Abstract
Leaflet movements in the mimosa-family tree Samanea saman stem from coordinated volume changes of cells in the leaf motor organs in the adaxial and abaxial motor cells (“flexors” and “extensors”). Shrinking, initiated by dissimilar light signals in extensors and in flexors, depends in both cell types on K+ efflux via depolarization-dependent potassium (KD) channels. To compare between flexor and extensor KD channels and to test for a possible interaction of these channels with the Ca2+-mobilizing phosphoinositide cascade evoked in these motor cells by the “shrinking signals,” we probed the channels with varying (5 nm–3 mm) cytosolic free-Ca2+ concentration ([Ca2+]cyt) in patch-clamped inside-out excised membrane patches. Ca2+ was not required for KD channel activation. [Ca2+]cyt of 600 nm decreased the mean number of open KD channels in flexors, as monitored at −30 mV. Detailed analysis revealed that in flexors millimolar [Ca2+]cyt decreased the maximum number of open channels, but simultaneously increased KD channel opening probability by negatively shifting the half-maximum-activation voltage by 40 to 50 mV. Thus, the promoting and the inhibitory effects at millimolar [Ca2+]cyt practically cancelled-out. In contrast to flexors, none of the gating parameters of the extensor KD channels were affected by [Ca2+]cyt. Irrespective of [Ca2+]cyt, the steady-state gating of extensor KD channels was slightly but significantly more voltage sensitive than that of flexors. The unitary conductances of flexor and extensor KD channels were similar and decreased by approximately 20% at millimolar [Ca2+]cyt. It is intriguing that the extensor KD channels were significantly less K+ selective than those in flexors.
Considerable insight into the regulation of plant K+-efflux channels (Kout or KD channels) has been achieved in one particularly well-studied model system, the stomatal guard cell (for reviews, see MacRobbie, 1998; Assmann and Shimazaki, 1999, and refs. therein), but even there the underlying mechanisms are not completely understood. To gain insight into the regulation of the KD channels we study another model system: the motor cells in a leaf-moving organ, the pulvinus, in the mimosa-family tree Samanea saman. The pulvinus moves leaves and leaflets by virtue of osmotic volume and turgor changes of its motor cells, resulting from the movement of ions, chiefly K+ and Cl−, into and out of the cells (Satter and Galston, 1981; Satter et al., 1988). Signals causing leaf unfolding (e.g. blue light) cause cell shrinking in the top (adaxial, flexor) one-half of the pulvinus and swelling in the bottom (abaxial, extensor) one-half. Signals causing leaf folding (e.g. red light followed by dark) cause the reverse responses. In both cell types, K+ is released passively from the shrinking cell into the apoplast (Moran et al., 1988; Satter et al., 1988; Lowen and Satter, 1989). In flexors, blue light (a “shrinking signal”) has been demonstrated recently to promote the opening of KD channels (Suh et al., 2000). KD channels open, presumably, in shrinking extensors as well. The abundance of KD channels in the S. saman motor cell membrane is quantitatively more than sufficient to conduct K+ fluxes needed to account for the osmotic changes. Moreover, KD channels are essential to the shrinking of the motor cells and hence, to pulvinar movements, as demonstrated by the arrest of movement by the KD channel blocker, tetraethylammonium (Moran et al., 1988).
In both cell types, the “shrinking signaling” (blue-light illumination of flexor protoplasts, or imposition of darkness on extensor protoplasts) results in the formation of 1,4,5-inositol trisphosphate (Kim et al., 1993, 1996), a second messenger in the phosphoinositide (PI) cascade (Berridge and Irvine, 1989; Berridge, 1997). According to the present paradigm on the roles of the PI cascade and Ca2+ mobilization in the shrinking of stomatal guard cells (Blatt et al., 1990; Gilroy et al., 1990; McAinsh et al., 1990; Irving et al., 1992; Lee et al., 1996), K+-efflux channels in guard cells are activated by depolarization resulting from Ca2+ activation of Cl− efflux (Schroeder and Hagiwara, 1989).
Although a similar paradigm is generally applicable to the shrinking of S. saman motor cells (Moran, 1990), depolarization is not the sole activator of K+ efflux in these (flexor) cells (Suh et al., 2000), nor is it in guard cells (Blatt, 1990; Lemtiri-Chlieh and MacRobbie, 1994), although the other effector is not known. Direct interaction with Ca2+ could be another plausible mode of regulation of K-efflux channels by the PI cascade. Cytosolic Ca2+ did promote the activation of K+-efflux channels in the plasma membrane in corn suspension cell protoplasts (Ketchum and Poole, 1991), in the alga Mougeotia (Lew et al., 1990), and in the alga Eremosphera viridis (Bauer et al., 1998). In guard cells, Kout channels were reported by some to be Ca2+ insensitive (Hosoi et al., 1988; Schroeder and Hagiwara, 1989; Lemtiri-Chlieh and MacRobbie, 1994), whereas others reported their inhibition by cytosolic Ca2+ of 200 nm (relative to 2 nm; Fairley-Grenot and Assmann, 1992). In addition, Ca2+ entry enhanced the rundown of outward-rectifying K+ channels in pulvinar cells of Mimosa pudica (Stoeckel and Takeda, 1995).
