Abstract
Gangliosides are glycosphingolipids concentrated in glycolipid-enriched membrane microdomains. Mainly restricted to the nervous system in healthy adult, complex gangliosides such as GD3 and GD2 have been shown to be involved in aggressiveness and metastasis of neuro-ectoderm derived tumors such as melanoma and neuroblastoma. GD3 synthase (GD3S), the key enzyme that controls the biosynthesis of complex gangliosides, was shown to be over-expressed in Estrogen Receptor (ER)-negative breast cancer tumors, and associated with a decreased overall survival of patients. We previously demonstrated that GD3S expression in ER-negative breast cancer cells induced a proliferative phenotype and an increased tumor growth. In addition, our results clearly indicate that Tumor Necrosis Factor (TNF) induced GD3S over-expression in breast cancer cells via NFκB pathway. In this study, we analyzed the effect of TNF on ganglioside biosynthesis and expression in breast cancer cells from different molecular subtypes. We showed that TNF up-regulated the expression of GD3S in MCF-7 and Hs578T cells, whereas no change was observed for MDA-MB-231. We also showed that TNF induced an increased expression of complex gangliosides at the cell surface of a small proportion of MCF-7 cells. These results demonstrate that TNF differentially regulates gangliosides expression in breast cancer cell lines and establish a possible link between inflammation at the tumor site environment, expression of complex gangliosides and tumor development.
Introduction
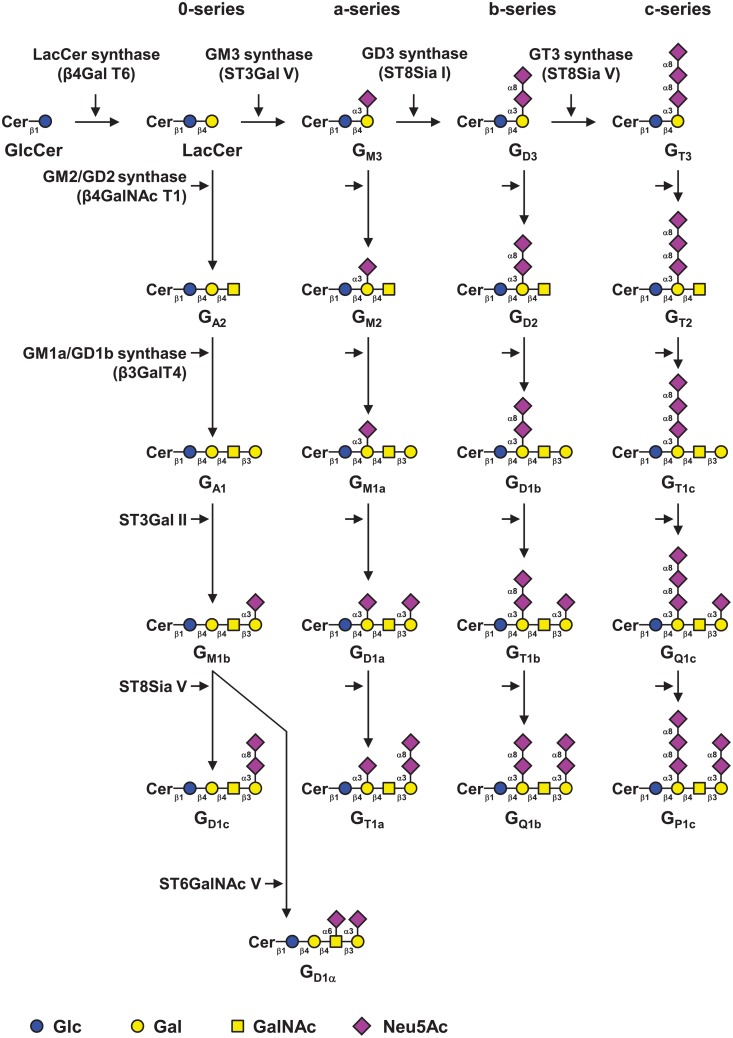
Gangliosides define as subclass of acidic glycosphingolipids (GSL) carrying one or more sialic acid residues in the carbohydrate moiety. Gangliosides are essential compounds of the outer leaflet of the plasma membrane, where they interact with phospholipids, cholesterol, and transmembrane proteins to form glycolipid-enriched microdomains [1] in which they interact with signaling molecules including receptors tyrosine kinases and integrins, and regulate signal transduction pathways involved in cell adhesion, proliferation, and recognition processes, [2–4]. The carbohydrate moiety of gangliosides is synthesized in the Golgi apparatus by specific glycosyltransferases (GT) and gangliosides are classified in four series according to the number of sialic acid residues linked to the lactosylceramide (Fig 1). Changes in ganglioside composition are observed between human tissues, complex gangliosides with two or more sialic acid residues being normally restricted to the nervous system [5,6]. Changes in the structure of gangliosides can also occur under pathological conditions [7–9] and a neo-expression of disialogangliosides such as GD2 and GD3 is observed in several cancers from neuroectoderm origin including melanoma and neuroblastoma, in which they play a key role in invasion and metastasis [10], and disialogangliosides are attractive targets for cancer immunotherapy [11,12].
Fig 1. Biosynthesis of gangliosides.
Gangliosides are classified in 4 series according to the number of sialic acid residues linked to lactosylceramide (LacCer) [22]. The 0-series gangliosides are directly synthesized from LacCer and the precursors of other series are synthesized by specific sialyltransferases: ST3Gal V (GM3 synthase), ST8Sia I (GD3 synthase) and ST8Sia V (GT3 synthase), respectively. The elongation of precursors is performed by the sequential action of N-acetyl-galactosaminyltransferase (β4GalNAc T1), galactosyltransferase (β3Gal T4) and sialyltransferases (ST3Gal II and ST8Sia V). Cer, ceramide. Adapted from [4].
In breast cancer, complex gangliosides GD3 and 9-O-acetyl-GD3 have been reported to be over-expressed in about 50% of invasive ductal breast carcinoma [13] and the GD3 synthase (GD3S) gene displayed higher expression among estrogen receptor negative breast cancer tumors [14], associated with poor pathohistological grading and a decreased free survival of patients [15]. We previously demonstrated that the expression of GD3S in breast cancer cells induced a proliferative phenotype and increased tumor growth due to the constitutive activation of c-Met receptor by GD2 ganglioside [16–18]. We also demonstrated that GD3S gene expression is up-regulated by TNF via the NFκB pathway and that estradiol repressed GD3S expression in estrogen receptor (ER) positive breast cancer cells by preventing NFκB nuclear translocation [19]. Moreover, GD2 ganglioside was recently identified as a new breast cancer stem cells specific marker [20].
