Abstract
Rates of H2O2 production by tobacco suspension cells inoculated with zoospores from compatible or incompatible races of the pathogen Phytophthora nicotianae were followed by direct measurement of oxygen evolution from culture supernatants following catalase addition. Rates of HO2./O2− production were compared by following the formation of the formazan of sodium, 3′-[1-[phenylamino-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate. In the incompatible interaction only, both reactive oxygen species (ROS) were produced by the cultured host cells in a minor burst between 0 and 2 h and then in a major burst between 8 and 12 h after inoculation. Absolute levels of H2O2 could not be accurately measured due to its metabolism by host cells, but results are consistent with the majority of H2O2 being formed via dismutation of HO2./O2−. The effects of inhibitors of endogenous Cu/Zn superoxide dismutase (diethyldithiocarbamate) and catalase (3-amino-1,2,4-triazole and salicylic acid) were also examined. Yields of ROS in the presence of the inhibitors diphenylene iodonium, allopurinol, and salicylhydroxamic acid suggest that ROS were generated in incompatible host responses by more than one mechanism.
Reactive oxygen species (ROS), in particular the superoxide anion (O2−), its conjugate acid, the perhydroxyl radical (HO2.), and their dismutation product hydrogen peroxide (H2O2) are produced in one or more bursts of oxidative activity during resistance expression in a wide range of host/pathogen interactions and have been implicated in stimulation of the hypersensitive response (HR) (Sutherland, 1991; Wojtaszek, 1997; Heath, 1998). The source of the oxidative burst(s) during host disease resistance responses in a number of plant-pathogen systems has been proposed to be an NAD(P)H oxidase (for review, see Low and Dwyer, 1994; Higgins et al., 1998; Bolwell, 1999). However, other research has indicated the possible involvement of xanthine oxidases (Montalbini and Della Torre, 1996) and peroxidases (Bolwell et al., 1998).
A corollary of the debate concerning the source of ROS is the question of whether H2O2 is generated via a HO2./O2− -dependent or -independent pathway. In an earlier study we estimated yields of HO2./O2− production during the incompatible responses of tobacco cells toward zoospores of the Oomycete pathogen Phytophthora nicotianae (Pn) (previously referred to as Phytophthora parasitica var nicotianae) (Able et al., 1998). However, this work did not monitor the generation of H2O2 during these responses.
In comparison with HO2./O2−, H2O2 is stable in aqueous solution at neutral pH. As a result of this stability, its presence is relatively easy to detect, and the majority of studies into the oxidative burst have concentrated on its detection (for review, see Baker and Orlandi, 1995; Low and Merida, 1996; Mehdy et al., 1996; Wojtaszek, 1997). Cytochemical studies have used the reaction of H2O2 with either CeCl2 (Czaninski et al., 1993; Bestwick et al., 1997) or 3,3-diaminobenzidine in the presence of peroxidase (Schroeder et al., 1996; Thordal-Christensen et al., 1997) to visualize sites of production using electron microscopy. However, these methods are unsuitable for either quantification or time-course studies (Schroeder et al., 1996), and 3,3-diaminobenzidine also reacts with HO2./O2− (Steinbeck et al., 1993).
Quantification of H2O2 has been based on either the quenching of chemiluminescent dyes (Lindner et al., 1988) or the reaction of fluorescent dyes with H2O2, resulting in either a gain or loss of fluorescence (Low and Heinstein, 1986; Levine et al., 1994). Potential problems with these techniques include a dependence upon peroxidases to detect H2O2 and the effect of variations in peroxidase concentration on the strength of fluorescence or light emissions. These techniques also lack absolute specificity for H2O2 (Yoshiki et al., 1996).
In this current study, we have measured H2O2 production by two methods. The first of these measures the loss of fluorescence by the reporter dye pyranine (Legendre et al., 1993), whereas the second method is based on the use of a Clark-type oxygen electrode. This electrode has been widely used in studies of respiration and photosynthesis (Trudgill, 1985; Halliwell and Gutteridge, 1999) and in studies of oxygen consumption during the oxidative burst in Pn-infected tobacco seedlings (Guest et al., 1989). The method as we have applied it directly measures the evolution of oxygen from H2O2 after the addition of catalase (CAT) to culture supernatants.
We are now able to report comparative yields of H2O2 and HO2./O2− in tobacco cell cultures challenged with zoospores from an incompatible race of Pn. Furthermore, putative-specific inhibitors of potential sources of these ROS have been evaluated for their ability to alter ROS yields in an attempt to identify the major production pathways during the HR.
RESULTS
H2O2 Production Measured Using Fluorescence
At 18 h after inoculation, supernatant from inoculated tobacco cells decreased the amount of fluorescence observed in the presence of pyranine. This decrease was not inhibitable by prior addition of CAT, indicating that H2O2 was not responsible for this loss of fluorescence. Supernatants from both uninoculated cells and inoculated (compatible and incompatible) cells significantly lowered fluorescence to levels below those observed in pyranine controls to which only buffer was added (30.2 fluorescence units). Supernatants from inoculated cells gave a relatively low emission in the absence of pyranine (results not shown).
H2O2 was then added to cells to determine whether the cells were capable of consuming H2O2. Eighteen hours after the addition of 100 μm H2O2 to tobacco cells, its presence was not detectable in culture supernatants. When 1 mm salicylhydroxamic acid (SHAM) was added at the same time as the H2O2 (to inhibit peroxidases), 18 h after addition some H2O2 was detected (a loss of approximately 5 fluorescence units). However, this loss of fluorescence is relatively small when compared with the loss of 25 units that occurs when 100 μm H2O2 is added directly to pyranine in supernatant in the absence of SHAM and measured immediately. This result indicates that while cell peroxidases contributed to H2O2 metabolism by the cells, other SHAM-independent processes were also involved. In addition, 1 mm SHAM interacted directly with pyranine reducing its fluorescence by approximately 2 units. While CAT and superoxide dismutase (SOD) did not react with pyranine directly, Mn(III)desferal progressively destroyed pyranine fluorescence and could not be used in fluorescence experiments.
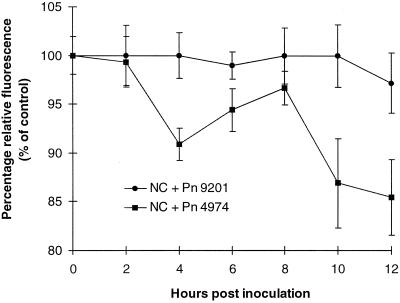
Since all interactions decreased the fluorescence of pyranine over time, relative fluorescence at each time point was determined using the fluorescence of control cells for that time point. Although relative fluorescence was unchanged after 12-h post inoculation in all interactions, a significant decrease in relative fluorescence did occur with supernatants from the incompatible interaction harvested between 2 and 4 h and between 8 and 10 h after zoospore addition. In contrast, little or no fluorescence loss occurred in compatible interactions (Fig. 1). In these experiments, CAT added at 0 h significantly prevented loss of fluorescence while SOD added at this time had no effect.
Figure 1.