In view of these different possibilities, and since the coupling of the PI cascade to KD channels in shrinking S. saman motor cells has not yet been resolved, the sensitivity of flexor and extensor KD channels to Ca2+ needs to be examined. Moreover, since the initiation of shrinking of S. saman motor cells is linked to a different photoreceptor in the flexors and extensors, the question arises whether, in each cell type, this cascade is linked differently also at the effector end, to the K+-efflux channels. In fact, a detailed comparison between the KD channels of the two cell types has not been carried out and in spite of their mutual resemblance noticed so far (Moran et al., 1988, 1990), these K+-efflux channels might not even be the same molecular entities in flexors and extensors. For example, the two outward-rectifying K+ channels, KCO1 and SKOR1, cloned recently from Arabidopsis, display superficially similar behavior in heterologous expression systems (e.g. are activated by depolarization exceeding the K+ reversal potential [Erev]), although they are encoded by genes from different potassium channel families (Czempinski et al., 1997; Gaymard et al., 1998). Such a possibility merits the comparison of the flexor and extensor K+-efflux channels.
To address the above questions, we examined the cytosolic [Ca2+] dependence of KD channels by patch clamp, in inside-out patches excised from both extensor and flexor cells. In this configuration, the [Ca2+] in the vicinity of the channel is controlled much more strictly than in the whole-cell configuration, where the channels in the plasma membrane are in a rather close proximity to the Ca2+-storing (and potentially Ca2+-releasing) organelles: vacuole, mitochondria, chloroplasts, and endoplasmic reticulum. We investigated the Ca2+ dependence of the outward-rectifying plant K+ channels in the plasma membrane at a single channel level. To our knowledge, this is the first such detailed analysis of higher plant K+-efflux channels in situ. This analysis revealed differences in three properties between the flexor and the extensor KD channels: in Ca2+ sensitivity, in K+ selectivity, and in voltage sensitivity.
RESULTS
Cytosolic Ca2+ Affects Flexor KD Channel Gating
The activity of single KD channels in a representative patch from an extensor protoplast, with 600 nm free Ca2+ at the cytoplasmic side, is shown in Figure 1. As “befits” KD channels, the channel activity increased with depolarization. At a saturating depolarization, eight channels were open simultaneously in this patch. From the linear unitary current-voltage relationships we were able to deduce the Erev of −79 mV (Fig. 1B), and the mean single-channel conductance (γS) of 17.5 pS (the slope of iS-EM, the idealized single-channel current-voltage relationship; Fig. 1C). From the proximity of Erev to EK (−79 and −78 mV, respectively), we concluded that these channels were K+ selective {for comparison, the respective equilibrium potentials of the other, potentially permeant, ions—Cl−, H+, and Ca2+—were: ECl, +117 mV; EH, +72 mV; and ECa, −13 to +244 mV, depending on the value of the cytosolic concentration of free Ca2+ ([Ca2+]cyt)}.
Figure 1.
Unitary outward K+ currents via KD channels versus EM, from a representative “inside-out” patch of a S. saman extensor cell protoplast, during a slow voltage ramp. A, A linearly increasing voltage ramp applied to the patch membrane. B, Three traces of current (superimposed) during the voltage ramp. Note the “up and down steps” signifying opening and closing of KD channels. Straight lines—fitted manually to open-channel currents—indicate idealized current levels through “n” (nos. to the right) simultaneously open KD channels. Note the increase of “n” with the increased depolarization. ILeak, Current recorded when all KD channels are closed. Erev, −79 mV. C, Initial analysis. IS, Mean of three current records; iS, idealized unitary current through a single open channel; ILeak, as in B; 0, the level of zero current. D, Voltage dependence of KD channel activation. The n̄ was calculated as a point-by-point ratio of currents in C (corrected for leak; Eq. 2). Note that n̄ increases with membrane depolarization. Dashed line, Boltzmann relationship (Eq. 4), with the following parameters (see “Materials and Methods”): n̄max = 5.9; E1/2= −37 mV; z = 1.4.
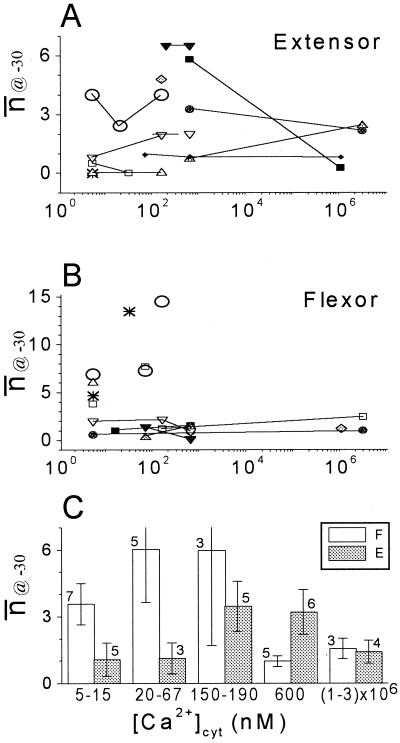
To evaluate their steady-state gating properties, we plotted the mean number of open channels (n̄), versus membrane potential (EM) and fitted these data with the Boltzmann relationship (Eq. 3; Fig. 1D). At a non-saturating potential of −30 mV, a fraction of KD channels was usually active. We quantified this activity in patches from 11 extensor and 10 flexor protoplasts, in different [Ca2+]cyt, in terms of n̄@-30, the n̄ at −30 mV, which was read off the Boltzmann curve (Fig. 1D). n̄@-30 values were then pooled into five groups of three to seven patches each, according to subranges of [Ca2+]cyt (Fig. 2; “Materials and Methods”). Data from individual patches were connected by lines, to reveal potential trends. We detected no consistent effect of [Ca2+]cyt in the individual patches (Fig. 2, A and B). However, among the averaged values of n̄@-30 at the different [Ca2+]cyt, flexor n̄@-30 values at 15 nm were significantly larger than those at 600 nm (Fig. 2C). Since, potentially, inhibitory effects of [Ca2+]cyt may have masked promoting effects of [Ca2+]cyt on KD channel opening, we took advantage of the resolution of single channel data to examine such effects separately on the individual gating properties of the KD channels. We tested the effect of [Ca2+]cyt on the classically defined steady-state properties of channel gating (the half-maximum-activation voltage, the mean number of channels open at saturation potentials, and the effective number of gating charges, z [Eq. 4]; Hille, 1992). In addition, we examined the properties of K+ permeation through the open channel pore (the γS and channel selectivity).
