Abstract
Background
Diffusion weighted imaging (DWI) has been widely used to investigate the integrity of white matter (WM; indexed by fractional anisotropy, FA) in alcohol dependence and cigarette smoking. These disorders are highly comorbid, yet cigarette use has often not been adequately controlled in neuroimaging studies of alcohol dependent populations. In addition, information on white matter deficits in currently drinking, nontreatment-seeking (NTS) individuals with alcohol dependence is limited. Therefore, the aim of this work was to investigate WM microstructural integrity in alcohol use disorder by comparing matched samples of cigarette smoking NTS and social drinkers (SD).
Methods
Thirty-eight smoking NTS and nineteen smoking SD subjects underwent DWI as well as structural magnetic resonance imaging. After an in-house preprocessing of the DWI data, FA images were analyzed with Tract-Based Spatial Statistics (TBSS). FA obtained from the TBSS skeleton was tested for correlation with recent alcohol consumption.
Results
Smoking NTS had lower FA relative to smoking SD, predominantly in the left hemisphere (p<0.05, family-wise error rate-corrected corrected across FA skeleton). Across the full sample, FA and number of drinks per week were negatively related (ρ=−0.348, p=0.008). Qualitative analyses of the structural connections through compromised white matter as identified by TBSS showed differential connectivity of gray matter in NTS compared to SD subjects of left frontal, temporal, and parietal regions.
Conclusions
NTS subjects had lower white matter FA than SD, indicating compromised white matter integrity in the NTS population. The inverse relationship of entire white matter skeleton FA with self-reported alcohol consumption supports previous evidence of a continuum of detrimental effects of alcohol consumption on white matter. These results provide additional evidence that alcohol dependence is associated with reduced white matter integrity in currently drinking nontreatment-seeking alcohol dependent individuals, after controlling for the key variable of cigarette smoking.
Keywords: alcohol use disorder, connectivity, diffusion tensor imaging, neuroimaging, tract-based spatial statistics
Introduction
An estimated 15 million people in the United States have an alcohol use disorder (AUD) (Department of Health and Human Sevices, 2015), which in 2010 cost the nation 249 billion dollars (Sacks et al., 2015). Additionally, in AUD, the prevalence of tobacco use has been estimated to be ~60–90%, as compared to ~10% in a light-drinking population (Falk et al., 2006, Kalman et al., 2005, Romberger and Grant, 2004). Numerous neuroimaging studies have reported deleterious effects of alcohol on regional white matter (WM) microstructure in AUD (Bagga et al., 2014, Müller-Oehring et al., 2009, Pfefferbaum et al., 2006, Pfefferbaum and Sullivan, 2002, Pfefferbaum et al., 2000, Yeh et al., 2009, Monnig et al., 2013, Pfefferbaum et al., 2014, Segobin et al., 2015). However, this established body of research typically does not address the potential confound of cigarette smoking in abnormalities of brain structure in AUD. There is also a body of literature that indicates cigarette smoking has detrimental effects on WM (Zhang et al., 2011, Savjani et al., 2014, Baeza-Loya et al., 2016, Zou et al., 2017). However, given that up to 85% of alcoholics are also smokers (Durazzo et al., 2007b, Romberger and Grant, 2004), it is still unclear what the relative contribution of chronic alcohol misuse is to deficits in WM. Some work in nontreatment-seeking (NTS) individuals with AUD has shown differences in brain volumes of NTS compared to light drinking controls (Cardenas et al., 2005), that could be, in part, attributed to smoking status (Durazzo et al., 2007a); but the effects of extensive alcohol misuse alone have not yet been isolated.
While research into WM microstructural abnormalities in detoxified/abstinent AUD is extensive, the same cannot be said for the NTS population. This is important, as findings from detoxified alcoholics may not generalize to community-dwelling NTS. Only 8.3% of those with an AUD have sought treatment (Department of Health and Human Sevices, 2015), which makes the NTS population the majority of individuals with AUD. Additionally, as noted above, the potential influence of comorbid cigarette use in AUD has not been addressed in neuroimaging studies of the NTS population. Thus, the overarching goal of this work was to determine if there are detectable effects of alcohol dependence on WM microstructure in a currently drinking NTS population, while controlling for cigarette smoking status. Based on existing literature in detoxified AUD subjects, we hypothesized that smoking NTS will have lower WM microstructural integrity (assessed with fractional anisotropy, FA) compared to social drinking (SD) controls. To test our hypothesis, we utilized Tract-Based Spatial Statistics (TBSS) to identify areas of compromised WM microstructure in NTS. We also applied a novel, WM tractography-based approach, to objectively identify the gray matter regions that could be targets of cortical projections that pass through any observed WM differences.
