Abstract
Objective
Serious mental illness (SMI) and Type II diabetes mellitus (DM2) have a high comorbidity, and both have a higher prevalence of anxiety disorders compared to the general population. Targeted Training in Illness Management (TTIM) is a group based self-management training approach which targets SMI and DM2 concurrently. This analysis examines data from a randomized controlled trial of TTIM intervention to examine the impact of comorbid anxiety on baseline psychiatric symptomatology and diabetic control, and on longitudinal treatment outcomes.
Methods
We conducted secondary analysis on data from a prospective, 60 week, randomized controlled trial testing TTIM vs treatment as usual (TAU) in 200 individuals with serious mental illness and diabetes. Primary outcomes included measures related to SMI symptoms, functional status, general health status and diabetes control. Measures were compared between those participants with anxiety disorders versus those without anxiety at baseline as well as over time using linear mixed effects analyses.
Results
Forty seven percent of the participants had one or more anxiety disorders. At baseline, those with an anxiety diagnosis had higher illness severity, depressive and other psychiatric symptomatology and disability. Diabetic control (HbA1c) was not significantly different at baseline. In the longitudinal analyses, no significant mean slope differences over time (group by time interaction effect) between those with anxiety diagnoses and those without in TAU group were found for primary outcomes. Within the TTIM arm, those with anxiety disorders had significantly greater improvement in mental health functioning. Those with anxiety comorbidity in the TTIM group demonstrated significantly lower HbA1c levels compared to no anxiety comorbidity, and also demonstrated a greater improvement in HbA1c over the first 30 weeks compared to those without anxiety comorbidity.
Conclusion
Comorbid anxiety in SMI and DM2 population is associated with increased psychiatric symptomatology and greater disability. Individuals from this population appear to experience greater improvement in functioning from baseline with the TTIM intervention. Anxiety comorbidity in the SMI and DM2 population does not appear to have a negative impact on diabetic control. These complex relationships need further study.
Introduction
It is known that patients with serious mental illness (SMI) have a higher prevalence of anxiety disorders when compared to the general population1–3. In one systematic review, 40.2% of patients with schizophrenia or schizoaffective disorder, 51.5% of bipolar disorder patients, and 55.6% of patients with depressive disorder expressed feeling frequent or constant anxiety4. Evidence supports that patients with SMI and a comorbid anxiety disorder not only have elevated depressive and psychotic symptoms compared to those without anxiety but also lower social functioning5 and quality of life6. The problem is compounded by the fact that these patients tend to have their anxiety disorders inadequately addressed or treated7.
Type II diabetes mellitus (DM2) is another positively correlated comorbidity for patients with SMI. With age/sex matched controls, patients with SMI had a higher prevalence of DM2 and greater overall illness burden8–14. This includes not only poor glycemic control but also worsening of disability, increased general complications, higher rate of mortality, and decreased quality of life15, 16. Studies have shown that second generation antipsychotics increase the chances of developing DM217–20, beyond the increased physiologic risk prior to the administration of pharmaceuticals21. Patients with both conditions often perceive barriers keeping them from proper disease management in the form of increased stress and isolation, lack of support from family and friends, and poor communication/integration with their healthcare team22.
People with type 2 diabetes mellitus have an increased risk of experiencing anxiety disorders and elevated anxiety symptoms23. In one systematic review generalized anxiety disorder was present in 14% and elevated symptoms of anxiety were present in 40% of patients with diabetes mellitus24. Numerous studies show that the presence of comorbid anxiety with diabetes mellitus is associated with poor glycemic control25. Diabetics with elevated anxiety and/or depression symptoms are less likely to report adhering to self-care recommendations26. Lifetime depression and anxiety with DM2 increased the risk of more severe psychological symptoms, hyperglycemia, and difficulties with healthy behavior27.
One study suggests that treating a patient with both DM2 and SMI, using cognitive remediation strategies and functional adaptation skills training, can lead to better outcomes28. Targeted Training in Illness Management (TTIM) is a group based self-management training approach which targets SMI and DM2 concurrently. TTIM uses psychoeducation, goal-setting, behavioral modeling, and care linkage applying the principles of social cognitive theory29. Added on to a normal treatment regimen, the TTIM approach has been shown to improve outcomes in randomized controlled trial (RCT) conditions in a population with serious mental illness and comorbid diabetes30.
Using interim baseline data from the TTIM study, we have previously reported relationships among comorbid anxiety, glucose control as measured by HbA1c level, and overall illness burden31. In a preliminary, cross-sectional sample of 157 patients, anxiety disorders were seen in 33.1% of participants with SMI and DM2, and were associated with increased severity of depressive symptoms and decreased function. At baseline, HbA1c levels were not significantly different in those with or without anxiety, and having multiple anxiety disorders was not associated with differences in DM2 control31.