Given the critical role of both GD2 ganglioside and inflammation in breast cancer aggressiveness [21], and in order to provide a general overview of the effect of inflammatory cytokines on ganglioside biosynthesis, we examined the effect of TNF on the expression of the main ganglioside-specific GT genes as well as cell surface gangliosides in breast cancer cells from different molecular subtypes.
Materials and methods
Antibodies
Anti-GM3 mAb GMR6 (mouse IgM), anti-GM2 mAb MK1-16 (mouse IgM) and anti-GD1b GGR12 (mouse IgG3) were purchased from AMS Biotechnology (Abingdon, UK). Anti-GD3 mAb R24 (mouse IgG3) and anti-GD2 mAb 14.G2a (mouse IgG2a) were purchased from Abcam (Cambridge, MA, USA). Fluorescein isothiocyanate (FITC)-conjugated cholera toxin B subunit from Vibrio cholerae used for GM1a expression analysis was from Sigma-Aldrich (Saint-Quentin Fallavier, France). Alexa Fluor® 488 donkey anti-mouse IgG (H+L) and Alexa Fluor® 488 anti-mouse IgM (μ-chain) were purchased from Molecular Probes Invitrogen (Cergy Pontoise, France).
Cell culture
The human breast cancer cell lines MCF-7 and MDA-MB-231 were obtained from LGC standards (Molsheim, France) and the American Type Cell Culture Collection (Rockville, MD, USA), respectively. The Hs578T human breast cancer cell line [23] was kindly provided by Dr Van Slambrouck (Department of Chemistry and Biochemistry, South Dakota State University, Brookings, SD 57007, USA). Cells were routinely grown in monolayer cultures in Dulbecco’s Modified Eagle Medium (DMEM) with 4.5 g/L glucose, 2 mM L-glutamine, supplemented with 10% fetal calf serum (FCS) and 100 μg/mL penicillin-streptomycin (Lonza, Verviers, Belgium) at 37°C in an atmosphere of 5% CO2. Cells were treated with 40 ng/mL TNF (Eurobio AbCys, Paris, France) during 12–24 h before qPCR analysis or during 48 h for other experiments.
RNA Extraction, cDNA Synthesis and Quantitative real-time Polymerase Chain Reaction (qPCR)
Total RNA was extracted from the different cell lines using the Nucleospin RNA II kit (Macherey-Nagel, Düren, Germany). The amount of extracted RNA was quantified using a DeNovix DS-11 spectrophotometer (DeNovix Inc., Wilmington, DE, USA) and the purity of the RNA was checked by the ratio of absorbance at 260 nm vs. 280 nm. Total RNA was subjected to reverse transcription using the Maxima First Strand cDNA Synthesis Kit (ThermoFisher Scientific, France) according to the protocol provided by the manufacturer. PCR reactions were performed using 2X SYBR® Green Universal QPCR Master Mix (ThermoFisher Scientific). Primer sequences (Eurogentec, Seraing, Belgium) used for the PCR reactions are given in Table 1. HPRT gene was used to normalize the expression of transcripts of interest. PCR reactions were performed using the Mx3005p Quantitative System (Stratagene) as previously described [16]. PCR conditions were as follows: 95°C for 30 s, Tm °C for 60 s, 72°C for 20 s (40 cycles). The analysis of amplification was performed using the Mx3005p software and relative quantification was performed using the method described by Pfaffl that takes in account the efficiency of each sequence amplification [24]. Student’s t-test was used for statistical analysis. A p-value < 0.05 was considered statistically significant.
Table 1. Primer pairs used for qPCR experiments.
| Gene | Glycosyltransferase | Sense primer Antisense primer | Tm (°C) |
|---|---|---|---|
| HPRT |
5’-GCCAGACTTTGTTGGATTTG-3’ 5’-CTCTCATCTTAGGCTTTGTATTTTG-3’ |
58 | |
| ST3GAL5 | GM3 synthase |
5’-ATCGGTGTCATTGCCGTTGT-3’ 5’-TTCATAGCAGCCATGCATTGA-3’ |
51 |
| ST8SIA1 | GD3 synthase |
5’-GCGATGCAATCTCCCTCCT-3’ 5’-TTGCCGAATTATGCTGGGAT-3’ |
60 |
| B4GALNT1 | GM2/GD2 synthase |
5’-CAGCGCTCTAGTCACGATTGC-3’ 5’-CCACGGTAACCGTTGGGTAG-3’ |
51 |
| B3GALT4 | GM1/GD1b synthase |
5’-TATGTGCTGTCAGCGTCTGCT-3’ 5’-ACAAAGACATCCTCTAATGGGAGAA-3’ |
60 |
Extraction and preparation of glycosphingolipids
Cultured cells were washed twice with ice-cold PBS and detached from T175 flasks with cell dissociation non-enzymatic solution. Cells were sonicated on ice in 200 μL of water. The resulting material was dried under vacuum and sequentially extracted by CHCl3/CH3OH (2:1, v/v), CHCl3/CH3OH (1:1, v/v) and CHCl3/CH3OH/H2O (1:2:0.8, v/v/v). Supernatants were pooled, dried and subjected to a mild saponification in 0.1 M NaOH in CHCl3/CH3OH (1:1) at 37°C for 2 h and then evaporated to dryness [25]. Samples were reconstituted in CH3OH/H2O (1:1, v/v) and applied to a reverse phase C18 cartridge (Waters, Milford, MA, USA) equilibrated in the same solvent. After washing with CH3OH/H2O (1:1, v/v), GSL were eluted by CH3OH, CHCl3/CH3OH (1:1, v/v) and CHCl3/CH3OH (2:1, v/v). The elution fraction was dried under nitrogen stream prior to structural analysis.
Mass spectrometry analysis of GSL
Prior to mass spectrometry analysis, GSL were permethylated according to Ciucanu and Kerek [26]. Briefly, compounds were incubated 2 h in a suspension of 200 mg/mL NaOH in dry DMSO (300 μL) and CH3I (200 μL). The methylated derivatives were extracted in CHCl3 and washed several times with water. The reagents were evaporated and the sample was dissolved in CHCl3 in the appropriate dilution. MALDI-MS and MS/MS analyses of permethylated GSL were performed on 4800 Proteomics Analyzer (Applied Biosystems, Framingham, MA, USA) mass spectrometer, operated in the positive reflectron mode. For MS acquisition, 5 μL of diluted permethylated samples in CHCl3 were mixed with 5 μL of 2,5-dihydroxybenzoic acid matrix solution (10 mg/mL dissolved in CHCl3/CH3OH (1:1, v/v)). The mixtures (1 μL) were then spotted on the target plate and air dried. MS survey data comprises a total of 50 sub-spectra of 1500 laser shots. Peaks observed in the MS spectra were selected for further MS/MS. CID MS/MS data comprises a total of 100 sub-spectra of 3000 laser shots. Two or more spectra can be combined post-acquisition with mass tolerance set at 0.1 Da to improve S/N ratio. The potential difference between the source acceleration voltage and the collision cell was set to 1 kV and argon was used as collision gas.