Figure 2.
The effect of [Ca2+]cyt on the average activity of KD channels at −30 mV, n̄@-30. Different symbols (connected by lines) denote data from different patches. A, Extensor cells. B, Flexor cells. C, Average values of n̄@-30 obtained from n patches at the indicated ranges of [Ca2+]cyt (±se). F, Flexor; E, extensor.
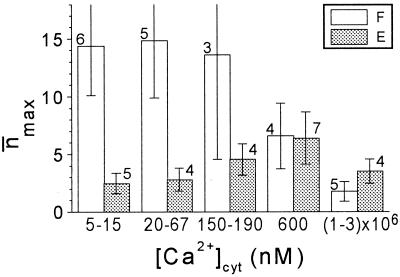
n̄max (n̄ at saturation potentials) varied considerably from patch to patch, in both cell types, much more than most of the other parameters (Fig. 3), resembling the same phenomenon already noted in broad bean guard cells (Ilan et al., 1994). In extensor cells, [Ca2+]cyt did not have any significant effect on n̄max. In flexor cells, n̄max was significantly smaller at [Ca2+]cyt of 1 to 3 mm than at 5 to 67 nm (Fig. 3), and this conclusion holds even if the data from two flexor patches with the most extreme values of n̄max at these low concentrations are ignored. Since n̄max is a product of the total number of channel proteins in the membrane (N), and the voltage-independent probability of their opening (fO; Ilan et al., 1996), either N or fO (or both) could be responsible for the [Ca2+]cyt-induced n̄max decrease. However, N and fO can be resolved only in very prolonged recordings in saturation voltages, which was impractical in our experiments. n̄max, averaged over the physiological [Ca2+]cyt of 20 to 600 nm, was approximately 11 in flexors, significantly more than approximately 5, in extensors (Table I). Since the flexors and extensors do not differ in size (Moran et al., 1988) or in the values of whole-cell steady-state current levels (not shown), they would be expected to have the same KD channel density. Further work is required to reconcile this with the observed difference in n̄max between the flexor and extensor membrane patches.
Figure 3.
The effect of [Ca2+]cyt on the n̄max. Mean values of n̄max obtained from n patches at the indicated ranges of [Ca2+]cyt (±se). F, Flexor; E, extensor.
Table I.
Steady-state properties of the Samanea KD channels
| Cell Type | Parameter
|
||||
|---|---|---|---|---|---|
| γS | Erev | n̄max | E1/2 | z | |
| pS | mV | mV | |||
| Extensor | 20.9 ± 0.8 (10) | −75 ± 1 (10) | 5.3 ± 2 (10) | −24 ± 8 (10) | 1.9 ± 0.3 (10) |
| Flexor | 19.6 ± 0.8 (8) | −78 ± 1 (8) | 11.2 ± 4 (8) | −14 ± 8 (8) | 1.2 ± 0.2 (8) |
Extensor and flexor cells compared at a physiological range of free [Ca2+]cyt (20–600 nm). Permeation properties (resulting from fit of Eq. 1 to the steady-state unitary current-voltage relationship, as in “Materials and Methods” and Fig. 1B). Gating properties (resulting from the fit of Eq. 4 to the steady-state voltage dependence of channel opening, as in “Materials and Methods” and Fig. 1C). Data are means ± se (no. of cells).
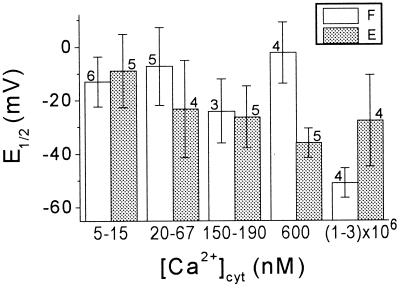
The values of half-maximum-activation potential (E1/2) varied only a little less than n̄max (Fig. 4). Nevertheless, at millimolar [Ca2+]cyt, E1/2 in flexor cells was significantly smaller than at 5 to 15 nm or at 600 nm (by approximately 40 and approximately 50 mV, respectively). In extensors cells, E1/2 did not seem to be affected (Fig. 4). Extensor and flexor cells did not differ significantly in the overall mean values of E1/2 at [Ca2+]cyt of 20 to 600 nm, (24 and 14 mV, respectively; Table I).