This manuscript reports the updated analysis of baseline data from the complete sample of 200 study participants in the TTIM study, and compares longitudinal data on primary outcomes between participants with anxiety disorders versus those without anxiety. Findings can help inform care approaches for individuals with serious mental illness and complex comorbidity.
Methods
TTIM is derived from the Life Goals Program, by Bauer and colleagues and the Diabetes Awareness and Rehabilitation Training by McKibbin and colleagues32–34. The delivery of TTIM in a 2-step process has been described in detail in an earlier publication29, 35. In step one, 12 weekly, group-format, in-person sessions (6-10 participants per group) are co-delivered by a nurse educator and a peer educator with serious mental illness and DM2. In step 2, over the 48-weeks following the group sessions, participants have brief (10-15 min) telephone maintenance sessions with peer educators and nurse educators. Telephone sessions occur every other week for the first 3 months, and monthly thereafter.
This project was a NIMH-funded prospective, 60 week, randomized controlled trial testing TTIM vs treatment as usual (TAU) in 200 individuals with serious mental illness and diabetes conducted in a safety-net health system primary care setting. Research assessments were conducted at baseline (prior to randomization), and at 13, 30 and 60 weeks. A detailed report on recruitment and retention methods for this study has been previously published36. Individuals were referred for participation in the study by their clinicians and individuals also self-referred in response to IRB-approved study advertisements. In addition to clinician referrals and self-referrals, eligible participants were sought via an IRB-approved protocol that identified individuals with SMI-DM in the medical records of a tertiary care teaching hospital. Individuals were identified from the health system’s electronic health record, either by having serious mental illness on their problem list or being treated with medication for serious mental illness (lithium, mood stabilizer, antipsychotic). Using an IRB approved process, these individuals were consecutively contacted and invited to participate in the study. All study participants had DM2 and schizophrenia, schizoaffective disorder, bipolar disorder or major depressive disorder confirmed by the Mini-International Neuropsychiatric Interview (MINI)37.
The RCT had multiple primary outcomes evaluating four domains: mental illness symptom severity, functioning, general health and DM control. Additional physical health outcomes included body mass index (BMI) and blood pressure.
Serious mental illness symptoms primary outcomes were Brief Psychiatric Rating Scale (BPRS), Montgomery-Asberg Depression Scale (MADRS) and Clinical Global Impression (CGI). The BPRS38 measures psychotic and non-psychotic symptoms in serious mental illness. Possible scores range from 7 to 126, with higher scores indicating greater symptom severity. The MADRS is a 10-item depression severity scale widely utilized in studies with patients with serious mental illness39. Possible scores range from 0 to 60 with higher scores indicating worse depression. The CGI is a broad measure of global psychopathology that evaluates illness severity on a 1 to 7 point continuum40. Possible scores range from 0 to 7, with higher scores indicating greater psychopathology.
Functional status was evaluated using Global Assessment of Functioning (GAF) and Sheehan Disability Scale (SDS). The GAF is a 100-point single-item scale that measures global functioning41. Possible scores range from 1 to 100, with higher scores indicating better functioning. The SDS measures role impairment in three domains (work/school; family life/home; social life)42. Possible scores range from 0 to 30, with higher scores indicating greater disability.
General Health Status outcome was the Short Form 36 Health Survey (SF-36), which is a self-report of general health43 divided into a physical component summary (PCS) and mental component summary (MCS). Norm-based scores are placed on the same metric with a mean of 50 and standard deviation of 10. Scores above 50 reflect higher functional status than the average population and scores below 50 reflect lower than average function.
DM control was evaluated with serum glycosylated hemoglobin (HbA1c) drawn at study baseline, 30 and 60 weeks. This indicates relative DM control over the past 3 months with scores ideally< 7.
Data Analysis
Data analyses were conducted in SAS software version 9.3, SPSS version 23, and R software version for 64-bit Windows operating system. The level of significance except where noted otherwise was α = .05.
For purposes of this study, all DSM-IV anxiety disorder diagnoses were included except for Obsessive Compulsive Disorder (OCD), based on agreement by study investigators that OCD patients were not representative of the anxiety cohort as a whole.
For baseline analysis, we report descriptive statistics, including means and standard deviation within SMI diagnosis groups, in a pooled sample of those in TAU or TTIM. We also report the p-value from the nonparametric Kruskal–Wallis one-way analysis of variance by ranks across diagnostic groups. In our longitudinal analyses for the primary outcomes, we evaluate potential differences in the group-by-time interaction effect using linear mixed effects analyses. These series of mixed effects models (separate for each outcome) also included main effects for group and time along with a random intercept. A mixed effects approach is particularly suited for dealing with missing data by a maximum likelihood algorithm under the assumption that the missingness is dependent on the data at hand.
Results
Sample Description
In this analysis all subjects were pooled, regardless of treatment assignment. Almost half of the participants, 94 out of 200 (47.0%), had one or more anxiety disorder, indicating a high prevalence of comorbid anxiety in this group of patients with SMI and DM2. Table 1 contains the proportion of participants with particular anxiety disorders. Prevalence of these anxiety disorders in reported US samples with schizophrenia, DM2 and the general US population have been provided for relative comparison. Generalized anxiety disorder (GAD) was the most common anxiety disorder in this study population.