Percentage relative pyranine fluorescence supernatant of inoculated cells as a measure of H2O2 production. Pyranine (10 μg mL−1) was added to the supernatant of tobacco cells at the time of sampling the supernatant. Relative fluorescence of the supernatants of cv ‘North Carolina’ (NC) cells inoculated with Pn 9201 (compatible) or Pn 4974 (incompatible) zoospores was determined by dividing by the fluorescence observed in unchallenged control treatments at the same time point. Data represent means ± se of n = 6 from three experiments.
To determine the amount of H2O2 detected in culture supernatants a calibration curve was produced with a line of best fit calculated (y = 29.6 − 0.73x with r = −0.94). On this basis, during the incompatible interaction and at 10-h post inoculation, approximately 10 nmol H2O2 0.1 g−1 cells was present.
Measurement of H2O2 Using an Oxygen Electrode
After 18 h, very little H2O2 was detectable in any interaction when using the Clark-type oxygen electrode. When 90 nmol of H2O2 was added to 0.1 g of unchallenged cells, it was metabolized by the cells within 4 h. Twenty-five nanomoles of H2O2 was metabolized within 30 min. One mm SHAM did not alter this consumption of H2O2.
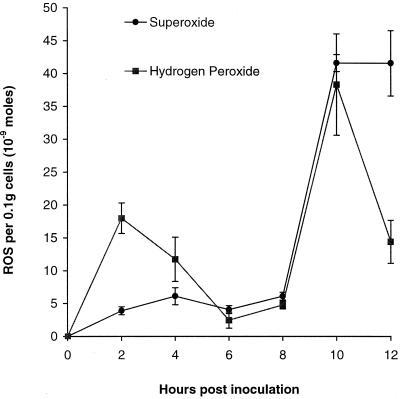
Despite the fact that H2O2 was being metabolized by the cells, the rate of synthesis by incompatibly responding cells was high enough such that the levels of H2O2 present at different times after zoospore challenge indicated relative rates of production (Fig. 2). As was the case with the fluorescence measurements, H2O2 was not detected at any time in control treatments or during compatible interactions. H2O2 was produced in the incompatible interaction in two bursts between 0 and 2 h and again between 8 and 10 h. At 10-h post inoculation, 38.4 ± 7.7 × 10−9 mol H2O2 0.1 g−1 cells was detected (approximately 4 times the levels estimated using the loss of pyranine fluorescence). These patterns of production are consistent with those seen for HO2./O2− generation under these experimental conditions (Fig. 2; Able et al., 1998). At 10-h post inoculation, 41.7 ± 1.3 × 10−9 mol HO2./O2− 0.1 g−1 cells was present.
Figure 2.
ROS production during the incompatible interaction. HO2./O2− was detected using Mn(III)desferal-inhibitable XTT reduction and H2O2 estimated using an oxygen electrode. Data represent means ± se of n = 18 from six experiments for HO2./O2− and n = 8 from three experiments for H2O2 data.
Effect of Scavengers on ROS Production and the HR
In the incompatible interaction, the addition of SOD significantly increased the amount of H2O2 detected at 10 h from 38.4 ± 7.7 × 10−9 mol H2O2 0.1 g−1 cells to 50.0 ± 6.1 × 10−9 mol of H2O2 whereas Mn(III)desferal significantly lowered the level detected to 18.0 ± 4.4 × 10−9 mol H2O2 0.1 g−1 cells. The addition of CAT to challenged cells at 0 h completely prevented accumulation of H2O2 whereas SOD and Mn(III)desferal inhibited production of HO2./O2− (Able et al., 1998).
Sodium,3′-[1-[phenylamino-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene-sulfonic acid hydrate (XTT, 5 × 10−4 m) was added to the supernatant from incompatible cells at 10-h post inoculation to determine whether any HO2./O2− was still present. No significant reduction of XTT occurred indicating that, as expected, none of the transient HO2./O2− remained.
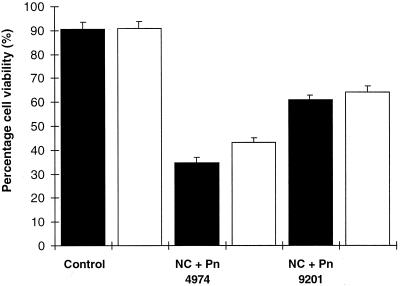
Whereas CAT significantly improved the viability of cells undergoing an HR (Fig. 3), it did not improve viability to the same degree as the HO2./O2− scavengers did. SOD and Mn(III)desferal maintained cell viability in the incompatible interaction at levels not significantly different from a compatible interaction (Able et al., 1998). To establish further whether H2O2 plays a role in the HR, H2O2 was added to unchallenged cells. After 6 h of exposure to 100 μm H2O2 the viability of cells (0.1 g) in the wells had decreased to values similar to those observed in a compatible interaction (55.4% ± 2.8%, n = 8). The addition of 2 mm H2O2 only decreased cell viability to 47.1% ± 3.4%. However, at a lower concentration of cells the effect of 100 μm H2O2 on viability was more severe with 0.01 g of cells maintaining a viability of only 24.15% ± 2.39% (n = 4) after 6 h of treatment. The addition of 1 mm H2O2 to 0.01 g of cells decreased cell viability to 12.58% ± 1.90%.
Figure 3.
The effect of CAT on viability of cells. Viability of cv NC2326 control cells and cv NC2326 cells inoculated with zoospores from Pn 4974 (incompatible) or Pn 9201 (compatible) was determined after 12 h. White bars represent the addition of 400 units of CAT. Black bars indicate the absence of CAT. Data represent means ± se of n = 8 from three experiments.
CAT had no effect on XTT reduction (results not shown). This indicates that H2O2 neither contributes to the reduction of XTT nor has any effect on the production of HO2./O2− during the incompatible interaction.
The Effects of CAT Inhibition
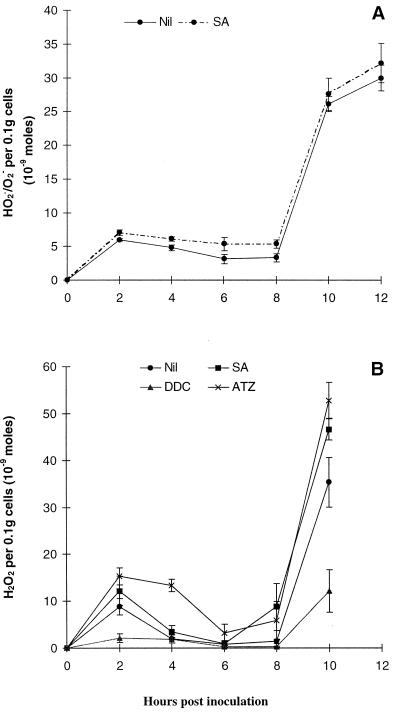
There was no significant effect of 1 mm salicylic acid (SA) on the HR. Although SA decreased the viability of cells by 12 h from 61.9% ± 1.1% to 57.3% ± 1.4% during the incompatible interaction, a similar effect was observed in the controls where the viability was reduced from 89.2% ± 0.6% to 84.7% ± 0.8% in the presence of SA. The addition of 1 mm SA had minimal effect on HO2./O2− production in the incompatible interaction (Fig. 4A), however, there was a moderate but significant increase in H2O2 (Fig. 4B). This may reflect the inhibition by SA of endogenous CAT activity thus increasing the steady-state concentration of H2O2.
