Abstract
Introduction
Programmed death ligand 1 (PD-L1) testing of non-small cell lung cancer (NSCLC) specimens helps select patients most likely to respond to immune checkpoint inhibitors. PD-L1 immunohistochemical testing is approved for formalin-fixed, paraffin-embedded (FFPE) surgical pathology specimens; however, the testing performance on FFPE cytology cell block specimens is unknown.
Materials and Methods
The study is a retrospective cohort analysis of advanced stage NSCLC patients treated at our institution where tumor PD-L1 expression using the clone 22C3 pharmDx kit on the Dako Automated Link 48 platform was performed on either cytology cell block or surgical pathology specimens. Concomitant tumor mutation biomarkers were also collected, as well as tumor clinicopathologic characteristics and clinical outcome data following pembrolizumab treatment.
Results
232 patient tumor specimens were tested for PD-L1 expression (94 on cytology cell block and 138 on surgical pathology specimens). No significant differences in PD-L1 tumor proportion score (TPS) were observed between cytology and surgical pathology groups, with both patient cohorts containing ~35% of tumors showing TPS ≥50%. Although few in number, patients with PD-L1 TPS ≥50% based on cytology vs. surgical pathology who received treatment with pembrolizumab demonstrated similar response and disease control rates.
Conclusions
In this cohort of advanced NSCLC patients with standard of care PD-L1 testing performed on either FFPE cytology cell blocks or FFPE surgical pathology specimens, similar patterns were observed in population tumor PD-L1 expression patterns, concomitant driver mutations, and clinical response to palliative pembrolizumab in selected patients with TPS ≥50%.
Keywords: non-small cell lung cancer (NSCLC), adenocarcinoma, PD-L1, cytology cell block, biopsy
Introduction
The treatment and management of patients with advanced non-small-cell lung cancer (NSCLC) has seen dramatic advances over the past decade, with the development of precision systemic therapies directed towards somatic mutations and rearrangements in epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), and B-Raf proto-oncogene, serine/threonine kinase (BRAF), thus providing patients in these molecularly defined subsets significant benefits from treatment with oral tyrosine kinase inhibitors (TKIs) [1]. More recently, immune checkpoint inhibitor therapy in NSCLC through programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) blockade using agents such as pembrolizumab has also led to significant improvements in response rate and progression free survival [2, 3].
In 2016, the U.S. Food and Drug Administration (FDA) approved pembrolizumab as first-line palliative systemic therapy in advanced NSCLC patients whose tumors demonstrate high PD-L1 expression (tumor proportion score [TPS] ≥50%) and with no actionable EGFR or ALK aberrations [4]. This was on the basis of a phase III clinical trial showing superior response rates, survival, and toxicity profile in this selected group of patients when compared with conventional platinum doublet chemotherapy [3]. In platinum-refractory advanced NSCLC, pembrolizumab has similarly demonstrated superior survival, response rate, and toxicity profile as compared to docetaxel in tumors with any PD-L1 expression (TPS ≥ 1%) [5]. Further, pembrolizumab is now approved for use in combination with platinum-pemetrexed in all advanced non-squamous NSCLCs with any level of PD-L1 expression. Accordingly, the most recent National Comprehensive Cancer Network (NCCN) guidelines now recommend that PD-L1 testing be performed on tumor samples in all patients with advanced NSCLC to facilitate optimal therapeutic stratification [6].
Currently, PD-L1 testing takes the form of immunohistochemical (IHC) staining of tumor samples, with the TPS reported as the percentage of tumor cells demonstrating any membranous or cytoplasmic staining. There are currently multiple different PD-L1 IHC assays that have been developed as companion or complementary diagnostic assays for various immunotherapy agents, each with varying antibody clones, IHC detection systems, scoring cutoffs, testing of tumor cells vs. tumor-infiltrating immune cells, and performance metrics. This has led to ongoing uncertainties regarding the ability to interchange and cross-compare results between different testing platforms [1, 7]. Notably, to date all of the large scale clinical trial data used to justify approval of immune checkpoint inhibitors in this setting has been on the basis of PD-L1 IHC performed on formalin-fixed, paraffin-embedded (FFPE) surgical pathology tissue specimens.
In routine clinical practice, the reality is that the tumor specimen available for initial diagnostic evaluation and subsequent ancillary testing may often be a cytology specimen. This reflects the growing importance of minimally invasive tissue acquisition modalities (e.g. bronchoscopy or thoracentesis) in the day-to-day care of patients presenting with advanced lung cancers. Cytologic specimens with high quality cell block preparations have proven more than adequate substrates both initial diagnostic evaluation as well as downstream molecular testing in this clinical setting [8, 9]. Given the strong interventional pulmonology and cytopathology programs at our own institution, high quality cytology cell blocks represent over half of all diagnostic NSCLC specimens undergoing molecular testing [10, 11].
