Abstract
Purpose
Retinitis pigmentosa (RP), a group of inherited diseases characterized by the death of rod photoreceptors, followed by the death of cone photoreceptors, progressively leading to partial or complete blindness. Currently no specific treatment is available for RP patients. Sulforaphane (SFN) has been confirmed to be an effective antioxidant in the treatment of many diseases. In this study, we tested the therapeutic effects of SFN against photoreceptor degeneration in Pde6rd10 (rd10) mice.
Methods
rd10 mice and C57BL/6J wild-type (WT) mice were treated with SFN and saline, respectively, from P6 to P20. Electroretinography (ERG), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and western blot were tested, respectively, at P21 for the analysis of retinal function, retinal cell apoptosis or death and the protein express of GRP78/BiP (TUNEL) as a marker of endoplasmic reticulum stress.
Results
Compared to the saline group, the SFN-treated group showed significantly higher ERG a-wave and b-wave amplitudes, less photoreceptor death, and downregulation of GRP78/BiP.
Conclusions
Our data showed that SFN ameliorated the retinal degeneration of rd10 mice, which is possibly related to the downregulation of GRP78 expression.
Keywords: Antioxidant, Sulforaphane, Retinitis pigmentosa, Endoplasmic reticulum stress, rd10 mice
Introduction
Retinitis pigmentosa (RP) is a class of inherited diseases with the progressive degeneration of photoreceptor cells. Although various treatments have been used to protect retinal cells, such as antioxidants, gene or stem cell therapy,(1-3) no effective medications are available for RP treatment due to the limited therapeutic benefits or side effects. (4)
Many mutant genes involved in the pathogenesis of RP have been determined.(5) However, the effective treatments targeting these genetic defects have yet to be reported. Pde6b (cGMP phosphodiesterase 6B, rod receptor, beta polypeptide) encodes the beta subunit of phosphodiesterase (PDE), a peripheral membrane enzyme involved in the phototransduction cascade in rod photoreceptors. Mutations in PDE are associated with RP. The Pde6brd10 (rd10) mouse carries a missense mutation in exon 13 of the beta subunit of the rod phosphodiesterase gene (Pde6b).(6,7) This mutation also causes human autosomal recessive RP.(5,8) Progressive retinal outer nuclear layer (ONL) degeneration in rd10 mouse was observed around post-natal day 16 (P16), followed by a progressive reduction in visual function, determined by measuring rod and cone ERG a-wave and b-wave. Compared with rd1, the rd10 phenotype has a slower onset and milder retinal degeneration and may provide a better model of RP for testing drug therapy. Sulforaphane (SFN), presented in cruciferous plants, is an isothiocyanate with functions such as anti-apoptosis, antioxidant, and free radical scavenger.(9) In some previous studies, SFN showed therapeutic effects on neural protection, and could protect brains against hypoxic-ischemic injury(10) and relieve ischemia-reperfusion-induced retinal damage.(11,12) Our study investigated the protective effects of SFN on retinal photoreceptor cells in rd10 mice, which provides data for further developing SFN as a new therapeutic medication for RP.
Materials and Methods
rd10 mice (Stock Number: 004297) and C57BL/6J wild-type (WT) mice (Stock Number: 000664) were purchased from the Jackson Laboratory (Bar Harbor, Maine). All animal procedures were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic Foundation and were conducted in accordance with the regulation of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
SFN (Enzo Life Sciences, Farmingdale, NY) dissolved in saline (4 mg/ml) was administered by intraperitoneal injection (IP) to rd10 mice at 35 mg/kg daily from postnatal day (P)6 to P20. This dosage was a result of preliminary experiments performed by our group based on the increase of the body weight compared with the saline group (data not shown). The treatment was started from P6 because rd10 mice were reported to start retinal abnormality from P7.(13) Our analysis of the data was at P21 because the rd10 mice already show significant photoreceptor degeneration at P20.(14-16) To be more conservative, the treatment was performed daily because SFN level in the body is probably decreased significantly in 24 h.(17) An equal volume of saline was administrated to the vehicle group of rd10 mice. At P21, dark-adapted and light-adapted electroretinograms (ERGs) were recorded and then the eyeballs and retinas were harvested.
