Abstract
Potato common scab, which is caused by soil-borne Streptomyces species, is a severe plant disease that results in a significant reduction in the economic value of potatoes worldwide. Due to the lack of efficacious pesticides, crop rotations, and resistant potato cultivars against the disease, we investigated whether biological control can serve as an alternative approach. In this study, multiple Bacillus species were isolated from healthy potato tubers, and Bacillus amyloliquefaciens Ba01 was chosen for further analyses based on its potency against the potato common scab pathogen Streptomyces scabies. Ba01 inhibited the growth and sporulation of S. scabies and secreted secondary metabolites such as surfactin, iturin A, and fengycin with potential activity against S. scabies as determined by imaging mass spectrometry. In pot assays, the disease severity of potato common scab decreased from 55.6 ± 11.1% (inoculated with S. scabies only) to 4.2 ± 1.4% (inoculated with S. scabies and Ba01). In the field trial, the disease severity of potato common scab was reduced from 14.4 ± 2.9% (naturally occurring) to 5.6 ± 1.1% after Ba01 treatment, representing evidence that Bacillus species control potato common scab in nature.
Introduction
Potato is one of the most important crops worldwide but is easily affected by serious diseases such as late blight, bacterial wilt, soft rot, and common scab. Potato common scab can be caused by at least four gram-positive bacteria from the Streptomyces genus, including S. scabies, S. acidiscabies, S. turgidiscabies, and S. ipomoeae. Of these, S. scabies is the best characterized pathogen [1, 2]. The typical scab symptom on potato tubers is superficial, raised, deep-pitted corky lesions that affect tuber quality and marketability in fresh markets or processing operations. Scab symptoms are mainly caused by secreted toxins from Streptomyces species such as thaxtomins, concanamycin, borrelidin, or FD-891 [1, 3, 4]. Among these, thaxtomin A, a cellulose biosynthesis inhibitor, is well characterized and considered the main virulence factor of most Streptomyces species [5].
Because of the limited understanding of the genetic diversity of S. scabies and the genetic differences in various potato cultivars, developing effective control strategies for potato common scab is challenging [6–10]. Traditional control methods such as soil amendment/chemistry to lower soil pH, soil fumigation with chloropicrin, pre-sowing treatment of seed tubers with fluazinam or flusulfamide, and crop rotation are usually not efficacious and may harm the environment [6, 11, 12]. Research in biological control as an alternative approach is emerging. Several studies have used biocontrol agents, including non-pathogenic Streptomyces spp. [13–15], Pseudomonas spp. [16–18], and Bacillus spp. [19, 20], to combat potato common scab. Currently, only two studies have reported the effects of Bacillus spp. on potato common scab. Han et al. demonstrated that Bacillus sp. sunhua secreted iturin A and macrolactin A as potential antibacterial agents and inhibited the sporulation of S. scabies [19]. Meng et al. showed that Bacillus amyloliquefaciens BAC03 secretes LCI protein as an antibacterial product and increases plant height and tuber weight, in addition to reducing the disease severity of potato common scab in pot assays [20]. However, whether these two Bacillus species can control the scab pathogen Streptomyces species in agricultural fields is unclear.
Bacillus species, including B. subtilis and B. amyloliquefaciens, produce endospores, resulting in a long shelf life (~2 years), a desirable characteristic for a biocontrol agent. Although B. amyloliquefaciens is a close relative of B. subtilis, the secondary metabolites produced by the two species are distinct. For example, B. amyloliquefaciens FZB42, a commercial strain, dedicates 8.5% of the genome (~340 kb) to synthesize secondary metabolites, which is two-fold higher than that of the B. subtilis 168 isolate (4.5% of its genome; ~350 kb) [21–24]. Based on genomic analyses, FZB42 can secrete many secondary metabolites, including lipopeptides (surfactin, iturin, and fengycin), polyketides (macrolactin, bacillaene, and difficidin), and volatiles (acetoin/2,3-butandiol), which may directly suppress the growth of plant pathogens or elicit induced systemic resistance (ISR) of the plant host.
In this study, we isolated a biocontrol agent, B. amyloliquefaciens Ba01, from healthy potato tubers and showed its inhibitory effects on the growth and sporulation of the potato common scab pathogen S. scabies. The potential inhibitory mechanism of Ba01 against S. scabies is possibly through the secretion of lipopeptides such as surfactin, iturin A, and fengycin. Ba01 not only reduced the disease severity of potato common scab in pot assays, but also in scab naturally occurring in the field, representing a documented example that Bacillus species reduce the disease severity of potato common scab in nature.
Materials and methods
Strains, growth media, chemicals, and phylogenetic analysis
Bacillus species and S. scabies strains are listed in Table 1. Bacillus species isolated from healthy potato tubers with nutrient agar medium were identified by sequencing 16S rRNA with primers fD1 and rP2 [25] (Table A in S1 File) and the gyrase A gene with primers p-gyrA-F and p-gyrA-R [26] (Table A in S1 File). S. scabies strains isolated from scabby potato tubers with nutrient agar or SCNA medium were identified by sequencing 16S rDNA with primers fD1 and rP2 and by PCR-RFLP with amplification of the partial atpD gene with primers atpDPF and atpDPR [27] (Table A in S1 File). The sequence results were compared in the Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequence alignment and analysis of gene similarity were performed using the ClustalW program in the MEGA 7 program. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model [28]. Phylogenetic trees were drawn using the MEGA7 program [29].
Table 1. Strains used in this study.
| Strain | Species | Source |
|---|---|---|
| Ba01 | Bacillus amyloliquefaciens | Houli, Taiwan |
| Ba02 | Bacillus amyloliquefaciens | Houli, Taiwan |
| Ba03 | Bacillus amyloliquefaciens | Houli, Taiwan |
| Ba04 | Bacillus amyloliquefaciens | Houli, Taiwan |
| Bs01 | Bacillus subtilis | Douliu, Taiwan |
| B. sp. | Bacillus sp. | Dounan, Taiwan |
| PS01 | Streptomyces scabies | [41] |
| PS02 | Streptomyces scabies | [41] |
| PS07 | Streptomyces scabies | [41] |
| PS08 | Streptomyces scabies | [41] |
| YC1020 | Streptomyces scabies | [41] |
| YC1028 | Streptomyces scabies | [41] |
| A3(2) | Streptomyces coelicolor | [42] |
| CL2 | Streptomyces scabies | Dounan, Taiwan |
| CL3 | Streptomyces scabies | Dounan, Taiwan |
| CL4 | Streptomyces scabies | Dounan, Taiwan |
| CL5 | Streptomyces scabies | Dounan, Taiwan |
Media used in this study include Nutrient Agar medium (NA: HIMEDIA, India), Starch Casein Nitrate Agar medium (SCNA; 0.001% FeSO47.H2O, 0.002% CaCO3, 0.005% MgSO4.7H2O, 0.03% casein, 0.2% K2HPO4, 0.2% NaCl, 0.2% KNO3, 1% starch, and 1.8% agar), Yeast Malt Extract agar medium (YME medium; 0.4% yeast extract, 1% malt extract, 0.4% dextrose, and 2% agar), Streptomyces Sporulation Medium #1 (SSM1; 0.1% MgSO4.7H2O, 0.1% casein, 0.1% yeast extract, 1% soluble starch, 0.05% K2HPO4, and 2% agar), Luria-Bertani medium (LB; MDBio, Inc., Taiwan), and Mueller Hinton medium (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The following chemical agents were used in this study: iturin A (Sigma-SI-I1774, St Louis, MO, USA), surfactin (Sigma-SI-S3523), and fengycin (Sigma-SMB00292).