Materials and Methods
Subjects
All procedures were approved by the Indiana University Institutional Review Board in accordance with the Belmont Report. Subjects were recruited from the community using advertisements in a local paper and social media. Written informed consent was obtained after confirmation that breath alcohol concentration (BrAC) was zero in all subjects and study procedures were explained. Subjects were 21–55 years old, and able to read, understand, and complete all procedures in English. This study was a retrospective analysis of magnetic resonance imaging (MRI) data from nineteen smoking social drinkers (SD) and thirty-eight smoking nontreatment-seeking (NTS) alcohol dependent participants. MRI data were acquired in subjects who participated in the positron emission tomography (PET) studies designed to investigate the dopamine system; PET data from most subjects in the current work have been published (Albrecht et al., 2013, Yoder et al., 2011b, Oberlin et al., 2015, Yoder et al., 2016, Yoder et al., 2011a). All subjects included in the present analyses were cigarette smokers. Exclusion criteria were: history or presence of any psychiatric, neurological, or other medical disorder, current use of any psychotropic medication, positive urine pregnancy test, positive urine toxicology screen for illicit substances, and contraindications for safety in the MRI scanner. The Semi-Structured Assessment for the Genetics of Alcoholism was administered to confirm presence or absence of AUD. NTS met DSM-IV criteria for alcohol dependence, had not received treatment within the past year, and were not actively seeking treatment. The following questionnaires were also administered: a medical history and demographics questionnaire, the 90-day Timeline Follow-Back for alcohol use (TLFB), Alcohol Dependence Scale (ADS), Fagerstrom Test for Nicotine Dependence (Pomerleau et al., 1994), Edinburgh Handedness Inventory, and an internally-developed substance use questionnaire.
Imaging
MRI imaging was done on a 3T Siemens Magnetom Trio with a 12-channel head coil array (Siemens, Erlangen, Germany). Diffusion-weighted imaging (DWI) data were acquired using monopolar Stejskal-Tanner diffusion weighting, single shell (b-value = 1000 s/mm2), and 48 distinct diffusion gradients (eight b = 0 images acquired first; GRAPPA with in-plane acceleration of 2). Echo time (TE) was 81ms, echo spacing 0.7ms, scan duration 8:10min, 128×128 matrix, anterior to posterior phase-encoding, 6/8 partial Fourier phase, 68 axial slices, and 2×2×2 mm3 isotropic voxels. There were minor variations in TE and scan duration, as the DWI sequence was incrementally adjusted over time to minimize bed vibration and image artifacts. A small subset of data (n = 7) were collected with one b = 0 volume. A high-resolution, T1-weighted, whole-brain magnetization prepared rapid gradient echo (MP-RAGE) image was acquired with the parameters optimized for the Alzheimer’s Disease Neuroimaging Initiative (http://adni.loni.usc.edu/): 9:14 min, no GRAPPA, matrix size 240×256, 160 sagittal slices, 2.91ms TE, 1.05×1.05×1.2 mm3 voxels.
Image Processing
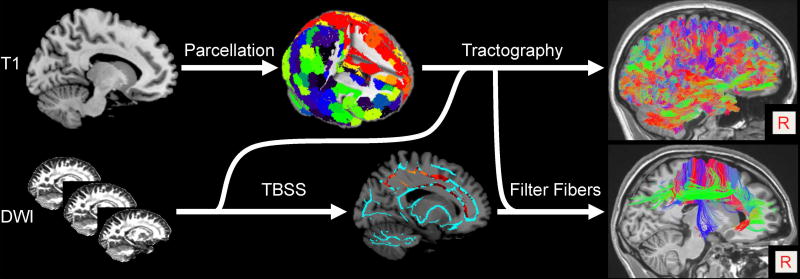
Processing was carried out with an in-house pipeline implemented in Matlab (MathWorks, version 2014b) that incorporated programs from the Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library (FSL version 5.0.9)(Jenkinson et al., 2012) and Camino (Cook et al., 2006) software suites. Figure 1 illustrates the key steps of the image processing.
Figure 1.
Overall processing scheme for isolating tractography-derived streamlines that passed through regions of significantly different FA in the TBSS group analyses (see text for details). Each subject’s gray matter (derived by segmenting the T1 image; top left) is divided into 278 functionally-derived regions (top middle, GM regions color-coded). Diffusion-weighted image (DWI) data (bottom left) are preprocessed to obtain diffusion tensor information and fractional anisotropy (FA) volumes. Anatomical and DWI data information are combined to perform deterministic tractography (top right). FA data are analyzed with tract-based spatial statistics (TBSS). The TBSS-derived significant clusters (bottom middle; orange-red) are used to “filter” the reconstructed fiber tracts to identify those that passed through the significant clusters (bottom right). The fiber tracts are color-coded to indicate their predominant orientation (left/right in red; anterior/posterior in green; inferior/superior in blue).
T1 preprocessing
Preprocessing steps included: (1) Denoising of each subjects’ T1-weighted image with an optimized nonlocal means filter for 3D MRI (Coupé et al., 2008), (2) Automated cropping and bias field correction (FSL robustfov and FAST), (3) Brain extraction (FSL-BET), and (4) Tissue-type (FSL-FAST) and subcortical structure (FSL-FIRST) segmentation.
Both gray and white matter (GM, WM) images from FSL-FAST were enlarged by a single modal dilation. The GM-WM spatial overlap provided an interface region that defined seed regions for tractography. A combined cerebrospinal fluid (CSF; from FSL-FAST) and subcortical GM (from FSL-FIRST) masks were used to minimize erroneous assignment of WM voxels as GM.