Table 1.
Specific Anxiety Disorders in the TTIM RCT participants
| Anxiety Disorder | TTIM RCT participants (N, %) | US mean, patients with schizophrenia7 | US mean, patients with DM224 | US mean, general population1 |
|---|---|---|---|---|
| Panic disorder | 31 (15.5%) | 9.8% | 1.3% | 2.7% |
| Agoraphobia | 37 (18.5%) | 5.4% | 4.6% | 0.8% |
| Social phobia | 35 (17.5%) | 14.9% | 7.3% | 8.7% |
| PTSD* | 28 (14.0%) | 12.4% | 1.2% | 3.5% |
| GAD** | 51 (25.5%) | 10.9% | 13.5% | 3.1% |
| One or more anxiety disorder | 94 (47.0%) | 38.3% | 14% | 18.4% |
PTSD=post-traumatic stress disorder
GAD=generalized anxiety disorder
Baseline Demographic and Clinical Differences
In this analysis all subjects were pooled, regardless of treatment assignment. No significant demographic differences with regards to age, gender, race and education were detected in those with and without anxiety disorders. No significant differences were found for SMI diagnosis (p=0.2006), SMI duration (p=0.3655), DM duration (p=0.1061), prevalence of HTN (p=0.4089), and use of insulin (p=0.0519). There were also no significant differences in Charlson Index (p=0.6095), a measure which categorizes medical comorbidity and predicts one year mortality44.
For SMI symptoms and severity, significant differences were found in CGI, MADRS and BPRS scores. Those with anxiety diagnosis had higher CGI scores (p=0.0006), higher MADRS scores (p=0.0003), and higher BPRS scores (p=0.0055), indicating higher illness severity, depressive symptoms and over-all psychiatric symptomatology respectively. SDS scores were significantly higher in anxiety participants (p=0.0002), indicating higher disability at baseline. Anxiety participants also had significantly lower SF-36 mental scores (p<0.0001), reflective of lower relative functional health status. Participants with comorbid anxiety had lower mean GAF score, but this does not reach statistical significance (p=0.1468).
No significant differences were found between the two groups in baseline HbA1c (p=0.2817), diabetes knowledge (p=0.7976), systolic BP (p=0.2494) and BMI (p=0.4880).
Table 2.
Baseline clinical characteristics of RCT participants (pooled sample) with and without a comorbid anxiety disorder diagnosis
| Variable | Anxiety Diagnosis (N) | Mean (SD) or N (%) | No Anxiety Diagnosis (N) | Mean (SD) or N (%) | P value |
|---|---|---|---|---|---|
|
| |||||
| DEMOGRAPHICS | |||||
|
| |||||
| Age (mean, SD) | 94 | 51.78 (9.96) | 106 | 53.47 (8.93) | 0.2285 |
|
| |||||
| Gender Female (N, %) | 64 | 68.09% | 64 | 60.38% | 0.2570 |
|
| |||||
| Race (N, %) | 0.1445 | ||||
| -Caucasian | 33 | 35.11% | 41 | 38.68% | |
| -African-American | 48 | 51.06% | 59 | 55.66% | |
| -Other | 13 | 13.83% | 6 | 5.66% | |
|
| |||||
| CLINICAL CHARACTERICTICS | |||||
|
| |||||
| Serious Mental Illness Diagnosis | 0.2006 | ||||
| Schizophrenia/Schizoaffective Disorder (N, %) | 21 | 22.34% | 28 | 26.42% | |
| Bipolar Disorder (N, %) | 32 | 34.04% | 24 | 22.64% | |
| Major Depressive Disorder (N, %) | 41 | 43.62% | 54 | 50.94% | |
|
| |||||
| Serious mental illness duration (mean years, SD) | 94 | 19.49 (13.27) | 105 | 17.57 (12.03) | 0.3655 |
|
| |||||
| DM duration (mean years, SD) | 92 | 9.56 (8.34) | 105 | 10.51 (7.24) | 0.1061 |
|
| |||||
| SERIOUS MENTAL ILLNESS SYMPTOM SEVERITY, FUNCTIONAL STATUS, GENERAL HEALTH, PHYSICAL BIOMARKERS | |||||
|
| |||||
| CGI (mean, SD) | 94 | 4.51 (0.92) | 106 | 4.07 (0.91) | 0.0006 |
|
| |||||
| MADRS (mean, SD) | 93 | 26.43 (9.13) | 106 | 21.95 (8.66) | 0.0003 |
|
| |||||
| BPRS (mean, SD) | 93 | 42.01 (10.02) | 106 | 38.23 (8.53) | 0.0055 |
|
| |||||
| GAF (mean, SD) | 94 | 50.32 (11.30) | 106 | 52.76 (11.48) | 0.1468 |
|
| |||||
| Sheehan Disability (mean, SD) | 94 | 19.41 (6.02) | 106 | 16.49 (5.98) | 0.0002 |
|
| |||||
| SF-36 (mean, SD) | |||||
| -Physical | 94 | 39.68 (9.21) | 106 | 39.47 (11.55) | 0.8794 |
| -Mental | 94 | 32.77 (9.74) | 106 | 39.61 (11.80) | <0.0001 |
|
| |||||
| HbA1c (mean, SD) | 92 | 7.80 (2.21) | 104 | 8.17 (2.38) | 0.2817 |
Differences in Longitudinal Outcomes
We evaluated on our primary outcomes whether there were significant slope differences over time (group by time interaction effect) within the treatment groups between those with and without anxiety disorders.