Figure 4.
The effect of inhibitors of endogenous CAT and SOD on ROS production during the incompatible interaction. The effect of 1 mm SA on HO2./O2− (A) and H2O2 (B) production and of 1 mm ATZ and 1 mm DDC on H2O2 production (B) was monitored. Data represent means ± se of n = 9 from two experiments for SA data and n = 6 from two experiments for ATZ and DDC results.
Addition of an alternative CAT inhibitor, 3-amino-1,2,4-triazole (ATZ), yielded similar results. One millimolar ATZ did not affect the reduction of XTT by challenged cells or the level of the HR (results not shown). However, 1 mm ATZ slightly increased H2O2 production at 2-, 4-, and 10-h post inoculation in the incompatible interaction (Fig. 4B).
SOD Inhibition by Diethyldithiocarbamate
When the SOD inhibitor diethyldithiocarbamate (DDC) was added to incompatible cells at the time of zoospore addition, the two bursts of H2O2 detected using the oxygen electrode were significantly reduced (Fig. 4B). There was no increase in H2O2 detection in control or compatible treatments.
Background reduction of XTT by unchallenged control cells was between 0.692 and 0.998 absorbance units in the presence of 1 mm DDC after 18 h, suggesting a direct reaction between XTT and DDC. This was confirmed by experiments conducted in the absence of host cells. When the incompatible interaction was carried out in the presence of DDC, the absorbance of reduced XTT after 18 h was usually greater than 3.0 units. To determine whether this significant increase in reduction of XTT in the presence of DDC was due to an increase in available HO2./O2− due to endogenous Cu/ZnSOD inhibition, 100 units of MnSOD, which is not inhibited by DDC, was added. However, MnSOD in the presence of DDC had no effect on the reduction of XTT in background control cells or in the incompatible interaction. The activity of MnSOD was confirmed using the xanthine/xanthine oxidase assay (Faulkner et al., 1994) and by its ability to inhibit XTT reduction in the incompatible interaction in the absence of DDC.
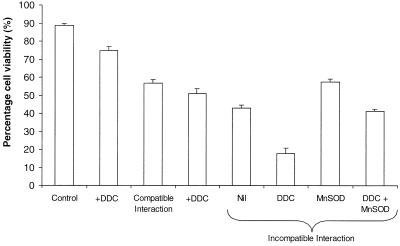
Mn(III)desferal could not be added in the presence of DDC as it reacted directly with DDC to form a reddish-brown precipitate. DDC (1 mm) significantly decreased the viability of cells in all interactions and the controls (between 5.8% and 13.9%). MnSOD significantly increased cell viability in the incompatible interaction when DDC was present. However, this improved viability was not significantly different from the viability of cells in the incompatible interaction without DDC and was significantly lower than the viability of incompatible cells (without DDC) in the presence of MnSOD (Fig. 5).
Figure 5.
The effect of Cu/Zn SOD inhibition on the viability of challenged cells. DDC (1 mm) and/or MnSOD (100 units) was added at time of zoospore (or water) addition, and the viability of cells assayed at 18-h post inoculation. Data represent means ± se of n = 6 from two experiments.
DDC did not directly react with nitroblue tetrazolium (NBT) in the absence of cells and so the localization of NBT reduction provided the opportunity to study the effects of DDC more thoroughly. When 1 mm DDC was added to control cells and to the incompatible interaction, NBT formazan (insoluble) was formed within the cytoplasm of cells in both treatments very rapidly such that it was difficult to distinguish between them. The NBT formazan was a light purple with patches of dark blue within the cytoplasm in lines leading from the inner surface of the cell membrane in all interactions. MnSOD did not inhibit this reduction of NBT in the cytoplasm.
Sources of ROS Generation during the Incompatible Interaction
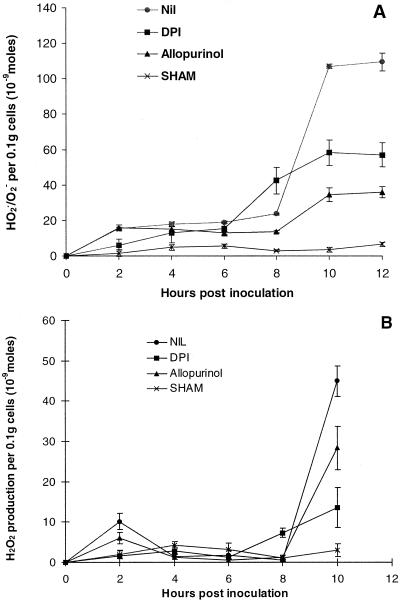
Diphenyleneiodonium (DPI), allopurinol, and SHAM were added to cell cultures during zoospore challenge to examine the role of NAD(P)H oxidases, xanthine oxidases, and peroxidases, respectively in HO2./O2− production and the HR. The concentrations shown in Figure 6 are the lowest at which maximal effects were observed (results not shown).
Figure 6.
ROS production during an incompatible interaction in the presence of possible source enzyme inhibitors. Twenty micromolar DPI, 500 μm allopurinol, or 2 mm SHAM was added at 0 h and HO2./O2− production (A) and H2O2 production (B) monitored over 12 and 10 h, respectively. Data represent means ± se of n = 9 from three experiments for A and of n = 8 from three experiments for B.
Inhibition of Possible Source Enzymes
During an incompatible response, while the addition of 20 μm DPI completely inhibited the first burst of ROS, the second burst was only partially inhibited (Fig. 6, A and B). The second burst also occurred earlier in the presence of DPI, between 6 and 10 h after inoculation. In the presence of 500 μm allopurinol, two bursts of HO2./O2− production still occurred in the incompatible interaction (Fig. 6A). However, the second burst was significantly reduced (approximately 50% of that observed in the absence of allopurinol). Allopurinol did however, partially inhibit both bursts of H2O2 production (Fig. 6B). SHAM (2 mm) completely inhibited both bursts of ROS production (Fig. 6, A and B). When SHAM was added to cells a yellow product formed within 2 h. Spectra of this product revealed that it interfered with the 470-nm peak of the XTT formazan. As it occurred in all interactions, the high backgrounds were accounted for by subtracting appropriate control treatment values when determining HO2./O2− production.
The level of inhibition of XTT reduction after 12 h was the same, irrespective of whether DPI, allopurinol, or SHAM was added at the time of inoculation or 8 h later, just before the second HO2./O2− burst (results not shown). However, HO2./O2− production was unaffected by DPI, allopurinol, or SHAM if added after the second burst (at 10 h after inoculation). In the presence of DPI or SHAM, added at the time of inoculation, hyphal growth in the wells was observed to be more extensive than in the absence of these inhibitors in the incompatible interaction.