DWI preprocessing
Diffusion images were visually inspected for signal dropout and significant head motion artifacts. Only datasets that passed visual inspection were included. DWI images were denoised with a local principal component analysis filter (Manjón et al., 2013). The eight b = 0 volumes were spatially registered (FSL-FLIRT dof6) to the first volume and averaged to optimize image quality and minimize effects of head motion. Motion correction of each DWI volume was achieved with linear registration (FLIRT dof6) to the reference b = 0 volume. Eddy current correction was performed with FSL eddy correct (Jenkinson and Smith, 2001). The b = 0 volume was co-registered (dof6 and WM boundary-based registration [BBR]) to the preprocessed T1 image and this transformation was subsequently applied to the DWI data. Voxel-wise calculation of fractional anisotropy (FA) and tensor modeling were conducted using multi-tensor fitting in Camino, where each voxel was classified as either isotropic, single-tensor (anisotropic, Gaussian), or crossing fibers (anisotropic, non-Gaussian). At multiple points throughout the preprocessing steps, images were visually inspected for quality assurance.
Tract-Based Spatial Statistics (TBSS)
Voxelwise statistical analysis of the FA data was performed with FSL’s Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006). Each subject’s FA data were aligned into a common space using the nonlinear registration (FNIRT), which uses a b-spline representation of the registration warp field. Next, a mean FA volume was created and thinned to form an FA skeleton that represented the centers of all tracts in the sample. Each subject's aligned FA data were then projected onto the skeleton, and the resultant datasets served as input into the voxelwise statistics algorithm. Group differences were interrogated with FSL’s randomise permutation testing. Contrasts were generated after 10,000 permutations with Threshold-Free Cluster Enhancement (TFCE) (Smith and Nichols, 2009), and were corrected for multiple comparisons (family-wise error; FWE). The anatomic locations of significant clusters were used as a starting point for post-hoc, qualitative tractography analyses (2.6). Cluster size, peak voxel significance and the corresponding coordinate, as well as the mean FA value of each significant cluster were extracted with the FSL cluster tool. Additionally, the mean FA value from each subject’s FA skeleton was extracted using Matlab. These extracted average FA values were tested for a relationship with recent alcohol use by nonparametric regressions (Spearman’s rho) in SPSS 24. Nonparametric testing was utilized because, across all subjects, recent alcohol use (drinks/week) was non-normally distributed.
Connectivity Analyses
Deterministic Tractography
Deterministic tractography conducted in Camino with the Fiber Assignment by Continuous Tracking (FACT) algorithm used a single seed per voxel at the GM-WM interface region. Fiber assignment began in a seed voxel, with 1 mm increments (1 step per voxel). Except for two-tensor voxels, fiber tracking followed the major diffusion gradient from voxel-to-voxel except when the turning angle exceeded 45° in 5 steps, which resulted in termination of tracking. Encountering a two-tensor voxel led to fiber duplication, with each of the two fibers followed one of the tensor directions. Streamlines were restricted to WM voxels where FA > 0.1, and were terminated when they reached a GM voxel on either end. Very short (< 8 mm) or long (> 180 mm) streamlines were discarded.
Tract (Streamline) Isolation
To isolate fibers passing though voxels with significant group differences in FA, the TBSS output images were transformed into each subject’s native space with FSL tbss_deproject. Camino procstreamlines projected the cluster maps onto each subject’s tractography results, and constrained the tractography data to fibers that passed through the significant clusters (e.g., TBSS p-value volumes; Figure 1). To perform quality checks of modeled streamlines, fiber data were converted to trk format for visualization in TrackVis (Wang et al., 2007).
Gray Matter Parcellation
Each subject’s gray matter was subdivided into 278 regions based on a functionally-derived parcellation (Shen et al., 2013). This allowed us to locate gray matter sources of streamlines passing through white matter areas of significantly different FA. The parcellation was applied to each subject’s data by the following steps: 1) spatial transformation of the native T1 volume into MNI space (FSL: flirt-dof6, flirt-dof12, fnirt); 2) application of the individual inverse transformation parameters to the MNI template parcellation; and 3) masking the resultant native-space parcellation with each subject’s GM mask from FSL-FAST. For quality assurance, the final parcellations were overlaid on the respective T1 volumes for visual inspection.
Connectivity Visualization
The number of streamlines that connected any pair of gray matter regions was obtained from a connectivity matrix generated with the Camino conmat function. Connections were assessed separately for NTS and SD. For each group, a region-by-region connectivity matrix was generated with the conservative requirement that, to be counted, a given connection must be present in all subjects within that group. GM regions that were connected via the significant cluster areas were visualized by an overlay of the connected GM regions for each group onto a Colin 27 (CH2) MNI brain template.
Other Statistical Analyses
Group differences in sample characteristics were assessed with independent-samples t-tests or χ2 tests (R; version 3.3.0).
Results
Subjects
Subject characteristics are presented in Table 1. NTS and SD were well-matched on all demographic characteristics and tobacco use. NTS reported significantly higher alcohol consumption and scored higher on the Alcohol Dependence Scale (ADS).
Table 1.