ERG tests were conducted as previously described.(18-20) Briefly, after overnight dark adaptation, mice were anesthetized by intraperitoneal injection of ketamine (80 mg/kg)/xylazine (16 mg/kg). The pupils were dilated with tropicamide ophthalmic solution (1%), phenylephrine hydrochloride ophthalmic solution (2.5%), and cyclopentolate hydrochloride ophthalmic solution (1%) and the corneal surface was anesthetized with proparacaine hydrochloride ophthalmic solution (0.5%). In dark-adapted session of ERG test, the flash luminance ranged from 3.6 to 2.1 log cd s/m2. In light-adapted session of ERG test, the flash luminance ranged from -0.8 to 1.9 log cd s/m2. The time for light-adaptation was 7 minutes before the first light-adapted ERG was recorded. The a-wave and b-wave amplitudes were used for the evaluation of the retinal function. The a-wave amplitude was measured from the baseline to the amplitude at 8 ms after the on-set of flash stimulation. The b-wave amplitude was measured from the a-wave trough to the b-wave peak.
Cell death (apoptosis) was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) with the In Situ Cell Death Detection Kit (Roche Applied Science, Indianapolis, IN). The expression of GRP78/BiP was determined by western blot as previously described.(20) Briefly, mouse eyes were fixed with 4% paraformaldehyde. After dehydration with 10%, 20% and 30% sucrose, samples were embedded in optimal cutting temperature compound (OCT) and cut sagittally passing through the optic nerve head (10 μm) and placed onto slides.
For TUNEL analysis, retinal sections were incubated with freshly prepared 0.1% Triton X-100/0.1% sodium citrate permeabilization solution. After rinsing with 1XPBS thrice, sections were incubated with the TUNEL reaction mixture for 60 min at 37°C in the dark. Sections were mounted with VECTASHIELD mounting medium with DAPI (Burlingame, CA), and visualized with the fluorescence microscope.
GRP78/BiP expression was detected by western blot. Total protein was extracted from retinas with Cell Lysis reagents according to the manufacturer's instruction (Thermo Fisher Scientific) and quantified using the bicinchoninic acid protein assay kit. Equal amounts of protein (40μg) from each sample were subjected to electrophoresis on a 10% SDS-polyacrylamide gel. After proteins were electroblotted to a polyvinylidene difluoride membrane, the membrane was blocked with phosphate buffered saline with TWEEN® 20 containing 5% dried non-fat milk at room temperature for 1 h, and incubated with primary antibodies (anti-GRP78/BiP, 1:2000, ab21685; anti-GAPDH, 1:2000, SC-25778) at 4°C overnight, followed by incubating with the goat-anti-rabbit horseradish peroxidase-conjugated secondary antibody for 2 h. After incubation, membrane was washed three times, and the antigen-antibody complexes were visualized by the enhanced chemiluminescence system (PerkinElmer).
Two-way analysis of variance (ANOVA) was used for the statistical analysis of ERG a-wave and b-wave amplitudes. The power analysis was conducted by the F-test of one-way ANOVA, where we considered numbers as outcome and groups as the factor. All other comparisons were made by one-way ANOVA. P< 0.05 was considered significant.