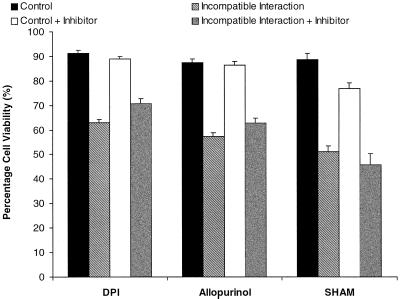
At 12 h after inoculation, DPI and allopurinol added at the time of zoospore addition had no significant effect on the viability of compatible cells or control cells yet significantly suppressed the HR (Fig. 7). When either SOD or Mn(III)desferal were added to the DPI or allopurinol-treated cells, there was no increase in protection. If DPI and allopurinol were added to incompatibly-challenged cells immediately before the second burst of HO2./O2− production (results not shown), their protective effect against the HR was only slightly diminished. Neither DPI or allopurinol significantly affected the HR when added after the second HO2./O2− burst (at 10-h post inoculation). In contrast SHAM did not reduce the HR (Fig. 7). However, SHAM decreased the viability of control (Fig. 7) and compatible cells (data not shown), suggesting some non-specific effects.
Figure 7.
The effect of possible source enzyme inhibitors on cell viability. Twenty micromolar DPI, 500 μm allopurinol, or 2 mm SHAM was added at 0 h and cell viability measured at 12 h post inoculation. Data represent means ± se of n = 8 from three experiments.
All three of the inhibitors were added to the xanthine/xanthine oxidase assay (Faulkner et al., 1994; Sutherland and Learmonth, 1997) to determine their ability to inhibit xanthine oxidase. Allopurinol inhibited uric acid formation as expected, whereas SHAM had no significant effect. When DPI (<1 μm) was added both cytochrome c (Cyt c) and XTT reduction were inhibited by more than 95%. Similar levels of inhibition of uric acid formation were also observed under these conditions indicating that DPI directly inhibits the activity of xanthine oxidase. As a result of this inhibitory ability, 20 μm DPI and 500 μm allopurinol were added simultaneously to an incompatible interaction and effects on HO2./O2− production and cell viability determined (Table I). DPI inhibited HO2./O2− production during the incompatible interaction to a greater extent than allopurinol but when both inhibitors were added together, the effect was not additive. HO2./O2− production occurred at the same level as when only DPI was added. In addition, there was no additive effect of DPI and allopurinol on the HR. Viability levels were similar to those observed when only DPI was present (Table I).
Table I.
The effect of the addition of allopurinol and DPI on ROS production and the HR
| Treatment | HO2./O2− | Cell Viability |
|---|---|---|
| 10−9 mol 0.1 g−1 cells | % | |
| Control | ||
| Nil | – | 87.1 ± 0.7 |
| 20 μm DPI | – | 82.2 ± 3.2 |
| 500 μm allopurinol | – | 83.9 ± 0.7 |
| 500 μm allopurinol + 20 μm DPI | – | 82.4 ± 0.7 |
| Incompatible interaction | ||
| Nil | 68.7 ± 10.9 | 38.6 ± 0.8 |
| 20 μm DPI | 7.78 ± 1.6 | 48.7 ± 1 |
| 500 μm allopurinol | 24.07 ± 6.8 | 45.7 ± 2.6 |
| 500 μm allopurinol + 20 μm DPI | 6.67 ± 1.5 | 48.8 ± 0.7 |
Five-hundred micromolar allopurinol and 20 μm DPI were added at the time of Pn 4974 zoospore (incompatible) or water (control) addition to NC cells. Data represent means ± se of n = 8 from two experiments.
Effects of Exogenous NADP+/NADPH
Exogenous NADP+ (1 mm) significantly lowered the HO2./O2− production in an incompatible interaction by approximately 50% (Table II) while having no significant effect on control or compatible cells. NADP+ also inhibited H2O2 production and slightly suppressed the HR. SOD and Mn(III)desferal significantly suppressed the HR in the presence of NADP+ (increasing cell viability from 44.6% ± 1.5% to 56.4% ± 1.0% in the presence of Mn(III)desferal).
Table II.
The effect of NADP+ and NADPH addition on ROS production and the HR
| ROS Production in the Incompatible Interaction
|
Percentage Cell Viability
|
|||||
|---|---|---|---|---|---|---|
| HO2./O2−a
|
H2O2b | Control | Incompatible interaction | |||
| XTT | Cyt c | |||||
| 10−9 mol 0.1 g−1 cells | % | |||||
| ±NADP+ (1 mm) | Nil | 48.0 ± 9.1 | – | 34.8 ± 3 | 88.7 ± 1.2 | 39.0 ± 1.2 |
| NADP+ | 24.6 ± 4.9 | – | 15.2 ± 1.1 | 80.9 ± 0.6 | 44.6 ± 1.5 | |
| ±NADPH (1 mm) | Nil | — | 14.6 ± 1.2 | 34.8 ± 3 | 84.6 ± 1.8 | 42.5 ± 0.8 |
| NADPH | — | 19.7 ± 0.7 | 67.3 ± 3.8 | 87.0 ± 3.5 | 32.9 ± 2.4 | |
One millimolar NADP+ or 1 mM NADPH was added at the time of Pn 4974 zoospore (incompatible) or water (control) addition to NC cells. Data represent means ± se of n = 12 from three experiments.
Determined from Mn(III)desferal inhibitable XTT or Cyt c reduction.
Based on O2 evolution.
The effect of exogenous NADPH (1 mm) on HO2./O2− production was difficult to measure due to a slow, direct reduction of XTT by this level of NADPH in the absence of cells. As Cyt c appeared to be much less susceptible to this direct reduction, Cyt c inhibition by SOD was used as an indicator of HO2./O2− production in the presence of exogenous NADPH. It must be noted, however, that as Cyt c is a much less sensitive assay (Able et al., 1998), the levels of radical measured are much lower than those that could be potentially detected when using XTT. The presence of NADPH in the incompatible interaction did not significantly alter the Cyt c reduction observed nor did it alter viability in control or susceptible cells. However, exogenous NADPH significantly decreased cell viability in the incompatible interaction (Table II). SOD and Mn(III)desferal significantly suppressed the HR in the presence of NADPH [increasing viability from 32.9% ± 2.4% to 57.1% ± 0.7%, n = 12 in the presence of Mn(III)desferal]. One millimolar NADPH significantly increased the amount of H2O2 detected at 10-h post inoculation in the incompatible interaction (Table II).
DISCUSSION
Dynamics of H2O2 and HO2./O2− Production
H2O2 was produced in a burst between 0 and 2 h and in a second more intensive burst between 8- and 10-h post inoculation in resistant tobacco cells inoculated with an incompatible race of Pn. These trends reflect those observed when HO2./O2− production was followed (Fig. 2; Able et al., 1998).
Two methods were used to measure H2O2 in this system. The loss or gain of fluorescence of a reporter dye has been widely used for the detection of H2O2 in plant-pathogen systems (Low and Heinstein, 1986; Legendre et al., 1993; Levine et al., 1994), whereas the detection of H2O2 by the evolution of oxygen after the addition of CAT is often used in respiration and photosynthesis studies (Trudgill, 1985; Halliwell and Gutteridge, 1999). Clark-type oxygen electrodes have previously been used to measure total oxygen consumption in plant-pathogen systems (Guest et al., 1989; Vera-Estrella et al., 1992, 1993), while CAT has been used as an indicator of whether H2O2 is a component of this oxygen consumption (Doke and Miura, 1995). To our knowledge, an oxygen electrode has not previously been used in plant pathological studies to quantify H2O2 production.