Disk diffusion assays
Disk diffusion assays were used to test anti-S. scabies activity of six Bacillus strains, including four B. amyloliquefaciens isolates, one B. subtilis isolate, and one Bacillus sp. isolate. Bacillus species were grown overnight at 37°C in LB liquid medium, and the cell concentrations were calculated by measuring OD600 (1 OD600 = ~2x107 CFU/mL). S. scabies isolates were grown on solid SSM1 medium at 28°C for 14 days. Spores were collected with cell scrapers and washed twice with ddH2O before determining spore concentration by serial dilution. Then, 100 μL of 107 CFU/mL S. scabies cells were spread on YME solid medium, and two 6-mm disks were placed on the surface of each agar plate. Then, 3 μL of 1 OD600 Bacillus cells or ddH2O were placed on the disk, and plates were cultured at 28°C for five days before being photographed.
Determination of minimum and fractional inhibitory concentrations
Minimum inhibitory concentration (MIC) indices were determined following the CLSI-M07-A9 protocol. Freshly collected spores of S. scabies PS07 were diluted with Mueller Hinton medium, and 50 μL were added to each well to a final concentration at 5x105 CFU/mL in a 96-well plate format. The compounds were two-fold serially diluted with Mueller Hinton medium, and 50 μL of each dilution were added to the wells containing spores, yielding a total volume of 100 μL per well. Iturin A concentrations ranged from 0.125 to 64 μg/mL, while surfactin and fengycin concentrations ranged from 0.25 to 64 μg/mL. The plates were incubated at 28°C for 48 h. The MIC was defined as the lowest concentration of a compound that completely inhibited the growth of S. scabies PS07 as detected by the unaided eye.
The fractional inhibitory concentration (FIC) of compounds was determined via checkerboard titration assays. Freshly collected spores of S. scabies PS07 were diluted with Mueller Hinton medium, and 50 μL were added to each well of a 96-well plate to a final concentration of 5x105 CFU/mL. The two compounds to be assessed were two-fold serially diluted with Mueller Hinton medium, and 25 μL of each compound were added to the wells containing spores, yielding a total volume of 100 μL per well. Iturin A concentrations ranged from 1 to 64 μg/mL, while surfactin and fengycin concentrations ranged from 0.25 to 64 μg/mL. The plates were incubated at 28°C for 48 h. The MIC or FIC of compounds, either alone or in combination, was defined as the lowest concentration of each compound that completely inhibited the growth of S. scabies PS07 as detected by the unaided eye. The FIC index was calculated by the following formula: FIC = (MIC of compound A combined) / (MIC of compound A alone) + (MIC of compound B combined) / (MIC of compound B alone). For calculation purposes, an MIC >64 μg/mL was assumed to be 128 μg/mL.
Scanning electron microscopy
The agar plates from the disk diffusion assay were cultured at 25°C for five days, and the undifferentiating and non-inhibition zones were excised and fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) at 4°C for 24 h. The samples were then rinsed three times with cold 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h each time, soaked in 2% osmium tetroxide in sodium cacodylate buffer at 4°C for 5 h, and rinsed again. After fixation, the samples were dehydrated in a series of ethanol concentrations (50%, 70%, 80%, 90%, and 95%) at 4°C for 10 min each and in 100% acetone twice (first at 4°C for 15 min and then at room temperature for 15 min). The samples were then critical point dried in liquid CO2, mounted on metal stubs for gold coating, and observed under the scanning electron microscope JEOL JSM 6510 at 15 kV.
Imaging mass spectrometry
S. scabies PS07 was initially inoculated in a vertical line on 2% YME solid agar plates. After 12 h, B. amyloliquefaciens Ba01 was inoculated in a horizontal line on the same plate for an additional 24 h, and the region in which the two microbes interacted, as well as the individual regions with compounds secreted from each microbe, were excised and transferred to an indium tin oxide-coated glass target plate. Pure, serially diluted surfactin, iturin A, and fengycin were used as standards to determine the compound concentration secreted by Ba01. Surfactin and fengycin were two-fold serially diluted with methanol from 2,500 μg/mL to 39.1 μg/mL and from 312.5 μg/mL to 4.9 μg/mL, respectively, while iturin A was two-fold serially diluted with 50% ethanol from 10,000 μg/mL to 156.3 μg/mL. Then, 1 μL of each diluted compound was dropped onto the YME agar. The universal matrix powder (1:1 mixture of α-cyano-4-hydroxycinnamic acid and 2, 5-dihydroxybenzoic acid) was sprinkled on the top of samples. After covering with matrix, the samples were exposed to air overnight at 37°C until dried completely.
Imaging mass spectrometry (IMS) data were collected on a Bruker Autoflex Speed MALDI TOF/TOF spectrometer at the Agricultural Biotechnology Research Center, Academia Sinica and analyzed by Bruker Compass Version 1.2 Software Suite [30]. For the samples used in this study, linear positive ion mode was applied with 85% laser power and 333.3 Hz laser frequency. The 1,100 μm of raster interval in the X and Y dimensions were applied, and each raster summed up to 500 shots. The detection mass range was set from m/z 100 to 2125.
Pot assays
Pot assays were conducted to examine the biocontrol activity of Ba01 against S. scabies PS07. S. scabies was grown in solid SSM1 medium at 25°C for 14 days, and the spores were collected by cell scrapers and washed twice with ddH2O. Potato (cultivar: Kennebec) tuber pieces with a bud were air dried and planted in nursery pots filled with sterilized soil (1:1 peat moss:King Root plant substrate) at 25°C for three weeks until seedlings emerged. Each potato seedling was transferred to a seven-inch pot in a greenhouse with the temperature maintained between 18°C and 22°C. Five-week-old potato plants were then inoculated with S. scabies PS07 by mixing 50 mL (2x109 CFU/mL) of inoculum with the soil. For B. amyloliquefaciens Ba01 treatment, Ba01 was grown overnight in LB liquid medium at 37°C and rinsed twice with ddH2O. Then, 50 mL containing 2.8 x109 Ba01 cells were applied to the treatments. Each of the five treatments included three potato plants, for a total of 15 plants: (a) mock control without PS07 or Ba01; (b) PS07 only; (c) Ba01 only; (d) PS07 and Ba01 inoculated on the same day; and (e) PS07 inoculated first for 14 days, followed by inoculation of Ba01. Potato plants were watered twice weekly, and fertilizer (HYPONeX 2) was applied weekly beginning the fifth week after planting. Potato tubers were harvested 12 weeks after planting, and the disease severity in each treatment was calculated as follows, originally described by Wanner et al. [13]: Σ (percentage coverage by lesions x predominant lesion type x number of tubers with these scores) / (18 x total number of potato tubers evaluated) x 100. Lesion types were divided into four degrees: 0 = no symptoms, 1 = superficial, 2 = raised, and 3 = pitted. Percentage coverage by lesions for each tuber was classified in seven degrees: 0 = no scab, 1 = 0.1% to 2%, 2 = 2.1% to 5%, 3 = 5.1% to 10%, 4 = 10.1% to 25%, 5 = 25.1% to 50%, and 6 = ≥50%. Disease incidence was calculated by determining the proportion of tubers with >5% scab coverage from collected potato tubers.