Flow cytometry analysis
Cells were plated in 6-well plates (2.5 × 105 cells/well). The next day, cells were treated with 40 ng/mL TNF for 48 h. Cells were detached by trypsin and incubated for 30 min at 4°C with anti-gangliosides mAbs: anti-GM3 (1:100), anti-GM2 (1:100), anti-GD3 (1:100), anti-GD2 (1:100) or anti-GD1b (1:100), all diluted in PBS containing 0.5% bovine serum albumin (BSA; Sigma-Aldrich, Lyon, France). After washing with PBS/0.5% BSA, cells were incubated for 30 min on ice with Alexa Fluor® 488 anti-mouse IgM or IgG (dilution 1:500 in PBS/0.5% BSA). Controls were performed using secondary antibodies alone. To analyze GM1a expression, cells were incubated with a FITC-conjugated cholera toxin B subunit from Vibrio cholerae (1:1000) for 30 min at 4°C. Controls were performed using non-stained cells. Cells were then subjected to flow cytometry analysis using a FACScalibur flow cytometer from Becton Dickinson (Le-Pont-de-Claix, France).
Immunofluorescence staining
MCF-7 cells were plated on glass coverslips (ThermoFisher Scientific) in 6-well plates (2 × 105 cells/well). The next day, cells were treated with 40 ng/mL TNF for 48 h. Cells were then washed with DPBS and fixed 15 min in 4% paraformaldehyde at room temperature. After washing, cells were permeabilized in 0.5% Triton X-100 and blocked 1 h in a blocking buffer containing 2% BSA, 2% FCS and 0.2% gelatin in PBS. Cells were incubated for 1 h at room temperature with anti-GM3 (1:100), anti-GM2 (1:100), anti-GD3 (1:100), anti-GD2 (1:100) or anti-GD1b (1:50) antibodies diluted in blocking buffer. After three washes with PBS, cells were incubated 1 h with appropriated secondary antibodies (dilution 1:1000 in blocking buffer) and with the FITC-conjugated cholera toxin B subunit from Vibrio cholerae for GM1a analysis (1:4000). Cells were washed with PBS then with deionized water, and finally mounted in Fluorescent mounting medium (Dako, Carpenteria, CA, USA). The nuclei were stained with DAPI. Slides were examined under a Zeiss LSM 700 confocal microscope. The image acquisition characteristics were the same throughout the different conditions to ensure the comparability of the results and to allow the comparison of the fluorescence levels. For statistical analysis, images of ≥ 100 cells for each condition were collected based on the DAPI signal and were then analyzed for GD3 and GD2 expression using an automated algorithm on ImageJ software. Anova was used for statistical analysis. A p-value < 0.05 was considered statistically significant.
Results
MS analysis of GSL of breast cancer cells
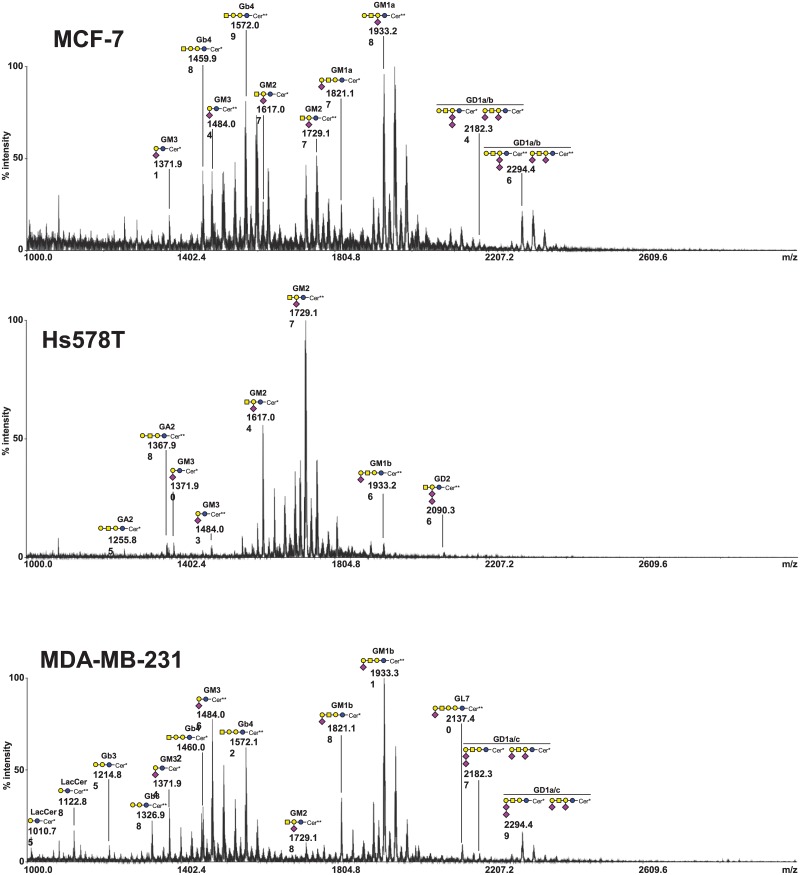
The composition of total GSL was determined by MALDI-TOF mass spectrometry after permethylation in three commonly used breast cancer cell lines, i.e. the luminal-A MCF-7 cells, the basal-like MDA-MB-231 cells, whose ganglioside composition was already analyzed [16], and the basal-like Hs578T cells, whose ganglioside profile appeared to be quite different from the two other cell lines (Fig 2 and Table 2). Two ceramide isoforms are commonly expressed in human tissues due to the substitution of the sphingosine moiety by palmitic acid C16:0 or lignoceric acid C24:0. In MCF-7 cells, an additional ceramide isoform was observed with a cerotic acid C26:0 linked to sphingosine. As expected, MCF-7 essentially expressed a-series gangliosides, mainly GM1a, whereas MDA-MB-231 cells mainly expressed GM1b as confirmed by fragmentation of permethylated GSL using MALDI-TOF/TOF mass spectrometry (data not shown). GM3 and GM2 were also expressed in both cell lines but in lower amounts. Both cell lines also expressed low amounts of disialogangliosides. Signals at m/z 2182.34 and 2294.46 corresponding to GD1 isomers with 3 hexoses, one N-acetylhexosamine and 2 N-acetylneuraminic acid residues, were confirmed as and mixture of GD1a (90%) and GD1b (10%) in MCF-7 cells and a mixture of GD1a (40%) and GD1c (60%) in MDA-MB-231 cells. Both cell lines also expressed neutral GSL from globo-series such as Gb3 and Gb4. These results are in agreement with previous published data [18,27]. The GSL composition of Hs578T cells was never previously determined and appeared relatively simpler compared to the two other cell lines. Hs578T cells mainly expressed the monosialoganglioside GM2 but very low amounts of GM3 and GM1b. Neutral GSL from globo-series such as Gb3 and Gb4 was also almost absent and only a low amount of GA2 was detected. Finally, Hs578T cells also expressed a low amount of the disialoganglioside GD2 as indicated by the signal at m/z 2090.1.