The two methods give different estimates of H2O2 production. The fluorescence method detected less than half the amount detected using the oxygen electrode and produced higher variability between replicates. Some studies have used fluorescence-based assays in the presence of stirred cells (Low and Heinstein, 1986; Legendre et al., 1993; Levine et al., 1994), which may introduce additional artifacts such as mechanically induced responses (Yahraus et al., 1995) or direct interference with the fluorescence signal. In this study only culture supernatants were assayed in order to avoid these possibilities. Based on its greater sensitivity, reduced opportunity for artifacts and lower relative mean ses, the oxygen electrode-based assay appears more suitable for measurement of H2O2 in the tobacco/Pn interaction than does the fluorescence assay.
The modest but significant increase in H2O2 production detected by the oxygen electrode in the presence of SOD suggests that normally not all HO2./O2− is dismutated to H2O2 but that some is consumed by other processes in competition with spontaneous dismutation. The decreases in H2O2 detected in the presence of Mn(III)desferal support the theory that Mn(III)desferal does not act by dismutating HO2./O2− to H2O2 and O2 (Beyer and Fridovich, 1989). When H2O2 production was measured using the fluorescence based assay, SOD had no effect. We note that in several other studies based on fluorescence detection, the presence of SOD did increase the amount of H2O2 detected (Murphy and Auh, 1992; Levine et al., 1994).
Although H2O2 may be the dismutation product of HO2./O2−, direct measurement of HO2./O2− is the only true indicator of its involvement in disease resistance. We previously developed a sensitive, specific, and quantitative assay for HO2./O2−, which has been applied to cultured tobacco cells challenged with Pn zoospores (Able et al., 1998). Estimates of HO2./O2− at the height of the oxidative burst (9.25 × 10−9 mol HO2./O2− min−1 mg−1 protein or 1.18 × 10−14 mol HO2./O2− cell−1 min−1) were somewhat higher but within the same order of magnitude as measured by other authors (Moreau and Osman, 1989 [a correction of Doke, 1983a, 1983b]; Ivanova et al., 1991). Due to the nature of the assay system and the metabolism of H2O2 by cells, an estimate of the total quantities of H2O2 produced during the 18-h post inoculation period could not be obtained. Steady-state levels of H2O2 fluctuated throughout the experiment reflecting the balance between prevailing rates of production and metabolism. At the height of the defense response of incompatible cells (8- to 10-h post inoculation), the net rate of production was 1.04 × 10−14 mol H2O2 cell−1 min−1 (averaged over this period) as measured using the oxygen electrode. These levels are slightly lower but broadly comparable with those observed in soybean cells elicited with poly-galacturonic acid, where at the height of the defense response, the production of 3 × 10−14 mol of H2O2 cell−1 min−1 was measured using pyranine (Legendre et al., 1993).
Using the XTT assay, 1.18 × 10−14 mol HO2./O2− cell−1 min−1 were produced at the height of the defense response in tobacco cells challenged with incompatible zoospores. Assuming virtually all HO2./O2− detected in the XTT assay was dismutated to H2O2, then at the height of the defense response 0.59 × 10−14 mol H2O2 cell−1 min−1 would be produced. This represents approximately half the amount of H2O2 detected using the oxygen electrode. There are two conclusions to be considered. First, given the errors involved in making these estimates by quite different methodologies, it could be concluded that these results are entirely consistent with the hypothesis that all the H2O2 formed is the result of HO2./O2− dismutation. However, since cells are actively breaking down H2O2, the true level of H2O2 generated by the burst will always be higher than that estimated (which represents the steady state). Thus, an alternative conclusion is that levels of H2O2 are not being fully accounted for by dismutation of the HO2./O2− as estimated by the XTT assay. First, XTT may not scavenge all radical produced because some of the radical might dismutate to H2O2 before contact with XTT. Second, a proportion of XTT formazan is accumulated by the host cells and is not harvested in the supernatant. The third possibility is that there exists an alternative, independent pathway for H2O2 production. This is supported by the inability of Mn(III)desferal to completely inhibit H2O2 generation. The significantly larger quantities of H2O2 (relative to HO2./O2−) produced during the first 2-h post inoculation (Fig. 2) would also suggest that an alternate pathway for H2O2 production might be present. Nevertheless, the evidence supports the conclusion that at least the major pathway for H2O2 production is via HO2./O2− dismutation.
CATs
SA is known to be involved in the induction of systemic acquired resistance (Ryals et al., 1995) and possibly the HR (Shirasu et al., 1997). However, the mode of action of SA and its relationship to H2O2 production during disease resistance have not been clearly established. After detailed analyses of inhibition by SA of tobacco CATs at levels of H2O2 observed during defense responses (<1 μm), Durner and Klessig (1996) suggested that H2O2 increases are downstream of SA induction in the chain of events and are a result of the inhibition of CAT. In contrast, Bi and colleagues (1995) reported that although SA levels increased, no changes to CAT activity occurred in tobacco plants inoculated with Pseudomonas syringae. H2O2 has been reported to act upstream of SA (Neuenschwander et al., 1995) in tobacco plants expressing systemic acquired resistance, whereas SA has also been reported to have no involvement in H2O2 production whatsoever in tissues of tobacco plants expressing systemic acquired resistance against tobacco mosaic virus (Ryals et al., 1995). In our study, the increase of H2O2 production by tobacco cells challenged by incompatible Pn zoospores in the presence of SA suggests that SA may have inhibited endogenous CATs as proposed by Durner and Klessig (1996). SA has also been shown to increase SOD levels within 2 h of treatment in planta (Rao et al., 1997), and therefore an increase in H2O2 due to increased SOD activity might be expected.
Consistent with these results, the addition of the CAT inhibitor ATZ also moderately increased the detected quantities of H2O2. Notably, these increased levels of H2O2 were not accompanied by any change to HO2./O2− yield or cell viabilities under incompatible challenge.
SODs
The Cu/ZnSOD inhibitor, DDC, increased reduction of NBT and XTT in all interactions by a process insensitive to the addition of MnSOD. This suggests that DDC interacts with other redox elements in cells (in addition to its inhibition of Cu/ZnSOD) and that these interactions led to a HO2./O2−-independent reduction of NBT and XTT. However, it must be noted that MnSOD may not have been an adequate substitute for endogenous Cu/ZnSOD due to its inability to penetrate the cell wall and access either the plasmalemma or intracellular sites of HO2./O2− generation.
DDC did lower the amount of H2O2 accumulated in tobacco cells inoculated with incompatible zoospores of Pn. This result is consistent with findings in other interactions (Levine et al., 1994; Auh and Murphy, 1995) and suggests that endogenous SOD significantly increases the yield of H2O2 to levels above that which would result from spontaneous dismutation of HO2./O2− alone. The decrease in H2O2 in the presence of DDC supports the hypothesis that other processes in the cell (which consume HO2./O2−) effectively compete with the spontaneous dismutation reaction in the absence of active SOD. The concomitant increase in the level of HR implies that these other processes may be more important in inducing the HR than the production of H2O2 itself.
