Abstract
Proposals to increase research reproducibility frequently call for focusing on effect sizes instead of p values, as well as for increasing the statistical power of experiments. However, it is unclear to what extent these two concepts are indeed taken into account in basic biomedical science. To study this in a real-case scenario, we performed a systematic review of effect sizes and statistical power in studies on learning of rodent fear conditioning, a widely used behavioral task to evaluate memory. Our search criteria yielded 410 experiments comparing control and treated groups in 122 articles. Interventions had a mean effect size of 29.5%, and amnesia caused by memory-impairing interventions was nearly always partial. Mean statistical power to detect the average effect size observed in well-powered experiments with significant differences (37.2%) was 65%, and was lower among studies with non-significant results. Only one article reported a sample size calculation, and our estimated sample size to achieve 80% power considering typical effect sizes and variances (15 animals per group) was reached in only 12.2% of experiments. Actual effect sizes correlated with effect size inferences made by readers on the basis of textual descriptions of results only when findings were non-significant, and neither effect size nor power correlated with study quality indicators, number of citations or impact factor of the publishing journal. In summary, effect sizes and statistical power have a wide distribution in the rodent fear conditioning literature, but do not seem to have a large influence on how results are described or cited. Failure to take these concepts into consideration might limit attempts to improve reproducibility in this field of science.
Introduction
Biomedical research over the last decades has relied heavily on the concept of statistical significance–i.e. the probability that an effect equal to or larger than that observed experimentally would occur by chance under the null hypothesis–and classifying results as “significant” or “non-significant” on the basis of an arbitrary threshold (usually set at p < 0.05) has become standard practice in most fields. This approach, however, has well-described limitations that can lead to erroneous conclusions when researchers rely on p values alone to judge results [1–6]. First of all, p values do not measure the magnitude of an effect, and thus cannot be used by themselves to evaluate its biological significance [7]. Moreover, the predictive value of a significance test is heavily influenced by factors such as the prior probability of the tested hypothesis, the number of tests performed and their statistical power [8]; thus, similar p values can lead to very different conclusions in distinct scenarios [1].
Recent calls for improving research reproducibility have focused on reporting effect sizes and confidence intervals alongside or instead of p values [6–9] and for the use of both informal Bayesian inference [10] and formal data synthesis methods [11] when aggregating data from multiple studies. The concepts of effect size and statistical power are central for such approaches, as how much a given experiment will change a conclusion or an effect estimate will depend on both. However, it is unclear whether they receive much attention from authors in basic science publications. Discussion of effect sizes seems to be scarce, and recent data has shown that sample size and power calculations are very rare in the preclinical literature [12,13]. The potential impact of these omissions is large, as reliance on the results of significance tests without consideration of statistical power can decrease the reliability of study conclusions [14].
Another issue is that, if effect size is not taken into account, it is difficult to adequately assess the biological significance of a given finding. As p values will be low even for small effect sizes if sample size is large, biologically trivial effects can be found to be statistically significant. In preclinical studies, overlooking effect sizes will thus lead to inadequate assessment of therapeutic potential, whereas in basic research it will cause difficulties in dissecting essential biological mechanisms from peripheral modulatory influences [15]. The wealth of findings in the literature will thus translate poorly into better comprehension of phenomena, and the abundance of statistically significant findings with small effect sizes can eventually do more harm than good. This problem is made much worse when many of these studies have low positive predictive values due to insufficient power, leading a large fraction of them to be false positives [8,14,16–18].
To analyze how effect sizes and statistical power are taken into account in the description and publication of findings in a real-case scenario of basic biomedical science, we chose to perform a systematic review of articles on learning of rodent fear conditioning, probably the most widely used behavioral task to study memory in animals [19]. Focusing on this task provides a major advantage in the fact that the vast majority of articles use the same measure to describe results (i.e. percentage of time spent in freezing behavior during a test session). As effect sizes are comparable across studies, studying their distribution allows one to estimate the statistical power of individual experiments to detect typical differences.
Our first objective in this study is to analyze the distribution of effect sizes and statistical power in a large sample of articles using different interventions, showing how they are related to the outcome of statistical significance tests. Next, we will study whether these two measures are correlated, in order to look for evidence of publication bias and effect size inflation. We will also correlate effect sizes and variances with different aspects of experimental design, such as species, sex and type of conditioning, as well as with indicators of risk of bias. To inquire whether effect size and power are taken into consideration by authors when interpreting findings, we will evaluate whether they correlate with effect size inferences made by readers based on textual descriptions of results in the articles. Finally, we will analyze whether mean effect size and power correlate with article-level metrics, such as number of citations and impact factor of the publishing journal, to explore how they influence the publication of results.
Results
Article search and inclusion
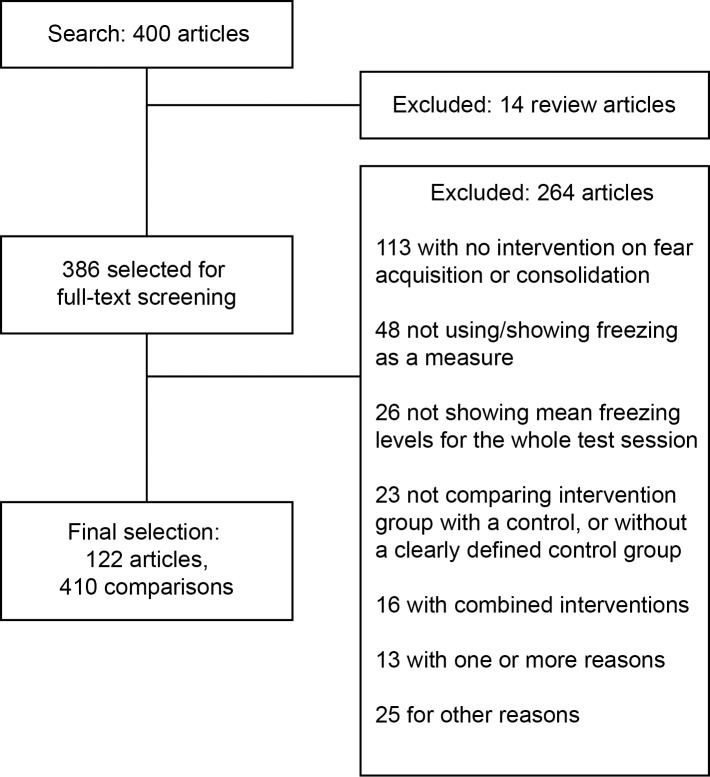
As previously described in a protocol published in advance of full data collection [20], we performed a PubMed search for fear conditioning articles published online in 2013. The search process (Fig 1) yielded 400 search hits, of which 386 were original articles that were included if they fulfilled pre-established criteria (see Methods). Two investigators examined all included articles, and agreement for exclusions measured on a double-screened sample of 40 articles was 95%. This led to a final sample of 122 articles and 410 experiments, used to build the database provided as S1 Data.
Fig 1. Study flow diagram.
Our PubMed search yielded 400 results, of which 14 were excluded based on initial screening of titles and abstracts and 386 were selected for full-text analysis. This led to the inclusion of 122 articles, containing a total of 410 comparisons (i.e. individual experiments). The main reasons for exclusion are listed in the figure, in compliance with the PRISMA statement [21].
Distribution of effect sizes among experiments
For each experiment, we initially calculated effect size as the relative difference (i.e. percentage of change) in the freezing levels of treated groups when compared to controls. As shown in Fig 2A, this leads interventions that enhance memory acquisition (i.e. those in which freezing is significantly higher in the treated group) to have larger effect sizes than those that impair it (i.e. those in which freezing is significantly lower in the treated group) due to an asymmetry that is inherent to ratios. To account for this and make effect sizes comparable between both types of interventions, we used a normalized effect size, with difference expressed as a percentage of the highest freezing value between groups (Fig 2B) [11].
Fig 2. Distribution of effect sizes.
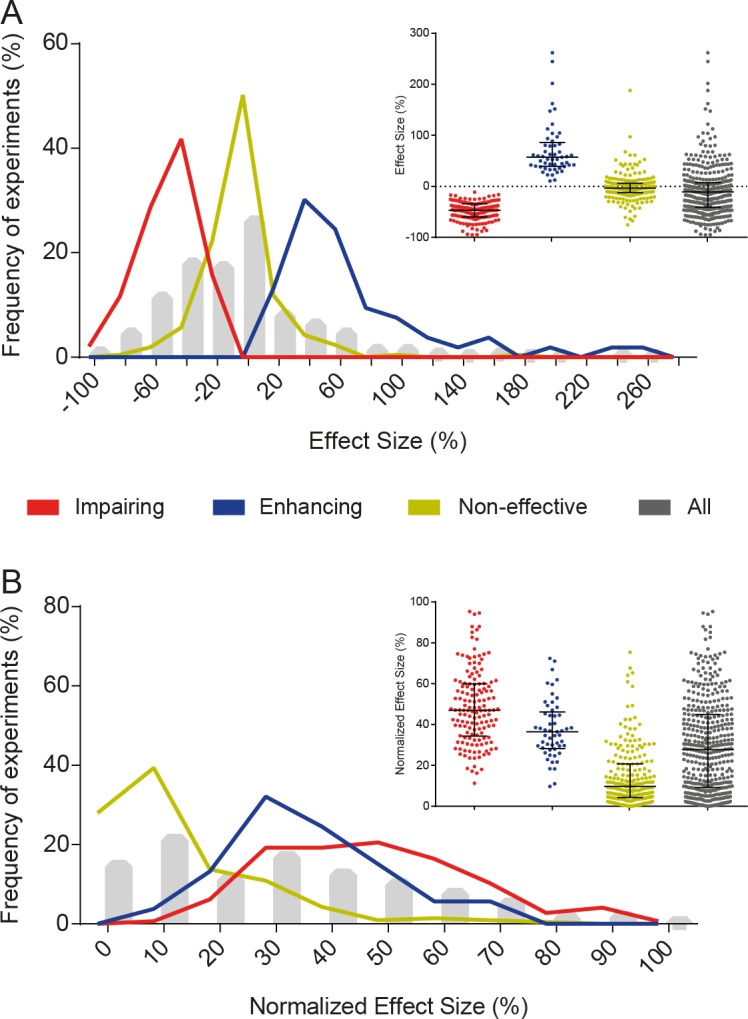
(A) Distribution of effect sizes, calculated as % of control group freezing. Interventions were divided into memory-impairing (-48.6 ± 18.1%, n = 146), memory-enhancing (71.6 ± 53.2%, n = 53) or non-effective (-1.8 ± 26.2%, n = 211) for visualization purposes, according to the statistical significance of the comparison performed in the article. Additionally, the whole sample of experiments is shown in grey (-9.0 ± 47.5% [-13.6 to -4.4], n = 410). Values are expressed as mean ± SD [95% confidence interval]. Lines and whiskers in the inset express median and interquartile interval. (B) Distribution of normalized effect sizes, calculated as % of the group with the highest mean (i.e. control group for memory-impairing interventions, or treated group for memory-enhancing interventions).