Fig 2. Mass spectrometry (MALDI-TOF) analysis of permethylated GSL isolated from MCF-7, Hs578T and MDA-MB-231 cells.
Blue circle, Glc; yellow circle, Gal; yellow square, GalNAc; purple diamond, Neu5Ac. Only masses corresponding to N-palmitoyl- (C16:0) or N-lignoceroyl- (C24:0) 2-amino-4-octadecene-1,3-diol (sphingosine) are indicated. Cer*: Ceramide d18:1/C16:0; Cer**: Ceramide d18:1/C24:0.
Table 2. Compositional assignments of singly charged sodiated molecular ions [M + Na]+, observed in MALDI-TOF mass spectrometry spectra of permethylated GSL from MCF-7, Hs578T and MDA-MB-231 cells.
| Fatty acids | GSL | Calculated mono-isotopic molecular masses | MCF-7 | Hs578T | MDA-MB-231 |
|---|---|---|---|---|---|
| 16:0 | LacCer | 1010.7485 | - | - | 1010.75 |
| 24:0 | LacCer | 1122.8743 | - | - | 1122.88 |
| 16:0 | Gb3 | 1214.8483 | - | - | 1214.85 |
| 24:0 | Gb3 | 1326.9741 | - | - | 1326.98 |
| 16:0 | GA2 | 1255.8748 | - | 1255.85 | - |
| 24:0 | GA2 | 1367.9736 | - | 1367.98 | - |
| 16:0 | GM3 | 1371.9222 | 1371.91 | 1371.90 | 1371.94 |
| 24:0 | GM3 | 1484.0480 | 1484.04 | 1484.03 | 1484.06 |
| 16:0 | Gb4 | 1459.9746 | 1459.98 | 1459.96 | 1460.02 |
| 24:0 | Gb4 | 1572.1004 | 1572.09 | 1572.08 | 1572.12 |
| 16:0 | GM2 | 1617.0485 | 1617.07 | 1617.04 | 1617.10 |
| 24:0 | GM2 | 1729.1743 | 1729.17 | 1729.17 | 1729.18 |
| 16:0 | GD3 | 1733.0958 | - | - | - |
| 24:0 | GD3 | 1845.2216 | - | - | - |
| 16:0 | GM1a/b | 1821.1483 | 1821.17 (GM1a) |
1821.18 (GM1b) |
|
| 24:0 | GM1a/b | 1933.2741 | 1933.28 (GM1a) |
1933.26 (GM1b) |
1933.31 (GM1b) |
| 16:0 | GD2 | 1978.2222 | - | 1978.24 | - |
| 24:0 | GD2 | 2090.3480 | - | 2090.36 | - |
| 16:0 | GT3 | 2094.2695 | - | - | - |
| 24:0 | GT3 | 2206.3953 | - | - | - |
| 16:0 | GL7 | 2025.2481 | - | - | - |
| 24:0 | GL7 | 2137.3739 | - | - | 2137.40 |
| 16:0 | GD1a/b | 2182.3219 | 2182.34 (GD1a/b) |
- | 2182.37 (GD1a/c) |
| 24:0 | GD1a/b | 2294.4477 | 2294.46 (GD1a/b) |
- | 2294.49 (GD1a/c) |
Only masses corresponding to N-palmitoyl- (C16:0) or N-lignoceroyl- (C24:0) 2-amino-4-octadecene-1,3-diol (sphingosine) are indicated. Assignments were confirmed by mass spectrometry fragmentation of permethylated GSL by MALDI-TOF/TOF (data not shown). LacCer, lactosylceramide; Gb, globoside.
Effect of TNF on the expression of ganglioside GTs in breast cancer cell lines
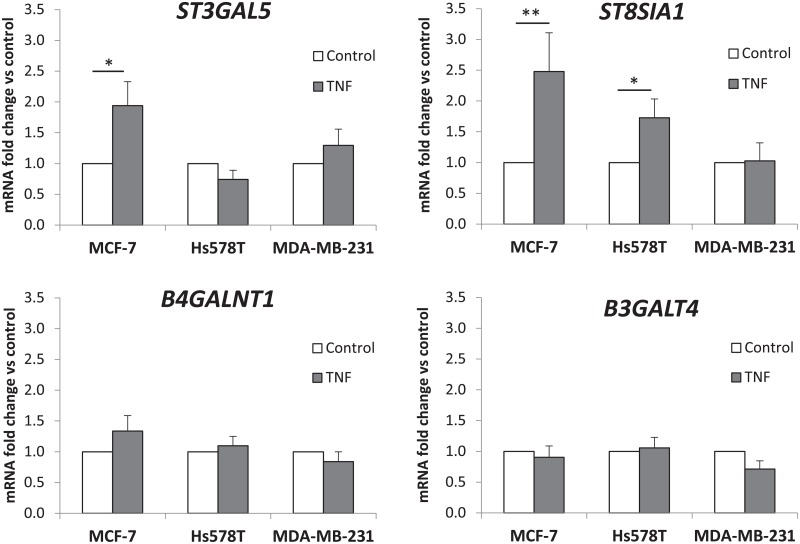
The effect of TNF on the expression of glycosyltransferase genes involved in ganglioside biosynthesis was determined by qPCR for the three cell lines. The mRNA levels for each GT gene, i.e. ST3GAL5 encoding the GM3 synthase, ST8SIA1 encoding the GD3 synthase, B4GALNT1 encoding the GM2/GD2 synthase and B3GALT4 encoding the GM1/GD1b synthase, were normalized to the expression of HPRT and reported to the expression of GT genes in non-treated cells (Fig 3). Our results confirmed the increased expression of ST8SIA1 after TNF treatment in MCF-7 and Hs578T as previously reported [19] but no change in the expression of ST8SIA1 was observed in MDA-MB-231 cells. We also confirmed by qPCR the expression of TNFR1, the gene encoding the TNF receptor 1, in the three cell lines (data not shown). TNF treatment also increased the expression of ST3GAL5 in MCF-7 but no significant change of ST3GAL5 expression was observed in both Hs578T and MDA-MB-231 cells. In parallel, no significant change of B4GALNT1 and B3GALT4 gene expression under TNF treatment was observed in the three cell lines. These results indicate that TNF treatment could increase the biosynthesis of b-series gangliosides, especially in MCF-7 cells, by increasing the expression of two essential GT genes (ST3GAL5 and ST8SIA1) whereas TNF had no effect on the expression of these genes in MDA-MB-231 cells.
