in which the different components of the same prosthesis were treated for removal of the microbial biofilm by using different methods perhaps should be reconsidered in light of this information [4, 5].
In this randomized study, we found no difference in sensitivity between DTT and sonication for detection of PJI, and both tests were more sensitive than standard tissue cultures. Although our data suggest that the use of DTT may be more effective than sonication when aseptic loosening of the implant is suspected preoperatively, further studies focused on such patients will be necessary to confirm these data. Cultures of sonication or DTT fluid should be considered important additions to routine tissue cultures along with other clinical laboratory criteria in the complex workflow for PJI diagnosis.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Istituto Ortopedico Rizzoli, Bologna, Italy; and The Great Romagna Hub Laboratory, Pievesestina, Italy.
References
- 1.Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010;48:1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergin PF, Doppelt JD, Hamilton WG, Mirick GE, Jones AE, Sritulanondha S, Helm JM, Tuan RS. Detection of periprosthetic infections with use of ribosomal RNA-based polymerase chain reaction. J Bone Joint Surg Am. 2010;92:654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Pozo JL, Patel R. Clinical practice: infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drago L, Romano CL, Mattina R, Signori V, De Vecchi E. Does dithiothreitol improve bacterial detection from infected prostheses? A pilot study. Clin Orthop Relat Res. 2012;470:2915–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drago L, Signori V, De Vecchi E, Vassena C, Palazzi E, Cappelletti L, Romano D, Romano CL. Use of dithiothreitol to improve the diagnosis of prosthetic joint infections. J Orthop Res. 2013;31:1694–1699. [DOI] [PubMed] [Google Scholar]
- 6.Esteban J, Gomez-Barrena E, Cordero J, Martin-de-Hijas NZ, Kinnari TJ, Fernandez-Roblas R. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J Clin Microbiol. 2008;46:488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holinka J, Bauer L, Hirschl AM, Graninger W, Windhager R, Presterl E. Sonication cultures of explanted components as an add-on test to routinely conducted microbiological diagnostics improve pathogen detection. J Orthop Res. 2011;29:617–622. [DOI] [PubMed] [Google Scholar]
- 8.Janz V, Wassilew GI, Hasart O, Tohtz S, Perka C. Improvement in the detection rate of PJI in total hip arthroplasty through multiple sonicate fluid cultures. J Orthop Res. 2013;31:2021–2024. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 suppl):61–65.e61. [DOI] [PubMed] [Google Scholar]
- 10.Marin M, Garcia-Lechuz JM, Alonso P, Villanueva M, Alcala L, Gimeno M, Cercenado E, Sanchez-Somolinos M, Radice C, Bouza E. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neut D, van Horn JR, van Kooten TG, van der Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopaedic joint implants. Clin Orthop Relat Res. 2003;413:261–268. [DOI] [PubMed] [Google Scholar]
- 12.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 13.Parvizi J, Gehrke T; International Consensus Group on Periprosthetic Joint Infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29:1331. [DOI] [PubMed] [Google Scholar]
- 14.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009;47:1878–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portillo ME, Salvado M, Alier A, Martinez S, Sorli L, Horcajada JP, Puig L. Advantages of sonication fluid culture for the diagnosis of prosthetic joint infection. J Infect. 2014;69:35–41. [DOI] [PubMed] [Google Scholar]
- 16.Puig-Verdie L, Alentorn-Geli E, Gonzalez-Cuevas A, Sorli L, Salvado M, Alier A, Pelfort X, Portillo ME, Horcajada JP. Implant sonication increases the diagnostic accuracy of infection in patients with delayed, but not early, orthopaedic implant failure. Bone Joint J. 2013;95:244–249. [DOI] [PubMed] [Google Scholar]
- 17.Société de Pathologie Infectieuse de Langue Française (SPILF); Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT); Groupe de Pathologie Infectieuse Pédiatrique (GPIP); Société Française d'Anesthésie et de Réanimation (SFAR); Société Française de Chirurgie Orthopédique et Traumatologique (SOFCOT); Société Française d'Hygiène Hospitalière (SFHH); Société Française de Médecine Nucléaire (SFMN); Société Française de Médecine Physique et de Réadaptation (SOFMER); Société Française de Microbiologie (SFM); Société Française de Radiologie (SFR-Rad); Société Française de Rhumatologie (SFR-Rhu). Recommendations for bone and joint prosthetic device infections in clinical practice (prosthesis, implants, osteosynthesis). Societe de Pathologie Infectieuse de Langue Francaise. Med Mal Infect. 2010;40:185–211. [DOI] [PubMed] [Google Scholar]
- 18.Trampuz A, Piper KE, Hanssen AD, Osmon DR, Cockerill FR, Steckelberg JM, Patel R. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol. 2006;44:628–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. [DOI] [PubMed] [Google Scholar]