Results
ERGs were used to compare outer retinal function of mice after SFN treatment. Figures 1a and 1b showed the typical ERG waveforms of both WT mice and rd10 mice in different flash intensities under dark-adapted and light-adapted conditions respectively. Figures 1c and 1d show the luminance-response curve of a-wave and b-wave in dark-adapted condition, while Figure 1e shows the luminance-response curve of b-wave in light-adapted condition. In all of these data, the difference of the SFN group and saline group of the WT mice was not significant (p>0.05). Compared with the saline group or the SFN group of the WT mice, the a-wave and b-wave amplitudes were significantly lower in vehicle-treated rd10 mice in dark-adapted and light-adapted conditions (p<0.01). However, after the treatment with SFN, the a-wave and b-wave amplitudes significantly increased. The differences between the SFN group and the saline group of rd10 mice were significant (dark-adapted a-wave: p<0.001; dark-adapted b-wave: p=0.002; and light-adapted b-wave: p=0.008). The ERG amplitudes of the rd10 mice were still significantly lower than those of the WT mice (SFN group and saline group, respectively) in dark-adapted condition (a-wave: p<0.01; b-wave: p<0.05). However, the differences of the ERG b-wave amplitudes between the rd10 mice and the WT mice (SFN group and saline group, respectively) were not significant (p>0.05). These data indicate that SFN ameliorated the functional loss of retinal rod and cone photoreceptor cells in rd10 mice.
Figure 1.
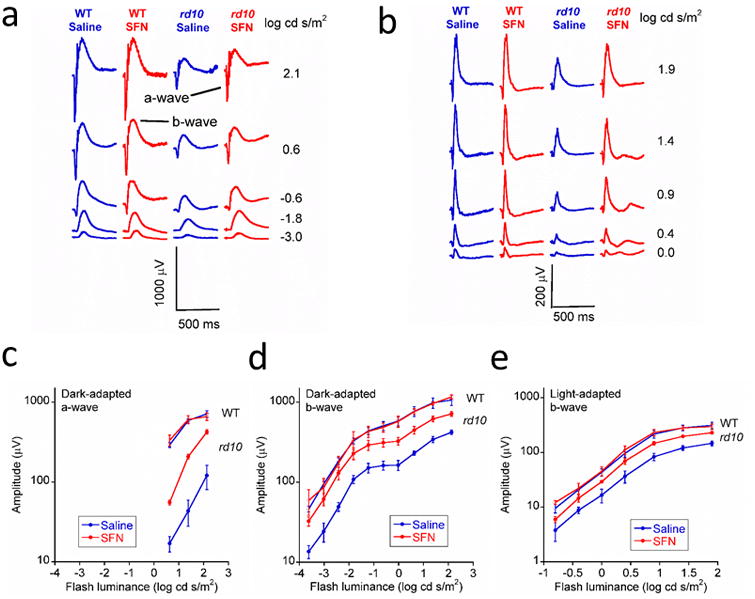
SFN treatment ameliorated the reduction of ERG amplitudes in rd10 retinas. ERG results obtained from rd10 mice treated with SFN (n = 4) and saline (n = 4), and WT mice treated with SFN (n = 3) and saline (n = 3). a. Typical dark-adapted ERG waveforms. b. Typical light-adapted ERG waveforms. c. Luminance-response curves of dark-adapted ERG a-wave amplitude. d. Luminance-response curves of dark-adapted ERG b-wave amplitude. e. Luminance-response curves of light-adapted ERG b-wave amplitude. The error bars indicate standard errors. Under both dark-adapted and light-adapted conditions, the ERG a-wave and b-wave amplitudes were significantly higher in SFN-treated group than in the saline group in rd10 retinas in both dark-adapted and light-adapted conditions (p< 0.01).
Apoptosis of retinal cells were tested by TUNEL. Figure 2a presents representative TUNEL images in the SFN and saline treated rd10 retinas. The number of TUNEL-positive cells was significantly decreased in the SFN group compared with the saline group (Figure 2b, p<0.01).
Figure 2.