Fig 3. Effect of TNF on GTs expression in MCF-7, Hs578T and MDA-MB-231 cell lines.
Cells were treated with 40 ng/mL TNF for 12-24h. ST3GAL5, ST8SIA1, B4GALNT1 and B3GALT4 mRNA expression was determined by qPCR. Results were normalized to the expression of HPRT and reported to the expression of GTs in non-treated cells (control). Each bar represents the mean +/- S.E.M. of n ≥ 3 experiments. *: p < 0.05, **: p < 0.01 vs. untreated (control).
Flow cytometry analysis of gangliosides at the cell surface of breast cancer cells
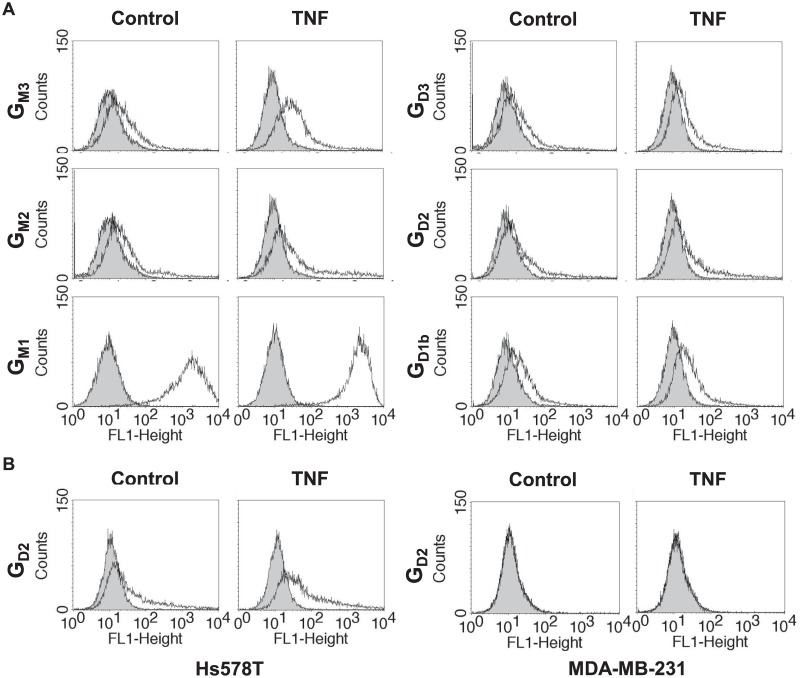
To establish the ganglioside profile of control and TNF-treated cells, we analyzed the composition of gangliosides at the cell surface using specific anti-ganglioside mAbs or cholera toxin B subunit for GM1a detection. The results presented in Fig 4A showed that MCF-7 cells expressed a limited number of gangliosides of the a-series, mainly GM1a. Low amounts of GM3 and GM2 were also detected confirming the results of MS analysis. Moreover, we were able to confirm the presence of low amount of GD1b but also of GD3 and GD2 in a very limited subpopulation of MCF-7 cells, whereas these b-series gangliosides were not detected by MS analysis. In agreement with the increased expression of GM3 synthase transcripts observed by qPCR, GM3 and GM2 expression was increased at the cell surface of MCF-7 cells after TNF treatment. Importantly and also in agreement with qPCR analysis, the subpopulation expressing GD3 and GD2 also appeared to be higher in MCF-7 treated cells than in control cells. In parallel, we analyzed the expression of gangliosides at the surface of Hs578T cells. Interestingly we could only detect an increased expression of GD2 in TNF-treated cells (Fig 4B). Finally, MDA-MB-231 cells presented a ganglioside pattern similar to MCF-7 cells (data not shown), and no change of ganglioside expression was observed at their cell surface after TNF treatment, in agreement with qPCR analysis. These results indicate that the increased expression of GT genes induced by TNF in both MCF-7 and Hs578T cells led to an increased expression of disialoganglioside GD2 at the cell surface.
Fig 4. Flow cytometry analysis of the effect of TNF on gangliosides expression at the surface of breast cancer cell lines.
MCF-7 (A), Hs578T and MDA-MB-231 (B) cells were treated with 40 ng/mL TNF for 48h. Control and treated cells were incubated with anti-GM3, GM2, GD3, GD2 or GD1b specific antibodies and revealed with and Alexa Fluor® 488 anti-mouse IgG or IgM. GM1a ganglioside was revealed with the FITC-conjugated cholera toxin B subunit from Vibrio cholerae. The grey peaks correspond to negative controls with anti-mouse IgG or IgM secondary antibody alone or with non-stained cells as control for GM1 signal. Results are from one experiment representative of 3 independent experiments.