Subject Characteristics.
| SD | NTS | p-value | |
|---|---|---|---|
| N | 19 | 38 | |
| Age | 37.8 ± 8.6 | 38.6 ± 8.1 | n.s. |
| Gender | 3 F | 7 F | n.s. |
| Race | 6 AA | 17 AA | n.s. |
| Ethnicity | 0 HL | 0 HL | |
| Education (years) | 12.8 ± 2.3 | 12.7 ± 1.9 | n.s. |
| Handedness | 19 R | 36 R; 2 A | |
| Drinks/DD | 3.4 ± 1.4 | 8.8 ± 3.3 | < 0.05 |
| Drinks/Week | 6.4 ± 7.5 | 38.4 ± 18.9 | < 0.05 |
| ADS | 3.9 ± 2.8 | 12.5 ± 5.4 | < 0.05 |
| Fagerstrom (smokers only) | 4.4 ± 1.4 | 4.3 ± 2.1 | n.s. |
Data are mean ± standard deviation.
ADS: Alcohol Dependency Scale. SD: social drinkers. NTS: nontreatment-seeking alcoholics. AA: African-American. HL: Hispanic Latino. R: right. A: ambidextrous. n.s.: not significant. DD: drinking day.
TBSS: SD vs. NTS
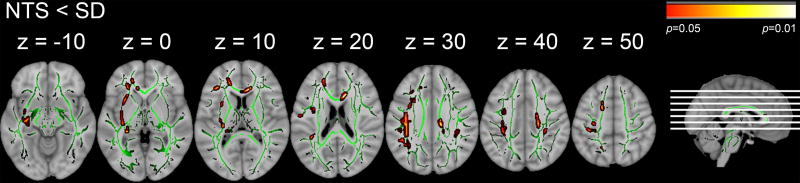
NTS had lower FA compared to SD, predominantly in the left hemisphere (p<0.05 TFCE, FWE-corrected; Figure 2). Thirty-three significant clusters were identified. Data for the largest (exceeding 90 mm3) and likely most relevant clusters are presented in Table 2. For a full list of clusters, the reader is referred to Supplementary Table 1.
Figure 2.
TBSS results. Areas of significant group differences in FA (p < 0.05, FWE-corrected, red-yellow colors) superimposed on the mean fractional anisotropy (FA) skeleton shown in green. Smoking social drinkers (SD) compared to nontreatment seekers NTS. Axial slice positions are illustrated in the sagittal section. For better visualization, significant voxels within the white matter skeleton were thickened with tbss_fill.
Table 2.
Characteristics of significant clusters from the NTS < SD TBSS contrast.
| Cluster* | Cluster Size (mm3) |
FA SD |
FA NTS |
Peak p-value (FWE) |
Peak MNI coordinates# (mm) (x, y, z) |
|---|---|---|---|---|---|
| 1 | 1298 | 0.59 ± 0.07 | 0.54 ± 0.09 | 0.028 | −39, −12, 28 |
| 2 | 417 | 0.55 ± 0.10 | 0.47 ± 0.12 | 0.045 | −31, −24, −7 |
| 3 | 340 | 0.60 ± 0.09 | 0.53 ± 0.13 | 0.044 | −19, 42, 4 |
| 4 | 319 | 0.57 ± 0.10 | 0.52 ± 0.10 | 0.045 | 14, −20, 30 |
| 5 | 296 | 0.58 ± 0.08 | 0.53 ± 0.09 | 0.045 | −33, −20, −1 |
| 6 | 282 | 0.52 ± 0.08 | 0.44 ± 0.07 | 0.044 | 12, 27, 14 |
| 7 | 258 | 0.35 ± 0.09 | 0.27 ± 0.10 | 0.042 | 34, −38, 33 |
| 8 | 202 | 0.57 ± 0.07 | 0.52 ± 0.10 | 0.044 | −45, −46, 30 |
| 9 | 168 | 0.52 ± 0.09 | 0.44 ± 0.08 | 0.046 | −30, 8, 5 |
| 10 | 133 | 0.55 ± 0.08 | 0.48 ± 0.08 | 0.048 | −36, −54, 26 |
| 11 | 122 | 0.67 ± 0.06 | 0.61 ± 0.08 | 0.046 | −30, 12, 32 |
| 12 | 120 | 0.78 ± 0.08 | 0.73 ± 0.09 | 0.042 | −27, −3, 42 |
Eleven clusters (< 90 mm3) were omitted (See Supplementary Table 1 for full list).
Coordinates are in Montreal Neurological Institute (MNI) space.
TBSS: tract-based spatial statistics. SD: social drinkers. NTS: nontreatment-seekers. FA: fractional anisotropy. FWE: Family-wise error. MNI: Montreal Neurological Institute.