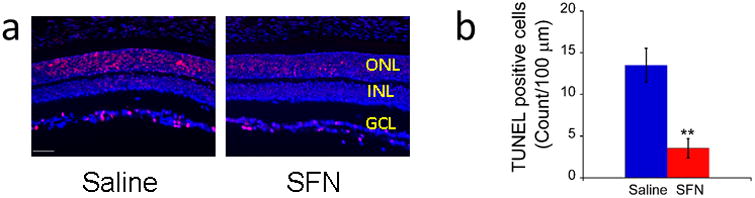
SFN treatment reduced cellular apoptosis in rd10 retinas. a. Representative images (20×) of TUNEL assay in rd10 mice treated with saline or SFN. The red spots indicate the TUNEL-positive cells. Scale bar indicates 50 μm. b. Number of TUNEL positive cells in a 100-μm-long retinal section adjacent to optic nerve head (mean ± SD, n = 3 per group). **p< 0.01vs. saline group.
ER stress in retinal cells was tested by measuring protein expression of GRP78/BiP by western blot. The western blot data showed that the protein expression of GRP78/BiP was significantly down-regulated in rd10 mice after the treatment of SFN (Figure 3a and 3b, p< 0.05).
Figure 3.
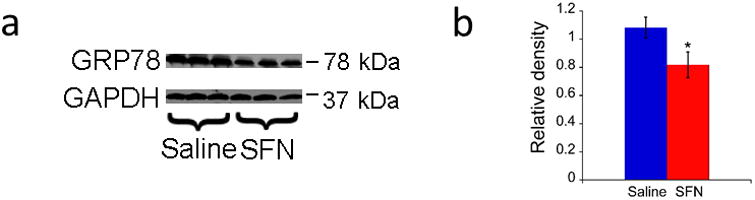
SFN treatment down-regulated GRP78 in rd10 retinas. a. Representative images of western blot for GRP78 in rd10 mice. b. Relative density of western blot for GRP78 (mean ± SD, n = 3 per group). The error bars indicate standard deviations. * p< 0.05vs. saline group.
Discussion
In this study, the protective effects of SFN against RP were investigated in rd10 mouse model. Our results showed that SFN preserved retinal function with a scheme of early treatment. Our ERG data shows that the cone function of the SFN group in rd10 mice is still lower than those of the WT groups, but the differences were not significant, while the rod function of the SFN group in rd10 mice was still significantly low than those of the WT groups. This difference between rod and cone function is similar to the data observed in our previous study (20), which is related to the earlier degeneration of rod cells than cone cells. (21) Our analysis suggests that SFN may exert its protective effects by reducing cell apoptosis which may be related to the inhibition of endoplasmic reticulum (ER) stress pathways. Apoptosis is the final common pathological pathway in the photoreceptor degeneration of RP.(22) ER is an important intracellular organelle for protein synthesis and folding.(23) Many physiopathological abnormalities such as the expression of mutant protein(24) and redox imbalance(25) would induce the aggregation of unfolded and misfolded proteins in ER, followed by ER stress. ER stress is related to a variety of diseases, including diabetes,(26,27) cancer,(28,29) age-related macular degeneration(30) and neurodegenerative diseases.(31-33) Under normal physiological condition, GRP78/BiP protein combines with ER-localized signal transducers IRE1, PERK and ATF6, which mediate unfolded protein response (UPR) to ER stress.(24) UPR triggers multiple cellular responses, including the increase of the ability of protein folding, the inhibition of protein synthesis, and ER-associated protein degradation of the unfolded or misfolded protein, for the homeostasis of cells. During UPR, GRP78/BiP is separated from IRE1, PERK and ATF6 and activates these three ER stress transducers to ameliorate ER stress. However, when UPR cannot alleviate ER stress by self-regulation, pro-apoptotic mechanisms play the predominant role, which causes apoptosis of cells.(34,35) The present study found that a sustained dose of SFN significantly down-regulated the protein expression of GRP78/BiP in ER in rd10 retina, suggesting that SFN may inhibit ER stress and thereby reduce photoreceptor apoptosis. The data of this study confirm the protective effect of SFN in a relatively early stage of an RP mouse model. Later stage of the RP mouse model can be further studied to confirm the SFN treatment effect.