Ethics statement
The field trial was conducted on private land owned by Mr. Rong Su, who permitted and assisted us in performing the biological control experiments using B. amyloliquefaciens Ba01 against potato common scab. Because the land was privately owned, specific permission was not required. This land did not house endangered or protected species.
Field trial
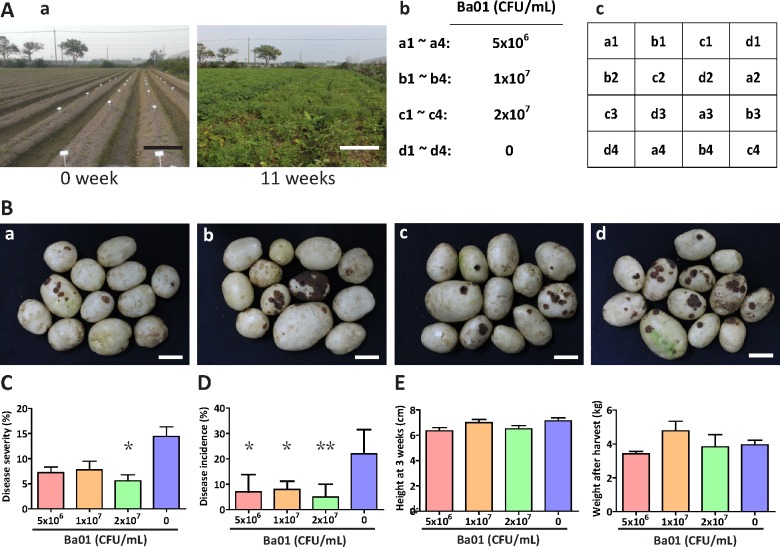
The 11-week trial was conducted in an agricultural field with naturally occurring potato common scab from December 2, 2015 to February 17, 2016 in Dounan, Taiwan (23.669919, 120.463699). The temperature in Dounan was between 15°C and 21°C during the experiment. The field was divided into 16 blocks (four blocks per treatment) with a randomized complete block design. Each block contained 20 potato plants, and 10 protective potato plants were located between blocks. Treatments were as follows: (A) 5x106 CFU/mL Ba01, (B) 1x107 CFU/mL Ba01, (C) 2x107 CFU/mL Ba01, and (D) mock (no Ba01). Ba01 was applied at 0, 2, and 3 weeks after planting. The Ba01 cells in a volume of 200 mL were poured directly onto the soil that covered the tuber buds at week 0, and 400 mL of Ba01 were applied 2 and 3 weeks after planting. We randomly collected 25 potato tubers from each block to evaluate the disease severity and incidence of each block, and four blocks of the same treatment were used to determine the mean ± standard error and compared to other treatments based on Tukey’s test.
Tuber slice assays
Tuber slice assays were used to test the pathogenicity of multiple S. scabies strains isolated from the field trial, as well as the biocontrol activity of Ba01 against these S. scabies strains. The procedures used have been described previously by Loria et al. [31], with minor modifications. In brief, the surface of potato tuber was sterilized with 1% NaOCl and cores (1.2 cm) of pith tissue were removed from the tubers. The cores were then sliced into pieces (0.25 cm thick) and placed on moist filter paper in glass petri dishes. Three tuber pieces were used for each treatment. Test strains were grown on solid SSM1 medium for 14 days at 28°C, and agar disks with the sporulating colony were inverted onto the tuber pieces. Tuber pieces were incubated in moist glass petri dishes at 28°C for six days in the dark and photographed.
Results
B. amyloliquefaciens inhibited the growth and sporulation of S. scabies
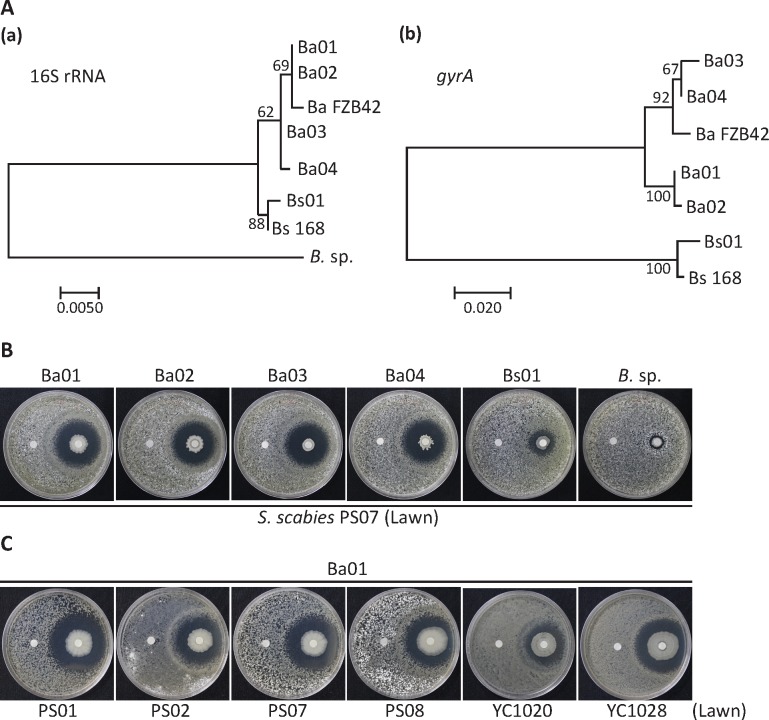
Bacillus strains, including four B. amyloliquefaciens isolates (Ba01, Ba02, Ba03, and Ba04), a B. subtilis (Bs01) isolate, and a Bacillus sp. isolate with species unidentified were used to test anti-S. scabies activity in vitro. The 16S rRNA and gyrA gene sequences of Bacillus strains were analyzed to determine phylogenetic assignments. Ba01 clustered closely with Ba02, and Ba03 clustered closely with Ba04. Bs01 and Bacillus sp. strains were distinct from Ba01~04 (Fig 1A). In the disk diffusion assays, Ba01, Ba02, Ba03, and Ba04 effectively inhibited the growth of S. scabies PS07, while B. subtilis Bs01 and Bacillus sp. demonstrated subtle inhibition (Fig 1B). Due to the similar activity of the four B. amyloliquefaciens isolates, we chose Ba01 to conduct further experiments. We tested the antibacterial activity of Ba01 against multiple S. scabies isolates (Fig 1C) and found that clear and undifferentiating inhibition zones were formed (Fig 2A and 2B). Based on morphologic observations from scanning electron microscopy, S. scabies PS07 hyphae were spiral and formed sporulation septa with constrictions (Fig 2C), while hyphae in the undifferentiating zone displayed vegetative septa without constrictions (Fig 2D).
Fig 1. B. amyloliquefaciens Ba01 showed antibacterial activity against S. scabies causing potato common scab.
A. Phylogenetic trees based on either the (a) 16S rRNA sequence or (b) gyrA gene sequence showed the evolutionary relationships between Bacillus isolates. The numbers at the nodes represent bootstrap values. The scale bars indicating the numbers of substitutions per nucleotide position were (a) 0.005 and (b) 0.02. B. Multiple Bacillus isolates exhibited a diverse degree of antibacterial activity against S. scabies PS07. Disk diffusion assays were used to test the antibacterial activity of Bacillus species against S. scabies. In this experiment, 106 S. scabies spores in 100 μL were spread on solid YME medium, and 3 μL of 1 OD600 (~6x104 cells) of Bacillus isolates were loaded on the right disk, while 3 μL of ddH2O were loaded on the left disk as a control. C. Ba01 was selected to test its antibacterial activity against multiple S. scabies isolates. S. scabies isolates were spread on solid YME medium, and 3 μL of 1 OD600 of Ba01 were added to the right disk and ddH2O to the left disk. All plates were incubated at 28°C for five days and photographed.