Figure 4.
The effect of [Ca2+]cyt on the E1/2. Mean values of E1/2 obtained from n patches at the indicated ranges of [Ca2+]cyt (±se). F, Flexor; E, extensor.
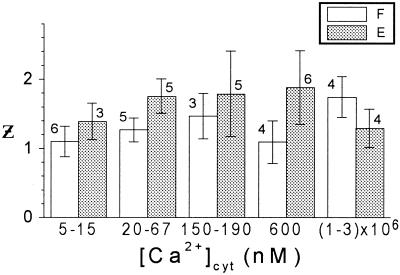
In contrast, the values of z varied very little among patches of one cell type and they did not change with the [Ca2+]cyt (Fig. 5). At the range of 20 to 600 nm, z was 1.9 in extensor cells and 1.2 in flexor cells (Table I), indicating that, although in both cell types the gating process involved the cross-membranal movement of at least two electrical charges, the gating of extensor KD channels was slightly but significantly more voltage sensitive than that of flexor KD channels.
Figure 5.
The effect of [Ca2+]cyt on the effective number of charges, z. Mean values of z obtained from n patches at the indicated ranges of [Ca2+]cyt (±se). F, Flexor; E, extensor.
The Effect of [Ca2+]cyt on KD Channel Selectivity and Conductance
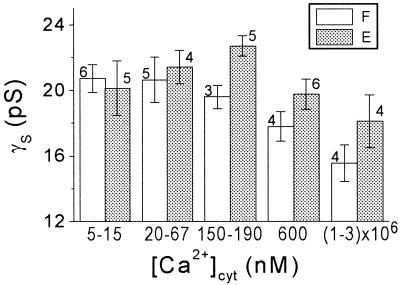
The two cell types did not differ with respect to the mean values of γS (the unitary conductance) at any one of the concentration ranges (Fig. 6). At the range of physiological [Ca2+]cyt of 20 to 600 nm, the mean γS was approximately 20 pS (Table I). High [Ca2+]cyt decreased γS slightly (by approximately 20%) in both cell types (Fig. 6). Thus in extensor cells at [Ca2+]cyt of 600 nm, γS was smaller than at 150 to 190 nm, and in flexor cells at millimolar [Ca2+]cyt, γS was smaller than at [Ca2+]cyt 190 nm (P < 0.05). This decrease of γS could be due to open-channel block by Ca2+ (e.g. Vergara and Latorre, 1983).
Figure 6.
The effect of [Ca2+]cyt on the γS. Mean values of γS obtained from n patches at the indicated ranges of [Ca2+]cyt (±se). F, Flexor; E, extensor.
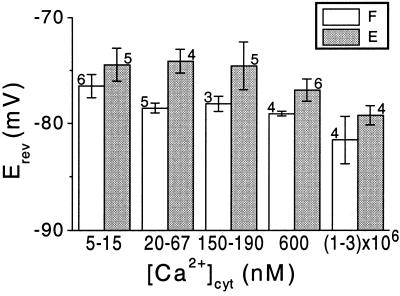
The mean values of Erev (of the iS), determined at five ranges of [Ca2+]cyt in flexor cells, were largely indistinguishable from the predicted K+ Nernst potential (EK) of −78 mm (Fig. 7). In extensor cells, a small though significant deviation of 4 mV from EK could be noted at the lower [Ca2+]cyt (20–67 nm; Fig. 7). When averaged over the physiological [Ca2+]cyt range of 20 to 600 nm, the mean Erev of extensor cells (−75 mV), was also significantly more positive than that of flexor cells (which was equal to EK; see also Table I).
Figure 7.
The effect of [Ca2+]cyt on the Erev of the unitary currents. Mean values of Erev obtained from n patches at the indicated ranges of [Ca2+]cyt (±se). F, Flexor; E, extensor.
Summary
KD channels in both cell types did not require [Ca2+]cyt for their activity. However, in flexor cells (but not in extensors), the steady-state gating properties were affected by the higher [Ca2+]cyt. In addition to the different sensitivity to cytosolic Ca2+, flexors and extensors differed perceptibly in two more details: in the steepness of their voltage dependence (z) and in their K+ selectivity.
DISCUSSION
Differentiation between Flexor and Extensor KD Channels Based on Ca2+ Effects
No information exists, as yet, about the molecular identity of the S. saman K+-efflux channels. Therefore, their functional characterization in planta is needed to provide the database against which such identification will ultimately need to be tested. In particular, sensitivity to Ca2+ may be a useful criterion for revealing possible differences between S. saman K+-efflux channels in the two cell types, and between them and other Kout channels. Contrary to extensor cells, a gating-promoting effect (a negative shift of E1/2) was indeed resolved in flexor cells at millimolar [Ca2+]cyt (Fig. 4). This negative shift of E1/2 could be theoretically attributed to non-specific screening of negative surface charges at the internal side of the membrane by the roughly thousand-fold increase of [Ca2+]cyt. We deem it unlikely, however, since, in addition to the high ionic strength of the “internal” solutions, the maximum increase in total divalent ion concentration in our experiments was less than 3-fold (2 mm Mg2+ was present in all the “internal” solutions). Both the high ionic strength and the small change in total divalent ion concentration would predict a negligible E1/2 shift (Gilbert and Ehrenstein, 1969; Kell and DeFelice, 1988).