As a result of potential limitations in tumor specimen availability for testing, PD-L1 IHC testing is now increasingly being performed on FFPE cytology cell blocks in routine clinical practice. At this time, only a few studies have been published on PD-L1 expression on cytology cell block vs. surgical pathology biopsy material, generally showing good concordance between paired cytologic and histologic specimens [12–14]. To provide a different but complementary line of evidence that PD-L1 testing could be successfully and reliably performed on cytology cell block preparations in clinical practice, we report our real-world experience with PD-L1 IHC testing using the FDA-approved companion diagnostic clone 22C3 pharmDx kit (Agilent Technologies, Santa Clara, CA) performed on either FFPE cytology cell block or FFPE surgical pathology specimens from a large cohort of patients with advanced NSCLC treated at our institution. We further present clinical outcome data for patients treated with pembrolizumab on the basis of high tumor PD-L1 expression as assayed on cytology cell block vs. surgical pathology specimens.
Materials and Methods
Patient and Clinical Characteristics
Consecutive patients with a diagnosis of lung cancer followed at Beth Israel Deaconess Medical Center whose tumors underwent evidence-based PD-L1 testing and genotyping were recorded through an ongoing institutional review board-approved study, as previously described [10, 11, 15]. Clinical characteristics, pathologic data, tumor genotype, and PD-L1 TPS information was collected from retrospective chart extraction. The site of sampling (lung, lymph node, pleura, brain, bone/soft tissue, liver, or other) and type of specimen were extracted from the pathology reports. Surgical pathology specimens included core needle biopsies, transbronchial biopsies, and large resection specimens; cytology specimens included endobronchial ultrasound guided transbronchial needle aspirates (EBUS-TBNA), fine needle aspirates (FNA), pleural fluid, and bronchial washing/lavage specimens. Molecular/PD-L1 testing requests were initiated by the treating oncologist, and were performed on the best available clinical specimen at the time of the request. There was no a priori preference for testing of a surgical pathology specimen or cytology cell block specimen. The specimen selection was based on either qualitative tumor cellularity statements included in the pathology/cytology report, or by comparative review by a pathologist when the tumor cellularity was not clear from the report and multiple specimens were available to choose from.
Tissue processing
As previously described, surgical pathology and cytology cell block specimens were generated and processed per standard pathology protocols [15, 16]. Briefly, surgical pathology specimens were fixed in 10% neutral buffered formalin (range 3–30 hours based on specimen size and when the specimen arrived in grossing room) prior to automated tissue processing and paraffin embedding. Cytology aspirates from EBUS-TBNA or FNA specimens were collected directly into a methanol–water fixative (CytoLyt; Hologic Corp., Marlborough, MA), with residual material remaining after preparing a ThinPrep slide used to create a cell block via either a plasma-thrombin method or Histogel method (Thermo Scientific Richard-Allan Scientific, Waltham, MA) before formalin-fixation (range 3–6 hours) and paraffin embedding using standard laboratory techniques [17]. Cell concentrates from fresh centrifuged pleural fluid specimens had cell blocks prepared using either a plasma-thrombin or Histogel method before formalin-fixation (range 3–6 hours) and paraffin embedding. All surgical pathology specimens and cytology cell block specimens were processed on automated tissue processors using standard laboratory tissue processing programs based on tissue size. Resulting hematoxylin/eosin and unstained slides were cut at 4 micron thick sections.
Tumor PD-L1 Analysis
PD-L1 IHC testing with proper controls using the PD-L1 clone 22C3 pharmDx kit on the Dako Automated Link 48 platform (Dako, Carpenteria, CA) was performed at Integrated Oncology/LabCorp (New York, NY). The PD-L1 TPS was calculated as the percentage of at least 100 viable tumor cells with complete or partial membrane staining, with tumor cells assessed morphologically by comparison to a serially cut Hematoxylin and Eosin stained slide. Tumor PD-L1 expression was divided into three clinically relevant TPS groups: <1% (no expression), 1–49% (low expression), and ≥50% (high expression). The TPS interpretations that were provided by the commercial vendor’s pathologists were the values used in this study, with verification by slide review by a board certified cytopathologist and surgical pathologist with subspecialty expertise in pulmonary pathology (PVL).
Tumor Genetic Analyses
Tumor genotype was determined by analyzing EGFR (Sanger sequencing or SNaPShot multiplex PCR of exons 18–21), ALK (florescence in situ hybridization [FISH] using the Vysis ALK Break Apart Probe kit), ROS1 (FISH break-apart probe), and KRAS (Sanger sequencing or SNaPshot multiplex PCR of codons 12–13) in tumor samples, as previously described [11, 15, 18]. These tests were outsourced to a commercial vendor (Integrated Oncology/LabCorp, New York, NY) by our hospital bundled within the rapid tumor genotype panel.