Acknowledgments
This study was supported by Knights Templar Eye Foundation Pediatric Ophthalmology Grant, Eversight Eye and Vision Research Grant, NIH P30EY025585 and Research to Prevent Blindness. The authors thank Dr. Neal S. Peachey of Department of Ophthalmic Research, Cleveland Clinic Foundation, and Dr Glenn Lobo of Department of Medicine, Medical University of South Carolina, for reviewing the manuscript.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103(30):11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pang JJ, Boye SL, Kumar A, Dinculescu A, Deng W, Li J, Li Q, Rani A, Foster TC, Chang B, Hawes NL, Boatright JH, Hauswirth WW. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci. 2008;49(10):4278–4283. doi: 10.1167/iovs.07-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Y, Zhang Y, Liu X, Ghazaryan E, Li Y, Xie J, Su G. Recent advances of stem cell therapy for retinitis pigmentosa. Int J Mol Sci. 2014;15(8):14456–14474. doi: 10.3390/ijms150814456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng A, Li Y, Tsang SH. Personalized therapeutic strategies for patients with retinitis pigmentosa. Expert Opin Biol Ther. 2015;15(3):391–402. doi: 10.1517/14712598.2015.1006192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12(4):238–249. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007;47(5):624–633. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42(4):517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin ME, Sandberg MA, Berson EL, Dryja TP. Recessive mutations in the gene encoding the beta-subunit of rod phosphodiesterase in patients with retinitis pigmentosa. Nat Genet. 1993;4(2):130–134. doi: 10.1038/ng0693-130. [DOI] [PubMed] [Google Scholar]
- 9.Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P. Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid Med Cell Longev. 2013;2013:415078. doi: 10.1155/2013/415078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ping Z, Liu W, Kang Z, Cai J, Wang Q, Cheng N, Wang S, Wang S, Zhang JH, Sun X. Sulforaphane protects brains against hypoxic-ischemic injury through induction of Nrf2-dependent phase 2 enzyme. Brain Res. 2010;1343:178–185. doi: 10.1016/j.brainres.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Ambrecht LA, Perlman JI, McDonnell JF, Zhai Y, Qiao L, Bu P. Protection of retinal function by sulforaphane following retinal ischemic injury. Exp Eye Res. 2015;138:66–69. doi: 10.1016/j.exer.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Pan H, He M, Liu R, Brecha NC, Yu AC, Pu M. Sulforaphane protects rodent retinas against ischemia-reperfusion injury through the activation of the Nrf2/HO-1 antioxidant pathway. PLoS One. 2014;9(12):e114186. doi: 10.1371/journal.pone.0114186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stasheff SF, Shankar M, Andrews MP. Developmental time course distinguishes changes in spontaneous and light-evoked retinal ganglion cell activity in rd1 and rd10 mice. J Neurophysiol. 2011;105(6):3002–3009. doi: 10.1152/jn.00704.2010. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Fernandez de la Camara C, Hernandez-Pinto AM, Olivares-Gonzalez L, Cuevas-Martin C, Sanchez-Arago M, Hervas D, Salom D, Cuezva JM, de la Rosa EJ, Millan JM, Rodrigo R. Adalimumab Reduces Photoreceptor Cell Death in A Mouse Model of Retinal Degeneration. Sci Rep. 2015;5:11764. doi: 10.1038/srep11764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanlon N, Coldham N, Gielbert A, Kuhnert N, Sauer MJ, King LJ, Ioannides C. Absolute bioavailability and dose-dependent pharmacokinetic behaviour of dietary doses of the chemopreventive isothiocyanate sulforaphane in rat. Br J Nutr. 2008;99(3):559–564. doi: 10.1017/S0007114507824093. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Khor TO, Yang Q, Huang Y, Wu TY, Saw CL, Lin W, Androulakis IP, Kong AN. Pharmacokinetics and pharmacodynamics of phase II drug metabolizing/antioxidant enzymes gene response by anticancer agent sulforaphane in rat lymphocytes. Mol Pharm. 