Fig 2. B. amyloliquefaciens Ba01 inhibited the growth and sporulation of S. scabies PS07.
A. A disk diffusion assay was used to observe the antibacterial effects of Ba01 against S. scabies PS07 on solid YME medium. Clear (C) and undifferentiating (U) zones were observed around the disk loaded with the Ba01 isolate. B. The morphology of Ba01 from panel A was observed under a scanning electron microscope. C. S. scabies PS07 without Ba01 treatment (ddH2O treated) produced spiral hyphae and sporulation septa with constrictions. D. S. scabies PS07 in the undifferentiating zone exhibited vegetative/smooth hyphae and septa without constrictions. Resolution is 10,000X. The scale bar represents 1 μm.
Identification of secondary metabolites secreted from Ba01 that potentially inhibited S. scabies growth
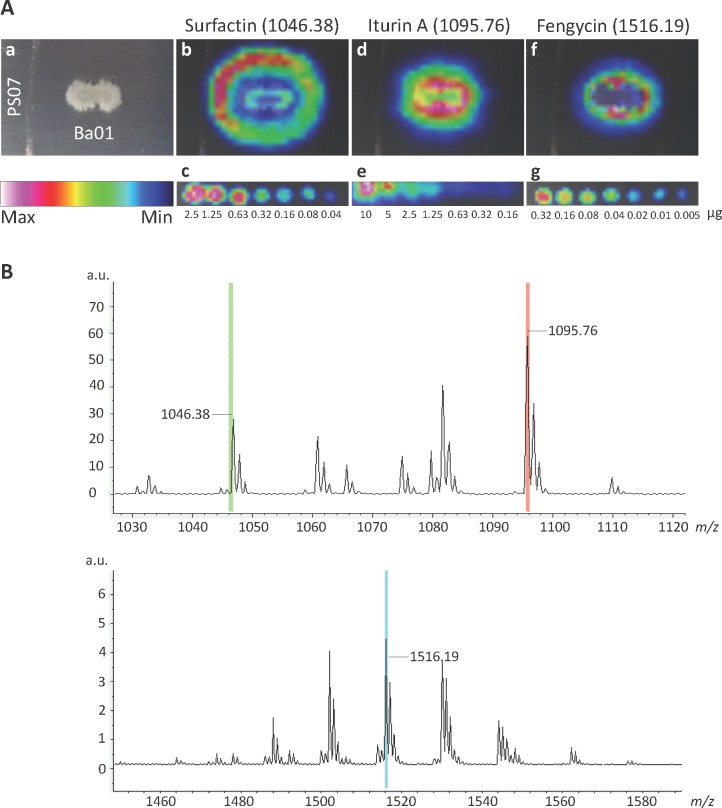
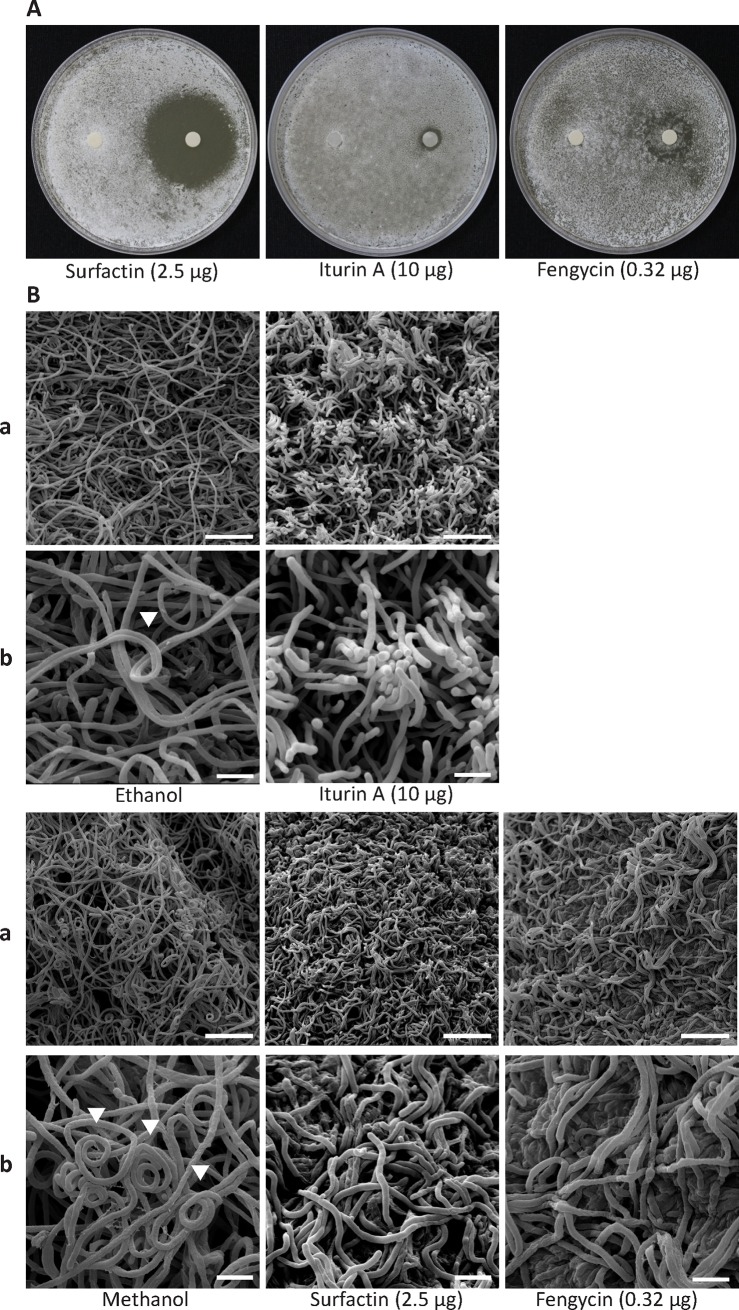
IMS is a powerful technique used to visualize the spatial distribution of various chemical compounds based on their molecular masses (e.g., m/z ratio) [30]. We used this technique to identify potential secondary metabolites secreted from Ba01 while it inhibited the growth of S. scabies PS07 on YME solid medium (Fig 3A). Three major peaks representing surfactin (m/z 1046.38), iturin A (m/z 1095.76), and fengycin (m/z 1516.19) were detected in the region in which Ba01 inhibited the growth of S. scabies. Of these, surfactin was secreted largely to neighboring regions at a maximum amount of 2.5 μg, as evidenced by density gradient and IMS spectra. Iturin A was secreted at the strongest intensity with a maximum amount of 10 μg, while fengycin was secreted at a maximum amount of 0.32 μg (Fig 3A and 3B). In order to confirm our findings, pure iturin A, surfactin, and fengycin were used to test the anti-S. scabies activity. Each of the three compounds demonstrated anti-S. scabies activity as evidenced by undifferentiating inhibition zones, but none of the compounds completely inhibited the growth of S. scabies (Fig 4A). Surfactin (2.5 μg) exhibited better inhibitory effects on the growth of S. scabies than iturin A (10 μg) or fengycin (0.32 μg) based on the disk diffusion assays (Fig 4A). Meanwhile, S. scabies treated with surfactin, iturin A, or fengycin exhibited defects in the formation of spiral hyphae, while S. scabies treated with iturin A demonstrated additional morphological defects such as clumped hyphae (Fig 4B). These results suggested that these three compounds identified by IMS inhibit the growth and differentiation of S. scabies. However, each compound was determined to have a MIC value >64 μg/mL against S. scabies (Table 2). The contrasting results between the disk diffusion assays (i.e., partial inhibition [undifferentiating] zone on solid medium) and the high MIC values of surfactin, iturin A, and fengycin in liquid medium against S. scabies might be because the definition of MIC endpoint requires the complete inhibition of growth, whereas some growth persisted even with extensive inhibition. Further experiments were performed to test if these three compounds demonstrated synergistic activity against S. scabies. However, synergistic activity was not detected based on determined FICs of 2, suggesting no interaction between compounds (Table 2).