A more likely explanation is a specific Ca2+ action, by binding. In lieu of any information about the molecular identity of the KD channel, the target of Ca2+ binding remains in the realm of speculation. If we assume direct binding of Ca2+ to the flexor KD channel, we need to assume also that this channel has Ca2+ binding domains, such as the “EF hands” in the Arabidopsis KCO1 channel (Czempinski et al., 1997). We may assume alternatively that Ca2+ affects the channel indirectly, via other Ca2+-activated proteins. Phosphorylation, for example, has been shown to cause E1/2 shifts in voltage-dependent outward-rectifying K+ channels (Esguerra et al., 1994; Levitan, 1994). Furthermore, phosphorylation can occur even in fragmented membranes (for example, extensor KD channels were regulated by phosphorylation in excised inside-out patches; Moran, 1996).
Difference in Selectivity between Flexor and Extensor KD Channels
We were surprised to discover that the extensor KD channels were less selective toward K+ than flexor channels (Fig. 7; Table I). This could be due, for example, to a partial permeability of the extensor KD channels to Ca2+ ions. For example, a departure of 4 mV from EK, with [Ca2+]cyt of 20 nm, may be accounted for by a Ca2+ permeability one-third (0.35) as large as the permeability to K+ (Lewis, 1979). The incomplete selectivity to K+ of the extensor KD channel resembles, in fact, that of the KD (Kout) channel in broad bean guard cells (Ilan et al., 1994) and the Kout channels of Arabidopsis mesophyll cells (Romano et al., 1998), where Erev values were also several mV above EK. Thus, although the Kin channel in guard cells did not conduct Ca2+ influx (Grabov and Blatt, 1998, 1999), this has not been excluded for the Kout channels in other plant systems. There are also various weakly Ca2+ permeant outward-rectifying K channels in animal systems (e.g. Hille, 1992). A variety of mechanisms could underlie this selectivity difference between flexor and extensor KD channels, such as mutations, mRNA editing or post-translational modifications, and this remains to be resolved.
For the S. saman motor system, the physiological implication of such permeability to Ca2+ would be, perhaps, the influx of Ca2+ through the KD channels open during the extensors shrinking phase, and enhancement of extensor shrinking via a positive feedback (activating more chloride channels, increasing depolarization, etc.). This, in turn, would enhance leaflet folding.
Although differing in selectivity, KD channels of both cell types were very similar in their unitary conductance (Fig. 6). To a first approximation, they were similar also in their kinetics (the latter was determined by fitting the activation and deactivation time courses of currents recorded in a whole-cell configuration with single exponentials, at [Ca2+]cyt of approximately 150 nm; data not shown).
Physiological Relevance of Ca2+ Effects on KD Channels
Since KD channels are essential to the shrinking of S. saman motor cells and therefore, to pulvinar movements (Moran et al., 1988), it could be expected that a significant Ca2+ effect on KD channels would be ultimately reflected in its effects on the movement of leaves. The reported observations in leaf-moving trees related to S. saman (M. pudica, Albizzia lophanta, Cassia fasciculata, and Robinia pseudoaccacia) all support a leaf-movement-enhancing role of Ca2+ (Campbell and Thompson, 1977; Roblin and Fleurat-Lessard, 1984; Moysset and Simon, 1989; Gomez and Simon, 1995). However, the overall effects of Ca2+ on KD channels in our experiments were either absent or minor. In fact, Ca2+ did not appear to be essential at all for the activity of the pulvinar KD channels, resembling the reported insensitivity of their counterparts in the guard cells. The promoting effect of the negative E1/2 shift in flexors at the highest concentrations of [Ca2+]cyt (if such a concentration could be reached in the vicinity of KD channels) would be probably offset by the decrease of γS and of n̄max. Thus in whole shrinking pulvinar cells in situ, KD channels are activated either via a Ca2+-independent mode, or, if [Ca2+]cyt is involved, an additional soluble cytosolic factor (absent in our experiments) may be required to mediate an activating action of Ca2+.
Moreover, at 600 nm in flexors, the apparent effect of [Ca2+]cyt on the gating of KD channel was a depression of activity: a small but significant decrease of n̄@-30 (relative to that at the lower concentration of 5–15 nm). This effect on n̄@-30 was consistent with the observation of rundown of pulvinar KD channels caused by Ca2+ influx in a S. saman close relative, M. pudica (Stoeckel and Takeda, 1995). Based on our findings in S. saman, it might be expected that increased cytosolic Ca2+ would decrease flexor KD channel activity and consequently impede flexor cell shrinking and leaf unfolding. This prediction is in conflict with the reported enhancement of leaf movements by Ca2+. Is it possible that [Ca2+]cyt level during flexor shrinking does not even reach the depressing concentration of 600 nm? This remains to be determined directly during the motor cell volume changes.
MATERIALS AND METHODS
Plant Material
Samanea saman (Jacq.) Merr. trees (recently referred to also as Pithecellobium saman [Jacq.] Benth.; Little and Wadsworth, 1964), were grown in a greenhouse under 16-h-light/8-h-dark schedule; leaves were harvested and protoplasts were isolated as described previously (Moran, 1996). The procedure for protoplast isolation has been further modified to include an additional rinse of the freshly chopped tissue pieces on a 20-μm mesh filter with solution containing 0.1% (w/v) polyvinylpyrrolidone to neutralize the possible effects of endogenous phenolics.