Correlations of FA with recent alcohol use
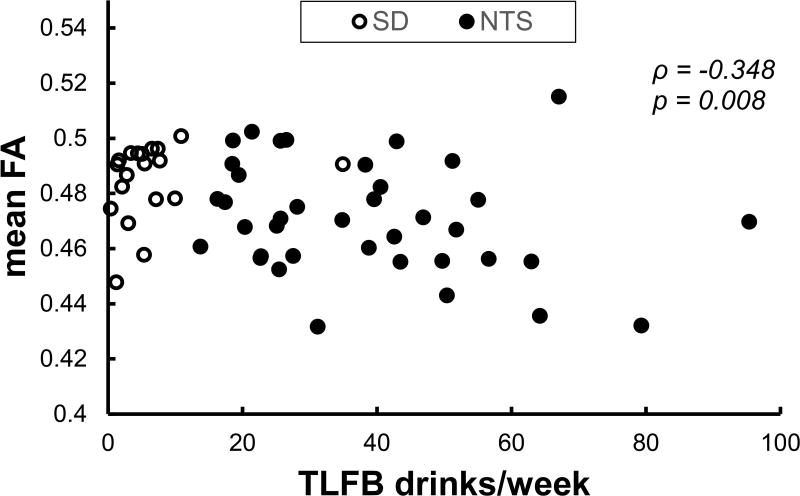
Across all subjects, number of drinks per week had a significant negative correlation with average FA from the TBSS skeleton (Spearman’s ρ = −0.348, p = 0.008; Figure 3).
Figure 3.
Correlation of mean FA obtained from the TBSS white matter skeleton with self-reported average number of drink per week in the past 90 days across the full sample. FA: fractional anisotropy. TFLB: Timeline Follow-Back. ρ: Spearman’s rho.
Structural Connectivity
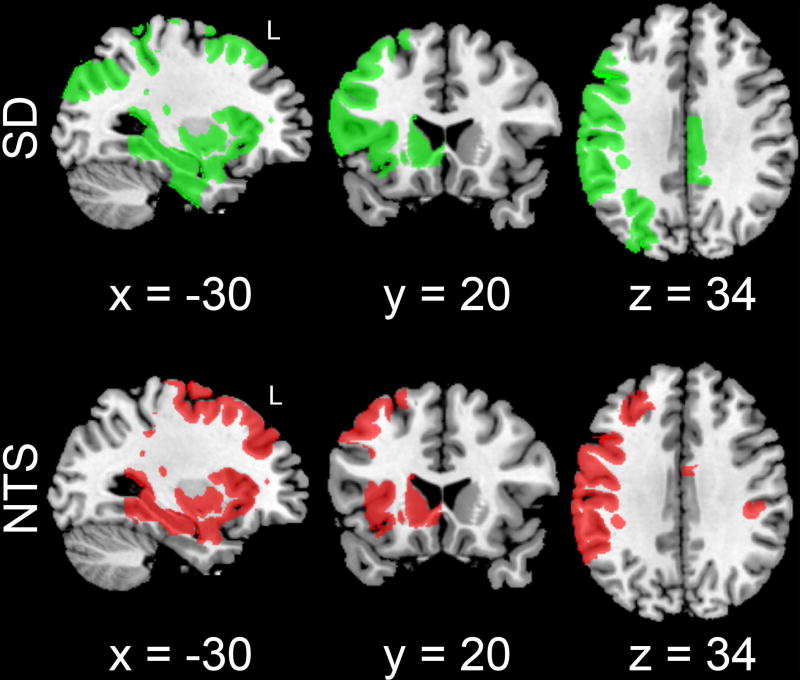
In SD, 42 of the 278 gray matter regions were connected though the significant clusters from the NTS < SD TBSS contrast, while NTS had 40 connected gray matter regions. Spatially, these regions were largely overlapping between groups, with some regions unique to each group. Figure 4 illustrates the connected GM regions for each group. There were qualitative differences in regional patterns of GM targets between groups (see Discussion).
Figure 4.
Spatial distribution of gray matter regions connected through TBSS-derived white matter clusters that showed significant group differences in FA. Connected regions are shown for both smoking social drinkers (SD, green) and smoking nontreatment-seeking alcohol use disorder subjects (NTS; red). Note that NTS have a qualitatively distinct spatial distribution of connected GM regions that are fewer in number compared to SD. Connected gray matter regions are overlaid on a Colin 27 (CH2) MNI template brain, with MNI-coordinates provided below each slice.
Discussion
This study found effects of alcohol dependence on WM microstructure in smoking nontreatment-seeking alcohol dependent individuals who had lower FA relative to smoking social drinkers. Not matching groups for smoking status is a potentially important confound that is often overlooked in neuroimaging studies of addiction. We used our results as a starting point for implementation of a novel approach to identify gray matter regions that could be the sources of denervation through the areas of compromised white matter (identified via TBSS). We also found that FA values in the whole study sample were negatively associated with recent drinking behavior.
The areas in which NTS had significantly lower FA compared to SD were primarily in the left hemisphere, notably, in the external capsule and the superior longitudinal fasciculus. FA deficits in regions that contain these tracts have been previously reported in AUD (Pfefferbaum et al., 2009, Yeh et al., 2009) and altered WM integrity in the tracts has been associated with altered cognitive function (Bagga et al., 2014, Trivedi et al., 2013). Also, detrimental effects of AUD on callosal WM have been reported to occur preferentially in the genu of the corpus callosum (Estruch et al., 1997, Pfefferbaum et al., 1996), and anterior WM tracts have also been shown to have greater deficits in FA (Pfefferbaum et al., 2009). Thus, the pattern of WM microstructural deficits that we observed in NTS are consistent with previous findings, and provide additional evidence that anterior WM and longer tracts, such as the superior longitudinal fasciculus, may be more vulnerable to the effects of alcohol. At this time, it is not clear why the results were apparently lateralized; however, it is possible that larger sample size may have revealed bilateral effects.