H2O2 Does Not Play a Major Direct Role in the HR
The addition of CAT to the system significantly increased the viability of cells but not to the same extent as the HO2./O2− scavengers [SOD and Mn(III)desferal]. Therefore, H2O2 appears to be at least partially involved in the HR. Although in soybean cells inoculated with Pseudomonas or treated with high concentrations of H2O2 (Levine et al., 1994) and in resistant tobacco plants inoculated with tobacco mosaic virus (Doke and Ohashi, 1988), H2O2 appeared responsible for an intensive HR, this does not appear to be the case in the tobacco-Pn system. Furthermore when an H2O2 burst was simulated in tobacco suspension cells using Glc oxidase, cell death was not induced (Dorey et al., 1999). H2O2 alone only had a significant effect when we added it to dilute suspensions of cells, probably as a result of their decreased collective scavenging capabilities (Baker and Orlandi, 1995). Furthermore, the concentrations of H2O2 generated by cells during the incompatible response were much lower than the levels of exogenous H2O2 required for cell death. In this study, the addition of 2 mm H2O2 was required to decrease cell viability by approximately 40%, yet less than picomolar levels of H2O2 are produced during the incompatible interaction and immediately before the HR. It is very unlikely that direct cellular damage by H2O2 is responsible for the HR in tobacco cells. This conclusion does not rule out the possibility that H2O2 is involved in the HR indirectly, via a signaling function that is only effective in concert with other cellular events specific to the host-pathogen interaction.
Sources of ROS in Resistance
Various enzyme systems may be responsible for the production of ROS during a disease resistance response. Many studies suggest that a membrane-bound NAD(P)H oxidase similar to that found in mammalian defense systems is responsible for the oxidative burst in plant pathogen systems (Doke, 1985; Levine et al., 1994; Auh and Murphy, 1995; Dwyer et al., 1996). Roles for cell wall peroxidases (Vera-Estrella et al., 1992; Bolwell et al., 1998) and purine oxidases (Montalbini and Della Torre, 1996) in the production of HO2./O2− during a defense response have also been proposed. Results within the Pn-tobacco system suggest that HO2./O2− is not produced by one enzyme but by a combination of the above. Inhibition of HO2./O2− production by SHAM would suggest a role for peroxidases. Inhibition rates of HO2./O2− by DPI and allopurinol support a major role for NAD(P)H oxidase and xanthine oxidases. Xanthine oxidases were recently reported to act as NAD(P)H oxidases (Harris and Massey, 1997).
The results of inhibitor studies such as these must be interpreted with considerable caution. DPI, used in this study to inhibit NAD(P)H oxidases, in our laboratory also inhibited the formation of uric acid by xanthine oxidase and may also inhibit peroxidases (Dème et al., 1994) and nitric oxide synthases (Wever et al., 1997). The observation that DPI and allopurinol did not have additive effects (Table I) indicates that DPI alone was able to efficiently inhibit both NAD(P)H-dependent and xanthine oxidases. Furthermore, DPI has recently been shown to inhibit SA accumulation in cells by an ROS-independent mechanism (Dorey et al., 1999).
SHAM, an inhibitor of cell wall-bound peroxidases (Van der Werf et al., 1991), also inhibits the alternative oxidase (Diethelm et al., 1990), xanthine oxidases (Rich et al., 1978) and lipoxygenase (Macri et al., 1995). However, in our laboratory SHAM did not inhibit uric acid formation by xanthine oxidase. SHAM may also increase the activity of NAD(P)H oxidase (Askerlund et al., 1987), esterases (Hsiao and Bornman, 1993), and some peroxidases (Bingham and Stevenson, 1995), although peroxidase activation by SHAM usually occurs only in illuminated green tissue (Diethelm et al., 1990). However, based on this logic, if the increased activity of these enzymes were responsible for HO2./O2− production in this system, HO2./O2− production might have increased in the presence of SHAM. The decomposition of SHAM by tobacco cells to an unknown product requires further investigation. The product of this decomposition could conceivably be responsible for some of the effects observed.
Several studies have claimed a requirement for NAD(P)H for HO2./O2− production to occur in tissue-cultured plant-pathogen systems (Doke and Chai, 1985; Vera-Estrella et al., 1992, 1993; Murphy and Auh, 1996). The enhancement by NAD(P)H and inhibition by NADP+ of HO2./O2− production in potatoes infected with Phytophthora infestans can be interpreted as evidence for the operation of an NAD(P)H oxidase in HO2./O2− production (Doke, 1985). A similar trend was observed in resistant tobacco cells inoculated with the incompatible race of Pn. However, it must also be recognized that peroxidases often require a similar ratio of NAD(P)H to NAD(P)+ to that required by NAD(P)H oxidases (Auh and Murphy, 1995). The effect of NAD(P)H may also be due to other unknown extracellular conditions since it is generally considered to be incapable of crossing the cell membrane (Schroeder et al., 1996). This is very significant because the source of a reductant for NAD(P)H oxidases located in the cell membrane is considered to be intracellular (Schroeder et al., 1996).
In summary, this study demonstrates the value of quantitative measurement of ROS production in dissecting the critical steps involved in the HR and the pathways that generate these reactive species. Our results indicate that HO2./O2− generation, which may occur via several pathways, is a critical factor leading to the HR in tobacco cells challenged by avirulent zoospores of Pn. By comparison, H2O2 generation (which occurs largely, but not completely, via HO2./O2−-dependent pathways) appears to play a minor role in the induction of the HR. This is particularly indicated by the effect of SOD addition in which the yield of HO2./O2− is reduced, and despite an increase in H2O2 the HR is largely prevented.
MATERIALS AND METHODS
The Assay System
Established suspension cell cultures of the near-isogenic tobacco (Nicotiana tabacum) cv Hicks (susceptible) and cv NC2326 (resistant) were inoculated with incompatible (race 0) and compatible (race 1) zoospores of Pn (Australian field isolates 4974 and 9201, respectively) using the microwell plate method detailed in Able et al. (1998). Cells (0.1 g) were placed in each well in a final volume of 2 mL of 5 mm phosphate buffer (pH 7.5) with 0.5% (w/v) Suc and incubated at 24°C and 100 rpm. Multiple wells of each treatment type were prepared to permit replication of measurements at each sampling time. Between two and four replicate wells were harvested at desired intervals, and the supernatants collected for spectrophotometric analysis.