No significant differences were found between those with anxiety diagnoses and those without in the TAU group for any of the primary outcome measures studied (all p values >0.05). (See Table 3)
Table 3.
Change in outcomes from baseline at 13, 30 and 60 week outcomes in individuals with and without a comorbid anxiety disorder diagnosis within the TTIM and TAU groups
| Variable | Baseline | 13-weeks | 30 weeks | 60 weeks | p-value |
|---|---|---|---|---|---|
| GAF | |||||
| TTIM A+ | 49.9/11.9 | 59.1/13.0 | 58.9/12.1 | 60.5/13.5 | 0.037 |
| A− | 53.4/10.1 | 60.0/12.0 | 61.5/14.0 | 61.6/12.8 | |
| TAU A+ | 50.7/10.8 | 54.4/11/1 | 51.4/12.3 | 54.7/11.5 | 0.822 |
| A− | 52.1/12.8 | 52.3/13.4 | 54.7/14.4 | 52.3/14.5 | |
| MADRS | |||||
| TTIM A+ | 24.7/10.2 | 16.6/9.8 | 19.2/9.7 | 17.5/9.7 | 0.075 |
| A− | 21.6/8.5 | 14.4/7.9 | 15.4/9.3 | 14.3/10.2 | |
| TAU A+ | 28.3/7.5 | 23.7/9.4 | 25.2/9.6 | 19.6/8.1 | 0.074 |
| A− | 22.3/8.9 | 19.5/10.1 | 17.9/9.8 | 17.8/9.2 | |
| BPRS | |||||
| TTIM A+ | 40.4/11.0 | 33.7/11.3 | 34.3/8.0 | 32.2/8.5 | 0.079 |
| A− | 37.2/8.5 | 30.7/6.6 | 31.9/7.5 | 31.9/9.6 | |
| TAU A+ | 43.6/8.8 | 38.7/8.8 | 38.7/8.2 | 36.6/9.1 | 0.263 |
| A− | 39.3/8.5 | 35.4/7.8 | 34.2/8.8 | 35.4/8.5 | |
| SF-36 Mental Scale | |||||
| TTIM A+ | 35.8/10.0 | 41.4/12.8 | 37.7/11.7 | 40.5/10.3 | 0.020 |
| A− | 38.4/11.1 | 44.4/9.3 | 43.6/11.4 | 43.7/11.7 | |
| TAU A+ | 29.8/8.5 | 36.1/12.4 | 33.1/11.1 | 36.8/10.4 | 0.990 |
| A− | 40.8/12.4 | 40.0/12.1 | 44.8/11.2 | 41.5/11.8 | |
| SF-36 Physical Scale | |||||
| TTIM A+ | 38.3/9.0 | 38.7/9.8 | 39.5/9.8 | 39.7/9.5 | 0.845 |
| A− | 40.3/11.0 | 41.6/11.1 | 40.2/12.2 | 39.6/12.6 | |
| TAU A+ | 41.1/9.3 | 38.8/8.9 | 42.0/10.9 | 41.0/8.3 | 0.570 |
| A− | 38.6/12.1 | 40.2/10.4 | 39.2/10.2 | 40.7/10.0 | |
| CGI | |||||
| TTIM A+ | 4.5/1.1 | 3.8/1.2 | 3.8/1.0 | 3.3/1.1 | 0.347 |
| A− | 4.5/0.8 | 3.5/1.1 | 3.6/1.1 | 3.2/1.1 | |
| TAU A+ | 4.5/0.8 | 4.1/1.0 | 4.4/0.9 | 4.0/0.9 | 0.812 |
| A− | 4.1/1.0 | 4.1/1.1 | 3.9/1.1 | 4.1/1.3 | |
| SDS | |||||
| TTIM A+ | 19.8/6.0 | 15.6/7.5 | 16.6/6.2 | 16.0/6.5 | 0.133 |
| A− | 16.3/5.2 | 12.9/6.0 | 13.7/8.3 | 14.1/8.1 | |
| TAU A+ | 19.0/6.0 | 17.2/6.1 | 18.6/6.7 | 16.5/6.6 | 0.554 |
| A− | 16.6/6.7 | 17.5/7.3 | 15.8/7.3 | 16.4/7.6 | |
| Hemoglobin A1c | |||||
| TTIM A+ | 7.8/2.1 | – | 7.4/1.7 | 7.6/1.5 | 0.030 |
| A− | 8.2/2.3 | – | 8.2/2.7 | 8.0/2.4 | |
| TAU A+ | 7.8/2.4 | – | 7.7/2.2 | 7.9/2.2 | 0.247 |
| A− | 8.1/2.5 | – | 7.9/1.9 | 7.6/1.7 | |
(A+: subjects with anxiety disorders
A−: subjects without anxiety disorders)
Within the TTIM subgroup there were significant group by time interaction effects over the 60 weeks between those with and without anxiety disorders for GAF, SF 36 Mental, and HbA1C (p-values 0.037, 0.020 and 0.030 respectively). Also, trends were seen for the MADRAS and BPRS (p-values 0.075 and 0.079 respectively) (See Table 3). No significant group by time interaction effects were found for CGI, SF36 General Health, and SDS.