To begin to understand possible changes in brain function secondary to WM disruption, we employed a novel filtered tractography approach, whereupon the putative GM endpoints of compromised WM tracts were identified. There were some regional differences in the connectivity patterns between NTS and SD. Connected GM regions that were only seen in the NTS group included areas in the frontal lobe and anterior cingulate gyrus. Connected regions that were only observed in SD included frontal lobe and middle/posterior cingulate gyrus, as well the left temporal, parietal, and occipital lobes. In NTS, the absence of connections to posterior regions in parietal and occipital lobes may be additional evidence that alcohol has detrimental effects on long WM tracts, such as the superior longitudinal fasciculus.
The observed WM deficits in NTS are likely to have functional ramifications in the brain. Recent research with functional MRI showed that functional connectivity of the cingulate cortex was associated with time to relapse in a recovering AUD sample (Zakiniaeiz et al., 2017). In addition, functional connectivity of the precuneus in response to alcohol cues was associated with the severity of alcohol dependence (Courtney et al., 2014). Thus, it may be the case that with continued alcohol misuse, structural differences in individuals with AUD may be related to functional deficits that alter brain function. In turn, this may contribute to the maintenance of AUD, and potentially increase the risk for relapse in those who attempt to quit drinking.
Resting state functional MRI has been used to parse the brain into networks with functional significance (Yeo et al., 2011), which assists with interpreting structural connectivity results. In the present study, regions with differential structural connectivity between NTS and SD predominantly were in the ventral and dorsal attention networks (Fox et al., 2006), frontoparietal network (Vincent et al., 2008), and the default mode network (Andrews-Hanna et al., 2010). Broadly speaking, these networks are involved in orientation of attention to internal and external stimuli, decision-making, and self-referential processes – all of which are relevant to addictive processes. Indeed, studies of AUD-related populations have reported altered function of these networks (Fryer et al., 2013, Wetherill et al., 2012, Chanraud et al., 2011). Thus, the observed structural differences in NTS in the present work are consistent with these functional findings. However, in this retrospective study, functional MRI data were not available, so we were unable to relate brain function to the observed structural differences.
There are other limitations to consider for this retrospective study. First, we were unable to test for any possible interaction of cigarette and alcohol use because a nonsmoking group was not available in this retrospective sample. We also lacked metrics on lifetime exposure to alcohol as well as continuous metrics of recent and lifetime smoking rates, which could have provided insight as to the putative cumulative effects of alcohol on WM and allowed for a more precise control for cigarette use. However, we believe that the observed negative association between recent drinking history and FA is likely related to lifetime alcohol exposure, as drinking patterns often solidify in early adulthood. Second, recent research suggests that tractography algorithms may be prone to false positive results (Maier-Hein et al., 2017). The approach we applied here was designed to reduce the likelihood of such effects via application of a conservative, deterministic tracking algorithm and by restriction of the seeding area to the GM/WM interface, rather than utilizing the full extent of all GM voxels in the brain. Finally, this approach only investigated WM connectivity through TBSS skeleton, which represents the center of core WM, and not the full extent of the WM tracts. In addition, no distinctions were made as to which specific regions were connected to one another. However, this approach provided qualitative insight into putative GM connections of compromised WM tracts in AUD. It also sets a premise for future work to investigate specific structural connectivity in AUD with regional- and/or network-based tractography approaches.
In conclusion, we used TBSS to assess the effects of alcohol dependence on WM integrity in currently-drinking subjects with alcohol dependence, while controlling for cigarette smoking status. We also found that recent drinking (a probable proxy for lifetime alcohol exposure) was inversely correlated with WM integrity. Additionally, we employed a novel, qualitative method to identify the GM regions that may be adversely affected by regions of significantly lower FA in NTS, such as the cingulate cortex and precuneus. The results strongly suggest that actively-smoking and drinking individuals with alcohol dependence have features of WM microstructural deficits. Presence of these deficits in NTS highlights the need for additional research on consequences of alcohol misuse in currently drinking, community-dwelling AUD populations, who represent the majority of individuals with the disorder.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Daniel Albrecht, James Walters, Karen Hile, Christine Herring, Lauren Federici, Cari Cox Lehigh, and Elizabeth Patton for assistance with recruitment and data collection, as well as Michele Beal and Courtney Robbins for assistance with MR imaging. We also thank Dr. Joey Contreras for assistance with the image processing pipeline.
Supported by ABMRF/The Foundation for Alcohol Research (KKY), NIAAA 5P60AA007611-25 (pilot P50 to KKY), NIAAA R21AA016901 (KKY), NIAAA R01AA018354 (KKY), the Indiana Clinical and Translational Sciences Institute (NIH TR000006, Indiana Clinical Research Center), and NIAAA AA07462 (T32).
Footnotes
The authors declare no potential conflict of interests.