Use of absolute differences in freezing instead of relative ones led to similar, but more constrained distributions (S1 Fig) due to mathematical limits on absolute differences. Freezing levels in the reference group correlated negatively with relative effect size and pooled coefficient of variation (i.e. the ratio between the sample size-weighted pooled SD and the pooled mean); however, normalization by the highest-freezing group reduced this effect (S2A–S2C Fig). Absolute effect size, on the contrary, showed a positive correlation with freezing levels in the control or highest-freezing group (S2D–S2F Fig). We also calculated effect sizes as standardized mean differences (i.e Cohen’s d, S3 Fig), but chose to use relative percentages throughout the study, as they are more closely related to the way results are expressed in articles.
All 410 experiments combined had a mean normalized effect size of 29.5 ± 22.4% (mean ± SD; 95% CI [27.4 to 31.7]). When results were divided according to the statistical comparison originally performed in the article, mean normalized effect size was 48.6 ± 18.1% for memory-impairing interventions, 37.6 ± 14.2% for memory-enhancing interventions and 14.4 ± 14.2% for non-effective interventions–i.e. those in which a significant difference between groups was not found. This does not imply that data in each of these groups represents effects coming from a different distribution, or that significant and non-significant results correspond to true positive or true negative effects. On the contrary, each group likely represents a mixture of heterogeneous effect size distributions, as sampling error and lack of statistical power can directly impact the chances of a result achieving statistical significance. Distribution of mean effect sizes at the article level showed similar results to those found at the level of experiments (S4 Fig).
The distribution of effect sizes shows that the vast majority of memory-impairing interventions cause partial reductions in learning, leaving the treated group with residual freezing levels that are higher than those of a non-conditioned animal. In fact, in all 35 memory-impairing experiments in which pre-conditioning freezing levels were shown for the treated group, these were lower than those observed in the test session–with p values below 0.05 in 25 (78%) out of the 32 cases in which there was enough information for us to perform an unpaired t test between sessions (S5 Fig). It is also worth noting that 26.5% of non-significant experiments had an effect size greater than 20%, suggesting that these experiments might have been underpowered. With this in mind, we went on to evaluate the distribution of statistical power among studies.
Distribution of statistical power among experiments
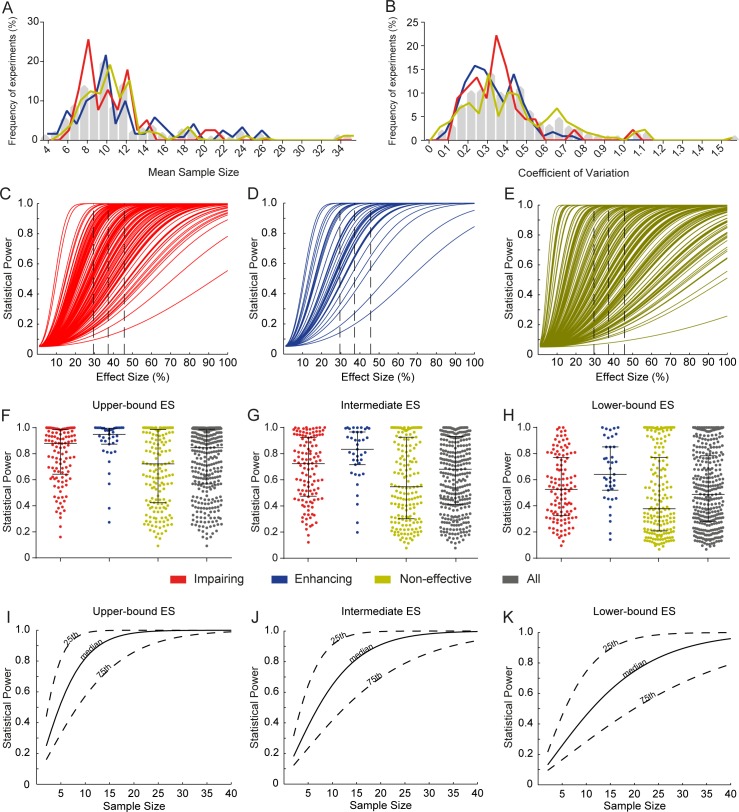
For analyzing statistical power, we first sought to evaluate the distribution of sample sizes and coefficients of variation (both of which are determinants of power). As shown in Fig 3A, most experiments had mean sample sizes between 8 and 12 animals/group, and this distribution did not vary between enhancing, impairing and non-effective interventions. On the other hand, higher coefficients of variation were more frequent among non-effective interventions (Fig 3B). This difference was partly explained by freezing levels in the reference group–which correlated negatively with coefficients of variation (S2G–S2I Fig) and were lower on average for non-significant experiments (49.3% vs. 52.9% in memory-impairing and 61.3% in memory-enhancing experiments).
Fig 3. Distribution of sample size, variation and statistical power.
(A) Distribution of mean sample size between groups. Gray bars show the distribution of the whole sample, while colored lines show distributions of impairing (n = 120), enhancing (n = 39) and non-significant (n = 177) experiments separately for visualization purposes. (B) Distribution of coefficients of variation (pooled standard deviation/pooled mean) for each type of experiment. (C) Distribution of statistical power for memory-impairing interventions: based on each experiment’s variance and sample size, power varies according to the difference to be detected for α = 0.05. Dashed lines show the three effect sizes used for point estimates of power in F, G and H. (D) Distribution of statistical power for memory-enhancing interventions. (E) Distribution of statistical power for non-effective interventions. (F) Distribution of statistical power to detect the upper-bound effect size of 45.6% (right dashed line on C, D and E) for impairing (red), enhancing (blue), non-significant (yellow) and all (grey) experiments. Lines and whiskers express median and interquartile interval. (G) Distribution of statistical power to detect the intermediate effect size of 37.2% (middle dashed line on C, D and E). (H) Distribution of statistical power to detect the lower-bound effect size of 29.5% (left dashed line on C, D and E). (I) Sample size vs. statistical power to detect the upper-bound effect size of 45.6%. Continuous lines use the 50th percentile of coefficients of variation for calculations, while dotted lines use the 25th and 75th percentiles. (J) Sample size vs. statistical power to detect the intermediate effect size of 37.2%. (K) Sample size vs. statistical power to detect the lower-bound effect size of 29.5%.
Based on each experiment’s variance and sample size, we built power curves to show how power varies according to the difference to be detected at α = 0.05 for each individual experiment (Fig 3C–3E). To detect the mean effect size of 45.6% found for nominally effective interventions (i.e. those leading to statistically significant differences between groups), mean statistical power in our sample was 0.75 ± 0.26; 95% CI [0.72–0.78] (Fig 3F). This estimate, however, is an optimistic, upper-bound calculation of the typical effect size of biologically effective interventions (from here on referred to as “upper-bound ES”): as only large effects will be detected by underpowered studies, basing calculations only on significant results leads to effect size inflation [14]. A more realistic estimate of effect size was obtained based only on experiments that achieved statistical power above 0.95 (n = 60) in the first analysis (and are thus less subject to effect size inflation), leading to a mean effect size of 37.2%. Predictably, mean statistical power to detect this difference (“intermediate ES”, Fig 3G) fell to 0.65 ± 0.28 [0.62–0.68]. Using the mean effect size of all experiments (“lower-bound ES”, 29.5%) led to an even lower power of 0.52 ± 0.29 [0.49–0.56] (Fig 3H), although this estimate of a typical effect size is likely pessimistic, as it probably includes many true negative effects.
Interestingly, using mean absolute differences instead of relative ones to calculate statistical power led to a smaller number of experiments with very low power (S6 Fig). This suggests that some of the underpowered experiments in the first analysis had low freezing levels in the reference group, as in this case even large relative differences will still be small when expressed in absolute terms for statistical analysis. Also of note is that, if one uses Cohen’s traditional definitions of small (d = 0.2), medium (d = 0.5) and large (d = 0.8) effect sizes [22] as the basis for calculations, mean power is 0.07 ± 0.01, 0.21 ± 0.07 and 0.44 ± 0.13, respectively (S7 Fig). These much lower estimates reflect the fact that effect sizes are typically much larger in rodent fear conditioning than in psychology experiments, for which this arbitrary classification was originally devised, and suggests that it might not be applicable to other fields of science.
A practical application of these power curves is that we were able to calculate the necessary sample size to achieve desired power for each effect size estimate, considering the median coefficient of variation (as well as the 25th and 75th quartiles) of experiments in our sample (Fig 3I–3K). Thus, for an experiment with typical variation, around 15 animals per group are needed to achieve 80% power to detect our ‘intermediate effect size’ of 37.2%, which we consider our more realistic estimate for a typical effect size in the field. Nevertheless, only 12.2% of comparisons in our sample had a sample size of 15 or above in each experimental group, suggesting that such calculations are seldom performed.
We also analyzed the distributions of statistical power at the level of articles instead of individual experiments. Results for these analyses are shown in S8 Fig, and are generally similar to those obtained for the experiment-level analysis, except that the long tail of non-significant experiments with large coefficients of variation is not observed. This suggests that experiments with large variation and low power are frequently found alongside others with adequate power within the same articles. It is unclear, however, whether this means that the low power of some experiments is a consequence of random fluctuations of experimental variance, or if these experiments use protocols that lead to larger coefficients of variation–for example, by generating lower mean levels of freezing (see S2 Fig).
Correlation between effect sizes and statistical power/sample size
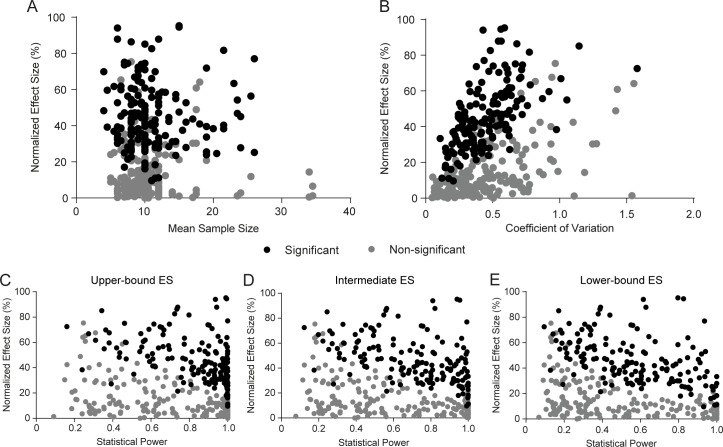
We next sought to correlate normalized effect size with sample size and statistical power for each experiment. The presence of a negative correlation between these variables has been considered an indirect measure of publication bias [23], as articles with low power or sample size will be subject to effect size inflation caused by selective reporting of significant findings [24]. In our analysis, no correlation was found between effect size and sample size (Fig 4A, r = 0.0007, p = 0.99); on the other hand, a positive correlation between effect size and coefficient of variation was observed (Fig 4B, r = 0.37, p<0.0001). Part of this correlation was mediated by the association of both variables with freezing levels (S2 Fig), but the correlation remained significant after adjustment for this variable (r = 0.32, p<0.001).