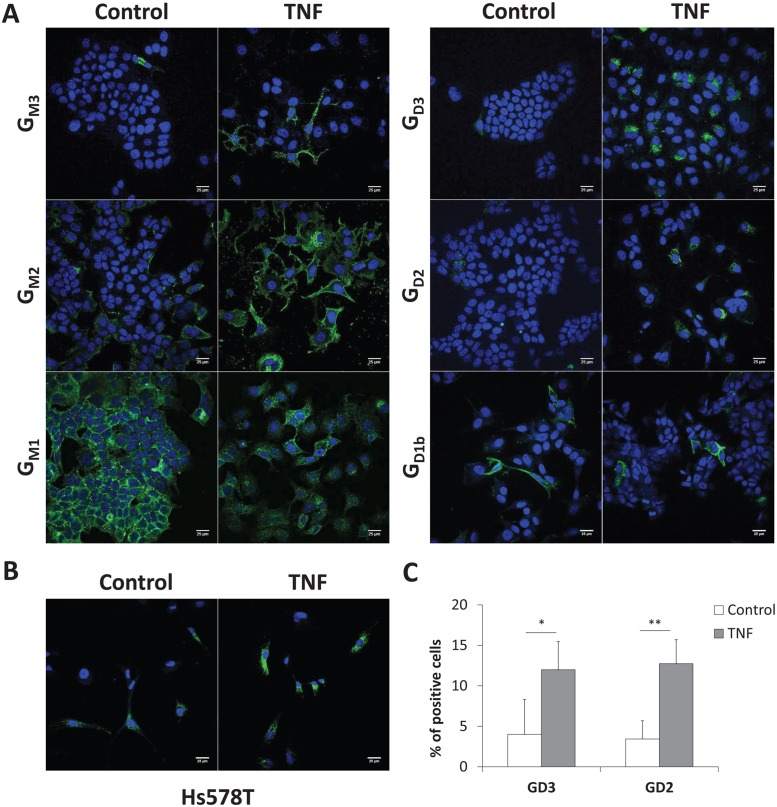
Immunocytochemistry analysis of gangliosides in MCF-7 cells
In order to confirm the results obtained by flow cytometry, control and TNF-treated MCF-7 were analyzed by immunofluorescence and confocal microscopy. As shown in Fig 5A, GM1a was highly expressed at the plasma membrane of MCF-7 cells, while lower amounts of GM3 and GM2 could be observed. The expression of GD3 and GD2 was also very low in control MCF-7 cells. After 48 h of incubation with TNF, GM3 and GM2 levels at the plasma membrane were higher, whereas no significant change was observed for GM1a, which remained highly expressed after TNF treatment. Finally, the analysis of GD2 expression in Hs578T cells allowed us to confirm the increased expression of GD2 at the cell surface of treated cells observed by flow cytometry (Fig 5B). To quantify variations in ganglioside expression in MCF-7 cells after TNF treatment, we analyzed the population expressing GD3 and GD2 using an automated algorithm on ImageJ software. For each condition, images of ≥ 100 control and treated-cells were collected based on the DAPI signal for total cell counting. In the control cell population, GD3 and GD2 expressing cells were estimated at 4.0 ± 4.0% and 3.4 ± 2.3% of total cells, respectively, whereas TNF treatment extended these populations to 12 ± 3.5% and 12.7 ± 3.0% (Fig 5C).
Fig 5. Immunocytochemistry and confocal microscopy analysis of gangliosides expression.
MCF-7 cells (A) and Hs578T cells (B) were treated with 40 ng/mL TNF for 48h. Control and treated cells were incubated with anti-GM3, GM2, GD3, GD2 or GD1b specific antibodies and revealed with Alexa Fluor® 488 conjugated anti-mouse IgG or IgM. GM1a ganglioside was revealed with the FITC-conjugated cholera toxin B subunit from Vibrio cholerae. The nuclei were counter stained with DAPI. Bars: 25 μm. For statistical analysis (C), images of ≥ 100 MCF-7 cells for each condition were collected based on the DAPI signal and were analyzed for GD3 and GD2 expression using an automated algorithm on ImageJ software. *: p < 0.05, **: p < 0.01 vs. untreated (control).
Discussion
Complex gangliosides from b-series are oncofetal markers of human tumors of neuroectoderm origin such as melanoma, glioblastoma and neuroblastoma where they play a functional role in tumor growth and metastasis by mediating cell proliferation, migration and angiogenesis [7]. Previous studies have shown that the expression of ganglioside-specific GTs was also altered in breast cancer tumors. In particular, the GD3S was shown to display higher expression among ER-negative breast cancer tumors [14], associated with a lower survival rate of patients [15]. In parallel, the gangliosides GD3, as well as its derivatives 9-O-acetyl-GD3 and 9-O-acetyl-GT3 are over-expressed in about 50% of invasive ductal carcinoma, whereas they show a very restricted expression in normal breast tissues, [13]. In that context, we previously showed that the ectopic expression of the GD3S in breast cancer cells induced the accumulation of b- and c-series gangliosides at the cell surface together with the acquisition of a proliferative phenotype and enhanced tumor growth [16,17], due to the specific and constitutive activation of c-Met receptor in the absence of ligand, and subsequent activation of the PI3K/Akt and Erk/MAPK pathways in GD2 expressing breast cancer cells [18]. Ganglioside GD2 was also identified as a putative marker of CD44hiCD24lo breast cancer stem cells (CSC) capable of initiating tumors, and several GT genes involved in GD2 biosynthesis (ST3GAL5, B4GALNT1, and ST8SIA1) are highly expressed in CSC [20]. Moreover, the induction of epithelial–mesenchymal transition (EMT) in transformed human mammary epithelial cells dramatically increased GD3S as well as GD2 expression [28], confirming the role of GD3S and GD2 in breast cancer progression.
Chronic inflammation in the tumor micro-environment is known to play an important role in cancer progression and NFκB was proposed as an important actor of inflammation-driven malignancy [21]. Several GTs were previously described to be regulated by TNF [29]. As an example, we showed that TNF up-regulates ST3GAL4 expression through an intronic ATF2-responsive element, resulting in sialyl-Lewisx antigen over-expression in lung epithelial cells [30]. Concerning GT genes involved in ganglioside biosynthesis, a functional NFκB binding site at -777/-762 pb upstream the ATG was shown to be essential for ST8SIA1 transcription in melanoma cells [31] and inflammatory cytokines including TNF and IL-6 enhanced GD3S gene expression in melanocytes [32]. We also reported that TNF enhanced GD3S expression in ER-negative breast cancer cells via NFκB pathway [19]. However, the effect of TNF on the expression of other ganglioside-specific GT genes in breast cancer cells was still unknown. In the present study, we examined the effect of TNF on the expression of the main ganglioside-specific GT genes as well as ganglioside expression in three breast cancer cells from different molecular subtypes. The most potent effect of TNF was observed for MCF-7 cells, in which TNF significantly enhanced the expression of genes encoding the GD3S (ST8SIA1) and also the GM3 synthase (ST3GAL5) that synthesizes GM3, the precursor of both GM2 and GD3. In contrast, the expression of the other ganglioside-specific GTs genes B4GALNT1 and B3GALT4 was not significantly modified after TNF treatment, showing that TNF controls the expression of enzymes involved in the first steps of a- and b-series biosynthesis in MCF-7 cells. It was previously reported that exposure of mouse mammary carcinoma cells to TGF-β and TNF induced EMT and generated cells with a CSC phenotype [33]. It was also reported that the mRNA levels for ST3GAL5, ST8SIA1, but also for B4GALNT1 and ST3GAL2 were increased in human breast CSC model induced through EMT [34]. The ER-positive MCF-7 cells belongs to luminal breast cancer subtype with an epithelial phenotype [35] and the changes in the expression of GT genes that we observe in this study could be therefore associated with TNF-induced EMT of MCF-7 cells.