At all points of measurement from baseline to 60 weeks, those with anxiety comorbidity had lower GAF values, reflecting relatively poor functioning, however the improvement in GAF in the anxiety comorbidity group with TTIM intervention was greater (10.6 points) than the improvement in GAF in participants without anxiety disorders (8.2 points). Those with anxiety comorbidity had lower SF-36 Mental scores at all points of measurement from baseline to 60 weeks, and also experienced a smaller increase in score (4.7 points) compared to those without anxiety (5.3 points).
Those with anxiety had higher MADRS and BPRS scores at all points compared to those without anxiety, however, experienced a greater improvement in BPRS (8.2 points vs 5.3 points). While not statistically significant in terms of the group by time interaction effect, the trend was still noted.
Those with anxiety comorbidity in the TTIM group had lower HbA1c values and less dispersion at baseline, 30 weeks and 60 weeks compared to those with no anxiety, indicative of better diabetic control. The change in HbA1c at 60 weeks from baseline was the same for those with and without anxiety disorders (0.2%). From baseline to 30 weeks, however, those with anxiety disorders experienced a decrease in mean HbA1c value while mean HbA1c value was unchanged in those without anxiety disorders. The difference in HbA1c between the two curves is greatest at 30 weeks (Figure 1).
Figure 1. HbA1c values in those with and without anxiety in the TTIM intervention arm.
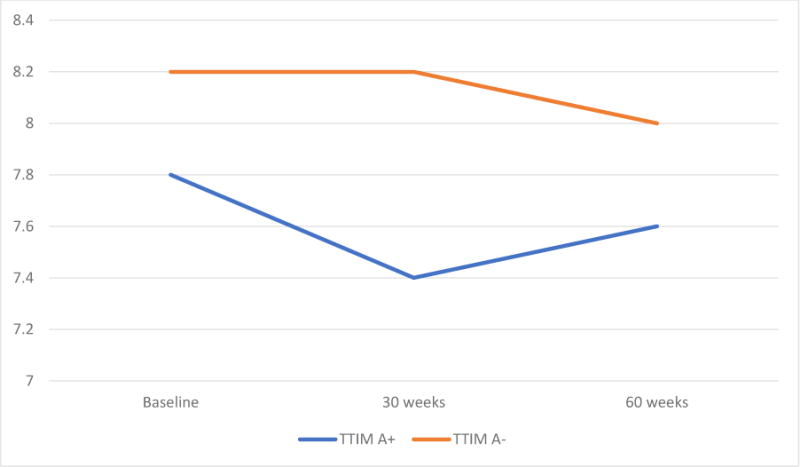
(A+: subjects with anxiety disorders
A−: subjects without anxiety disorders)
No significant slope differences over time between those with and without anxiety disorders in systolic BP and BMI were found (p-values 0.813 and 0.760 respectively).
Discussion
Findings from the pooled TTIM study samples show that anxiety disorders in individuals with SMI and DM2 are very common. While in our earlier preliminary baseline analysis, approximately one third of participants in the sample had a comorbid anxiety disorder, the results of the final analysis show an even greater burden with approximately half of the participants carrying one or more anxiety disorder diagnosis. While elevated prevalence rates of anxiety in the SMI population or DM2 population have been reported separately2, 3, 23, the findings from the TTIM study are novel in that they report findings from individuals with both SMI and DM2.
At baseline, comorbid anxiety was related to increased psychiatric symptomatology, particularly depression, and worse functioning and disability in the pooled samples. This is consistent with our previous preliminary analysis, as well as existing literature which shows that anxiety can exacerbate core symptoms of other psychiatric disorders related to worse prognosis5, 6.