Patient therapy with pembrolizumab based on PD-L1 IHC TPS and assessment of response
Patients whose tumors demonstrated high PD-L1 tumor expression (PD-L1 TPS ≥50%) and were selected by the treating oncologist to receive first-line pembrolizumab monotherapy were identified retrospectively from our cohort. The response to therapy was assessed according to Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 [19] from archived radiographic computed tomography (CT) scan data obtained during routine clinical care. The overall response rate (ORR) by RECIST and disease control rate (DCR) at 6 weeks were obtained in evaluable cases. Pembrolizumab was administered at the FDA-approved dose of 200mg given intravenously every 21 days as per the institutional standardized treatment template.
Statistical Methods
Statistics were performed using Microsoft Excel (Microsoft, Redmond, WA), the online statistical program vassarstats.net, and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Student’s t-test was used to compare means, and the Chi-squared test or Fischer’s exact test, depending on sample size, were used to compare categorical variables. Results were considered statistically significant if the p-value was less than 0.05.
Results
A total of 232 consecutive lung tumor specimens underwent PD-L1 testing at our institution during the study time period (November 2015 to May 2017). This patient cohort had a mean age of 67.5 years, 55% were women, 78% were current or former smokers, 78% were white, and 68% had advanced stage disease (Table 1). The majority of tested specimens were adenocarcinoma (72%), with tissue sampling originating most commonly from the lung (41%), lymph nodes (24%) or pleura (13%). PD-L1 IHC testing was performed on these clinically derived samples: 94 (40.5%) patients had their tumor tested using cytology cell block specimens and 138 (59.5%) patients with testing performed on surgical pathology biopsy or resection specimens (Table 1).
Table 1.
Clinical and pathologic characteristics of non-small cell lung carcinomas tested for PD-L1 immunohistochemistry (IHC) using the clone 22C3 pharmDx kit
| Characteristic | Overall (n=232) | Cytology Cell-block Specimen (n=94) | Surgical Pathology Specimen (n=138) | p-value |
|---|---|---|---|---|
| Age | ||||
| Average | 67.5 | 67.5 | 67.8 | 0.82 |
| Gender (n, %) | ||||
| Women | 128 (55.2%) | 52 (55.3%) | 76 (55.1%) | 0.97 |
| Men | 104 (44.8%) | 42 (44.7%) | 62 (44.9%) | |
| Smoking history (n, %) | ||||
| Never smoker | 51 (22.0%) | 22 (23.4%) | 29 (21.0%) | 0.70 |
| Smoker | 181 (78.0%) | 72 (76.6%) | 109 (79.0%) | |
| Ethnicity (n, %) | ||||
| White | 181 (78.0%) | 74 (78.7%) | 107 (77.5%) | 0.57 |
| Asian | 24 (10.3%) | 10 (10.6%) | 14 (10.1%) | |
| Black | 20 (8.6%) | 8 (8.5%) | 12 (8.7%) | |
| Hispanic | 6 (2.6%) | 1 (1.1%) | 5 (3.6%) | |
| Native American | 1 (0.4%) | 1 (1.1%) | 0 (0%) | |
| Stage (n, %) | ||||
| I–III | 75 (32.3%) | 25 (26.6%) | 50 (36.2%) | 0.12 |
| IV/recurrent | 157 (67.7%) | 69 (73.4%) | 88 (63.8%) | |
| Sample site (n, %) | ||||
| Lung | 94 (40.5%) | 22 (23.4%) | 72 (52.2%) | <0.0001 |
| Lymph node | 56 (24.1%) | 46 (48.9%) | 10 (7.2%) | |
| Pleura | 31 (13.4%) | 25 (26.6%) | 6 (4.3%) | |
| Bone/soft tissue | 26 (11.2%) | 0 (0%) | 26 (18.8%) | |
| Brain | 11 (4.7%) | 0 (0%) | 11 (8.0%) | |
| Liver | 10 (4.3%) | 0 (0%) | 10 (7.2%) | |
| Other | 4 (1.7%) | 1 (1.1%) | 3 (2.2%) | |
| Tumor type (n, %) | ||||
| Adenocarcinoma | 166 (71.6%) | 67 (71.3%) | 99 (71.7%) | 0.92 |
| Squamous cell carcinoma | 41 (17.7%) | 16 (17.0%) | 25 (18.1%) | |
| Non-small cell carcinoma-NOS/other | 25 (10.8%) | 11 (11.7%) | 14 (10.1%) |
P values provided for cytology cell block vs. surgical pathology for each variable. Age category means using t-test, and all other categories using chi-squared test.
The diagnostic cytology specimens included FFPE cell blocks from EBUS-TBNA (54/94; 57.4%), pleural fluid (26/94; 27.7%), fine needle aspirates (12/94; 12.8%), and bronchial lavage or bronchial brushing specimens (2/94; 2.1%). The diagnostic surgical pathology specimens included core needle biopsies (83/138; 60.1%), large resections (39/138; 28.3%), and transbronchial biopsies (16/138; 11.6%). As shown in Table 1, there were no statistically significant differences in patient age, gender, smoking history, ethnicity, clinical stage, or tumor type between the cytology and surgical pathology specimens in this cohort. However, cytology specimens were more frequently derived from lymph node aspirates (49%) or pleural effusions (27%), whereas surgical pathology specimens were more commonly derived from the primary lung mass (52%), or from extrathoracic metastases (bone/soft tissue, 19%; brain, 8%; or liver, 7%).