Authors Contribution
KKY conceptualized the study design, provided funding for the work, secured all regulatory approvals, supervised all aspects of the work, and provided intellectual input for the generation of this manuscript. EJC performed the image processing and data analyses, contributed to the computer code that facilitated processing and analyses, designed the novel qualitative approach in this work, drafted and edited the manuscript, and provided intellectual input. MD designed the MRI acquisition sequences; MD and JG drafted the computer code for processing and analyses, supervised processing and analyses, and provided intellectual input for this manuscript. TCD provided intellectual content to the data interpretation and editing of the manuscript. MEH assisted with image processing and provided intellectual contributions to the interpretation of the data and editing of the manuscript. All authors critically reviewed the manuscript and approved the final version for publication.
References
- Albrecht DS, Kareken DA, Yoder KK. Effects of smoking on D(2)/D(3) striatal receptor availability in alcoholics and social drinkers. Brain Imaging Behav. 2013;7:326–34. doi: 10.1007/s11682-013-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-Anatomic Fractionation of the Brain's Default Network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeza-Loya S, Velasquez KM, Molfese DL, Viswanath H, Curtis KN, Thompson-Lake DGY, Baldwin PR, Ellmore TM, De La Garza R, Salas R. Anterior cingulum white matter is altered in tobacco smokers. The American Journal on Addictions. 2016;25:210–214. doi: 10.1111/ajad.12362. [DOI] [PubMed] [Google Scholar]
- Bagga D, Sharma A, Kumari A, Kaur P, Bhattacharya D, Garg ML, Khushu S, Singh N. Decreased white matter integrity in fronto-occipital fasciculus bundles: relation to visual information processing in alcohol-dependent subjects. Alcohol. 2014;48:43–53. doi: 10.1016/j.alcohol.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Research: Neuroimaging. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel A-L, Pfefferbaum A, Sullivan EV. Disruption of Functional Connectivity of the Default-Mode Network in Alcoholism. Cerebral Cortex. 2011;21:2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PA, Bai Y, Nedjati-Gilani S, Seunarine KK, Hall MG, Parker GJ, Alexander DC. Camino: Open-Source Diffusion-MRI Reconstruction and Processing; 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine; 2006. p. 2759. [Google Scholar]
- Coupé P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An Optimized Blockwise Nonlocal Means Denoising Filter for 3-D Magnetic Resonance Images. Medical Imaging, IEEE Transactions on. 2008;27:425–441. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, London ED, Ray LA. The association between cue-reactivity in the precuneus and level of dependence on nicotine and alcohol. Drug and Alcohol Dependence. 2014;141:21–26. doi: 10.1016/j.drugalcdep.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Sevices, D. National Survey on Drug Use and Health [Online] Department of Health and Human Sevices; 2015. [Accessed 10 March 2017]. Available: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.htm#tab5-6a. [Google Scholar]
- Durazzo TC, Cardenas VA, Studholme C, Weiner MW, Meyerhoff DJ. Non-treatment-seeking heavy drinkers: Effects of chronic cigarette smoking on brain structure. Drug and Alcohol Dependence. 2007a;87:76–82. doi: 10.1016/j.drugalcdep.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol and Alcoholism. 2007b;42:174–185. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Estruch R, Nicolás JM, Salamero M, Aragón C, Sacanella E, Fernández-Solá J, Urbano-Márquez A. Atrophy of the corpus callosum in chronic alcoholism. Journal of the Neurological Sciences. 1997;146:145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H-Y, Hiller-Sturmhöfel S. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Research & Health: The Journal Of The National Institute On Alcohol Abuse And Alcoholism. 2006;29:162–171. [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Jorgensen KW, Yetter EJ, Daurignac EC, Watson TD, Shanbhag H, Krystal JH, Mathalon DH. Differential brain response to alcohol cue distractors across stages of alcohol dependence. Biological Psychology. 2013;92:282–291. doi: 10.1016/j.biopsycho.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-Morbidity of Smoking in Patients with Psychiatric and Substance Use Disorders. The American Journal on Addictions. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein KH, Neher PF, Houde J-C, Côté M-A, Garyfallidis E, Zhong J, Chamberland M, Yeh F-C, Lin Y-C, Ji Q, Reddick WE, Glass JO, Chen DQ, Feng Y, Gao C, Wu Y, Ma J, Renjie H, Li Q, Westin C-F, Deslauriers-Gauthier S, González JOO, Paquette M, St-Jean S, Girard G, Rheault F, Sidhu J, Tax CMW, Guo F, Mesri HY, Dávid S, Froeling M, Heemskerk AM, Leemans A, Boré A, Pinsard B, Bedetti C, Desrosiers M, Brambati S, Doyon J, Sarica A, Vasta R, Cerasa A, Quattrone A, Yeatman J, Khan AR, Hodges W, Alexander S, Romascano D, Barakovic M, Auría A, Esteban O, Lemkaddem A, Thiran J-P, Cetingul HE, Odry BL, Mailhe B, Nadar MS, Pizzagalli F, Prasad G, Villalon-Reina JE, Galvis J, Thompson PM, Requejo FDS, Laguna PL, Lacerda LM, Barrett R, Dell’acqua F, Catani M, Petit L, Caruyer E, Daducci A, Dyrby TB, Holland-Letz T, Hilgetag CC, Stieltjes B, Descoteaux M. The challenge of mapping the human connectome based on diffusion tractography. Nature Communications. 