Fig 3. Imaging mass spectrometry of Ba01 against S. scabies PS07.
A. (a) S. scabies PS07 was initially inoculated in a vertical line on a 2% YME solid agar plate. After 12 h, B. amyloliquefaciens Ba01 was inoculated in a horizontal line on the same plate for another 24 h. (b) The IMS image of an ion with m/z 1046.38 represents surfactin. (c) The image represents two-fold serial diluted surfactin as a standard control ranging from 2.5 to 0.04 μg. (d) The IMS image of an ion with m/z 1095.76 represents iturin A. (e) The image represents two-fold serial diluted iturin A as a standard control ranging from 10 to 0.16 μg. (f) The IMS image of an ion with m/z 1516.19 represents fengycin. (g) The image represents two-fold serial diluted fengycin as a standard control ranging from 0.32 to 0.005 μg/mL. Intensity gradients for surfactin, iturin A, and fengycin are normalized and illustrated by color histogram (maximum, white; minimum, black). B. The mass spectra of IMS regions include three major peaks: m/z 1046.38 (surfactin), 1095.76 (iturin A), and 1516.19 (fengycin).
Fig 4. Surfactin, iturin A, and fengycin inhibited the growth and formation of spiral hyphae of S. scabies PS07.
A. Disk diffusion assays were used to test the anti-S. scabies activity of surfactin, iturin A, and fengycin. Approximately 106 S. scabies spores were spread on YME solid agar, and a 6-mm disk containing iturin A (dissolved in ethanol), surfactin (dissolved in methanol), or fengycin (dissolved in methanol) were pressed on the surface of an agar plate and incubated at 28°C for five days. B. The morphology of S. scabies PS07 in the undifferentiating zone of panel A was observed under a scanning electron microscope. (a) The magnification is 5,000X, and the scale bar represents 10 μm. (b) The magnification is 15,000X, and the scale bar represents 2.5 μm.
Table 2. Minimum and fractional inhibitory concentrations of compounds against Streptomyces scabies PS07.
| Strain | MIC alone (μg/mL) | MIC combined (μg/mL) | *FIC index | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Iturin A | Surfactin | Fengycin | Iturin A, Surfactin | Iturin A, Fengycin | Surfactin, Fengycin | Iturin A + Surfactin | Iturin A + Fengycin | Surfactin + Fengycin | |
| PS07 | >64 | >64 | >64 | >64, >64 | >64, >64 | >64, >64 | 2 | 2 | 2 |
*FIC ≤0.5 (synergy); FIC >0.5 but ≤4 (no interaction); FIC >4 (antagonism)
Ba01 reduced the disease severity of potato common scab in pot assays
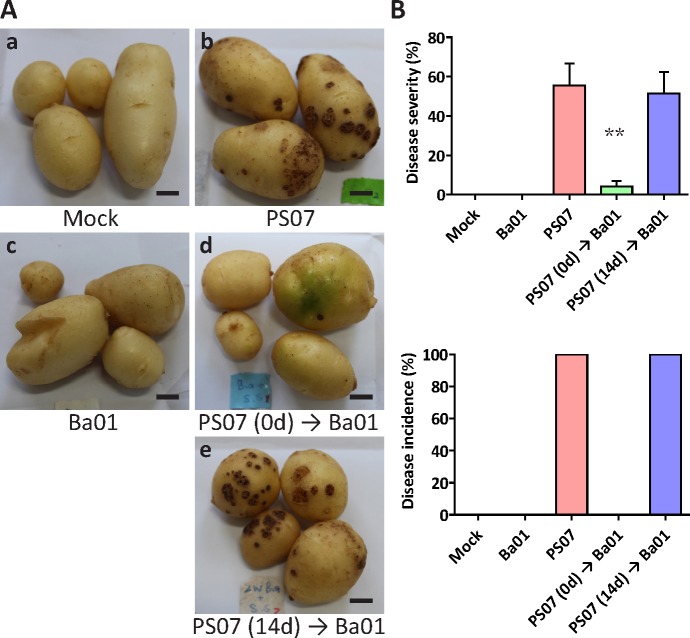
Pot assays were performed to test whether Ba01 can reduce the disease severity of potato common scab. Fifteen potato plants were divided into five treatments: (a) mock control without PS07 or Ba01; (b) PS07 only; (c) Ba01 only; (d) PS07 and Ba01 inoculated on the same day; and (e) inoculation of PS07 first, then Ba01 inoculation after 14 days. Potato tubers were harvested 12 weeks after planting, and the disease severity was calculated based on an index of percentage coverage by lesions multiplied by the index of predominant lesion type, divided by 18 [13]. Treatment with Ba01 reduced the disease severity of potato common scab from 55.6 ± 11.1% (inoculated with S. scabies only) to 4.2 ± 1.4% (inoculated with S. scabies and Ba01 on the same day) (Fig 5A and 5B) (P < 0.01, Tukey’s test). However, when Ba01 was applied two weeks after S. scabies inoculation, it did not reduce the disease severity or incidence (Fig 5A and 5B), indicating that Ba01 demonstrated preventive rather than therapeutic activity.
Fig 5. Ba01 reduced the disease severity of potato common scab in pot assays.
The growth conditions of S. scabies PS07, Ba01, and potato plants were described in the materials and methods. A. Three five-week-old potato plants were used for each treatment: (a) mock control without inoculation of S. scabies PS07 or Ba01; (b) inoculation of S. scabies PS07 only; (c) inoculation of Ba01 only; (d) inoculation of PS07 and Ba01 on the same day; and (e) inoculation of PS07 first, and then Ba01 inoculation after 14 days. The bar represents 1 cm. B. The disease severity and disease incidence of the potato common scab were reduced when potato plants were inoculated with PS07 and Ba01 on the same day. Data were expressed as the average of tubers collected from three potato plants ± standard error of the mean. P values were calculated using Tukey's test. Asterisks (**) indicate P < 0.01 as compared to the treatment of PS07 only.
Ba01 reduced the disease severity of potato common scab in the field
A field trial was conducted in an agricultural field with naturally occurring potato common scab (Fig 6A). Three treatments of Ba01 at 5x106, 1x107, and 2x107 CFU/mL, in addition to a mock control of 0 CFU/mL, in a randomized complete block design were used to test inhibitory activity against potato common scab disease (Fig 6A). Ba01 treatment at 2x107 CFU/mL significantly reduced disease severity from 14.4 ± 1.9% (no Ba01 treatment) to 5.6 ± 1.2% (P < 0.05; Tukey’s test) and decreased disease incidence from 21% to 5%. While Ba01 treatment at 5x106 and 1x107 CFU/mL did not reduce disease severity, disease incidence decreased from 21% to 7% and 8%, respectively (Fig 6B, 6C and 6D). Ba01 treatment did not stimulate plant growth or increase potato tuber yield (Fig 6E and 6F).