Fig 4. Correlations between effect size, variation and statistical power.
(A) Correlation between normalized effect size and mean sample size. No correlation is found (r = 0.0007, p = 0.99; r = -0.26, p = 0.64 after adjustment), although sample size variation is limited. (B) Correlation between normalized effect size and coefficient of variation. Correlation of the whole sample of experiments yields r = 0.37, p<0.0001* (n = 336; r = 0.32, p<0.001 after adjustment for freezing levels). (C) Correlation between normalized effect size and statistical power based on upper-bound effect size of 45.6%. Correlation of the whole sample of experiments yields r = -0.12, p = 0.03 (r = 0.11, p = 0.84 after adjustment for freezing levels), but distribution is skewed due to a ceiling effect on power. (D) Correlation between normalized effect size and statistical power based on intermediate effect size of 37.2%; r = -0.16, p = 0.003* (r = -0.16, p = 0.48 after adjustment). (E) Correlation between normalized effect size and statistical power based on lower-bound effect size of 29.5%; r = -0.21, p<0.0001* (r = -0.1, p = 0.06 after adjustment). Asterisks indicate significant results according to Holm-Sidak correction for 28 experiment-level correlations.
Because of this, negative correlations between effect size and power were observed for the three effect size estimates used (Fig 4C–4E), although they were larger for the lower-bound estimate (Fig 4E, r = -0.21, p<0.0001) than for the intermediate (Fig 4D, r = -0.16, p = 0.003) and upper-bound (Fig 4C, r = -0.12, p = 0.03) ones due to a ceiling effect on power. This negative correlation is observed even when power is calculated based on absolute differences (S9 Fig), for which the correlation between coefficients of variation and reference freezing levels is in the opposite direction of that observed with relative differences (see S2 Fig). This strongly suggests that the correlation represents a real phenomenon related to publication bias and/or effect size inflation, and is not merely due to the correlation of both variables with freezing levels. A correlation between effect size and power is also observed when both are calculated on the basis of standardized mean differences (i.e. Cohen’s d) (S10 Fig). In this case, the line separating significant and non-significant results for a given sample size is clearer, as significance is more directly related to standardized mean differences. Expressing effect sizes in Cohen’s d also makes effect size inflation in experiments with low sample size and power more obvious.
Interestingly, the correlation between effect size and power was driven by a scarcity of experiments with large effect size and high power. This raises the possibility that truly large effects are unusual in fear conditioning, and that some of the large effect sizes among low-powered experiments in our sample are inflated. On the other hand, a pattern classically suggesting publication bias–i.e. a scarcity of low-powered experiments with small effects [23]–is not observed. It should be noted, however, that our analysis focused on individual experiments within articles, meaning that non-significant results were usually presented alongside other experiments with significant differences; thus, this analysis does not allow us to assess publication bias at the level of articles.
Effects of methodological variables on the distributions of effect sizes and coefficients of variation
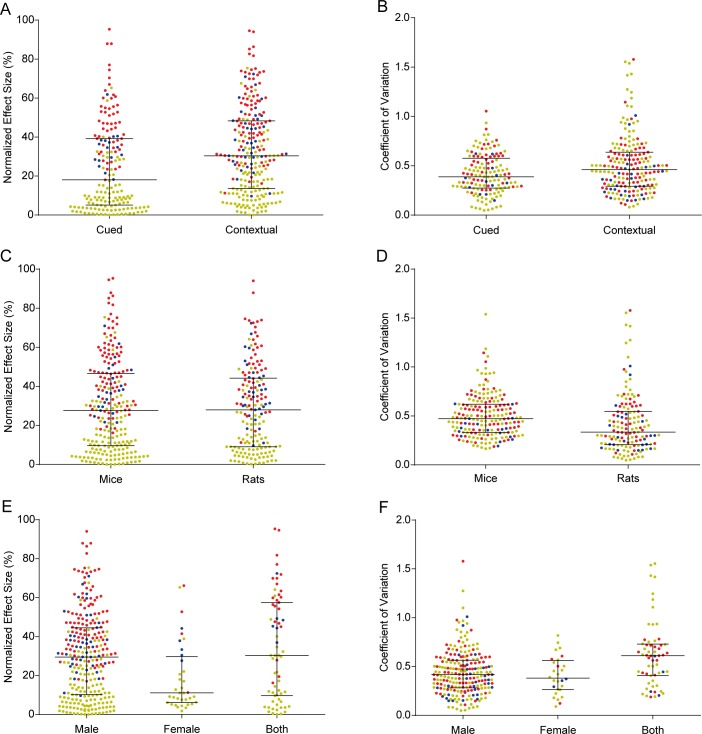
We next examined whether the distributions of effect sizes and coefficients of variation were influenced by type of conditioning, species or sex of the animals (Fig 5). Mean normalized effect size was slightly larger in contextual than in cued fear conditioning (33.2% vs. 24.4%, Student’s t test p<0.0001) and markedly larger in males than in females (30.3% vs. 18.9% vs. 34.2% for experiments using both, one-way ANOVA, p = 0.004), but roughly equivalent between mice and rats (29.8% vs. 29.1%, p = 0.76). Coefficients of variation were higher in contextual conditioning (0.51 vs. 0.41, Student’s t test p = 0.001), in experiments using animals of both sexes (0.62 vs. 0.44 in males and 0.41 in females, one-way ANOVA, p <0.0001), and in those using mice (0.50 vs. 0.42, Student’s t test, p = 0.008), although the latter difference was not statistically significant after correction for multiple comparisons. All of these associations should be considered correlational and not causal, as specific types of conditioning or animals of a particular species or sex might be more frequently used for testing interventions with particularly high or low effect sizes. Also of note is the fact that experiments on males were 7.7 times more common than those on females in our sample (277 vs. 36), indicating a strong preference of researchers for using male animals.
Fig 5. Effect sizes and coefficients of variation across different protocols, species and sexes.
Colors indicate memory-enhancing (red), memory-impairing (blue) or non-effective (yellow) experiments, all of which are pooled for analysis. Lines and whiskers express median and interquartile interval. (A) Distribution of effect sizes across cued (n = 171) and contextual (n = 239) conditioning protocols. Student’s t test, p<0.0001*. (B) Coefficients of variation across cued (n = 145) and contextual (n = 191) conditioning protocols. Student’s t test, p = 0.001*. (C) Distribution of effect sizes across experiments using mice (n = 237) or rats (n = 173). Student’s t test, p = 0.76. (D) Coefficients of variation across experiments using mice (n = 193) or rats (n = 143). Student’s t test, p = 0.008. (E) Distribution of effect sizes across experiments using male (n = 277), female (n = 36) or both (n = 67) sexes. One-way ANOVA, p = 0.004*; Tukey’s post-hoc test, male vs. female p = 0.01, male vs. both p = 0.40, female vs. both p = 0.003. 30 experiments were excluded from this analysis for not stating the sex of animals. (F) Coefficients of variation across experiments using male (n = 233), female (n = 28) or both (n = 60) sexes. One-way ANOVA, p<0.0001*; Tukey’s test, male vs. female p = 0.85, male vs. both p<0.0001, female vs. both p = 0.0006. For coefficient of variation analyses, 74 experiments were excluded due to lack of information on sample size for individual groups. Asterisks indicate significant results according to Holm-Sidak correction for 14 experiment-level comparisons.
We also examined whether effect sizes and coefficients of variation differed systematically according to the type, timing or anatomical site of intervention (S11 Fig). Effect sizes did not differ significantly between surgical, pharmacological, genetic and behavioral interventions (38.7% vs. 28.1% vs. 30.5% vs. 25.8% one-way ANOVA, p = 0.12), although there was a trend for greater effects with surgical interventions (which were uncommon in our sample). No differences were found between the mean effect sizes of systemic and intracerebral interventions (28.7% vs. 30.3%, Student’s t test, p = 0.45) or between those of pre- and post-training interventions (30.5% vs. 25.4%, Student’s t test, p = 0.07), although pre-training interventions had slightly higher coefficients of variation (0.49 vs 0.37, Student’s t test p = 0.0015). Coefficients of variation did not differ significantly between surgical, pharmacological, genetic and behavioral interventions (0.41 vs. 0.43 vs. 0.50 vs. 0.50, one-way ANOVA p = 0.08) or between systemic and intracerebral interventions (0.49 vs. 0.45, Student’s t test p = 0.15). Once again, these differences can only be considered correlational and not causal.
Risk of bias indicators and their relationship with effect size and power
As previous studies have shown that measures to reduce risk of bias are not widely reported in animal research [12,13], we investigated the prevalence of these measures in our sample of fear conditioning articles, and evaluated whether they were correlated with effect sizes or power. Table 1 shows the percentage of articles reporting 7 items thought to reduce risk of bias in animal studies, adapted and expanded from the CAMARADES checklist [25]. Although some items were reported in most articles (statement of compliance with animal regulations, adequate description of sample size, blinding), others were virtually inexistent, such as the presence of a sample size calculation (1 article) and compliance with the ARRIVE guidelines [26] (0 articles). Contrary to previous reports in other areas [27–30], however, no significant association was found between reporting of these indicators and either the percentage of significant experiments, the mean effect size of effective interventions or the mean statistical power of experiments in our sample (S12 Fig). The region of origin of the article also had no correlation with either of these variables (S13 Fig). Nevertheless, it should be noted that this analysis used only experiments on fear conditioning acquisition or consolidation, which were not necessarily the only results or the main findings presented in these articles. Thus, it is possible that other results in the article might have shown higher correlation with risk of bias indicators.