Patch-Clamp Experimental Procedure
Patch-clamp experiments were performed in a standard inside-out configuration (Hamill et al., 1981; Moran, 1996). Patch-clamp pipettes were prepared from borosilicate glass (catalog no. BF150–86–10, Sutter Instrument, Novato, CA) by a two-stage pull and fire polishing (both the micropipette puller and microforge were from Narashige [Tokyo]). The pipette was filled with an external solution. The bridge of the reference electrode was filled with an internal solution. After establishing a tight seal with the cell membrane, the bath was flushed with 10 volumes of the internal solution and the patch was excised into an inside-out configuration. The Ca2+ concentration of the bath solution was changed by flushing at least 10 volumes of the new solution. The order of Ca2+ concentrations applied was varied to eliminate systematic error due to possible time dependence (such as rundown or up-regulation). The iS current was filtered at 20 Hz (the −3db cutoff frequency of a four-pole Bessel filter), and digitized at a sampling rate of 50 Hz (Axon Instruments, Foster City, CA). To simplify comparisons with published experiments performed in different configurations, channel openings and current directed outward (with respect to the membrane) are shown as positive upward deflections from the closed-level (baseline) current. Likewise, in all of the experiments presented here, depolarization means increasing (more positive) potential at the cytoplasmic side.
The stimulation protocols were as follows. KD channels were activated by depolarization, applied in the form of ramps (Fig. 1A). The ramps were 40 s long and varied linearly with time from −80 to +40 mV. Channel activity was assumed to have attained a steady-state at each point during this slow rate of change of the EM (3 mV s−1). Between the depolarizations, the membrane was held for 20 s at a “resting” or “holding” potential of −80 or −100 mV (cytoplasmic side negative), at which KD channels were closed (Moran et al., 1988; Moran, 1996).
Analysis of Patch-Clamp Data
Determination of the Unitary Conductance and Erev in Inside-Out Patches
The slow voltage ramps and the resulting linear current-voltage (iS-EM) relationships between the iS and the EM served for the simultaneous determination of the Erev, the unitary conductance and the steady-state level of channel activity (Moran, 1996; Suh et al., 2000):
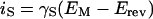 |
1 |
The Erev was obtained as the common zero-current intercept of several linear regressions (fitted by eye) to the different levels of open-channel current-voltage data points (Fig. 1B). γS, the slope of the idealized is-EM relationship of the single channel, was obtained by averaging the differences between the slopes of the linear regressions.
Characterization of Voltage-Dependent Gating in Single-Channel Patches
The total average current through the channels in the patch, IS, is the function of the average number of open KD channels, n̄, in the patch (Eq. 2):
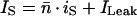 |
2 |
where ILeak is the linearly fitted baseline (leak current). -n at each EM value was calculated by dividing IS (after the subtraction of ILeak), point by point, by the idealized unitary open-channel current, iS (Fig. 1C). Provided that the channels are identical and statistically independent (Ehrenstein et al., 1970),
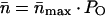 |
3 |
where PO is the voltage-dependent open probability of open channels.
The resulting steady-state n̄-EM relationship (reflecting the PO-EM relationship) was then fitted with the Boltzmann relationship
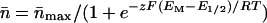 |
4 |
These Boltzmann parameter values were obtained for each treatment, 10 to 15 min after a change of solutions. The calculations and fit were performed on the data in the voltage range of −60 to +40 mV, using the commercially available program Origin (Microcal Software, Northampton, MA).
Statistics
Each characteristic parameter of the KD channels derived from single-channel data (γS, Erev, E1/2, etc.) initially was examined separately in each cell, at various [Ca2+]cyt concentrations (Fig. 2, A and B). We then grouped the data from all of the experiments in five concentration ranges, 5 to 15 nm, 20 to 67 nm, 150 to 190 nm, 600 nm, and 1 to 3 mm, to compare mean values (Fig. 2C). However, when comparing between flexor and extensor cells, we averaged the data from one “physiological” range of 20 to 600 nm (Table I). Whenever data were pooled together and averaged, a cell contributed no more than once to each average (a single value, or a mean, if there were more determinations than just one from a single patch in a given concentration range). Means are presented with their ses, with n, the number of cells averaged. Differences between means were deemed significant if, using a two-sided Student's t test, P < 0.05.
Solutions
The regular extracellular solution contained 5 mm K+, 9.5 mm MES, at pH 6.0, and 1 mm CaCl2, and was adjusted with sorbitol to osmolarity of 700 mOsm. The cytoplasmic surface was exposed to “internal solution”: 125 mm KCl, 20 mm HEPES, 1 mm MgATP and 1 mm MgCl2 (or 1 K2ATP and 2 mm MgCl2), 2 mm 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-K4, and variable total CaCl2. The desired concentrations of 600 nm free Ca2+, were calculated using the following equation:
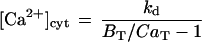 |
5 |
where CaT is the total concentration of Ca2+, BT is the total concentration of BAPTA, and kd is the dissociation constant of BAPTA of 200 nm (in the presence of 2 mm Mg and 0.1 m KCl; Pehtig et al., 1989). One or 3 mm Ca2+ was prepared by addition of 1or 3 mm Ca2+ in excess of 2 mm BAPTA. The osmolarity of the “internal solution” was adjusted with sorbitol to 750 mOsm. After addition of ATP and BABTA, the “internal solution” was adjusted with N-methylglucamine to pH 7.0 to 7.3 and used within a week of preparation. BAPTA was from Molecular Probes (Eugene, OR) or from Sigma (St. Louis). Other chemicals were from Sigma, Merck (Rahway, NJ), or BDH (AnalaR, Poole, UK).