While a statistically significant difference in GAF was not found at baseline, longitudinal data on the primary outcomes suggested those with anxiety in the TTIM group had lower GAF and SF-36 scores (p-values 0.037 and 0.020 respectively) over time compared to those without comorbid anxiety diagnoses, and trends were seen for higher MADRS and BPRS scores over time. A differential effect of the TTIM intervention needs to be pointed out. While both groups (with and without anxiety comorbidity) experienced improved psychiatric symptomatology with the TTIM intervention, the anxiety comorbidity group experienced a greater magnitude of improvement from baseline compared to no anxiety group in GAF (statistically significant p=0.037) and BPRS (not statistically significant but a trend p=0.079). The TTIM intervention is effective for participants both with and without anxiety comorbidity, but the magnitude of improvement may be larger in those with anxiety comorbidity.
As measured by HbA1c, we did not find evidence that comorbid anxiety is associated with worse diabetic control at baseline (p=0.2817). It is possible that anxiety makes participants with SMI and DM2 more aware about the potential risks of poorly controlled diabetes, accompanied by more consciousness of the need to control diet and weight. A negative association between anxiety and diabetic control in DM2 participants alone has been reported previously but inconsistently45–49.
In the longitudinal analyses, participants in the TTIM group with comorbid anxiety showed significantly lower HbA1c levels (p=0.030) and lower spread50 over time compared to those without anxiety. This is in the opposite direction of what we had expected. An inverted-U-shape relationship between levels of anxiety and functioning has been previously reported51, and it may be the case that in our sample, anxiety symptoms could potentially have helped to promote awareness and healthy behaviors relevant to diabetic control. For the first 30 weeks of the study, those with comorbid anxiety experienced a greater decline in HbA1c values (by 0.4%) compared to those without (0.0%), but the over-all change in HbA1c values between the two at 60 weeks was the same as it was at baseline. This may suggest that those with comorbid anxiety respond better to the TTIM intervention in the weeks after the intervention, but the differential effect is not sustained over time. Given that this is a post-hoc secondary analysis, more study would be required to better understand the relationship between anxiety, TTIM and diabetes control.
Several limitations to our study need to be pointed out. It was conducted at a single site; we did not have complete information about mental health treatments; psychotropic medications were not a specific focus of the intervention; and there are questions about generalizability of our sample to a general clinical population. Other confounding factors such as cognitive deficits, psychotic symptoms, and medication adverse effects, can independently influence diabetic control and adherence to treatment. Strengths of the study include the rigorous randomized control design, and well-characterized population with both SMI and DM2 diabetes.
Conclusion
Individuals with SMI and DM2 experience a very high prevalence of anxiety disorders. Comorbid anxiety disorders in this population is associated with worse psychiatric symptoms, lower functioning and greater disability. The TTIM intervention appears to lead to greater improvement in functioning from baseline in those with comorbid anxiety disorders. Anxiety comorbidity in the SMI and DM2 population does not appear to have a negative impact on diabetic control. There is a need for further study to better characterize these complex relationships.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R01MH085665. The project described was also supported by the National Center for Research Resources, Grant UL1RR024989, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000439. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Finally, the project described was also supported by Grant Number KL2TR000440 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Disclosures:
Dr. Sajatovic has received research support from Alkermes, Janssen, Merck, the Reinberger Foundation, the Reuter Foundation, and the Woodruff Foundation; serves as a consultant for Bracket, Health Analytics, Neurocrine, Otsuka, Prophase, Pfizer, Sunovion, and Supernus; receives royalties from Springer, Johns Hopkins University Press, Oxford Press, UpToDate, and Lexicomp; and participates in CME activities for the American Physician’s Institute, MCM Education, and CMEology.
Other authors report no disclosures.
Clinical Trials Registration: ClinicalTrials.gov: Improving outcomes for individuals with serious mental illness and diabetes (NCT01410357).
Contributor Information
Awais Aftab, Case Western Reserve University School of Medicine/University Hospitals Cleveland Medical Center, Cleveland, Ohio.
Chetan Bhat, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Douglas Gunzler, Center for Health Care Research and Policy. Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio.
Kristin Cassidy, Case Western Reserve University School of Medicine, University Hospitals Cleveland Medical Center, Cleveland, Ohio.
Charles Thomas, Center for Health Care Research and Policy. Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio.
Richard McCormick, Center for Health Care Research and Policy. Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio.
Neal V. Dawson, Professor of Medicine, Epidemiology & Biostatistics Center for Health Care Research and Policy. Case Western Reserve University, MetroHealth Medical Center, Cleveland, Ohio.
Martha Sajatovic, Professor of Psychiatry, Neurology and Epidemiology & Biostatistics Case Western Reserve University School of Medicine and Neurological Institute, University Hospitals Cleveland Medical Center, Cleveland, Ohio.