Fig 6. Ba01 reduced the severity of naturally occurring potato common scab in the field.
A. (a) An 11-week potato field trial was completed in Dounan, Taiwan. Bars = 100 cm. (b) Four treatments were treated with the Ba01 isolate at the concentrations indicated. (c) Each treatment had four blocks assigned by a randomized complete block design. B. Potato tubers were harvested from each Ba01 treatment: (1) Ba01 at 5x106 CFU/mL; (b) 1x107 CFU/mL; (c) 2x107 CFU/mL; and (d) water. Bars = 5 cm. C. The percentage of disease severity was calculated from 100 randomly selected tubers of each treatment. D. Disease incidence was calculated by determining the proportion of tubers with >5% scab coverage from 100 randomly selected tubers in each treatment. E. Potato plant height (left panel) and tuber weight (right panel) were not affected by Ba01 application. We randomly chose 25 potato tubers from each block to evaluate the disease severity and incidence of each block, and four blocks of the same treatment were used to determine the mean ± standard error and compare to other treatments. P values were calculated using Tukey's test. Asterisks *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively.
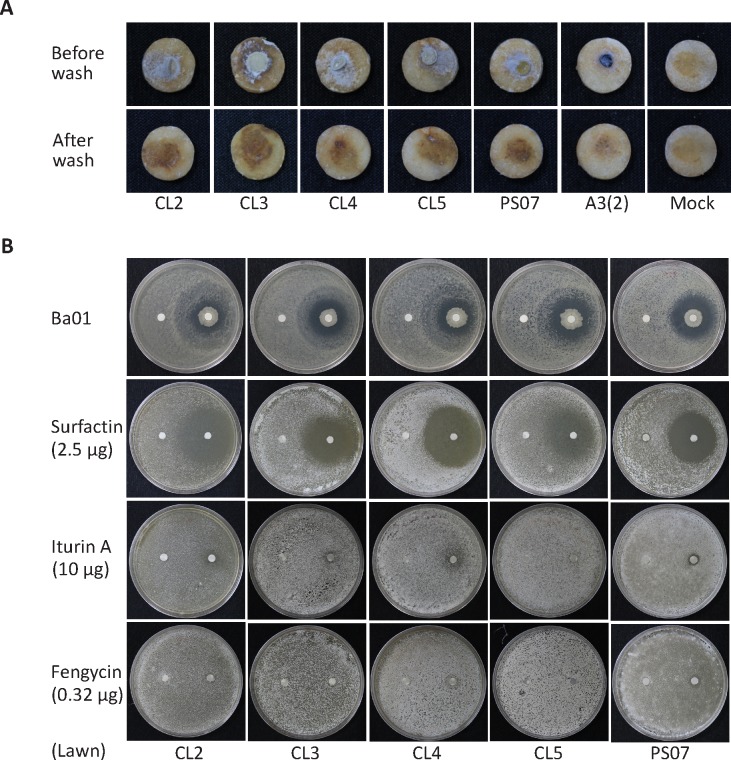
We isolated four Streptomyces isolates (CL2 to CL5) from scabby potato tubers in different experimental blocks and identified these strains as pathogenic S. scabies based on potato tuber slice assays (Fig 7A). Ba01 exhibited inhibitory effects toward four S. scabies isolates (CL2 to CL5), but these isolates were slightly less susceptible to Ba01 than PS07 based on disk diffusion assays. Interestingly, our data showed similar inhibitory activity of surfactin, iturin A, and fengycin against four S. scabies strains (CL2 to CL5) isolated from the field and S. scabies PS07 (Fig 7B), indicating that differential tolerance of S. scabies strains to Ba01 might be due to specific characteristics of S. scabies and not to the secretion of surfactin, iturin A, or fengycin.
Fig 7. Ba01 inhibits the growth of multiple S. scabies strains isolated from the field trial.
A. Potato slide assays were used to test the pathogenicity of S. scabies isolates. Agar disks with or without S. scabies spores were inverted onto potato tuber slices and incubated at 28°C for six days in the dark and photographed. Nonpathogenic Streptomyces coelicolor was used as a negative control, and a SSM1 agar disc was used as a mock control. B. Disc diffusion assays were used to test the anti-bacterial activity of Ba01 and three pure compounds against multiple S. scabies strains.
Discussion
Biological control agents have been extensively studied to combat plant pathogens in order to reduce environmental pollution, ecological disturbance due to pesticides used in fumigation, and pre-sowing tuber/seed treatments. In this study, we tested six Bacillus isolates and found that B. amyloliquefaciens Ba01 exhibited stronger inhibitory effects than B. subtilis or Bacillus sp. against the potato common scab pathogen. Additionally, S. scabies hyphae in the undifferentiated zone were still undifferentiated after 20 days, indicating that the hyphal growth of S. scabies was inhibited and was not simply due to growth delay. To our knowledge, only two studies have investigated the use of Bacillus isolates (Bacillus sp. sunhua and B. amyloliquefaciens BAC03) to control this plant pathogen [19, 20]. Our findings that surfactin, iturin A, and fengycin acted as antibiotics against S. scabies are partially supported by studies in which Han and colleagues showed that iturin A was responsible for combating Streptomyces species. In addition, these three compounds, controlled by the srf, bmy, and fen genes, respectively, have been shown to be secreted from B. amyloliquefaciens FZB42 [32]. Although surfactin and fengycin were not identified by Han et al. to be secreted by Bacillus sp. sunhua, these compounds have been detected in studies against other pathogens [33]. In this study, we provide evidence that Ba01 secretes surfactin, iturin A, and fengycin based on IMS analysis, while the other peaks seen on the spectra are derivatives of surfactin, iturin, or fengycin. Based on this powerful IMS technique, we also determined the secreted amount of these three compounds, which will aid in the investigation of which compound exerts the strongest effects. Ba01 produces a stronger inhibitory effect than any single pure compound (surfactin, iturin A, or fengycin), which indicates that compounds secreted from Ba01 might exert synergistic effects S. scabies. Further experiments may include disrupting a single gene (srfAD, ituD, or fenA), two genes (srfAD ituD, srfAD fenA, or ituD fenA), or three genes (srfAD ituD fenA) from the Ba01 isolate and testing the ability of these mutants to inhibit S. scabies growth and sporulation or their biocontrol efficacy against potato common scab in pot assays and field trials. Such experiments will provide additional evidence to show that synthesis of these compounds is required to inhibit the growth of S. scabies and reduce scab symptoms. However, several attempts to obtain these mutants via homologous recombination or in-frame deletion strategies were unsuccessful in the Ba01 isolate (S1 File), possibly due to the ‘wild’ nature of Ba01 (ie, low level of genetic competence and transformation amenability). Similar disruption techniques were successful in B. amyloliquefaciens FZB42 (data not shown), indicating that an efficient gene deletion is dependent on a specific isolate. In the future, disruption methods will need further modifications to disrupt genes in the Ba01 isolate.