ACKNOWLEDGMENTS
The authors are grateful to Dr. Stan Misler for helpful discussions, and to Hadas Shavit and Ling Yu for help in the preparation of protoplasts. Dr. Edna Schechtman's comments on the statistics are gratefully appreciated. The authors wish to thank Drs. Bernard Attali, Rainer Hedrich, Dirk Becker, Gerald Schoenknecht, and Bernd Mueller-Roeber for comments on an earlier version of the manuscript.
Footnotes
This work was supported by the German-Israeli Foundation for Scientific Research and Development (grant no. G 193–207.02/94 to N.M.) and by the United States-Israel Binational Agricultural Research and Development Fund (grant no. IS–2469–94CR to N.M.).
LITERATURE CITED
- Assmann SM, Shimazaki K. The multisensory guard cell: stomatal responses to blue light and abscisic acid. Plant Physiol. 1999;119:809–815. doi: 10.1104/pp.119.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CS, Plieth C, Hansen UP, Simonis W, Schoenknecht G. A steep Ca2+-dependence of a K+ channel in a unicellular green alga. J Exp Bot. 1998;49:1761–1765. [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blatt MR. Potassium channel currents in intact stomatal guard cells: rapid enhancement by abscisic acid. Planta. 1990;180:445–455. doi: 10.1007/BF00198799. [DOI] [PubMed] [Google Scholar]
- Blatt MR, Thiel G, Trentham DR. Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1990;346:766–769. doi: 10.1038/346766a0. [DOI] [PubMed] [Google Scholar]
- Campbell NA, Thompson WW. Effects of lantanum and ethylenediaminetetraacetate on leaf movements of Mimosa pudica. Plant Physiol. 1977;60:635–639. doi: 10.1104/pp.60.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czempinski K, Zimmermann S, Ehrhardt T, Mueller-Roeber B. New structure and function in plant K+ channels: KCO1, an outward rectifier with a steep Ca2+ dependency. EMBO J. 1997;16:2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein G, Lecar H, Nossal R. The nature of the negative resistance in bimolecular lipid membranes containing excitability-inducing material. J Gen Physiol. 1970;55:119–133. doi: 10.1085/jgp.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra M, Wang J, Foster CD, Adelman JP, North RA, Levitan IB. Cloned Ca2+-dependent K+ channel modulated by a functionally associated protein kinase. Nature. 1994;369:563–565. doi: 10.1038/369563a0. [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot KA, Assmann SM. Planta: 282–293. 1992. Whole-cell K+ current across the plasma membrane of guard cells from a grass: Zea mays. [DOI] [PubMed] [Google Scholar]
- Gaymard F, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud JB, Sentenac H. Identification and disruption of a plant Shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Ehrenstein G. Effect of divalent cations on potassium conductance of squid axons: determination of surface charge. Biophys J. 1969;9:447–463. doi: 10.1016/S0006-3495(69)86396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ. Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stomatal closure. Nature. 1990;346:769–771. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- Gomez LA, Simon E. Circadian rhythm of Robinia pseudoacacia leaflet movements: role of calcium and phytochrome. Photochem Photobiol. 1995;61:210–215. [Google Scholar]
- Grabov A, Blatt MR. Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA. 1998;95:4778–4783. doi: 10.1073/pnas.95.8.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR. A steep dependence of inward-rectifying potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells. Plant Physiol. 1999;119:277–287. doi: 10.1104/pp.119.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakman B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfluegers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA: Sinauer Associates; 1992. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi S, Lino M, Shimazaki KI. Outward-rectifying K+ channels in stomatal guard cell protoplasts. Plant Cell Physiol. 1988;29:907–911. [Google Scholar]
- Ilan N, Schwartz A, Moran N. External pH effects on the depolarization-activated K channels in guard cell protoplasts of Vicia faba. J Gen Physiol. 1994;103:807–831. doi: 10.1085/jgp.103.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N, Schwartz A, Moran N. External protons enhance the activity of the hyperpolarization-activated K channels in guard cell protoplast of Vicia faba. J Membr Biol. 1996;154:169–181. doi: 10.1007/s002329900142. [DOI] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc Natl Acad Sci USA. 1992;89:1970–1994. doi: 10.1073/pnas.89.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell MJ, DeFelice LJ. Surface charge near the cardiac inward-rectifier channel measured from single-channel conductance. J Membr Biol. 1988;102:1–10. doi: 10.1007/BF01875348. [DOI] [PubMed] [Google Scholar]
- Ketchum KA, Poole RJ. Cytosolic calcium regulates a potassium current in corn (Zea mays) protoplasts. J Membr Biol. 1991;119:277–288. doi: 10.1007/BF01868732. [DOI] [PubMed] [Google Scholar]