Table 1. Number of articles including quality assessment items.
| Quality assessment item | Randomization of allocation | Blinded or automated assessment | Sample size calculation | Exact sample size description | Statement of compliance with regulatory requirements | Statement on conflict of interest | Statement of compliance with ARRIVE |
|---|---|---|---|---|---|---|---|
| Number of articles (%) | 18/77 (23.4%) |
92/122 (75.4%) |
1/122 (0.8%) |
98/122 (80.3%) |
118/122 (96.7%) |
66/122 (54.1%) |
0/122 (0%) |
Percentages were calculated using all 122 articles, except in the case of randomization, which was calculated based on 77 articles, as it is not applicable to genetic interventions. In the case of blinding, 68 articles used automated analysis and 24 used blinded observers, totaling 92 articles scored for this item.
Correlation between effect sizes/statistical power and description of results
Given the wide distribution of effect sizes and statistical power in the literature on fear conditioning learning, we tried to determine whether these were taken into account by authors when describing results in the text. For each included comparison, we extracted the words or phrases describing the results of that experiment in the text or figure legends, and asked 14 behavioral neuroscience researchers to classify them according to the implicit information they contained about effect size. For comparisons with significant differences, terms were to be classified as implying strong (i.e. large effect size) or weak (i.e. small effect size) effects, or as neutral terms (i.e. those from which effect size could not be deduced). For non-significant differences, terms were to be classified as implying similarity between groups, as suggesting a trend towards difference, or as neutral terms (i.e. those from which the presence or absence of a trend could not be deduced). From the average of these classifications, we defined a score for each term (S1 and S2 Tables) and correlated these scores with the actual effect size and statistical power of experiments.
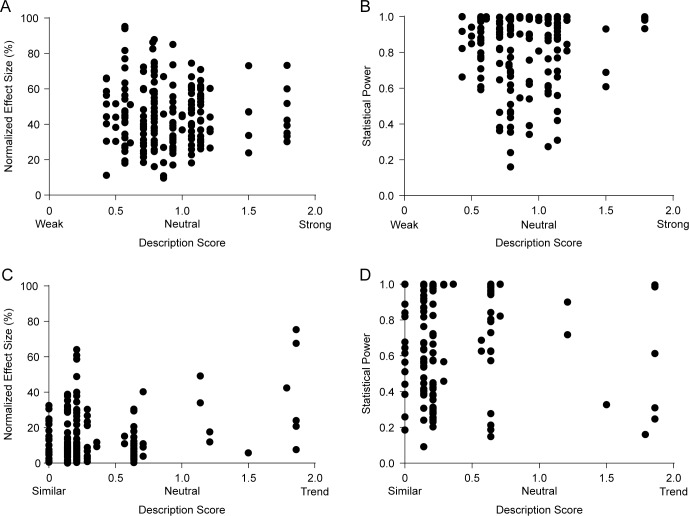
Agreement between researchers over classification was low, especially for terms describing significant differences: single measures intraclass correlation coefficients (reflecting the reliability of individual researchers when compared to the whole sample) were 0.234 for significant interventions and 0.597 for non-significant ones, while average measures coefficients (reflecting the aggregated reliability of the sample) were 0.839 and 0.962, respectively. This, along with a trend for the use of terms with little effect size information (“increase”, “decrease”, “significantly more”, “significantly less”, etc.), led most terms describing effective interventions to receive intermediate scores approaching 1 (i.e. neutral). For these interventions, no correlations were observed between this score and either effect size (r = -0.05, p = 0.48) or statistical power (r = 0.03, p = 0.73) (Fig 6A and 6B). For non-effective interventions, a significant correlation between description score and effect size was observed (Fig 6C, r = 0.28, p = 0.0002), as larger effect sizes were associated with terms indicating a trend for difference. Still, no correlation was observed between textual descriptions of results and power (Fig 6D, r = 0.03, p = 0.74). Moreover, statistical power was rarely mentioned in the textual description of results–the term “power” was used in this context in only 4 articles–suggesting that it is largely ignored when discussing findings, as shown in other areas of research [31].
Fig 6. Correlation between description of results and effect size/statistical power.
Description scores refer to the mean score given by 14 neuroscience researchers who rated terms as “weak” (0), “neutral” (1) or “strong” (2) in the case of those describing significant differences, or as “similar” (0), “neutral” (1) or “trend” (2) in the case of those describing non-significant ones. (A) Correlation between normalized effect size and description score for significant results. r = -0.05, p = 0.48 (n = 195). (B) Correlation between statistical power and description score for significant results. r = 0.03, p = 0.73 (n = 155). (C) Correlation between normalized effect size and description score for non-significant results. r = 0.28, p = 0.0002* (n = 174). (D) Correlation between upper-bound estimate of statistical power and description score for non-significant results. r = 0.03, p = 0.74 (n = 146). Asterisk indicates significant result according to Holm-Sidak correction for 28 experiment-level correlations.
Correlations of effect size, power and study quality with article citations
Finally, we investigated whether the percentage of significant experiments reported in each article, mean effect size for effective interventions, mean statistical power or a composite study quality score (aggregating the 7 risk of bias indicators described in Table 1) correlated with article impact, as measured by the number of citations (Fig 7) and the impact factor of the publishing journal (S14 Fig). None of the correlations was significant after adjustment for multiple comparisons, although a weak positive correlation was observed between study quality score and impact factor (r = 0.22, p = 0.01), driven by associations of higher impact factors with blinding (Student’s t test with Welch’s correction, p = 0.0001), conflict of interest reporting (Student’s t test with Welch’s correction, p = 0.03) and exact sample size description (Student’s t test, p = 0.03). It should be noted that the distribution of impact factors and citations is heavily skewed, limiting the use of linear correlations as planned in the original protocol–nevertheless, exploratory non-parametric analysis of the data confirmed the lack of significance of correlations. Once again, our data refers only to experiments on fear conditioning acquisition or consolidation–therefore, other data in the articles could feasibly account for the variation in impact factor and citations.
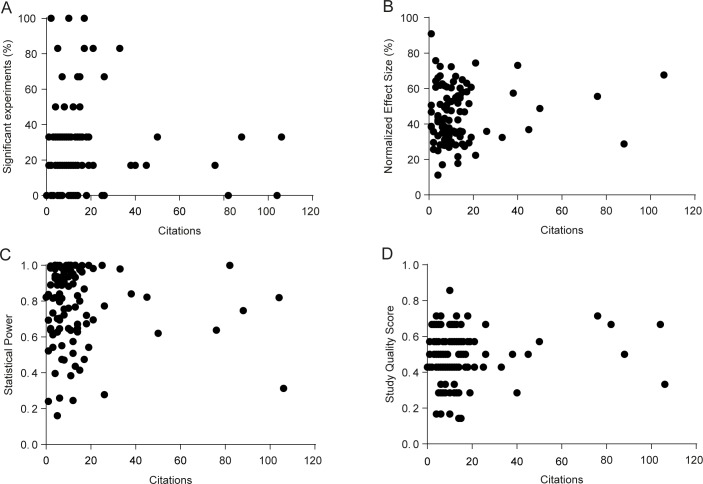
Fig 7. Correlation between citations and percentage of significant experiments, effect size and statistical power.
Citations were obtained for all articles on August 26th, 2016. (A) Correlation between % of significant results per article and citations. r = -0.03, p = 0.75 (n = 121). (B) Correlation between mean normalized effect size of effective interventions and citations. r = 0.097, p = 0.34 (n = 98). (C) Correlation between mean statistical power (upper-bound estimate) and citations. r = -0.08, p = 0.40 (n = 104). (D) Correlation between study quality score and citations. r = 0.09, p = 0.31 (n = 121). According to Holm-Sidak correction for 8 article-level correlations, none is significant.
Discussion
In light of the low reproducibility of published studies in various fields of biomedical research [32–34] which is thought by many to be a consequence of low statistical power and excessive reliance on significance tests [8,16] calls have been made to report effect sizes and confidence intervals alongside or in place of p values [4,6,7,9] and to increase statistical power [14,31,35]. However, it is unclear whether these proposals have had much impact on most fields of basic science. We have taken one particular memory task in rodents, in which outcomes and effect sizes are described in a standardized way and are thus comparable across studies, in order to analyze how these two concepts are dealt with in the study of fear learning.
Our first main finding is that most amnestic interventions targeting fear acquisition or consolidation cause partial effects, with residual freezing remaining significantly above pre-conditioning levels in 78% of the experiments with available data. Moreover, most of the large effect sizes in our sample were found in underpowered studies, suggesting that they could represent inflated estimates [24]. This is not necessarily unexpected: as fear memories depend on a well distributed network, both anatomically and molecularly [19], it seems natural that most interventions directed at a specific site or pharmacological target will modulate learning rather than fully block it. This creates a problem, however, when effect sizes are not considered in the analysis of experiments, as it is not possible to differentiate essential mechanisms of memory formation from modulatory influences on the basis of statistical significance alone. This can lead to a situation in which accumulating evidence, even if correct, can confuse rather than advance understanding, as has been suggested to occur in fields such as long-term potentiation [15] and apoptosis [36].
Matters are complicated further by the possibility that many of these findings are false positives and/or false negatives. The prevalence of both in relation to true positives and negatives depends crucially on statistical power, which in turn depends on sample size. Calculating the actual power of published experiments is challenging, as the difference used for the calculations should not be based on the observed results–which leads power analysis to become circular [18,37]. Thus, statistical power depend on expected effect sizes, which are arbitrary by nature–although they can sometimes be estimated from meta-analyses [14], which were not performed in this study due to the large variety of heterogeneous interventions. However, by considering the mean effect size for well-powered experiments in our sample, we arrived at an estimate of around 37.2% that might be considered “typical” for a published experiment with an intervention affecting fear conditioning acquisition or consolidation. Using the sample size and variation for each experiment, we found mean statistical power to detect this effect size to be 65% in our sample.
As sample size calculations are exceedingly rare, and insufficient power seems to be the norm in other fields of neuroscience as well [14,18], it is quite possible that classically used sample sizes in behavioral neuroscience (and perhaps in other fields of basic science) might thus be insufficient. Considering median variances and our intermediate effect size estimate, the ideal sample size to achieve 80% power would be around 15 animals per group. This number, however, was reached in only 12.2% of cases in our sample, as most experiments had sample sizes of 8 to 12, informally considered to be standard in the field. This seems to confirm recent models suggesting that current incentives in science favor the publication of underpowered studies [16,38], although they could also be due to restrictions on animal use imposed by ethical regulations. That said, average power in our sample for typical effect sizes was higher than those described in other areas of neuroscience by Button et al. [14]; however, this could reflect the fact that effect sizes in their study were calculated by meta-analysis, and might be smaller than those derived by our method of estimation, or underestimated due to the inclusion of negative results [18]. One should also note that the abovementioned power estimates were found to vary widely across subfields of neuroscience [18]–in this sense, the power distribution of fear conditioning studies seems to resemble those found for psychology and neurochemistry studies, in which a reasonable number of well-powered studies coexist with underpowered ones.