H2O2 Production
H2O2 was detected using the oxidative quenching of the fluorescent reporter dye, pyranine (8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt, Molecular Probes, Eugene, OR) as adapted from Low and Heinstein (1986) and Legendre et al. (1993). The loss of fluorescence by pyranine in the presence of H2O2 was measured using excitation and emission wavelengths of 403 and 514 nm, respectively, at a sensitivity of between 485 and 495 V (set automatically) at 24°C ± 1°C on a dual wavelength AMINCO-Bowman Series 2 Luminescence Spectrometer (SLM-AMINCO product line, Spectronic Instruments, Rochester, NY). Pyranine at a final concentration of 10 μg mL−1 was added to supernatant harvested from inoculated cells at varying times after zoospore addition. The loss of fluorescence was followed until the signal had decreased to a constant value. Mean values ± se were compiled from raw data 81 to 100 s after pyranine addition. All reagents added to the system were tested for any direct effects on pyranine fluorescence, while H2O2 standards were added to obtain a calibration curve. Oxygen evolved after the addition of CAT to the supernatant of inoculated cells was measured using a Rank Brothers Ltd. Biological Clark-type Oxygen Electrode (Cambridge, UK). For calibration purposes, nanomoles of oxygen at 100% air saturation at atmospheric pressure were determined from standard data tables (Lide, 1997). Zero oxygen content was achieved by the addition of a few crystals of sodium dithionite (Ajax Chemicals, Sydney). The chart recorder was then calibrated according to the difference in deflection between these two results. Supernatant (0.5 mL) from inoculated cells was stirred in the sealed electrode chamber with 0.5 mL of de-aerated 5 mm phosphate buffer (pH 7.5). After the initial stabilization, 1,000 units of CAT was injected through the capillary tube of the lid and evolution of oxygen recorded until the response reached a plateau.
HO2./O2− Measurement
HO2./O2− generation by the cells was detected by the addition of XTT (Diagnostic Chemicals, Charlottetown, Canada) to the wells at the time of zoospore addition (Able et al., 1998). The yield of HO2./O2− subsequently detected was determined from estimation of the XTT formazan produced (Sutherland and Learmonth, 1997; Able et al., 1998). When required, Cyt c and NBT were used as per Able et al. (1998).
Tobacco Cell Viability Assays
Viability was monitored using the hypertonic neutral red assay (O'Connell et al., 1985) as adapted by Able et al. (1998).
Modulation of the System
NAD(P)H (1 mm), NADP+ (1 mm), and the mammalian NAD(P)H oxidase inhibitor, DPI (0–100 μm) were added to determine whether a NAD(P)H oxidase-like enzyme is responsible for ROS production. Allopurinol (0–500 μm) and SHAM (ICN Chemicals, Costa Mesa, CA) (0–4 mm) were also added to inhibit xanthine oxidase and peroxidases, respectively.
Where required, 400 units of CAT were added to cells to remove H2O2, whereas 1 mm ATZ or 1 mm SA were added to inhibit endogenous CAT (Levine et al., 1994; Durner and Klessig, 1996). In selected experiments, either Cu/Zn SOD or Mn(III)desferal were added to remove HO2./O2−. Alternatively, the Cu/ZnSOD inhibitor, DDC (ICN Chemicals) (1 mm) was added in the absence or presence of 100 units of MnSOD. The activity of the HO2./O2− scavengers was confirmed using the xanthine/xanthine oxidase assay (Faulkner et al., 1994).
Statistical Analysis
Data were analyzed by appropriate Student's t tests or other analyses of variance using Microsoft Excel Version 5.0 and The SAS System for Windows 6.2 (SAS Institute, Cary, NC). Significant differences between individual treatments were determined using lsd or Neumann-Kuhls tests.
ACKNOWLEDGMENT
The authors would like to acknowledge Dr. Robert Learmonth for his assistance with spectrofluorometry.
Footnotes
This work was supported by the Australian Research Council (grant no. A19601127) and by a University of Southern Queensland PhD Scholarship (to A.J.A.).
LITERATURE CITED
- Able AJ, Guest DI, Sutherland MW. Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotianae. Plant Physiol. 1998;117:491–499. doi: 10.1104/pp.117.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerlund P, Larsson C, Widell S, Møller IM. NAD(P)H oxidase and peroxidase activities in purified plasma membranes from cauliflower inflorescences. Physiol Plant. 1987;71:9–19. [Google Scholar]
- Auh CK, Murphy TM. Plasma membrane redox enzyme is involved in the synthesis of O2− and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–1247. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Annu Rev Phytopathol. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer WF, Jr, Fridovich I. Characterization of a superoxide dismutase mimic prepared from desferrioxamine and manganese dioxide. Arch Biochem Biophys. 1989;271:149–156. doi: 10.1016/0003-9861(89)90265-8. [DOI] [PubMed] [Google Scholar]
- Bi YM, Kenton P, Mur L, Darby R, Draper J. Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J. 1995;8:235–245. doi: 10.1046/j.1365-313x.1995.08020235.x. [DOI] [PubMed] [Google Scholar]
- Bingham IJ, Stevenson EA. Causes and location of non-specific effects of SHAM on O2 uptake by wheat roots. Physiol Plant. 1995;93:427–434. [Google Scholar]
- Bolwell GP. Role of active oxygen species and NO in plant defense responses. Curr Opin Plant Biol. 1999;2:287–294. doi: 10.1016/S1369-5266(99)80051-X. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaninski Y, Sachot RM, Catesson AM. Cytochemical localization of hydrogen peroxide in lignifying cell walls. Ann Bot. 1993;72:547–550. [Google Scholar]
- Dème D, Doussiere J, De Sandro V, Dupuy C, Pommier J, Virion A. The Ca2+/NADPH-dependent H2O2 generator in thyroid plasma membrane: inhibition by diphenyleneiodonium. Biochem J. 1994;301:75–81. doi: 10.1042/bj3010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diethelm R, Miller MG, Shibles R, Stewart CR. Effect of salicylhydroxamic acid on respiration, photosynthesis, and peroxidase activity in various plant tissues. Plant Cell Physiol. 1990;31:179–185. [Google Scholar]
- Doke N. Generation of superoxide anion by potato tuber protoplasts during the hypersensitive response to hyphal wall components of Phytophthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Physiol Plant Pathol. 1983a;23:359–367. [Google Scholar]
- Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol. 1983b;23:345–357. [Google Scholar]
- Doke N. NADPH-dependent O2− generation in membrane fractions isolated from wounded potato tubers inoculated with Phytophthora infestans. Physiol Plant Pathol. 1985;27:311–322. [Google Scholar]
- Doke N, Chai HB. Activation of superoxide generation and enhancement of resistance against compatible races of Phytophthora infestans in potato plants treated with digitonin. Physiol Plant Pathol. 1985;27:323–334. [Google Scholar]
- Doke N, Miura Y. In vitro activation of NADPH-dependent O2− generating system in a plasma membrane-rich fraction of potato tuber tissues by treatment with an elicitor from Phytophthora infestans or with digitonin. Physiol Mol Plant Pathol. 1995;46:17–28. [Google Scholar]
- Doke N, Ohashi Y. Involvement of an O2− generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiol Mol Plant Pathol. 1988;32:163–175. [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S. Hydrogen peroxide from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol. 1999;121:163–171. doi: 10.1104/pp.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Klessig DF. Salicylic acid is a modulator of tobacco and mammalian catalases. J Biol Chem. 1996;271:28492–28501. doi: 10.1074/jbc.271.45.28492. [DOI] [PubMed] [Google Scholar]