- Kim HY, Cote GG, Crain RC. Potassium channels in Samanea saman protoplasts controlled by phytochrome and the biological clock. Science. 1993;260:960–962. doi: 10.1126/science.260.5110.960. [DOI] [PubMed] [Google Scholar]
- Kim HY, Cote GG, Crain RC. Planta 279–289. 1996. Inositol 1,4,5-trisphosphate may mediate regulation of K+ channels by light and darkness in Samanea saman motor cells. [DOI] [PubMed] [Google Scholar]
- Lee YS, Choi YB, Suh S, Lee J, Assmann SM, Joe CO, Kelleher JF, Crain RC. Abscisic acid-induced phosphoinositide turnover in guard cell protoplasts of Vicia faba. Plant Physiol. 1996;110:987–996. doi: 10.1104/pp.110.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EA. Role of calcium in the modulation of Vicia guard cell potassium channels by abscisic acid: a patch-clamp study. J Membr Biol. 1994;137:99–107. doi: 10.1007/BF00233479. [DOI] [PubMed] [Google Scholar]
- Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Lew RR, Serlin BS, Schauf CL, Stockton ME. Calcium activation of Mougeotia potassium channels. Plant Physiol. 1990;92:831–836. doi: 10.1104/pp.92.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little ERJ, Wadsworth FH. Common Trees of Puerto Rico and the Virgin Islands. U.S. Washington, DC: Department of Agriculture; 1964. [Google Scholar]
- Lowen CZ, Satter RL. Light-promoted changes in apoplastic potassium activity in the Samanea saman pulvinus, monitored with liquid membrane microelectrodes. Planta. 1989;179:421–427. doi: 10.1007/BF00397580. [DOI] [PubMed] [Google Scholar]
- MacRobbie EAC. Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM. Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature. 1990;343:186–188. [Google Scholar]
- Moran N. The role of ion channels in osmotic volume changes in Samanea motor cells analyzed by patch-clamp methods. In: Satter RL, Gorton HL, Vogelmann TC, editors. The Pulvinus: Motor Organ for Leaf Movement. Ed 1. Rockville, MD: American Society of Plant Physiologists; 1990. pp. 142–158. [Google Scholar]
- Moran N. Membrane-delimited phosphorylation enables the activation of the outward-rectifying K channels in a plant cell. Plant Physiol. 1996;111:1281–1292. doi: 10.1104/pp.111.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N, Ehrenstein G, Iwasa K, Mischke C, Bare C, Satter RL. Potassium channels in motor cells of Samanea saman: a patch-clamp study. Plant Physiol. 1988;88:643–648. doi: 10.1104/pp.88.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N, Fox D, Satter RL. Interaction of the depolarization-activated K channel of Samanea saman with inorganic ions: a patch-clamp study. Plant Physiol. 1990;94:424–431. doi: 10.1104/pp.94.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moysset L, Simon E. Role of calcium in phytochrome-controlled nyctinastic movements of Albizzia lophantha leaflets. Plant Physiol. 1989;90:1108–1114. doi: 10.1104/pp.90.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehtig R, Kuhn M, Payne R, Adler E, Chen T-H, Jaffe LF. On the dissociation constants of BAPTA-type calcium buffers. Cell Calcium. 1989;10:491–498. doi: 10.1016/0143-4160(89)90026-2. [DOI] [PubMed] [Google Scholar]
- Roblin G, Fleurat-Lessard P. A possible mode of calcium involvement in dark- and light-induced leaflet movements in Cassia fasciculata Michx. Plant Cell Physiol. 1984;25:1495–1499. [Google Scholar]
- Romano LA, Miedema H, Assmann SM. Ca2+-permeable, outwardly-rectifying K+ channels in mesophyll cells of Arabidopsis thaliana. Plant Cell Physiol. 1998;39:1133–1144. doi: 10.1093/oxfordjournals.pcp.a029314. [DOI] [PubMed] [Google Scholar]
- Satter RL, Galston AW. Mechanisms of control of leaf movements. Annu Rev Plant Physiol. 1981;32:83–110. [Google Scholar]
- Satter RL, Morse MJ, Lee Y, Crain RC, Cote G, Moran N. Light and clock-controlled leaflet movements in Samanea saman: a physiological, biophysical and biochemical analysis. Bot Acta. 1988;101:205–213. [Google Scholar]
- Schroeder JI, Hagiwara S. Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature. 1989;338:427–430. [Google Scholar]
- Stoeckel H, Takeda K. Calcium-sensitivity of the plasmalemmal delayed rectifier potassium current suggests that calcium influx in pulvinar protoplasts from Mimosa pudica L. can be revealed by hyperpolarization. J Membr Biol. 1995;146:201–209. doi: 10.1007/BF00238009. [DOI] [PubMed] [Google Scholar]
- Suh S, Moran N, Lee Y. Blue light activates depolarization-dependent K+ channels in flexor cells from Samanea saman motor organs via two mechanisms. Plant Physiol. 2000;123:833–843. doi: 10.1104/pp.123.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C, Latorre R. Kinetics of Ca2+-activated K+ channels from rabbit muscle incorporated into planar bilayers: evidence for a Ca2+ and Ba2+ blockade. J Gen Physiol. 1983;82:543–568. doi: 10.1085/jgp.82.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]