On the other hand, our statistical power to detect Cohen’s definitions of small, medium and large effects [22] were even lower than those recently reported in cognitive neuroscience studies by Szucs and Ioannidis (2017). That said, our data provides a strong cautionary note against the use of these arbitrary definitions, originally devised for psychology studies, in calculations of statistical power, as 88.7% of statistically significant experiments (or 48.2% of the whole sample) fell into the “large” category of Cohen’s original proposal. This suggests that laboratory studies in rodents have larger effects than those found in human psychology (an unsurprising finding, given the greater invasiveness of the interventions), as has also been found in meta-analyses studying similar treatments in laboratory animals and humans [39], demonstrating that what constitutes a small or large effect can vary between different fields of science.
An old-established truism in the behavioral neuroscience field–as well as in other fields of basic science–is that experiments in females tend to yield more variable results due to estrous cycle variations [40]. However, at least in our analysis, coefficients of variation were similar between experiments in males and females (and predictably higher in experiments using both), as has been found in other areas of science [41,42] suggesting that this belief is false. Nevertheless, adherence to it likely accounts for the vast preponderance of experiments on male animals, which were nearly 8 times more common than those in females in our sample–a sex bias greater than those described for most fields [43] although smaller than that recently reported for rodent models of anxiety [44]. Previous work in clinical [45] and preclinical [40,43] data has pointed out the drawbacks of concentrating experiments in male populations. However, despite calls for increasing the number of studies on females [46] this problem remains strikingly present in the fear learning field.
Concerning risk of bias indicators, the prevalence found in our sample was roughly similar to previous reports on animal studies for randomization and conflict of interest reporting [13] but were distinctly higher for blinded assessment of outcome, largely because 59% of articles used automated software to measure freezing, which we considered to be equivalent to blinded assessment. If one considers only articles reporting manual scoring of freezing, however, blinding was reported in 57% of cases, which is still higher than most areas of preclinical science [13]. As described previously in many fields [12,13,31] sample size calculations were almost non-existent, which helps to explain why many experiments are underpowered. Interestingly, although we analyzed a sample of papers published 3 years after the ARRIVE guidelines they were not mentioned in any of the articles, suggesting that their impact, at least in the field of behavioral neuroscience, was still rather limited at this time.
Contrary to previous studies, however, we did not detect an impact of these risk of bias indicators on article-level measures such as percentage of fear conditioning learning experiments with significant results, mean effect size of significant experiments and mean statistical power. This could mean that, compared to preclinical studies, bias towards positive results is lower in studies on fear learning. However, it seems more likely that, as we selected particular experiments within papers containing other results, we were not as likely to detect effects of bias on article-level measures. As basic science articles typically contain numerous results, it is perhaps less likely that all comparisons will be subject to bias towards positive findings. Moreover, the experiments in our sample probably included negative controls for other findings, which might have been expected to yield non-significant results. Thus, although our results do not indicate an impact of bias on article-level results, they should not be taken as evidence that this does not occur.
The same reasoning applies for the evaluation of publication bias, in which the experiments we analyzed could have been published along with positive ones. Nevertheless, we were still able to detect a negative correlation between effect size and statistical power, suggesting effect size inflation due to low statistical power to be present in studies on fear conditioning learning. Although the pattern we detected was less suggestive of actual publication bias, our capability to detect it was likely smaller due to the choice to use experiments within articles. Other methods to detect publication bias, such as the Ioannidis excess significance test [47] and the use of p-value distributions [48–50] were also considered, but found to be inappropriate for use with our methodology (in the first case due to the absence of a meta-analytic effect estimate, and in the second because exact p values were infrequently provided in articles).
One of the most interesting findings of our article was the lack of correlation of effect sizes inferred from textual description of results with the actual effect sizes of significant experiments, as well as with statistical power. Although this suggests that these measures are not usually considered in the interpretation of results, there are caveats to this data. First of all, agreement between what words describe a “strong” or “weak” effect between researchers evaluating them was strikingly low, suggesting that written language is a poor descriptor for quantitative data. Moreover, the fact that most terms used to describe differences were neutral to effect sizes (e.g. “significantly higher”, “significantly lower”, etc.) limited our ability to detect a correlation. That said, the high prevalence of neutral terms by itself is evidence that effect sizes are not usually taken into account when reporting results, as differences tend to be described in the text by their statistical significance only.
This point is especially important to consider in the light of recent calls for basic science to use data synthesis tools such as meta-analysis [11] and formal or informal Bayesian inference [2,8,10,51]. In both of these cases, the incremental effect of each new experiment on researchers’ beliefs on the veracity of a finding is dependent both on the effect size of the result and on its statistical significance. However, even exact p values were uncommonly reported in our sample, with the majority of articles describing p as being above or below a threshold value. This seems to suggest that researchers in the field indeed tend to consider statistical significance as a binary outcome, and might not be quite ready or willing to move towards Bayesian logic, which would require a major paradigm shift in the way results are reported and discussed.
An interesting question is that, if researches in the field indeed were to move away from null-significance hypothesis testing, the concept of statistical power as it is defined today would largely lose its meaning (as it is intrinsically linked to the idea of a significance threshold). Nevertheless, the necessity of adequate sample size for statistical robustness would remain–in this case, not in order to detect significant differences and prevent false-negatives and false-positives, but to estimate effect sizes with adequate precision. The current notion of statistical power to detect a given difference could thus be replaced with a desired confidence interval for the obtained result when performing sample size calculations–a formulation that might be useful in terms of differentiating biologically significant results from irrelevant ones.
Concerning article impact metrics, our results are in line with previous work showing that journal impact factor does not correlate with statistical power [14] or with most risk of bias indicators [13]. Furthermore, we showed that, in articles on fear conditioning, this lack of correlation also occurs for the percentage of significant experiments and the mean effect size for significant differences, and that it extends to citations measured over 2 subsequent years. That said, our article-level analysis was limited by the fact that, for many articles, the included experiments represented a minority of the findings. Moreover, most articles tend to cluster around intermediate impact factors (i.e. between 3 and 6) and relatively low (< 20) citation numbers. Thus, our methodology might not have been adequate to detect correlations between these metrics with article-wide effect size and power estimates.
The choice to focus on a particular type of experiment–in this case, interventions directed at rodent fear conditioning acquisition or consolidation–is both one of the main strengths and the major limitation of our findings. On one hand, it allows us to look at effect sizes that are truly on the same scale, as fear conditioning protocols tend to be reasonably similar across laboratories, and all included experiments described their results using the same metric. Thus, the studied effect sizes are not abstract and have real-life meaning. On the other hand, this decision limits our conclusions to this specific field of science, and also weakens our article-level conclusions, as most articles had only a fraction of their experiments analyzed.
Dealing with multiple experiments using different outcomes presents a major challenge for meta-research in basic science, and all alternatives present limitations. A radically opposite approach of converting all effect sizes in a field to a single metric (e.g. Pearson’s r, Cohen’s d, etc.) has been used by other researchers investigating similar topics in neuroscience and psychology [17,23,31,35]. Although normalizing effect sizes allows one to obtain results from a wider field, it also leads them to be abstract and not as readily understandable by experimental researchers. Moreover, this approach can lead to the aggregation of results from disparate types of experiments for which effect sizes are not in the same scale, leading to important distortions in calculating power for individual experiments. Finally, recent evidence indicates that, even within neuroscience, features such as statistical power have very different distributions across subfields [18], suggesting that surveys of individual areas are likely to be more reliable for studying them.
In our case, studying the concrete scenario of a specific methodology leads to more readily applicable suggestions for experimental researchers, such as the rule-of-thumb recommendation that the average number of animals per group in a fear conditioning experiments to achieve 80% power would be around 15 for typical effect sizes and variances. Our approach also allowed us to detect correlations between results and specific methodological factors (e.g. context vs. cued conditioning, female vs. male animals) that would not be apparent if multiple types of experiments were pooled together. Still, to provide more solid conclusions on the causal influence of these factors on experimental results, even our methodology has too wide a focus, as analyzing multiple interventions limits our possibilities to perform meta-analysis and meta-regression to control for confounding variables. Follow-up studies with more specific aims (i.e. meta-analyses of specific interventions in fear conditioning) are thus warranted to understand the variation between results in the field.
Finally, it is important to note that, while our study has led to some illuminating conclusions, they are inherently limited to the methodology under study. Thus, extrapolating our findings to other types of behavioral studies, not to mention other fields of science, requires data to be collected for each specific subfield. While this might appear herculean at first glance, it is easily achievable if scientists working within specific domains start to design and perform their own systematic reviews. Only through this dissemination of meta-research across different areas of science will we be able to develop solutions that, by respecting the particularities of individual subfields, will be accepted enough to have an impact on research reproducibility.
Materials and methods
The full protocol of data selection, extraction and analysis was initially planned on the basis of a pilot analysis of 30 papers, and was registered, reviewed and published ahead of full data extraction [20]. In brief, we searched PubMed for the term “fear conditioning” AND (“learning” OR “consolidation” OR “acquisition”) AND (“mouse” OR “mice” OR “rat” OR “rats”)” to obtain all articles published online in 2013. Titles and abstracts were first scanned for articles presenting original results involving fear conditioning in rodents that were written in English. Selected articles underwent full-text screening for selection of experiments that (a) described the effects of a single intervention on fear conditioning acquisition or consolidation, (b) had a clearly defined control group to which the experimental group is compared to, (c) used freezing behavior as a measure of conditioned fear in a test session and (d) had available data on mean freezing, SD or SEM, as well as on the significance of the comparison. Articles were screened by one of two investigators (C.F.D.C. or T.C.M.) for relevant data and were analyzed by the other–thus, all included experiments were dual-reviewed.
Only experiments analyzing the effect of interventions performed before or up to 6 hours after the training session (i.e. those affecting fear conditioning acquisition or its immediate consolidation) were included. Data on mean freezing and SD or SEM were obtained for each group from the text when available; otherwise, it was extracted using Gsys 2.4.6 software (Hokkaido University Nuclear Reaction Data Centre). When exact sample size for each group was available, the experiment was used for the analysis of effect size and statistical power–otherwise, only effect size was obtained, and the experiment was excluded from power analysis. For individual experiments, study design characteristics were also obtained, including species and sex of the animals, type of conditioning protocol, type, timing and site of intervention.