We tested whether surfactin, iturin A, and fengycin exhibited synergistic effects by determining the FIC, but did not observe synergistic activity between any two compounds (Table 2). Because the endpoint of FIC requires complete inhibition, the combination of any two compounds might partially inhibit growth but does not reach complete inhibition. Meanwhile, these results may also indicate that other compounds or proteins secreted from Ba01 may enhance the effects of surfactin, iturin A, or fengycin on S. scabies growth suppression. Previous studies showed that Bacillus species secrete compounds such as macrolactin A, bacillaene, and difficidin in the presence of plant pathogens [33–35]. Therefore, additional studies to identify other secondary metabolites or proteins secreted from B. amyloliquefaciens are warranted. Interestingly, several studies have demonstrated that B. amyloliquefaciens not only can suppress diseases but also promote plant growth [20, 36]. Nevertheless, in this study Ba01 only suppressed scab symptoms and did not promote potato plant growth or increase tuber weight.
In addition to secreting antibacterial compounds against S. scabies, it is possible that Ba01 elicits induced systemic resistance (ISR) of the potato plant. Previous studies have shown that Bacillus isolates can elicit ISR of various plant hosts, including tomato, bell pepper, muskmelon, watermelon, sugar beet, tobacco, cucumber, and loblolly pine, to combat pathogens [37–39]. For example, Chowdhury and colleagues found that cyclic lipopeptides and volatiles produced by B. amyloliquefaciens FZB42 can trigger ISR pathways and protect plants against pathogens [40]. Future studies involving pot experiments and field trials can address whether Ba01 triggers ISR signaling.
The less potent inhibitory effects of Ba01 against potato common scab in the field trial than those in the pot assays may be due to the complicated microbial community in the soil of the field trial. We isolated several S. scabies isolates from scabby potato tubers in the field and found that these isolates were pathogenic in potato tuber slice assays. However, these S. scabies isolates were less susceptible to Ba01 than S. scabies PS07, which was used in the pot assays, suggesting that S. scabies isolates in the field were relatively tolerant to Ba01 and that Ba01 demonstrates differential inhibitory effects against various S. scabies isolates. In the future, we may consider combining two or more B. amyloliquefaciens isolates in order to control multiple Streptomyces isolates in the field. Meanwhile, additional field tests with diverse moisture, temperature, soil pH, and environmental conditions can be conducted in order to test the efficacy of Ba01 in various situations. Although the Ba01 isolate can reduce disease severity in both pot assays and field trials, the application of Ba01 on potato plants via pouring of Ba01 solution requires extensive labor. This application strategy is challenging to farmers who grow potatoes due to the limited availability of manpower. Alternatively, an agricultural machine with an automatic pouring system can be developed to replace human workers, or the Ba01 isolate could be mixed with soil.
In summary, we present a report that B. amyloliquefaciens reduces symptoms of naturally occurring potato common scab. The potential mechanisms by which Ba01 inhibits the growth of S. scabies at least in part are through the secretion of surfactin, iturin A, or fengycin. The evidence that Ba01 inhibits the growth and sporulation of S. scabies and reduces scab symptoms in pot assays and field trials suggests that Ba01 is a potential biocontrol agent for controlling potato common scab.
Supporting information
(PDF)
Acknowledgments
We are grateful to Ms. Shang-Jie Yu for assistance with scanning electron microscopy, Mr. Rong Su for providing the potato field for the trial, Dr. Chih-Hung Huang for providing Streptomyces coelicolor A3(2) strain and Cecelia Wall for language edits. This work is financially supported by 104AS-10.7.3-BQ-B1(5), 105AS-10.6.3-BQ-B1, and 106AS-9.5.3.-BQ-B3(3) from the Bureau of Animal and Plant Health Inspection and Quarantine, and 102-230-B-002-041-MY2, 104-2320-B-002-063-MY3, and 106-2923-B-002-001-MY3 from the Ministry of Science & Technology in Taiwan.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is financially supported by 104AS-10.7.3-BQ-B1(5), 105AS-10.6.3-BQ-B1, and 106AS-9.5.3.-BQ-B3(3) from the Bureau of Animal and Plant Health Inspection and Quarantine, and 102-230-B-002-041-MY2, 104-2320-B-002-063-MY3, and 106-2923-B-002-001-MY3 from the Ministry of Science & Technology in Taiwan.
References
- 1.Loria R, Kers J, Joshi M. Evolution of plant pathogenicity in Streptomyces. Annu Rev Phytopathol. 2006;44:469–87. doi: 10.1146/annurev.phyto.44.032905.091147 [DOI] [PubMed] [Google Scholar]
- 2.Lambert DH, Loria R, Labeda DP, Saddler GS. Recommendation for the conservation of the name Streptomyces scabies. Request for an Opinion. Int J Syst Evol Microbiol. 2007;57(Pt 10):2447–8. doi: 10.1099/ijs.0.65275-0 [DOI] [PubMed] [Google Scholar]
- 3.Bignell DR, Seipke RF, Huguet-Tapia JC, Chambers AH, Parry RJ, Loria R. Streptomyces scabies 87–22 contains a coronafacic acid-like biosynthetic cluster that contributes to plant-microbe interactions. Mol Plant Microbe Interact. 2010;23(2):161–75. doi: 10.1094/MPMI-23-2-0161 [DOI] [PubMed] [Google Scholar]
- 4.Bignell DR, Fyans JK, Cheng Z. Phytotoxins produced by plant pathogenic Streptomyces species. J Appl Microbiology. 2014;116(2):223–35. [DOI] [PubMed] [Google Scholar]
- 5.King RR, Calhoun LA. The thaxtomin phytotoxins: sources, synthesis, biosynthesis, biotransformation and biological activity. Phytochemistry. 2009;70(7):833–41. doi: 10.1016/j.phytochem.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 6.Dees MW, Wanner LA. In search of better management of potato common scab. Potato Res. 2012; 55:249–68. [Google Scholar]
- 7.St-Onge R, Goyer C, Coffin R, Filion M. Genetic diversity of Streptomyces spp. causing common scab of potato in eastern Canada. Syst Appl Microbiol. 2008;31(6–8):474–84. doi: 10.1016/j.syapm.2008.09.002 [DOI] [PubMed] [Google Scholar]
- 8.Wanner LA. A survey of genetic variation in Streptomyces isolates causing potato common scab in the United States. Phytopathology. 2006;96(12):1363–71. doi: 10.1094/PHYTO-96-1363 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Bignell DR, Zuo R, Fan Q, Huguet-Tapia JC, Ding Y, et al. Promiscuous pathogenicity islands and phylogeny of pathogenic Streptomyces spp. Mol Plant Microbe Interact. 2016;29(8):640–50. doi: 10.1094/MPMI-04-16-0068-R [DOI] [PubMed] [Google Scholar]
- 10.Huguet-Tapia JC, Lefebure T, Badger JH, Guan D, Pettis GS, Stanhope MJ, et al. Genome content and phylogenomics reveal both ancestral and lateral evolutionary pathways in plant-pathogenic Streptomyces Species. Appl Environ Microbiol. 2016;82(7):2146–55. doi: 10.1128/AEM.03504-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin RP, Honeycutt CW, Griffin TS, Olanya OM, Halloran JM, He Z. Effects of different potato cropping system approaches and water management on soilborne diseases and soil microbial communities. Phytopathology. 2011;101(1):58–67. doi: 10.1094/PHYTO-04-10-0100 [DOI] [PubMed] [Google Scholar]