TNF treatment also significantly enhanced the expression of GD3S gene in Hs578T but the expression of the other GTs was not modified. The triple negative Hs578T cells were previously shown to over-express GD3S when treated by TNF [19]. In contrast with observations made in MCF-7 cells, TNF had no effect on the expression of the GM3 synthase. Surprisingly, no change in the expression of all GTs genes tested was observed for the triple negative MDA-MB-231 cells, which are of the same molecular subtype than Hs578T cells, and known to express the TNF receptor 1 (TNFR1) [36]. We also confirmed the expression of TNFR1 by qPCR in the three cell lines (data not shown). These results indicate that inflammation differently modulates the expression of ganglioside-specific GT genes in breast cancer cells.
The effect of TNF treatment on cell surface ganglioside expression was in parallel analyzed by flow cytometry and confocal microscopy. As expected, an increased expression of GM3 and GM2 was observed in TNF-treated MCF-7 cells that fitted well with the increased of GM3 synthase gene expression. GM2 is the precursor for the biosynthesis of GM1a and the high and maybe saturated expression of GM1a in control conditions could also explain the accumulation of GM2 under TNF treatment. In renal carcinoma cells, TNF increased the expression of GM2 by enhancing the mRNA level of B4GALNT1 encoding the GM2/GD2 synthase, and resulted in T cell death and immune dysfunction [37]. As a result of the increased expression of GD3S, a significant increase of GD2 was observed by flow cytometry in both TNF-treated MCF-7 and Hs578T cells and confocal microscopy analysis allowed us to confirm the expression of GD2 in about 13% of MCF-7 cells. Confocal microscopy also revealed an intracellular labeling of GD3 presumably in the Golgi apparatus, showing the presence of the GD2 precursor in TNF treated cells. These results demonstrate for the first time that TNF can modify the expression of gangliosides and increase GD2 expression at the cell surface of MCF-7 and Hs578T breast cancer cells. It was previously shown that TNF/TGFβ treatment of mouse mammary carcinoma cells induced EMT and generated breast cancer stem cells with a claudin-low molecular subtype characterized by the expression of mesenchymal and stem cell-associated markers, and associated with a poor prognosis [33]. According to the role of complex gangliosides, especially GD2, in breast cancer cells EMT and aggressiveness, the increased expression of GD2 in breast cancer cells under TNF treatment could establish a link between the presence of pro-inflammatory cytokines at the tumor site environment, expression of complex gangliosides and EMT, resulting in more aggressive cells with increased tumorigenicity and increased resistance to treatment.
Acknowledgments
This work was supported by the University of Lille and La Ligue Régionale contre le Cancer. We are indebted to the Research Federation FRABio (Univ. Lille, CNRS, FR 3688, FRABio, Biochimie Structurale et Fonctionnelle des Assemblages Biomoléculaires) for providing the scientific and technical environment conducive to achieving this work.
Abbreviations
- BSA
bovine serum albumin
- CSC
cancer stem cells
- DAPI
4',6-diamidino-2-phénylindole
- DMEM
Dulbecco’s Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- EMT
epithelial–mesenchymal transition
- ER
estrogen receptor
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GD3S
GD3 synthase
- GSL
glycosphingolipids
- GT
glycosyltransferase
- HPRT
hypoxanthine phosphoribosyltransferase
- MALDI-TOF
Matrix Assisted Laser Desorption Ionisation—Time of Flight
- MS
mass spectrometry
- PBS
phosphate buffered saline
- qPCR
quantitative real-time polymerase chain reaction
- TNF
tumor necrosis factor
- TNFR1
tumor necrosis factor receptor 1
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the University of Lille and La Ligue Régionale contre le Cancer grant.
References
- 1.Hakomori SI. The glycosynapse. Proc Natl Acad Sci USA. 2002;99: 225–232. doi: 10.1073/pnas.012540899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todeschini AR, Hakomori SI. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim Biophys Acta. 2008;1780: 421–433. doi: 10.1016/j.bbagen.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furukawa K, Ohkawa Y, Yamauchi Y, Hamamura K, Ohmi Y, Furukawa K. Fine tuning of cell signals by glycosylation. J Biochem. 2012;151: 573–578. doi: 10.1093/jb/mvs043 [DOI] [PubMed] [Google Scholar]
- 4.Julien S, Bobowski M, Steenackers A, Le Bourhis X, Delannoy P. How Do Gangliosides Regulate RTKs Signaling? Cells. 2013;2: 751–767. doi: 10.3390/cells2040751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groux-Degroote S, Guérardel Y, Delannoy P. Gangliosides: structure, biosynthesis, analysis and roles in cancer. ChemBioChem. 2017;18: 1146–1154. doi: 10.1002/cbic.201600705 [DOI] [PubMed] [Google Scholar]
- 6.Guérardel Y, Groux-Degroote S, Delannoy P. Gangliosphyngolipids: their structure and biological roles In: Cipolla L, editor. Carbohydrate Chemistry: State-of-the-art and challenges for drug development. Imperial College Press, London, UK; 2015. pp. 35–56. [Google Scholar]
- 7.Birklé S, Zeng G, Gao L, Yu RK, Aubry J. Role of tumor-associated gangliosides in cancer progression. Biochimie. 2003;85: 455–463. [DOI] [PubMed] [Google Scholar]
- 8.Prokazova NV, Bergelson LD. Gangliosides and atherosclerosis. Lipids. 1994;29: 1–5. [DOI] [PubMed] [Google Scholar]
- 9.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease—A review. J Lipid Res. 2008;49: 1157–1175. doi: 10.1194/jlr.R800007-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa K, Hamamura K, Aixinjueluo W, Furukawa K. Biosignals modulated by tumor-associated carbohydrate antigens: Novel targets for cancer therapy. Ann N Y Acad Sci. 2006;1086: 185–198. doi: 10.1196/annals.1377.017 [DOI] [PubMed] [Google Scholar]
- 11.Krengel U, Bousquet PA. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front Immunol. 2014;5: 325 doi: 10.3389/fimmu.2014.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabu C, McIntosh R, Jurasova Z, Durrant L. Glycans as targets for therapeutic antitumor antibodies. Future Oncol. 2012;8: 943–960. doi: 10.2217/fon.12.88 [DOI] [PubMed] [Google Scholar]