References
- 1.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preti A, Vrublevska J, Veroniki AA, Huedo-Medina TB, Fountoulakis KN. Prevalence, impact and treatment of generalised anxiety disorder in bipolar disorder: a systematic review and meta-analysis. Evid Based Ment Health. 2016;19:73–81. doi: 10.1136/eb-2016-102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpov B, Joffe G, Aaltonen K, et al. Anxiety symptoms in a major mood and schizophrenia spectrum disorders. Eur Psychiatry. 2016;37:1–7. doi: 10.1016/j.eurpsy.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Pallanti S, Quercioli L, Hollander E. Social anxiety in outpatients with schizophrenia: a relevant cause of disability. Am J Psychiatry. 2004;161:53–8. doi: 10.1176/appi.ajp.161.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Braga RJ, Mendlowicz MV, Marrocos RP, Figueira IL. Anxiety disorders in outpatients with schizophrenia: prevalence and impact on the subjective quality of life. J Psychiatr Res. 2005;39:409–14. doi: 10.1016/j.jpsychires.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Achim AM, Maziade M, Raymond E, Olivier D, Merette C, Roy MA. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37:811–21. doi: 10.1093/schbul/sbp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regenold WT, Thapar RK, Marano C, Gavirneni S, Kondapavuluru PV. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J Affect Disord. 2002;70:19–26. doi: 10.1016/s0165-0327(01)00456-6. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell AJ, Malone D. Physical health and schizophrenia. Curr Opin Psychiatry. 2006;19:432–7. doi: 10.1097/01.yco.0000228767.71473.9e. [DOI] [PubMed] [Google Scholar]
- 10.van Winkel R, De Hert M, Van Eyck D, et al. Prevalence of diabetes and the metabolic syndrome in a sample of patients with bipolar disorder. Bipolar Disord. 2008;10:342–8. doi: 10.1111/j.1399-5618.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell AJ, Dinan TG. Schizophrenia: a multisystem disease? J Psychopharmacol. 2010;24:5–7. doi: 10.1177/1359786810382059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoepf D, Potluri R, Uppal H, Natalwala A, Narendran P, Heun R. Type-2 diabetes mellitus in schizophrenia: increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry. 2012;27:33–42. doi: 10.1016/j.eurpsy.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39:306–18. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chwastiak LA, Davydow DS, McKibbin CL, et al. The effect of serious mental illness on the risk of rehospitalization among patients with diabetes. Psychosomatics. 2014;55:134–43. doi: 10.1016/j.psym.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiore V, Marci M, Poggi A, et al. The association between diabetes and depression: a very disabling condition. Endocrine. 2015;48:14–24. doi: 10.1007/s12020-014-0323-x. [DOI] [PubMed] [Google Scholar]
- 16.Gunzler D, Sajatovic M, McCormick R, et al. Psychosocial Features of Clinically Relevant Patient Subgroups With Serious Mental Illness and Comorbid Diabetes. Psychiatr Serv. 2017;68:96–9. doi: 10.1176/appi.ps.201500554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo JJ, Keck PE, Jr, Corey-Lisle PK, et al. Risk of diabetes mellitus associated with atypical antipsychotic use among patients with bipolar disorder: A retrospective, population-based, case-control study. J Clin Psychiatry. 2006;67:1055–61. doi: 10.4088/jcp.v67n0707. [DOI] [PubMed] [Google Scholar]
- 18.Guo JJ, Keck PE, Jr, Corey-Lisle PK, et al. Risk of diabetes mellitus associated with atypical antipsychotic use among Medicaid patients with bipolar disorder: a nested case-control study. Pharmacotherapy. 2007;27:27–35. doi: 10.1592/phco.27.1.27. [DOI] [PubMed] [Google Scholar]
- 19.Harley C, Li H, Corey-Lisle P, L’Italien GJ, Carson W. Influence of medication choice and comorbid diabetes: the cost of bipolar disorder in a privately insured US population. Soc Psychiatry Psychiatr Epidemiol. 2007;42:690–7. doi: 10.1007/s00127-007-0222-z. [DOI] [PubMed] [Google Scholar]
- 20.Erickson SC, Le L, Zakharyan A, et al. New-onset treatment-dependent diabetes mellitus and hyperlipidemia associated with atypical antipsychotic use in older adults without schizophrenia or bipolar disorder. J Am Geriatr Soc. 2012;60:474–9. doi: 10.1111/j.1532-5415.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Rizo C, Kirkpatrick B, Fernandez-Egea E, Oliveira C, Bernardo M. Abnormal glycemic homeostasis at the onset of serious mental illnesses: A common pathway. Psychoneuroendocrinology. 2016;67:70–5. doi: 10.1016/j.psyneuen.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blixen CE, Kanuch S, Perzynski AT, Thomas C, Dawson NV, Sajatovic M. Barriers to Self-management of Serious Mental Illness and Diabetes. Am J Health Behav. 2016;40:194–204. doi: 10.5993/AJHB.40.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith KJ, Beland M, Clyde M, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74:89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. J Psychosom Res. 2002;53:1053–60. doi: 10.1016/s0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RJ, Grigsby AB, Freedland KE, et al. Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med. 2002;32:235–47. doi: 10.2190/KLGD-4H8D-4RYL-TWQ8. [DOI] [PubMed] [Google Scholar]