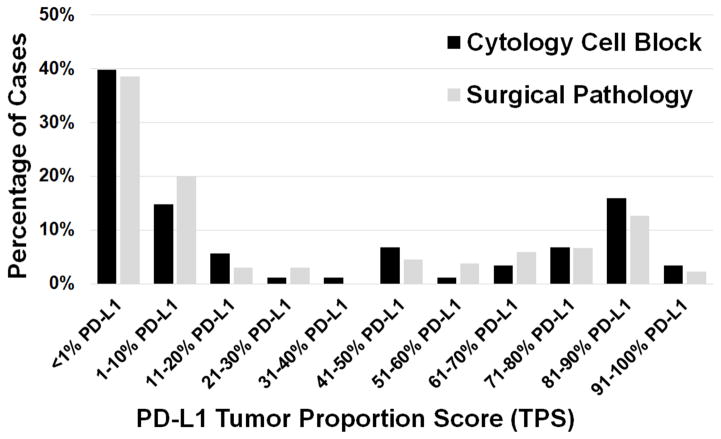
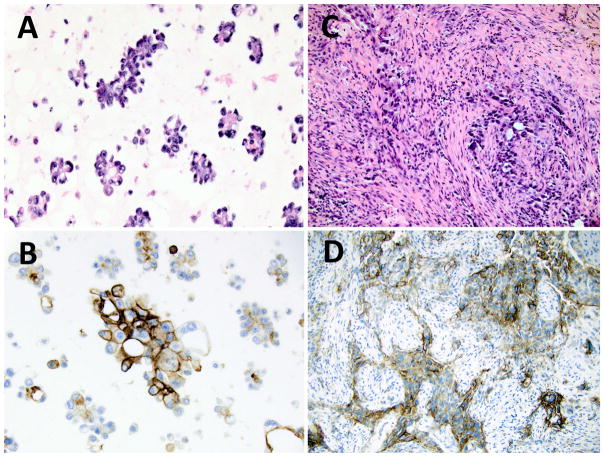
The results of PD-L1 immunohistochemical testing are summarized in Table 2. Overall, PD-L1 testing yielded an interpretable result in over 96% (223/232) of specimens. Of the 232 total cases, 87 (37.5%) showed no PD-L1 expression (TPS <1%), 55 (23.7%) showed low expression (TPS 1–49%), and 81 (34.9%) showed high expression (TPS ≥50%). When analyzed by specimen type, there was no statistically significant difference observed in the proportions of the clinically relevant groups of negative, low, or high PD-L1 TPS for tumors tested on cytology cell block specimens compared to tumors tested on surgical pathology specimens (Table 2). A finer breakdown of the cytology cell block vs. surgical pathology specimens with successful PD-L1 testing (n=223, 88 cytology cell block specimens, 135 surgical pathology specimens) into bins of increasing 10% expression increments also demonstrated a very similar overall distribution of PD-L1 expression, with no statistically significant difference noted in the distribution of values between groups (p=0.57, ns) (Figure 1). Figure 2 provides examples of high PD-L1 expressing tumors (TPS ≥50%) as assessed on a cytology cell block as compared to a surgical pathology specimen; both patients went on to receive pembrolizumab therapy (Figure 3).
Table 2.
PD-L1 expression stratified by specimen type and clinically relevant tumor proportion score (TPS) cutoff values
| PD-L1 TPS | Overall (n=232) | Cytology Cell-block Specimen (n=94) | Surgical Pathology Specimen (n=138) | p-value |
|---|---|---|---|---|
| <1% PD-L1 TPS | 87 (37.5%) | 35 (37.2%) | 52 (37.7%) | 0.4 |
| 1–49% PD-L1 TPS | 55 (23.7%) | 20 (21.3%) | 35 (25.3%) | |
| ≥50% PD-L1 TPS | 81 (34.9%) | 33 (35.1%) | 48 (34.8%) | |
| Failed analysis | 9 (3.9%) | 6 (6.4%) | 3 (2.2%) |
P-value via Fisher’s exact test.
Figure 1.
PD-L1 expression stratified by specimen type and increasing 10%-increments of tumor proportion score (TPS).
Figure 2.
PD-L1 immunohistochemical staining of lung adenocarcinoma specimens with a TPS ≥50%. A) Formalin fixed, paraffin embedded cell block preparation from a malignant pleural effusion showing clusters of malignant cells, positive for TTF-1 and Napsin-A (not shown), consistent with metastatic lung adenocarcinoma (H&E stain, 400× original magnification). C) Formalin fixed, paraffin embedded core biopsy of a lung mass showing an infiltrative poorly differentiated NSCLC, positive for TTF-1 and Napsin-A (not shown), consistent with a solid variant lung adenocarcinoma (H&E stain, 400× original magnification). PD-L1 IHC using the 22C3 pharmDx showing strong membranous staining in the majority of tumor cells in the cytology cell block (B) and surgical pathology core biopsy (D) specimens.