- 13.Marquina G, Waki H, Fernandez LE, Kon K, Carr A, Valiente O, et al. Gangliosides expressed in human breast cancer. Cancer Res. 1996;56: 5165–5171. [PubMed] [Google Scholar]
- 14.Ruckhäberle E, Rody A, Engels K, Gaetje R, von Minckwitz G, Schiffmann S, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112: 41–52. doi: 10.1007/s10549-007-9836-9 [DOI] [PubMed] [Google Scholar]
- 15.Ruckhäberle E, Karn T, Rody A, Hanker L, Gätje R, Metzler D, et al. Gene expression of ceramide kinase, galactosyl ceramide synthase and ganglioside GD3 synthase is associated with prognosis in breast cancer. J Cancer Res Clin Oncol. 2009;135: 1005–1013. doi: 10.1007/s00432-008-0536-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazet A, Groux-Degroote S, Teylaert B, Kwon KM, Lehoux S, Slomianny C, et al. GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biol Chem. 2009;390: 601–609. doi: 10.1515/BC.2009.054 [DOI] [PubMed] [Google Scholar]
- 17.Cazet A, Lefebvre J, Adriaenssens E, Julien S, Bobowski M, Grigoriadis A, et al. GD3 synthase expression enhances proliferation and tumor growth of MDA-MB-231 breast cancer cells through c-Met activation. Mol Cancer Res. 2010;8: 1526–1535. doi: 10.1158/1541-7786.MCR-10-0302 [DOI] [PubMed] [Google Scholar]
- 18.Cazet A, Bobowski M, Rombouts Y, Lefebvre J, Steenackers A, Popa I, et al. The ganglioside GD2 induces the constitutive activation of c-Met in MDA-MB-231 breast cancer cells expressing the GD3 synthase. Glycobiology. 2012;22: 806–816. doi: 10.1093/glycob/cws049 [DOI] [PubMed] [Google Scholar]
- 19.Bobowski M, Vincent A, Steenackers A, Colomb F, Van Seuningen I, Julien S, et al. Estradiol represses the GD3 synthase gene ST8SIA1 expression in human breast cancer cells by preventing NFκB binding to ST8SIA1 promoter. PLoS One. 2013;8: e62559 doi: 10.1371/journal.pone.0062559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battula VL, Shi Y, Evans KW, Wang RY, Spaeth EL, Jacamo RO, et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J Clin Invest. 2012;122: 2066–2078. doi: 10.1172/JCI59735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katanov C, Lerrer S, Liubomirski Y, Leider-Trejo L, Meshel T, Bar J, et al. Regulation of the inflammatory profile of stromal cells in human breast cancer: prominent roles for TNF-α and the NFκB pathway. Stem Cell Res Ther. 2015;6: 87 doi: 10.1186/s13287-015-0080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svennerholm L. Ganglioside designation. Adv Exp Med Biol. 1980;125: 11 [DOI] [PubMed] [Google Scholar]
- 23.Hackett AJ, Smith HS, Springer EL, Owens RB, Nelson-Rees WA, Riggs JL, et al. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J Natl Cancer Inst 1977;58: 1795–1806. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW. A new mathematical model for relative quantification in realtime RT-PCR. Nucleic Acids Res 2001;29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnaar RL. Isolation of glycosphingolipids. Methods Enzymol. 1994;230: 348–370. [DOI] [PubMed] [Google Scholar]
- 26.Ciucanu I, Kerek F. Rapid and simultaneous methylation of fatty and hydroxy fatty acids for gas-liquid chromatographic analysis. J Chromatogr A. 1984;284: 179–185. [Google Scholar]
- 27.Steenackers A, Cazet A, Bobowski M, Rombouts Y, Lefebvre J, Guérardel Y, et al. Expression of GD3 synthase modifies ganglioside profile and increases migration of MCF-7 breast cancer cells. C R Chimie. 2012;15: 3–14. [Google Scholar]
- 28.Sarkar TR, Battula VL, Werden SJ, Vijay GV, Ramirez-Peña EQ, Taube JH, et al. GD3 synthase regulates epithelial-mesenchymal transition and metastasis in breast cancer. Oncogene. 2015;34: 2958–2967. doi: 10.1038/onc.2014.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dewald JH, Colomb F, Bobowski-Gerard M, Groux-Degroote S, Delannoy P. Role of Cytokine-Induced Glycosylation Changes in Regulating Cell Interactions and Cell Signaling in Inflammatory Diseases and Cancer. Cells. 2016;5: E43 doi: 10.3390/cells5040043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colomb F, Krzewinski-Recchi MA, Steenackers A, Vincent A, Harduin-Lepers A, Delannoy P, et al. TNF up-regulates ST3GAL4 and sialyl-Lewisx expression in lung epithelial cells through an intronic ATF2-responsive element. Biochem J. 2017;474: 65–78. doi: 10.1042/BCJ20160602 [DOI] [PubMed] [Google Scholar]
- 31.Kang NY, Kim CH, Kim KS, Ko JH, Lee JH, Jeong YK, et al. Expression of the human CMP-NeuAc:GM3 alpha2,8-sialyltransferase (GD3 synthase) gene through the NF-kappaB activation in human melanoma SK-MEL-2 cells. Biochim Biophys Acta. 2007;1769: 622–630. doi: 10.1016/j.bbaexp.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 32.Miyata M, Ichihara M, Tajima O, Sobue S, Kambe M, Sugiura K, et al. UVB-irradiated keratinocytes induce melanoma-associated ganglioside GD3 synthase gene in melanocytes via secretion of tumor necrosis factor α and interleukin 6. Biochem Biophys Res Commun. 2014;445: 504–510. doi: 10.1016/j.bbrc.2014.02.038 [DOI] [PubMed] [Google Scholar]
- 33.Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL. TGFβ/TNFα-Mediated Epithelial-Mesenchymal Transition Generates Breast Cancer Stem Cells with a Claudin-Low Phenotype. Cancer Res. 2011;71: 4707–4719. doi: 10.1158/0008-5472.CAN-10-4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang YJ, Ding Y, Levery SB, Lobaton M, Handa K, Hakomori SI. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc Natl Acad Sci USA. 2013;110: 4968–4973. doi: 10.1073/pnas.1302825110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13: 215 doi: 10.1186/bcr2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyari HRE, Rawling T, Chen Y, Sudarmana W, Bourget K, Dwyer JM, et al. A novel synthetic analogue of ω-3 17,18-epoxyeicosatetraenoic acid activates TNF receptor-1/ASK1/JNK signaling to promote apoptosis in human breast cancer cells. FASEB J. 2017;31: 5246–5257. doi: 10.1096/fj.201700033R [DOI] [PubMed] [Google Scholar]
- 37.Raval G, Biswas S, Rayman P, Biswas K, Sa G, Ghosh S, et al. TNF-alpha induction of GM2 expression on renal cell carcinomas promotes T cell dysfunction. J Immunol. 2007;178: 6642–6652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.