From each comparison, we also obtained the description term used by the authors in the results session of the paper. Classification of the terms used to describe effects (S1 and S2 Tables) was based on a blinded assessment of words or phrases by a pool of 14 researchers who were fluent or native speakers of English and had current or past experience in the field of behavioral neuroscience. Categories were given a score from 0 to 2 in order of magnitude (i.e. 0 = weak, 1 = neutral, 2 = strong for significant results; 0 = similar, 1 = neutral, 2 = trend for non-significant results), and the average results for all researchers was used as a continuous variable for analysis.
Apart from experiment-level variables, we also extracted article-level data such as impact factor of the journal in which it was published (based on the 2013 Journal Citations Report), number of citations (obtained for all articles on August 26th 2016), country of origin (defined by the corresponding author’s affiliation) and the 7 risk of bias indicators described on Table 1. For article-level correlations, we compiled these measures into a normalized score.
After completion of data extraction, all calculations and analyses were performed according to the previously specified protocol. Specific details of calculations (as well as the raw data used) are presented as S1 Data. After this, the following additional analyses were performed in an exploratory fashion:
-
(a)
To confirm that residual freezing levels after memory-impairing interventions were indeed above training values, demonstrating that most amnestic intervention have partial effects, we extracted pre-conditioning freezing levels from training sessions when these were available. These levels were obtained for pre-shock periods only, and separated as baseline values for contextual (i.e. freezing in the absence of tone) or tone conditioning (i.e. freezing in the presence of a tone, but before shock), as displayed in S5 Fig. These were compared to the corresponding test session values for treated groups in memory-impairing interventions by an unpaired t test based on the extracted means, SD or SEM and sample size when these were available.
-
(b)
In the original protocol, only the mean of all effective interventions (i.e. upper-bound effect size) was planned as a point estimate to be used for power calculations, although we acknowledged this to be optimistic [20]. We later decided to perform power calculations based on the mean effect size of the experiments achieving power above 0.95 on the first analysis (i.e. intermediate effect size) to avoid effect size inflation, as we reached the conclusion that this would provide a more realistic estimate. Additionally, we calculated power based on the mean effect size of the whole sample of experiments as a lower-bound estimate, and presented all three estimates in the results section and figures.
-
(c)
In order to evaluate whether the distribution of effect sizes and statistical power varied if effect sizes were defined as absolute differences in freezing levels instead of relative ones, we repeated the analyses in Figs 2–4 using absolute differences in S1, S6 and S9 Figs. This proved to be particularly important to demonstrate that correlations between effect sizes and power were not the consequence of a confounding association of both variables with coefficients of variation. We also repeated power and correlation analyses using effect sizes as standardized mean differences (e.g. Cohen’s d) in S7 and S10 Figs)
-
(d)
To further evaluate the possible impact of the negative correlation between coefficients of variation and freezing levels on our results, we decided to use freezing levels as a covariate in the correlations shown in Fig 4. We also checked whether adding freezing levels as a covariate influenced the statistical analyses in Figs 5 and 6 and S11 Fig, but as this did not have a significant impact on the results in these figures, we only reported the originally planned analyses.
-
(e)
All of our planned analyses were parametric; after extraction, however, it was clear that some of the data deviated from a normal distribution (especially in the case of power estimates, citation counts and impact factor). Because of this, we performed additional non-parametric analyses for the correlations of citations and impact factor with percentage of significant results, mean normalized effect size, statistical power and study quality score.
-
(f)
In the protocol, we had planned to test correlations between normalized effect sizes and statistical power, mean sample size and absolute freezing levels (using the group with the highest freezing). After analyzing the results, we also decided to correlate normalized effect sizes with coefficients of variation (as this, rather than sample size, seemed to explain the lower power of non-significant results), additional power estimates (as using our original estimate led to a ceiling effect) and different estimates of freezing based on the control group or on the mean freezing of both groups (to compare these forms of normalization with the one we chose).
-
(g)
Due to the correlation of study quality assessment with journal impact factor, we performed an exploratory analysis of the correlation of this metric with each of the individual quality assessment items by performing a Student’s t test (corrected for unequal variances by Welch’s correction) between the impact factors of studies with and without each item.
-
(h)
Because of the additional analyses above, we adjusted the number of comparisons/correlations used as the basis of the Holm-Sidak correction for multiple comparisons. The total numbers used for each correction were 14 for experiment-level comparisons, 17 for article-level comparisons, 28 for experiment-level correlations and 8 for article-level correlations, leading to significance thresholds between 0.003 and 0.05.
Supporting information
All extracted and calculated data are present in the .accdb file. Each spreadsheet is connected to the level above it by an automatic field code. The complete description of each variable is presented in the database info PDF file.
(ZIP)
(A) Distribution of effect sizes for experiments, expressed as the absolute difference in freezing between groups. Interventions were divided into memory-impairing (-24.4 ± 10.6, n = 146), memory-enhancing (21.6 ± 8.6, n = 53) or non-effective (-1.09 ± 7.7, n = 211) for graphical purposes, according to the statistical significance of the comparison as informed by authors. Additionally, the whole sample is shown in grey (-6.5 ± 17.7 [-8.2 to -4.7], n = 410). Values are expressed as mean ± SD [95% confidence interval]. Line and whiskers in the inset express median and interquartile interval. (B) Distributions of normalized effect sizes for articles, calculated as the absolute differences between groups. Interventions were divided into memory-impairing (24.4 ± 10.6, n = 146), memory-enhancing (21.6 ± 8.6, n = 53) or non-effective (5.8 ± 5.2, n = 211). Additionally, the whole sample of experiments is shown in grey (14.5 ± 12.0 [13.3 to 15.6], n = 410).
(TIF)
(A) Correlation between the highest mean freezing between both groups and relative effect size, normalized as the % of freezing levels in the highest group. r = -0.20, p<0.0001* (n = 410). (B) Correlation between mean freezing in the control group and normalized effect size. r = -0.25, p<0.0001*. (C) Correlation between mean freezing between groups and normalized effect size. r = -0.45, p<0.0001*. (D) Correlation between the highest mean freezing between both groups and effect size expressed as absolute difference in freezing between groups. r = 0.28, p<0.0001*. (E) Correlation between mean freezing in the control group and absolute effect size. r = 0.18, p = 0.0003*. (F) Correlation between mean freezing between groups and absolute effect size. r = -0.01, p = 0.81. (G) Correlation between highest mean freezing and coefficient of variation (sample size-weighted pooled standard deviation/pooled mean) of both groups. r = -0.69, p<0.0001* (n = 336, as only experiments with exact sample sizes were used to calculate coefficients of variation). (H) Correlation between mean freezing in the control group and coefficient of variation. r = -0.65, p<0.0001*. (I) Correlation between mean freezing between groups and coefficient of variation. r = -0.72, p<0.0001*. Asterisks indicate significant results according to Holm-Sidak correction for 28 experiment-level correlations.
(TIF)
Distribution of effect sizes, expressed as standardized mean differences calculated on the basis of pooled standard deviations (i.e. Cohen’s d). Interventions were divided into memory-impairing (1.5 ± 0.7, n = 120), memory-enhancing (1.4 ± 0.5, n = 39) or non-effective (0.4 ± 0.3, n = 177) for graphical purposes, according to the statistical significance of the comparison as informed by authors. Additionally, the whole sample of experiments is shown in grey (0.9 ± 0.7 [0.8 to 1.0], n = 336). Values are expressed as mean ± SD [95% confidence interval]. Line and whiskers in the inset express median and interquartile interval.
(TIF)
(A) Distribution of mean effect sizes for articles, calculated as percentage of control group freezing. The mean effect size for each type of experiment for each article is shown in the figure–thus, each article can contribute to more than one category if it contains more than one type of experiment (although they are only counted once in the “all” columns). Interventions were divided into memory-impairing (-44.5 ± 18.6%, n = 71), memory-enhancing (75.9 ± 55%, n = 27) or non-effective (1.2 ± 21.8, n = 82). Additionally, the whole sample of articles is shown in grey (-6.1 ± 48% [-14.8 to 2.5], n = 122). Line and whiskers in the inset express median and interquartile interval. (B) Distributions of mean normalized effect sizes for articles, calculated as the percentage of the group with the highest mean. Interventions were divided into memory-impairing (46.2 ± 16.7%, n = 71), memory-enhancing (41.2 ± 12.2%, n = 27) or non-effective (13.6 ± 10.6, n = 82). Additionally, the whole sample of articles is shown in grey (31.5 ± 18.4% [28.2 to 34.8], n = 122). Values are expressed as mean ± SD [95% confidence interval].
(TIF)
(A) Mean freezing (%) in response to context during training and testing for control (left) and treated (right) groups (n = 25 experiments for which baseline freezing to context–i.e. freezing percentage before any footshocks were applied–was reported for the training session). (B) Mean freezing (%) in response to the first tone during training and in response to the average of all tones during testing, for control (left) and treated (right) animals (n = 10 experiments for which baseline freezing to tone–i.e. freezing percentage to the first tone before pairing with footshock–was reported for the training session). Mean freezing is higher in the test than in the training session in all 35 experiments, with p < 0.05 in 25 (78%) out of the 32 cases in which there was enough information (e.g. exact sample size) to perform an unpaired t test between sessions.
(TIF)
(A) Distribution of statistical power for memory-impairing interventions: based on each experiment’s variance and sample size, power varies according to the absolute difference to be detected for α = 0.05. Dashed lines show the three effect sizes used for point estimates of power in D, E and F. (B) Distribution of statistical power for memory-enhancing interventions. (C) Distribution of statistical power for non-effective interventions. (D) Distribution of statistical power to detect the upper-bound absolute effect size of 23.0% (i.e. mean of statistically significant experiments; right dashed line on A, B and C) for impairing (red), enhancing (blue), non-significant (yellow) and all (grey) experiments. Lines and whiskers express median and interquartile interval. (E) Distribution of statistical power to detect the intermediate absolute effect size of 17.1% (i.e. mean of significant experiments powered at 95% in the analysis described in D; middle dashed line on A, B and C). (F) Distribution of statistical power to detect the lower-bound absolute effect size of 14.5% (i.e. mean of all experiments; left dashed line on A, B and C).
(TIF)
(A) Distribution of statistical power calculated for Cohen’s d = 0.8 (i.e. large effects). for memory-impairing (red, 0.46 ± 0.15, n = 120), memory-enhancing (blue, 0.42 ± 0.10, n = 39), non-effective (yellow, 0.44 ± 0.12, n = 177) and all (grey, 0.44 ± 0.13, n = 336) experiments. (B) Distribution of statistical power calculated for Cohen’s d = 0.5. for memory-impairing (0.22 ± 0.07), memory-enhancing, (0.20 ± 0.05) non-effective (0.21 ± 0.07) and all experiments, (0.21 ± 0.07). (C) Distribution of statistical power calculated for Cohen’s d = 0.2 for memory-impairing (0.08 ± 0.01), memory-enhancing (0.07 ± 0.01), non-effective (0.07 ± 0.01) and all experiments, (0.08 ± 0.01). Lines and whiskers represent median and interquartile interval.