2017;8:1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjón JV, Coupé P, Concha L, Buades A, Collins DL, Robles M. Diffusion Weighted Image Denoising Using Overcomplete Local PCA. PLoS ONE. 2013;8:e73021. doi: 10.1371/journal.pone.0073021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Caprihan A, Yeo RA, Gasparovic C, Ruhl DA, Lysne P, Bogenschutz MP, Hutchison KE, Thoma RJ. Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychology of Addictive Behaviors. 2013;27:455–465. doi: 10.1037/a0027168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Oehring EM, Schulte T, Fama R, Pfefferbaum A, Sullivan EV. Global–Local Interference is Related to Callosal Compromise in Alcoholism: A Behavior-DTI Association Study. Alcoholism: Clinical and Experimental Research. 2009;33:477–489. doi: 10.1111/j.1530-0277.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Albrecht DS, Herring CM, Walters JW, Hile KL, Kareken DA, Yoder KK. Monetary discounting and ventral striatal dopamine receptor availability in nontreatment-seeking alcoholics and social drinkers. Psychopharmacology (Berl) 2015;232:2207–16. doi: 10.1007/s00213-014-3850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial Profile of White Matter Microstructural Integrity in Recovering Alcoholic Men and Women. Biological Psychiatry. 2006;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the Corpus Callosum in Older Alcoholic Men: A Magnetic Resonance Imaging Study. Alcoholism: Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV. Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry. 2009;65:680–90. doi: 10.1016/j.biopsych.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, Zahr NM, Sullivan EV. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. The Lancet Psychiatry. 2014;1:202–212. doi: 10.1016/S2215-0366(14)70301-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Microstructural but Not Macrostructural Disruption of White Matter in Women with Chronic Alcoholism. NeuroImage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In Vivo Detection and Functional Correlates of White Matter Microstructural Disruption in Chronic Alcoholism. Alcoholism: Clinical and Experimental Research. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF. Reliability of the fagerstrom tolerance questionnaire and the fagerstrom test for nicotine dependence. Addictive Behaviors. 1994;19:33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomedicine & Pharmacotherapy. 2004;58:77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 National and State Costs of Excessive Alcohol Consumption. American Journal of Preventive Medicine. 2015;49:e73–e79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Savjani RR, Velasquez KM, Thompson-Lake DGY, Baldwin PR, Eagleman DM, De La Garza R, II, Salas R. Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug and Alcohol Dependence. 2014;145:134–142. doi: 10.1016/j.drugalcdep.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Segobin S, Ritz L, Lannuzel C, Boudehent C, Vabret F, Eustache F, Beaunieux H, Pitel A-L. Integrity of white matter microstructure in alcoholics with and without Korsakoff's syndrome. Human Brain Mapping. 2015;36:2795–2808. doi: 10.1002/hbm.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage. 2013;82:403–415. doi: 10.1016/j.neuroimage.2013.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Trivedi R, Bagga D, Bhattacharya D, Kaur P, Kumar P, Khushu S, Tripathi RP, Singh N. White matter damage is associated with memory decline in chronic alcoholics: a quantitative diffusion tensor tractography study. Behav Brain Res. 2013;250:192–8. doi: 10.1016/j.bbr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a Frontoparietal Control System Revealed by Intrinsic Functional Connectivity. Journal of Neurophysiology. 2008;100:3328. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Proc. Intl. Soc. Mag. Reson. Med. 2007;15:3720. [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF. Frontoparietal connectivity in substance-naïve youth with and without a family history of alcoholism. Brain Research. 2012;1432:66–73. doi: 10.1016/j.brainres.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Dzemidzic M, Normandin MD, Federici LM, Graves T, Herring CM, Hile KL, Walters JW, Liang T, Plawecki MH, O'connor S, Kareken DA. Differences in IV alcohol-induced dopamine release in the ventral striatum of social drinkers and nontreatment-seeking alcoholics. Drug and Alcohol Dependence. 2016;160:163–169. doi: 10.1016/j.drugalcdep.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, Zheng Q-H, Mock BH, O’connor SJ, Herring CM. Reliability of striatal [11C]raclopride binding in smokers wearing transdermal nicotine patches. European Journal of Nuclear Medicine and Molecular Imaging. 2011a;39:220–225. doi: 10.1007/s00259-011-1965-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Albrecht DS, Kareken DA, Federici LM, Perry KM, Patton EA, Zheng QH, Mock BH, O'connor S, Herring CM. Test-retest variability of [11C]raclopride-binding potential in nontreatment-seeking alcoholics. Synapse. 2011b;65:553–61. doi: 10.1002/syn.20874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, Constable RT. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. NeuroImage: Clinical. 2017;13:181–187. doi: 10.1016/j.nicl.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. NeuroImage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Murray DE, Durazzo TC, Schmidt TP, Murray TA, Meyerhoff DJ. Effects of abstinence and chronic cigarette smoking on white matter microstructure in alcohol dependence: Diffusion tensor imaging at 4 T. Drug and Alcohol Dependence. 2017;175:42–50. doi: 10.1016/j.drugalcdep.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.