- 12.Wilson C, Ransom L, Pemberton B. The relative importance of seed‐borne inoculum to common scab disease of potato and the efficacy of seed tuber and soil treatments for disease control. J Phytopathol. 1999;147:13–8. [Google Scholar]
- 13.Wanner LA, Kirk WW, Qu XS. Field efficacy of nonpathogenic Streptomyces species against potato common scab. J Appl Microbiol. 2014;116(1):123–33. doi: 10.1111/jam.12336 [DOI] [PubMed] [Google Scholar]
- 14.Hiltunen LH, Ojanpera T, Kortemaa H, Richter E, Lehtonen MJ, Valkonen JP. Interactions and biocontrol of pathogenic Streptomyces strains co-occurring in potato scab lesions. J Appl Microbiol. 2009;106(1):199–212. doi: 10.1111/j.1365-2672.2008.03992.x [DOI] [PubMed] [Google Scholar]
- 15.Eckwall EC, Schottel JL. Isolation and characterization of an antibiotic produced by the scab disease-suppressive Streptomyces diastatochromogenes strain PonSSII. J Ind Microbiol Biotechnol. 1997;19(3):220–5. [DOI] [PubMed] [Google Scholar]
- 16.St-Onge R, Gadkar VJ, Arseneault T, Goyer C, Filion M. The ability of Pseudomonas sp. LBUM 223 to produce phenazine-1-carboxylic acid affects the growth of Streptomyces scabies, the expression of thaxtomin biosynthesis genes and the biological control potential against common scab of potato. FEMS Microbiol Ecol. 2011;75(1):173–83. doi: 10.1111/j.1574-6941.2010.00992.x [DOI] [PubMed] [Google Scholar]
- 17.Arseneault T, Goyer C, Filion M. Biocontrol of potato common scab is associated with high Pseudomonas fluorescens LBUM223 populations and phenazine-1-carboxylic acid biosynthetic transcript accumulation in the potato geocaulosphere. Phytopathology. 2016;106(9):963–70. doi: 10.1094/PHYTO-01-16-0019-R [DOI] [PubMed] [Google Scholar]
- 18.Arseneault T, Goyer C, Filion M. Pseudomonas fluorescens LBUM223 Increases Potato Yield and Reduces Common Scab Symptoms in the Field. Phytopathology. 2015;105(10):1311–7. Epub 2015/05/12. doi: 10.1094/PHYTO-12-14-0358-R [DOI] [PubMed] [Google Scholar]
- 19.Han JS, Cheng JH, Yoon TM, Song J, Rajkarnikar A, Kim WG, et al. Biological control agent of common scab disease by antagonistic strain Bacillus sp. sunhua. J Appl Microbiol. 2005;99(1):213–21. doi: 10.1111/j.1365-2672.2005.02614.x [DOI] [PubMed] [Google Scholar]
- 20.Meng QX, Jiang HH, Hanson LE, Hao JJ. Characterizing a novel strain of Bacillus amyloliquefaciens BAC03 for potential biological control application. J Appl Microbiol. 2012;113(5):1165–75. doi: 10.1111/j.1365-2672.2012.05420.x [DOI] [PubMed] [Google Scholar]
- 21.Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Sussmuth R, et al. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol. 2009;140(1–2):27–37. doi: 10.1016/j.jbiotec.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 22.Chen XH, Koumoutsi A, Scholz R, Borriss R. More than anticipated—production of antibiotics and other secondary metabolites by Bacillus amyloliquefaciens FZB42. J Mol Microbiol Biotechnol. 2009;16(1–2):14–24. doi: 10.1159/000142891 [DOI] [PubMed] [Google Scholar]
- 23.Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, et al. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat Biotechnol. 2007;25(9):1007–14. doi: 10.1038/nbt1325 [DOI] [PubMed] [Google Scholar]
- 24.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56(4):845–57. doi: 10.1111/j.1365-2958.2005.04587.x [DOI] [PubMed] [Google Scholar]
- 25.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S, Moholkar VS, Goyal A. Isolation, identification, and characterization of a cellulolytic Bacillus amyloliquefaciens strain SS35 from rhinoceros dung. ISRN Microbiol. 2013;2013:728134 doi: 10.1155/2013/728134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corrêa DB, Salomã A, Rodrigues-neto J, Harakava R, Destéfano SAL. Application of PCR-RFLP technique to species identification and phylogenetic analysis of Streptomyces associated with potato scab in brazil based on partial atpD gene sequences. Eur J Plant Pathol. 2015;142(1):1–12. [Google Scholar]
- 28.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10(3):512–26. doi: 10.1093/oxfordjournals.molbev.a040023 [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih CJ, Chen PY, Liaw CC, Lai YM, Yang YL. Bringing microbial interactions to light using imaging mass spectrometry. Nat Prod Rep. 2014;31(6):739–55. doi: 10.1039/c3np70091g [DOI] [PubMed] [Google Scholar]
- 31.Loria R, Bukhalid R, Creath R, Leiner R, Olivier M, Steffens J. Differential production of thaxtomins by pathogenic Streptomyces species in vitro. Phytopathology. 1995;85:537–41. [Google Scholar]
- 32.Koumoutsi A, Chen X-H, Henne A, Liesegang H, Hitzeroth G, Franke P, et al. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol. 2004;186(4):1084–96. doi: 10.1128/JB.186.4.1084-1096.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chowdhury SP, Hartmann A, Gao X, Borriss R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—a review. Front Microbiol. 2015;6:780 doi: 10.3389/fmicb.2015.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X-H, Vater J, Piel J, Franke P, Scholz R, Schneider K, et al. Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. J Bacteriol. 2006;188(11):4024–36. doi: 10.1128/JB.00052-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider K, Chen X-H, Vater J, Franke P, Nicholson G, Borriss R, et al. Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. J Nat Prod. 2007;70(9):1417–23. doi: 10.1021/np070070k [DOI] [PubMed] [Google Scholar]
- 36.Yuan J, Ruan Y, Wang B, Zhang J, Waseem R, Huang Q, et al. Plant growth-promoting rhizobacteria strain Bacillus amyloliquefaciens NJN-6-enriched bio-organic fertilizer suppressed Fusarium wilt and promoted the growth of banana plants. J Agric Food Chem. 2013;61(16):3774–80. doi: 10.1021/jf400038z [DOI] [PubMed] [Google Scholar]
- 37.Choudhary DK, Johri BN. Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol Res. 2009;164(5):493–513. doi: 10.1016/j.micres.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 38.Kloepper JW, Ryu CM, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94(11):1259–66. doi: 10.1094/PHYTO.2004.94.11.1259 [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Gutierrez L, Zeriouh H, Romero D, Cubero J, de Vicente A, Perez-Garcia A. The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate- and salicylic acid-dependent defence responses. Microb Biotechnol. 2013;6(3):264–74. doi: 10.1111/1751-7915.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chowdhury SP, Uhl J, Grosch R, Alqueres S, Pittroff S, Dietel K, et al. Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol Plant Microbe Interact. 2015;28(9):984–95. doi: 10.1094/MPMI-03-15-0066-R [DOI] [PubMed] [Google Scholar]
- 41.Lin C, Feng RY, Tsai CH, Chen YL. The fungicide tebuconazole inhibits potato common scab caused by Streptomyces scabies. J Plant Medicine. 2017;59(3):31–7. [Google Scholar]
- 42.Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature. 2002;417(6885):141–7. doi: 10.1038/417141a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.