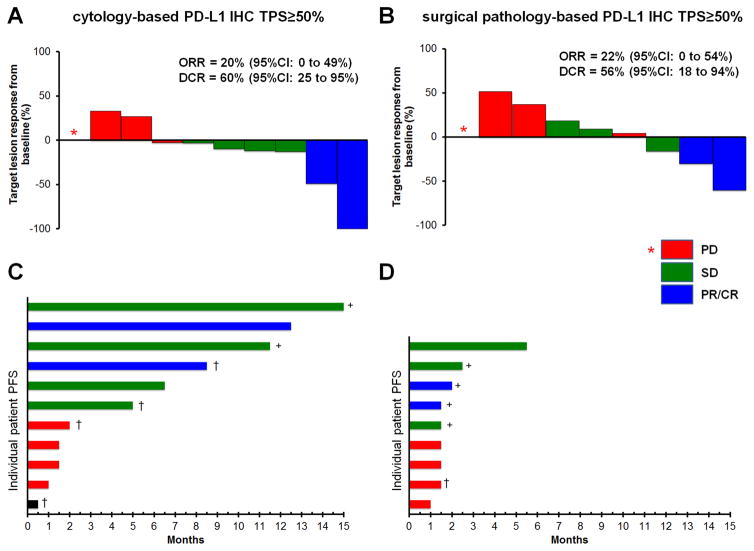
Figure 3.
Clinical outcomes with pembrolizumab in cytology cell block and surgical pathology tested specimens displaying PD-L1 TPS ≥50%. A) Patients with cytology cell block or B) surgical pathology specimens with high PD-L1 expression; Waterfall plots demonstrating best response of target lesions as a percentage decreased/increase from baseline as determined by RECIST to pembrolizumab. In tumors with progression only in non-target lesions, an asterisks (*) with no bar is used. C) Patients with cytology cell blocks or D) surgical pathology specimens with high PD-L1 expression; Swimmers plots of type/duration of response to pembrolizumab monotherapy (duration of pembrolizumab to progression or death was rounded to the nearest half month). Ongoing disease control is indicated by a plus (+) sign, disease-related death is indicated with a dagger (†). Red bars, progressive disease (PD); green bars, stable disease (SD); blue bars, partial response/complete response (PR/CR); black bar, no follow-up scan. PFS, progression free survival; ORR, overall response rate; DCR, disease control rate.
The presence of oncogenic driver mutations identified in this cohort of patients was stratified both by PD-L1 TPS as well as by specimen type (Table 3). Of the 223 specimens with successfully completed molecular and PD-L1 testing, 30 (14%) had EGFR mutations, 60 (27%) had KRAS mutations, 7 (3%) had an ALK rearrangement, and 2 (1%) had a ROS1 rearrangement. Stratifying on the basis of specimen type, there was no statistically significant difference observed in either the overall distribution of EGFR/KRAS/ALK/ROS1 alterations or the distribution of genomic alterations according to negative, low, or high PD-L1 TPS (Table 3).
Table 3.
Driver oncogene mutations stratified by PD-L1 Tumor Proportion Score (TPS) and by specimen type
| Oncogene mutation (overall cohort) | Total (n=223) | <1% PD-L1 TPS (n=87) | 1–49% PD-L1 TPS (n=55) | ≥50% PD-L1 TPS (n=81) |
|---|---|---|---|---|
| EGFR | 30 (13.5%) | 14 (16.1%) | 10 (18.2%) | 6 (7.4%) |
| ALK | 7 (3.1%) | 2 (2.3%) | 0 (0%) | 5 (6.2%) |
| ROS1 | 2 (0.9%) | 1 (1.1%) | 1 (1.8%) | 0 (0%) |
| KRAS | 60 (27.0%) | 19 (21.8%) | 13 (23.6%) | 28 (34.6%) |
| Oncogene mutation (cytology cell block) | Total (n=88) | <1% PD-L1 TPS (n=35) | 1–49% PD-L1 TPS (n=20) | ≥50% PD-L1 TPS (n=33) |
| EGFR | 12 (13.6%) | 6 (17.1%) | 5 (25.0%) | 1 (3.0%) |
| ALK | 4 (4.5%) | 1 (2.9%) | 0 (0%) | 3 (9.1%) |
| ROS1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| KRAS | 24 (27.3%) | 9 (25.7%) | 4 (20.0%) | 11 (33.3%) |
| Oncogene mutation (surgical pathology) | Total (n=135) | <1% PD-L1 TPS (n=52) | 1–49% PD-L1 TPS (n=35) | ≥50% PD-L1 TPS (n=48) |
| EGFR | 18 (13.3%) | 8 (15.4%) | 5 (14.3%) | 5 (10.4%) |
| ALK | 3 (2.2%) | 1 (1.9%) | 0 (0%) | 2 (4.2%) |
| ROS1 | 2 (1.5%) | 1 (1.9%) | 1 (2.9%) | 0 (0%) |
| KRAS | 36 (26.7%) | 10 (19.2%) | 9 (25.7%) | 17 (35.4%) |
Statistical comparisons for cytology cell block vs. surgical pathology specimens: total, p = 0.62; <1% PD-L1 TPS, p = 1.0; 1–49% PD-L1 TPS, p=0.64; ≥50% PD-L1 TPS, p=0.39; Fisher exact test.