- 26.Smith KJ, Pedneault M, Schmitz N. Investigation of anxiety and depression symptom co-morbidity in a community sample with type 2 diabetes: Associations with indicators of self-care. Can J Public Health. 2016;106:e496–501. doi: 10.17269/CJPH.106.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitworth SR, Bruce DG, Starkstein SE, Davis WA, Davis TM, Bucks RS. Lifetime depression and anxiety increase prevalent psychological symptoms and worsen glycemic control in type 2 diabetes: The Fremantle Diabetes Study Phase II. Diabetes Res Clin Pract. 2016;122:190–7. doi: 10.1016/j.diabres.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Wykes TL, Lee AA, McKibbin CL, Laurent SM. Self-Efficacy and Hemoglobin A1C Among Adults With Serious Mental Illness and Type 2 Diabetes: The Roles of Cognitive Functioning and Psychiatric Symptom Severity. Psychosom Med. 2016;78:263–70. doi: 10.1097/PSY.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blixen C, Perzynski A, Kanuch S, et al. Training peer educators to promote self-management skills in people with serious mental illness (SMI) and diabetes (DM) in a primary health care setting. Prim Health Care Res Dev. 2015;16:127–37. doi: 10.1017/S1463423614000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sajatovic M, Gunzler D, Kanuch S, et al. A 60-week Prospective Randomized Controlled Trial of Targeted Training in Illness Management vs. Treatment as Usual in Individuals with Serious Mental Illness and Diabetes Mellitus. Psychiatric Services. In Press. [Google Scholar]
- 31.Bajor LA, Gunzler D, Einstadter D, et al. Associations between comorbid anxiety, diabetes control, and overall medical burden in patients with serious mental illness and diabetes. Int J Psychiatry Med. 2015;49:309–20. doi: 10.1177/0091217415589307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandura A. Organisational applications of social cognitive theory. Australian journal of management. 1988;13:275–302. [Google Scholar]
- 33.Bauer MS, McBride L. Structured group psychotherapy for bipolar disorder: The life goals program. Springer Publishing Company; 2003. [Google Scholar]
- 34.McKibbin CL, Patterson TL, Norman G, et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophr Res. 2006;86:36–44. doi: 10.1016/j.schres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Lawless ME, Kanuch SW, Martin S, et al. A Nursing Approach to Self-Management Education for Individuals With Mental Illness and Diabetes. Diabetes Spectr. 2016;29:24–31. doi: 10.2337/diaspect.29.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanuch S, Cassidy M, Kristin A, et al. Recruiting and Retaining Individuals with Serious Mental Illness and Diabetes in Clinical Research: Lessons Learned from a Randomized, Controlled Trial. Journal of Health Disparities Research and Practice. 2016;9:8. [PMC free article] [PubMed] [Google Scholar]
- 37.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 4-57. [PubMed] [Google Scholar]
- 38.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological reports. 1962;10:799–812. [Google Scholar]
- 39.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 40.Guy W. Public Health Service, Alcohol, Drug Abuse, Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research. Rockville, MD: US Department of Health, Education, and Welfare; 1976. The clinical global impression scale The ECDEU assessment manual for psychopharmacology revised; pp. 218–222. (DHEW Publ No ADM 76-338). Vol. [Google Scholar]
- 41.Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale-reliability and validity of the Global Assessment of Functioning (GAF) Br J Psychiatry. 1995;166:654–9. doi: 10.1192/bjp.166.5.654. [DOI] [PubMed] [Google Scholar]
- 42.Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27:93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD. [DOI] [PubMed] [Google Scholar]
- 43.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 44.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005;43:607–15. doi: 10.1097/01.mlr.0000163658.65008.ec. [DOI] [PubMed] [Google Scholar]
- 45.Balhara YP, Sagar R. Correlates of anxiety and depression among patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2011;15:S50–4. doi: 10.4103/2230-8210.83057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196:116–21. doi: 10.1192/bjp.bp.109.067512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalra B, Kalra S, Balhara YP. Psychological assessment and management in diabetes. J Pak Med Assoc. 2013;63:1555–7. [PubMed] [Google Scholar]
- 48.Trento M, Raballo M, Trevisan M, et al. A cross-sectional survey of depression, anxiety, and cognitive function in patients with type 2 diabetes. Acta Diabetol. 2012;49:199–203. doi: 10.1007/s00592-011-0275-z. [DOI] [PubMed] [Google Scholar]
- 49.Trento M, Trevisan M, Raballo M, et al. Depression, anxiety, cognitive impairment and their association with clinical and demographic variables in people with type 2 diabetes: a 4-year prospective study. J Endocrinol Invest. 2014;37:79–85. doi: 10.1007/s40618-013-0028-7. [DOI] [PubMed] [Google Scholar]
- 50.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171–82. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- 51.Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 2010;17:522–30. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]