(TIF)
(A) Distribution of mean sample size within articles. Gray bars show the distribution of mean sample size for the whole sample of articles (10.8 ± 4.2 [10.1–11.4], n = 149), while lines show the distribution of the mean sample size of memory-impairing (red, 11 ± 4.2, n = 60), memory-enhancing (blue, 10.1 ± 3.1, n = 19) and non-effective (yellow, 10.7 ± 4.4, n = 70) interventions within each article. Each article can contribute to more than one category if it contains more than one type of experiment, although they are counted only once in the full distribution. Values are expressed as mean ± SD [95% confidence interval]. (B) Frequency distribution of mean coefficients of variation for the whole sample of articles (0.44 ± 0.23 [0.41–0.48], n = 149) and for memory-impairing (0.49 ± 0.23, n = 60), memory-enhancing (0.38 ± 0.14, n = 19) and non-effective interventions (0.42 ± 0.23, n = 70) within each article. (C) Statistical power distribution across articles. Based on each experiment’s variance and sample size, mean power varies according to the difference to be detected for α = 0.05. C.i, C.ii and C.iii show the distribution of statistical power curves for memory-impairing, memory enhancing and non-effective interventions within articles, respectively. Vertical dotted lines mark the effect sizes estimates used for the power calculations in D, E and F. (D) Statistical power calculated based on the upper-bound effect size of 45.6% for the mean of memory-impairing, memory-enhancing and non-effective interventions (using the mean power for each type of experiment in each article) or for all experiments in each article. Line and whiskers express median and interquartile interval. Mean statistical power is 0.77 ± 0.21 [0.73–0.81] (n = 105) for the whole sample, 0.79 ± 0.21 (n = 60) for memory-impairing, 0.89 ± 0.12 (n = 19) for memory-enhancing and 0.74 ± 0.23 (n = 70) for non-effective interventions. (E) Same as D, but using the intermediate effect size of 37.2% for calculations. Mean statistical power is 0.67 ± 0.24 [0.63–0.72] (n = 105) for the whole sample, 0.68 ± 0.24 (n = 60) for memory-impairing, 0.79 ± 0.15 (n = 19) for memory-enhancing and 0.65 ± 0.26 (n = 70) for non-effective interventions. (F) Same as D and E, but using the lower-bound effect size estimate of 29.5%. Mean statistical power is 0.55 ± 0.24 [0.50–0.60], n = 105 for the whole sample, 0.54 ± 0.24 (n = 60) for memory-impairing, 0.64 ± 0.18 (n = 19) for memory-enhancing and 0.54 ± 0.28 (n = 70) for non-effective interventions.
(TIF)
(A) Correlation between absolute effect size and mean sample size. No significant correlation is found (r = -0.05, p = 0.34), although this is largely due to the presence of two outliers. (B) Correlation between absolute effect size and coefficient of variation. Correlation of the whole sample of experiments yields r = 0.02, p = 0.70 (n = 336). (C) Correlation between absolute effect size and statistical power based on upper-bound effect size of 23.0%. Correlation of the whole sample of experiments yields r = -0.24, p<0.0001*. (D) Correlation between absolute effect size and statistical power based on intermediate effect size of 17.1%; r = -0.27, p<0.0001*. (E) Correlation between absolute effect size and statistical power based on lower-bound effect size of 14.5%; r = -0.28, p<0.0001*. Asterisks indicate significant results according to Holm-Sidak correction for 28 experiment-level correlations.
(TIF)
(A) Correlation between effect size expressed in Cohen’s d and mean sample size. Correlation of the whole sample of experiments yields r = -0.15, p = 0.007, suggesting the presence of effect size inflation and/or publication bias. Grey line indicates the effect size needed to achieve p < 0.05 in a two-sample t test with equal numbers of animals in both groups for each sample size. Although significant results are invariably above the line, non-significant results can be observed on both sides due to the use of different statistical tests (e.g. post-hoc tests corrected for multiple comparisons) or different sample sizes between groups (B) Correlation between Cohen’s d and coefficient of variation. The negative correlation (r = -0.14, p = 0.01) is mathematically expected, as higher coefficients of variation will lead effect sizes to be smaller when expressed as Cohen’s d. (C) Correlation between Cohen’s d and statistical power to detect an effect size of d = 0.8. Correlation of the whole sample of experiments yields r = -0.16, p = 0.0025*. (D) Correlation between Cohen’s d and statistical power based on effect size of d = 0.5; r = -0.15, p = 0.0045. (E) Correlation between Cohen’s d and statistical power based on effect size of d = 0.2; r = -0.15, p = 0.0047. Asterisks indicate significant results according to Holm-Sidak correction for 28 experiment-level correlations.
(TIF)
Colors indicate memory-enhancing (red), memory-impairing (blue) or non-effective (yellow) experiments, all of which are pooled together in the analysis. Line and whiskers express median and interquartile interval. (A) Distribution of effect sizes across surgical (n = 22), pharmacological (n = 159), genetic (n = 188) and behavioral (n = 41) interventions. One-way ANOVA, p = 0.12. (B) Coefficients of variation across surgical (n = 18), pharmacological (n = 128), genetic (n = 158) and behavioral (n = 32) interventions. One-way ANOVA, p = 0.08. (C) Distribution of effect sizes across systemic (n = 194) and intracerebral (n = 216) interventions. Student’s t test, p = 0.45. (D) Coefficients of variation across systemic (n = 157) and intracerebral (n = 179) interventions. Student’s t test, p = 0.15. (E) Distribution of effect sizes across interventions applied pre- (n = 333) or post-training (n = 77). Student’s t test, p = 0.07. (F) Coefficients of variation across interventions applied pre- (n = 272) or post-training (n = 64). Student’s t test, p = 0.0015*. For all coefficient of variation analyses, 74 experiments were excluded due to lack of information on sample size for individual groups. Asterisks indicate significant results according to Holm-Sidak correction for 14 experiment-level comparisons.
(TIF)
(A) Percentage of significant results in articles with (n = 18) and without (n = 59) randomization. Line and whiskers express median and interquartile interval. Student’s t test, p = 0.13. (B) Mean normalized effect size of significant results in articles with (n = 14) and without (n = 47) randomization. Student’s t test, p = 0.79. (C) Mean statistical power (upper-bound estimate) in articles with (n = 16) and without (n = 49) randomization. Student’s t test, p = 0.33. (D) Percentage of significant results in articles with (n = 92) and without (n = 30) blinded/automated assessment of freezing. Student’s t test, p = 0.65. (E) Mean normalized effect size of significant results in articles with (n = 73) and without (n = 25) blinded/automated assessment. Student’s t test, p = 0.06. (F) Mean statistical power in articles with (n = 81) and without (n = 24) blind/automated assessment. Student’s t test, p = 0.17. (G) Percentage of significant results in articles with (n = 66) and without (n = 56) statement of conflict of interest. Student’s t test, p = 0.12. (H) Mean normalized effect size of significant results in articles with (n = 52) and without (n = 46) statement of conflict of interest. Student’s t test, p = 0.72. (I) Mean statistical power in articles with (n = 56) and without (n = 49) statement of conflict of interest. Student’s t test, p = 0.78. (J) Percentage of significant results per article with (n = 98) and without (n = 24) exact sample size description for fear conditioning experiments. Student’s t test, p = 0.63. (K) Mean normalized effect size of significant results for articles with (n = 76) and without (n = 22) sample size description. Student’s t test, p = 0.33. Sample size varies for each of the three variables, as not all papers have significant results or exact sample sizes allowing power calculations. On panels G-I, white circles indicate papers with a conflict of interest stated, while black circles indicate papers that stated no conflict of interest. According to Holm-Sidak correction for 17 article-level comparisons, none of the differences is significant.
(TIF)
The region of origin of articles were defined according to the affiliation of the corresponding author (or authors) of each article. Line and whiskers express median and interquartile interval. (A) Distribution of mean effect size for significant results across regions of origin. One-way ANOVA p = 0.49. (B) Percentage of significant results per paper across regions of origin. One-way ANOVA p = 0.34 (C) Distribution of mean statistical power (upper-bound estimate) across regions of origin. One-way ANOVA p = 0.98. (D) Distribution of study quality scores across regions of origin. One-way ANOVA p = 0.11. According to Holm-Sidak correction for 17 article-level comparisons, none of the differences is significant.
(TIF)
Impact factors were obtained from the 2013 Journal Citation Report. (A) Correlation between mean normalized effect size and impact factor. r = -0.05, p = 0.63 (n = 98). (B) Correlation between % of significant results per article and impact factor. r = -0.08, p = 0.37 (n = 121). (C) Correlation between mean statistical power (upper-bound estimate) and impact factor. r = 0.05, p = 0.62 (n = 104). (D) Correlation between study quality score and impact factor. r = 0.22, p = 0.01 (n = 121). According to Holm-Sidak correction for 8 article-level correlations, none of them are significant.
(TIF)
Volunteers were asked to judge each term on the left column as representing a strong, neutral or weak effect. Each term was given a score (strong, 2; neutral, 1; weak, 0) by each respondent and the mean score for each term (right column) was calculated based on an average of all 14 researchers. Single-measures intraclass correlation coefficient (reflecting agreement among researchers) was .234, while average-measures intraclass correlation coefficient (reflecting the aggregated reliability of the obtained means) was .839. Terms are ordered by score from weakest to strongest.