A total of 20 patients from the testing cohort whose tumors demonstrated high PD-L1 expression (TPS ≥50%) were selected for first-line treatment with evidence-based pembrolizumab during the study period: 11 with high tumor PD-L1 expression obtained from cytology specimens and 9 with high tumor PD-L1 expression obtained from surgical pathology specimens. As shown in Figure 3A and 3B, 19 were evaluable using RECIST with an ORR (confirmed and unconfirmed) of 2/10 (20%, 95%CI: 0–49%) in the cytology-obtained PD-L1 cohort and 2/9 (22%, 95%CI: 0–54%) in the surgical pathology-obtained PD-L1 cohort (p=1.0). The DCR at 6 weeks was 6/10 (60%, 95%CI: 25–95%) in the cytology-obtained PD-L1 cohort and 5/9 (56%, 95%CI: 18–94%) in the surgical pathology specimen-obtained PD-L1 cohort (p=1.0). Importantly, the cytology-obtained PD-L1 cohort included cases with sustained partial response or stable disease for over 12 months (Figure 3C). By chance, the follow-up of response duration for the surgical pathology specimen-obtained PD-L1 cohort has been of shorter duration relative to the cytology cell block testing cohort, though many patients continue to receive pembrolizumab (Figure 3D).
Discussion
Immune checkpoint inhibitors are now integral to the care of treatment-naïve and previously treated patients with advanced NSCLC, with notable and durable improvements in clinical outcomes and toxicity profiles as compared with conventional cytotoxic chemotherapy in defined subsets of patients. At present, tumor PD-L1 IHC remains the best vetted biomarker for prediction of therapeutic response to PD-1/PD-L1 blockade, with most published experience describing use of FFPE surgical pathology specimens with less known about the use of cytology specimens. Here, we provide a new line of evidence exploring use of the FDA-approved 22C3 pharmDx IHC platform for assessment of tumor PD-L1 expression on lung cancer specimens using FFPE cytology cell block specimens obtained in routine clinical practice. Our population-based data indicates that results of PD-L1 IHC testing and clinical outcomes with pembrolizumab use on the basis of immunologic profiling obtained from FFPE cytology cell block specimens is similar to those seen with FFPE surgical pathology specimens.
To date, very few studies have compared PD-L1 IHC performance on cytologic vs. surgical pathology specimens. A recent study of 86 paired cytology-surgical pathology specimens obtained from the same tumor/patient showed a very high correlation coefficient (R2 between 0.87 and 0.95) between cytology and surgical pathology specimens, assayed with both the 28-8pharmDx and 22C3pharmDx antibody clones/kits [12]. A published abstract investigating 40 paired cytology-surgical pathology specimens similarly described a strong correlation coefficient (R2 of 0.70) of PD-L1 tumor staining using the E1L3N antibody clone with excellent intraobserver agreement [13]. PD-L1 in clinical practice is being performed on cell blocks derived from EBUS-TBNA procedures [14], and a high concordance of PD-L1 tumor expression by IHC has been reported in in paired EBUS-TBNA cytology and transbronchial biopsy specimens [20]. All these studies incorporate paired samples for PD-L1 testing, with both cytology and surgical pathology specimens obtained from the same patient tumor for parallel testing. As such, these paired biopsy studies provide good direct evidence that the technical performance of PD-L1 testing via IHC is similar if using surgical pathology or cytology specimens derived from the same tumor/patient.