- Dwyer SC, Legendre L, Low PS, Leto TL. Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim Biophys Acta. 1996;1289:231–237. doi: 10.1016/0304-4165(95)00156-5. [DOI] [PubMed] [Google Scholar]
- Faulkner KM, Stevens RD, Fridovich I. Characterization of Mn(III) complexes of linear and cyclic desferrioxamines as mimics of superoxide dismutase activity. Arch Biochem Biophys. 1994;310:341–346. doi: 10.1006/abbi.1994.1176. [DOI] [PubMed] [Google Scholar]
- Guest DI, Upton JCR, Rowan KS. Fosetyl-Al alters the respiratory response in Phytophthora nicotianae-infected tobacco. Physiol Mol Plant Pathol. 1989;34:257–265. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Ed 3. Oxford: Clarendon Press; 1999. [Google Scholar]
- Harris CM, Massey V. The reaction of reduced xanthine dehydrogenase with molecular oxygen. J Biol Chem. 1997;272:8370–8379. doi: 10.1074/jbc.272.13.8370. [DOI] [PubMed] [Google Scholar]
- Heath MC. The enigmatic hypersensitive response: induction, execution, and role. Physiol Mol Plant Pathol. 1998;55:1–3. [Google Scholar]
- Higgins VJ, Lu H, Xing T, Gelli A, Blumwald E. The gene-for-gene concept and beyond: interactions and signals. Can J Plant Pathol. 1998;20:150–157. [Google Scholar]
- Hsiao KC, Bornman CH. Salicylhydroxamic acid mimics esterase-like action. J Exp Bot. 1993;44:1847–1849. [Google Scholar]
- Ivanova DG, Gughova NV, Merzlyak MN, Rassadina GV. Effect of Phytophthora infestans infection on superoxide dismutase dependent cytochrome c reducing activities of leaves as related to resistance of potato plants to late blight. Plant Sci. 1991;78:151–156. [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS. Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol. 1993;102:233–240. doi: 10.1104/pp.102.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Lide DR. CRC Handbook of Chemistry and Physics. Ed 78. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- Lindner WA, Hoffmann C, Grisebach H. Rapid elicitor-induced chemiluminescence in soybean cell suspension cultures. Phytochemistry. 1988;27:2501–2503. [Google Scholar]
- Low PS, Dwyer SC. Comparison of the oxidative burst signalling pathways of plants and human neutrophils. In: Daniels MJ, Downie JA, Osbourn AE, editors. Advances in Molecular Genetics of Plant Microbe Interactions. Vol. 3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 361–369. [Google Scholar]
- Low PS, Heinstein PF. Elicitor stimulation of the defense responses in cultured plant cells monitored by fluorescent dyes. Arch Biochem Biophys. 1986;249:472–479. doi: 10.1016/0003-9861(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense: function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Macri F, Braidot E, Petrussa E, Vianello A. Lipoxygenase activity of plasmalemma and its relation to plant cell senescence and stress response. Acta Phytopathol Entomol Hung. 1995;30:81–87. [Google Scholar]
- Mehdy MC, Sharma YK, Sathasivan K, Bays NW. The role of activated oxygen species in plant disease resistance. Physiol Plant. 1996;98:365–374. [Google Scholar]
- Montalbini P, Della Torre G. Evidence of a two-fold mechanism responsible for the inhibition by allopurinol of the hypersensitive response induced in tobacco by tobacco necrosis virus. Physiol Mol Plant Pathol. 1996;48:273–287. [Google Scholar]
- Moreau RA, Osman SF. The properties of reducing agents released by treatment of Solanum tuberosum with elicitors from Phytophthora infestans. Physiol Mol Plant Pathol. 1989;35:1–10. [Google Scholar]
- Murphy TM, Auh CK. Phytophthora elicitor inhibits ferricyanide reduction by cultured rose cells. Environ Exp Bot. 1992;32:487–496. [Google Scholar]
- Murphy TM, Auh CK. The superoxide synthases of plasma membrane preparations from cultured rose cells. Plant Physiol. 1996;110:621–629. doi: 10.1104/pp.110.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenschwander U, Vernooij B, Friedrich L, Uknes S, Kessmann H, Ryals J. Is hydrogen peroxide a second messenger of salicylic acid in systemic acquired resistance? Plant J. 1995;8:227–233. [Google Scholar]
- O'Connell RJ, Bailey JA, Richmond DV. Cytology and physiology of infection of Phaseolus vulgaris by Colletotrichum lindemuthianum. Physiol Plant Pathol. 1985;27:75–98. [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB. Influence of salicylic acid on H2O production, oxidative stress and H2O2-metabolizing enzymes. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich PR, Wiegand NK, Blum H, Moore AL, Bonner WD., Jr Studies on the mechanism of inhibition of redox enzymes by substituted hydroxamic acids. Biochim Biophys Acta. 1978;525:325–337. doi: 10.1016/0005-2744(78)90227-9. [DOI] [PubMed] [Google Scholar]
- Ryals J, Lawton KA, Delaney TP, Friedrich L, Kessmann H, Neuenschwander U, Uknes S, Vernooij B, Weymann K. Signal transduction in systemic acquired resistance. Proc Natl Acad Sci USA. 1995;92:4202–4205. doi: 10.1073/pnas.92.10.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder AT, Martin G, Low PS. A comparison of methods for determination of the oxidative burst in whole plants. In: Stacey G, Mullin B, Gresshoff PM, editors. Biology of Plant-Microbe Interactions. St. Paul, MN: International Society for Molecular Plant-Microbe Interactions; 1996. pp. 15–20. [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck MJ, Khan AU, Appel WH, Jr, Karnovsky MJ. The DAB-Mn++ cytochemical method revisited: validation of specificity for superoxide. J Histochem Cytochem. 1993;41:1659–1667. doi: 10.1177/41.11.8292156. [DOI] [PubMed] [Google Scholar]
- Sutherland MW. The generation of oxygen radicals during host plant responses to infection. Physiol Mol Plant Pathol. 1991;39:79–94. [Google Scholar]
- Sutherland MW, Learmonth BA. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radic Res. 1997;27:283–289. doi: 10.3109/10715769709065766. [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Trudgill PW. Oxygen consumption. In: Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985. pp. 329–342. [Google Scholar]
- Van der Werf A, Raaimakers D, Poot P, Lambers H. Evidence for a significant contribution by peroxidase-mediated oxygen uptake to root respiration of Brachypodium pinnatum. Planta. 1991;183:347–352. doi: 10.1007/BF00197732. [DOI] [PubMed] [Google Scholar]
- Vera-Estrella R, Blumwald E, Higgins VJ. Effect of specific elicitors of Cladosporium fulvum on tomato suspension cells: evidence for the involvement of active oxygen species. Plant Physiol. 1992;99:1208–1215. doi: 10.1104/pp.99.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella R, Blumwald E, Higgins VJ. Non-specific glycopeptide elicitors of Cladosporium fulvum: evidence for involvement of active oxygen species in elicitor-induced effects on tomato cell suspensions. Physiol Mol Plant Pathol. 1993;42:9–22. doi: 10.1104/pp.99.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever RMF, Van Dam T, Van Rijn HJM, De Groot F, Rabelink TJ. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem Biophys Res Commun. 1997;237:340–344. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahraus T, Chandra S, Legendre L, Low PS. Evidence for a mechanically induced oxidative burst. Plant Physiol. 1995;109:1259–1266. doi: 10.1104/pp.109.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki Y, Kinumi M, Kahara T, Okubo K. Chemiluminescence of soybean saponins in the presence of active oxygen species. Plant Sci. 1996;116:125–129. [Google Scholar]