2012;9(10):2819–2827. doi: 10.1021/mp300130k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke JD, Hsu A, Williams DE, Dashwood RH, Stevens JF, Yamamoto M, Ho E. Metabolism and tissue distribution of sulforaphane in Nrf2 knockout and wild-type mice. Pharm Res. 2011;28(12):3171–3179. doi: 10.1007/s11095-011-0500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu M, Sturgill-Short G, Ganapathy P, Tawfik A, Peachey NS, Smith SB. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp Eye Res. 2012;96(1):124–131. doi: 10.1016/j.exer.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M, Kang K, Bu P, Bell BA, Kaul C, Qiao JB, Sturgill-Short G, Yu X, Tarchick MJ, Beight C, Zhang SX, Peachey NS. Deficiency of CC chemokine ligand 2 and decay-accelerating factor causes retinal degeneration in mice. Exp Eye Res. 2015;138:126–133. doi: 10.1016/j.exer.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang K, Tarchick MJ, Yu X, Beight C, Bu P, Yu M. Carnosic acid slows photoreceptor degeneration in the Pde6b(rd10) mouse model of retinitis pigmentosa. Sci Rep. 2016;6:22632. doi: 10.1038/srep22632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piano I, Novelli E, Gasco P, Ghidoni R, Strettoi E, Gargini C. Cone survival and preservation of visual acuity in an animal model of retinal degeneration. Eur J Neurosci. 2013;37(11):1853–1862. doi: 10.1111/ejn.12196. [DOI] [PubMed] [Google Scholar]
- 22.Chang GQ, Hao Y, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11(4):595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 23.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 24.Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16(6):653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maity S, Basak T, Bhat A, Bhasin N, Ghosh A, Chakraborty K, Sengupta S. Cross-compartment proteostasis regulation during redox imbalance induced ER stress. Proteomics. 2014;14(15):1724–1736. doi: 10.1002/pmic.201300449. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009;583(9):1521–1527. doi: 10.1016/j.febslet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang SX, Sanders E, Wang JJ. Endoplasmic reticulum stress and inflammation: mechanisms and implications in diabetic retinopathy. J Ocul Biol Dis Infor. 2011;4(1-2):51–61. doi: 10.1007/s12177-011-9075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav RK, Chae SW, Kim HR, Chae HJ. Endoplasmic reticulum stress and cancer. J Cancer Prev. 2014;19(2):75–88. doi: 10.15430/JCP.2014.19.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke HJ, Chambers JE, Liniker E, Marciniak SJ. Endoplasmic reticulum stress in malignancy. Cancer Cell. 2014;25(5):563–573. doi: 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Libby RT, Gould DB. Endoplasmic reticulum stress as a primary pathogenic mechanism leading to age-related macular degeneration. Adv Exp Med Biol. 2010;664:403–409. doi: 10.1007/978-1-4419-1399-9_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13(3):385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 32.Matus S, Glimcher LH, Hetz C. Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Curr Opin Cell Biol. 2011;23(2):239–252. doi: 10.1016/j.ceb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Torres M, Matamala JM, Duran-Aniotz C, Cornejo VH, Foley A, Hetz C. ER stress signaling and neurodegeneration: At the intersection between Alzheimer's disease and Prion-related disorders. Virus Res. 2015;207:69–75. doi: 10.1016/j.virusres.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rana T, Shinde VM, Starr CR, Kruglov AA, Boitet ER, Kotla P, Zolotukhin S, Gross AK, Gorbatyuk MS. An activated unfolded protein response promotes retinal degeneration and triggers an inflammatory response in the mouse retina. Cell Death Dis. 2014;5:e1578. doi: 10.1038/cddis.2014.539. [DOI] [PMC free article] [PubMed] [Google Scholar]