In this study, we approached the question from a different angle, aiming to provide an additional line of indirect evidence that cytology cell block and surgical pathology specimens behave similarly as substrates for 22C3 pharmDx PD-L1 testing in advanced NSCLC as it pertains to prediction of response to therapy with pembrolizumab. This large cohort of patients represents the real-world experience of therapeutic stratification and management of patients with advanced stage NSCLC on the basis of diagnostic and ancillary testing performed on available tumor samples: either cytology cell block or surgical pathology specimens. Importantly, each case reported here had PD-L1 testing performed on either cytology cell block or surgical pathology specimens; no paired sample testing was done. As such, one would hypothesize that if cytology FFPE cell block preparations for technical, sampling, or interpretative reasons behaved in a significantly different manner than FFPE surgical pathology specimens, then this bias would be reflected in differences in PD-L1 TPS staining patterns or distribution between cytology cell block and surgical pathology tested cohorts. However, we show in a sufficiently large cohort of patients with PD-L1 testing performed on either cytology cell blocks (n=94) or surgical pathology specimens (n=138) that there were no differences in overall clinical characteristics (Table 1), tumor PD-L1 expression on the basis of TPS of <1%/1–49%/≥50% (Table 2), overall distribution of PD-L1 expression when examined in 10% increments (Figure 1), and concomitant oncogenic driver mutations (Table 3). Although the numbers of treated patients are relatively small, we for the first time provide early evidence that there was also a similar clinical response to pembrolizumab in selected patients with TPS ≥50% based on either a cytology cell block testing or surgical pathology specimen testing (Figure 3). Admittedly, because the non-paired cytology cell block and surgical pathology specimens in these cohorts are from different patient-tumors and have no direct relationship to one another, we are unable to draw direct conclusions on specimen comparability. However, in aggregate the findings presented here support the notion that from a clinical utility standpoint, FFPE cytology cell block specimens may be equivalent to FFPE surgical pathology specimens for PD-L1 biomarker testing.
Currently, PD-L1 expression serves as the best biomarker for the selection of patients whose tumors may most likely respond to immune checkpoint inhibitor therapy. Spatial and temporal heterogeneity of tumor PD-L1 expression, PD-L1 expression on tumor-infiltrating lymphocytes vs. tumor cells, differences in PD-L1 expression between primary and metastatic foci, and different antibody clones and IHC platforms all contribute to the complexity in accurately and reproducibly assessing this biomarker, especially when basing treatment decisions on small biopsy specimens. While the results from the BLUEPRINT project [7] and others will help clarify many of these biomarker testing issues, the clinician currently must make treatment decisions with the best data available. It remains to be seen whether tumor mutation burden, either in conjunction with or in lieu of PD-L1 TPS, may be a better biomarker for the selection of patients who may respond to immune checkpoint inhibitory therapy [21].
In our series, although the number of patients receiving pembrolizumab therapy is relatively low, the observed ORR of approximately 20% (in both the cytology and surgical pathology cohorts) is lower than the approximately 40% ORR reported in clinical trials of NSCLC in tumors with PD-L1 TPS ≥50%. This warrants further exploration in a larger, prospective manner and might be explained by the clinical reality of selecting patients with poorer performance status and greater co-morbidities who are often excluded from clinical trials. However, the confidence intervals for ORR and DCR at 6 weeks are concordant with data previously reported in the KEYNOTE studies [2, 3]. Further, multiple PD-L1 high expression cases identified on the basis of cytology specimens had durable partial response/disease control in excess of one year – a hallmark of PD-1/PD-L1 blockade in selected cases of advanced NSCLC.
In clinical practice, PD-L1 testing of NSCLC specimens is now a mandatory component of the therapeutic stratification schema to best pair patient and disease characteristics with optimal therapies, particularly for advanced/recurrent disease. As such, testing must be performed on a specimen that is both adequate and readily available. In many circumstances, the most accessible option may be a cytologic specimen, either from the primary tumor or a metastatic site. Although we report overall comparable performance and outcome metrics when comparing PD-L1 testing on FFPE cytology cell blocks to FFPE surgical pathology specimens, one should not necessarily infer that all cytology specimens are adequate substrates for testing [22]. Alcohol-fixed direct smears, cytospin preparations, or cell blocks prepared by other methods are all cytology-type specimens that might also serve as adequate testing substrates, pending adequate validation studies. As with any immunohistochemical test, a laboratory should perform adequate validation for cytology cell block specimens, as a number of pre-analytic factors could potentially contribute to false negative results as compared to the gold standard of FFPE surgical pathology specimens on which most IHC protocols were initially developed [23].
In conclusion, immune checkpoint inhibitor therapy has shown great promise in providing robust and durable responses in some patients with NSCLC. Much work is ongoing to determine how to best select these patients, both with respect to the proper biomarker to test as well as what substrates the testing should be performed on. Cytology cell block specimens have proven utility for diagnostic and ancillary testing in NSCLC, with further studies needed to identify how best to use these specimens for PD-L1 testing in the setting of lung cancer.
Supplementary Material
Highlights.
PD-L1 immunohistochemical testing is the best current biomarker test for selecting patients with non-small cell lung cancer for immune checkpoint inhibitor therapy.
It is not known if cytology specimens can be used as testing substrates for PD-L1.
In a large cohort of lung cancer patients, cytology cell block specimens show similar testing characteristics to surgical pathology biopsy specimens.
Acknowledgments
Funding/Grant Support: This work was funded in part through an American Cancer Society grant RSG 11–186 (DBC), National Cancer Institute grant P50CA090578 (DBC), and internal donations to Beth Israel Deaconess Medical Center.
Footnotes
Author Contributions
VFT, DR, MS, DBC, PVL: Conceptualization, methodology, formal analysis, investigation, resources, data curation, writing- original draft and review and editing, visualization.
BPG: Methodology, formal analysis, investigation, data curation, visualization.
Conflict of interest: DBC has received consulting fees and honoraria from Pfizer, Boehringer Ingelheim and Ariad pharmaceuticals; outside the submitted work. PVL has received consulting fees from Gala Therapeutics; outside the submitted work. No other conflict of interest is stated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soo RA, Stone ECA, Cummings KM, et al. Scientific Advances in Thoracic Oncology 2016. J Thorac Oncol. 2017;12:1183–1209. doi: 10.1016/j.jtho.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4. [assessed 9/7/2017];Pembrolizumab (KEYTRUDA) Checkpoint Inhibitor. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm526430.htm.
- 5.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) [assessed 9/7/2017];Non-Small Cell Lung Cancer, Version 8. 2017 doi: 10.6004/jnccn.2017.0050. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. [DOI] [PubMed]
- 7.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–222. doi: 10.1016/j.jtho.2016.11.2228. [DOI] [PubMed] [Google Scholar]
- 8.VanderLaan PA. Fine-needle aspiration and core needle biopsy: An update on 2 common minimally invasive tissue sampling modalities. Cancer. 2016;124:862–870. doi: 10.1002/cncy.21742. [DOI] [PubMed] [Google Scholar]
- 9.Roy-Chowdhuri S, Aisner DL, Allen TC, et al. Biomarker Testing in Lung Carcinoma Cytology Specimens: A Perspective From Members of the Pulmonary Pathology Society. Arch Pathol Lab Med. 2016 Apr 15; doi: 10.5858/arpa.2016-0091-SA. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.DiStasio M, Chen Y, Rangachari D, et al. Molecular Testing Turnaround Time for Non-Small Cell Lung Cancer in Routine Clinical Practice Confirms Feasibility of CAP/IASLC/AMP Guideline Recommendations: A Single-center Analysis. Clin Lung Cancer. 2017;18:e349–e356. doi: 10.1016/j.cllc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Rangachari D, VanderLaan PA, Le X, et al. Experience with targeted next generation sequencing for the care of lung cancer: insights into promises and limitations of genomic oncology in day-to-day practice. Cancer Treat Commun. 2015;4:174–181. doi: 10.1016/j.ctrc.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skov B, Skov T. Paired Comparison of PD-L1 Expression on Cytologic and Histologic Specimens From Malignancies in the Lung Assessed With PD-L1 IHC 28–8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol. 2017;25:453–459. doi: 10.1097/PAI.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 13.Russell-Goldman E, Sholl LM, Vivero M. Cytology-histologic correlation of PD-L1 immunohistochemistry in lung carcinomas. Mod Pathol. 2017;30(suppl 2):114A. doi: 10.1002/cncy.21973. [DOI] [PubMed] [Google Scholar]
- 14.Stoy S, Rosen L, Murgu S. The Use of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Cytology Specimens for Programmed Death Ligand 1 Immunohistochemistry Testing in Non-Small Cell Lung Cancer. J Bronchology Interv Pulmonol. 2017;24:181–183. doi: 10.1097/LBR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 15.VanderLaan PA, Yamaguchi N, Folch E, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39–44. doi: 10.1016/j.lungcan.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folch E, Yamaguchi N, VanderLaan PA, et al. Adequacy of lymph node transbronchial needle aspirates using convex probe endobronchial ultrasound for multiple tumor genotyping techniques in non-small-cell lung cancer. J Thorac Oncol. 2013;8:1438–1444. doi: 10.1097/JTO.0b013e3182a471a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Fortune KA, Randolph ML, Wu HH, Cramer HM. Improvements in cell block processing: The Cell-Gel method. Cancer. 2017;125:267–276. doi: 10.1002/cncy.21814. [DOI] [PubMed] [Google Scholar]
- 18.Rangachari D, VanderLaan PA, Shea M, et al. Correlation between Classic Driver Oncogene Mutations in EGFR, ALK, or ROS1 and 22C3-PD-L1 ≥50% Expression in Lung Adenocarcinoma. J Thorac Oncol. 2017;12:878–883. doi: 10.1016/j.jtho.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Sakakibara R, Inamura K, Tambo Y, et al. EBUS-TBNA as a Promising Method for the Evaluation of Tumor PD-L1 Expression in Lung Cancer. Clin Lung Cancer. 2017;18:527–534. doi: 10.1016/j.cllc.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mino-Kenudson M. Programmed death-ligand 1 immunohistochemistry testing for non-small cell lung cancer in practice. Cancer. 2017;125:521–528. doi: 10.1002/cncy.21873. [DOI] [PubMed] [Google Scholar]
- 23.Gown AM. Diagnostic immunohistochemistry: what can go wrong and how to prevent it. Arch Pathol Lab Med. 2016;140:893–898. doi: 10.5858/arpa.2016-0119-RA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.