(DOCX)
Volunteers were asked to judge each term on the left column as representing similar means between both groups, a trend of difference or no information on the presence of a trend (neutral). Each term was given a score (trend, 2; neutral, 1; similar, 0) by each respondent and the mean score for each term (right column) was calculated based on an average of all 14 researchers. Single-measures intraclass correlation coefficient (reflecting agreement among researchers) was .597, while average-measures intraclass correlation coefficient (reflecting the aggregated reliability of the obtained means) was .962. Terms are ordered by score from most similar to most associated with a trend.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work is supported by FAPERJ grants E-26/111.277/2014 and E-26/201.544/2014 to O.B.A., by RCUK NC3Rs grant NC/L000970/1 to M.R.M., by CNPq scholarships to T.C.M. and C.F.D.C, and by a FAPERJ supplementary scholarship to C.F.D.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nuzzo R. Statistical errors. Nature. 2014;506: 150–152. doi: 10.1038/506150a [DOI] [PubMed] [Google Scholar]
- 2.Colquhoun D. An investigation of the false discovery rate and the misinterpretation of p-values. R Soc Open Sci. 2014;1: 140216–140216. doi: 10.1098/rsos.140216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman N, Krzywinski M. Points of significance: P values and the search for significance. Nat Methods. 2016;14: 3–4. [Google Scholar]
- 4.Halsey LG, Curran-Everett D, Vowler SL, Drummond GB. The fickle P value generates irreproducible results. Nat Methods. 2015;12: 179–185. doi: 10.1038/nmeth.3288 [DOI] [PubMed] [Google Scholar]
- 5.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. 2016;70: 129–133. [Google Scholar]
- 6.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31: 337–350. doi: 10.1007/s10654-016-0149-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev. 2007;82: 591–605. doi: 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- 8.Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2: 696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trafimow D, Marks M. Editorial. Basic Appl Soc Psych. 2015;37: 1–2. [Google Scholar]
- 10.Goodman SN, Fanelli D, Ioannidis JPA. What does research reproducibility mean? Sci Transl Med. 2016;8: 1–6. [DOI] [PubMed] [Google Scholar]
- 11.Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, et al. Meta-analysis of data from animal studies: A practical guide. J Neurosci Methods. 2014;221: 92–102. doi: 10.1016/j.jneumeth.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One. 2009;4: e7824 doi: 10.1371/journal.pone.0007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macleod MR, Lawson McLean A, Kyriakopoulou A, Serghiou S, de Wilde A, Sherratt N, et al. Risk of bias in reports of in vivo research: a focus for improvement. PLoS Biol. 2015;13: e1002273 doi: 10.1371/journal.pbio.1002273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Button KS, Ioannidis JP, Mokrysz C, Nosek B, Flint J, Robinson ESJ, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14: 365–76. doi: 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- 15.Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nat Neurosci. 1999;2: 597–604. doi: 10.1038/10154 [DOI] [PubMed] [Google Scholar]
- 16.Higginson AD, Munafò MR. Current incentives for scientists lead to underpowered studies with erroneous conclusions. PLoS Biol. 2016;14: e2000995 doi: 10.1371/journal.pbio.2000995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szucs D, Ioannidis JPA. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol. 2017;15: e2000797 doi: 10.1371/journal.pbio.2000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nord CL, Valton V, Wood J, Roiser JP. Power-up: a reanalysis of “power failure” in neuroscience using mixture modelling. J Neurosci. 2017;37: 3592–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24: 897–931. doi: 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- 20.Moulin TC, Carneiro CFD, Macleod MR, Amaral OB. Protocol for a systematic review of effect sizes and statistical power in the rodent fear conditioning literature. Evid Based Preclin Med. 2016;3: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6: e1000097 doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.) New York: Academic Press; 1977. [Google Scholar]
- 23.Kuhberger A, Fritz A, Scherndl T. Publication bias in psychology: A diagnosis based on the correlation between effect size and sample size. PLoS One. 2014;9: e105825 doi: 10.1371/journal.pone.0105825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ioannidis JPA. Why most discovered true associations are inflated. Epidemiology. 2008;19: 640–648. doi: 10.1097/EDE.0b013e31818131e7 [DOI] [PubMed] [Google Scholar]
- 25.Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30: 433–9. doi: 10.1016/j.tins.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 26.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8: e1000412 doi: 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macleod MR, van der Worp HB, Sena ES, Howells DW, Dirnagl U, Donnan GA. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke. 2008;39: 2824–9. doi: 10.1161/STROKEAHA.108.515957 [DOI] [PubMed] [Google Scholar]
- 28.Vesterinen HM, Sena ES, Ffrench-Constant C, Williams A, Chandran S, Macleod MR. Improving the translational hit of experimental treatments in multiple sclerosis. Mult Scler. 2010;16: 1044–55. doi: 10.1177/1352458510379612 [DOI] [PubMed] [Google Scholar]
- 29.Rooke EDM, Vesterinen HM, Sena ES, Egan KJ, Macleod MR. Dopamine agonists in animal models of Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17: 313–320. doi: 10.1016/j.parkreldis.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 30.Currie GL, Delaney A, Bennett MI, Dickenson AH, Egan KJ, Vesterinen HM, et al. Animal models of bone cancer pain: Systematic review and meta-analyses. Pain. 2013;154: 917–926. doi: 10.1016/j.pain.2013.02.033 [DOI] [PubMed] [Google Scholar]
- 31.Sedlmeier P, Gigerenzer G. Do studies of statistical power have an effect on the power of studies? 1989;105: 309–316. [Google Scholar]
- 32.Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9: 4–15. doi: 10.1080/17482960701856300 [DOI] [PubMed] [Google Scholar]
- 33.Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10: 712 doi: 10.1038/nrd3439-c1 [DOI] [PubMed] [Google Scholar]
- 34.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483: 531–3. doi: 10.1038/483531a [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psychol. 1962;65: 145–53. [DOI] [PubMed] [Google Scholar]
- 36.Lazebnik Y. Can a biologist fix a radio?—Or, what I learned while studying apoptosis. Cancer Cell. 2002;2: 179–82. [DOI] [PubMed] [Google Scholar]
- 37.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994; 121: 200–206. [DOI] [PubMed] [Google Scholar]
- 38.Smaldino PE, McElreath R. The natural selection of bad science. R Soc Open Sci. 2016;3: 160384 doi: 10.1098/rsos.160384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63: 1118–1126. doi: 10.1016/j.biopsych.2008.01.012 [DOI] [PubMed] [Google Scholar]
- 40.Wald C, Wu C. Of mice and women: The bias in animal models. Science. 2010;327: 1571–1572. doi: 10.1126/science.327.5973.1571 [DOI] [PubMed] [Google Scholar]
- 41.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117: 1–5. doi: 10.1016/j.pain.2005.06.020 [DOI] [PubMed] [Google Scholar]
- 42.Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40: 1–5. doi: 10.1016/j.neubiorev.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 43.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35: 565–572. doi: 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohammad F, Ho J, Woo JH, Lim CL, Poon DJJ, Lamba B, et al. Concordance and incongruence in preclinical anxiety models: Systematic review and meta-analyses. Neurosci Biobehav Rev. 2016;68: 504–529. doi: 10.1016/j.neubiorev.2016.04.011 [DOI] [PubMed] [Google Scholar]
- 45.Wizemann TM. Sex-specific reporting of scientific research Washington: National Academies Press; 2012. [PubMed] [Google Scholar]
- 46.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509: 282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4: 245–253. doi: 10.1177/1740774507079441 [DOI] [PubMed] [Google Scholar]
- 48.Ridley J, Kolm N, Freckelton RP, Gage MJG. An unexpected influence of widely used significance thresholds on the distribution of reported P-values. J Evol Biol. 2007;20: 1082–1089. doi: 10.1111/j.1420-9101.2006.01291.x [DOI] [PubMed] [Google Scholar]
- 49.Simonsohn U, Nelson LD, Simmons JP. p -Curve and Effect Size. Perspect Psychol Sci. 2014;9: 666–681. doi: 10.1177/1745691614553988 [DOI] [PubMed] [Google Scholar]
- 50.de Winter JC, Dodou D. A surge of p-values between 0.041 and 0.049 in recent decades (but negative results are increasing rapidly too). PeerJ. 2015;3: e733 doi: 10.7717/peerj.733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagenmakers EJ. A practical solution to the pervasive problems of p values. Psychon Bull Rev. 2007;14: 779–804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All extracted and calculated data are present in the .accdb file. Each spreadsheet is connected to the level above it by an automatic field code. The complete description of each variable is presented in the database info PDF file.
(ZIP)
(A) Distribution of effect sizes for experiments, expressed as the absolute difference in freezing between groups. Interventions were divided into memory-impairing (-24.4 ± 10.6, n = 146), memory-enhancing (21.6 ± 8.6, n = 53) or non-effective (-1.09 ± 7.7, n = 211) for graphical purposes, according to the statistical significance of the comparison as informed by authors. Additionally, the whole sample is shown in grey (-6.5 ± 17.7 [-8.2 to -4.7], n = 410). Values are expressed as mean ± SD [95% confidence interval]. Line and whiskers in the inset express median and interquartile interval. (B) Distributions of normalized effect sizes for articles, calculated as the absolute differences between groups. Interventions were divided into memory-impairing (24.4 ± 10.6, n = 146), memory-enhancing (21.6 ± 8.6, n = 53) or non-effective (5.8 ± 5.2, n = 211). Additionally, the whole sample of experiments is shown in grey (14.5 ± 12.0 [13.3 to 15.6], n = 410).
(TIF)
(A) Correlation between the highest mean freezing between both groups and relative effect size, normalized as the % of freezing levels in the highest group. r = -0.20, p<0.0001* (n = 410). (B) Correlation between mean freezing in the control group and normalized effect size. r = -0.25, p<0.0001*. (C) Correlation between mean freezing between groups and normalized effect size. r = -0.45, p<0.0001*. (D) Correlation between the highest mean freezing between both groups and effect size expressed as absolute difference in freezing between groups. r = 0.28, p<0.0001*. (E) Correlation between mean freezing in the control group and absolute effect size. r = 0.18, p = 0.0003*. (F) Correlation between mean freezing between groups and absolute effect size. r = -0.01, p = 0.81. (G) Correlation between highest mean freezing and coefficient of variation (sample size-weighted pooled standard deviation/pooled mean) of both groups. r = -0.69, p<0.0001* (n = 336, as only experiments with exact sample sizes were used to calculate coefficients of variation). (H) Correlation between mean freezing in the control group and coefficient of variation. r = -0.65, p<0.0001*. (I) Correlation between mean freezing between groups and coefficient of variation. r = -0.72, p<0.0001*. Asterisks indicate significant results according to Holm-Sidak correction for 28 experiment-level correlations.
(TIF)
Distribution of effect sizes, expressed as standardized mean differences calculated on the basis of pooled standard deviations (i.e. Cohen’s d). Interventions were divided into memory-impairing (1.5 ± 0.7, n = 120), memory-enhancing (1.4 ± 0.5, n = 39) or non-effective (0.4 ± 0.3, n = 177) for graphical purposes, according to the statistical significance of the comparison as informed by authors. Additionally, the whole sample of experiments is shown in grey (0.9 ± 0.7 [0.8 to 1.0], n = 336). Values are expressed as mean ± SD [95% confidence interval]. Line and whiskers in the inset express median and interquartile interval.
(TIF)
(A) Distribution of mean effect sizes for articles, calculated as percentage of control group freezing. The mean effect size for each type of experiment for each article is shown in the figure–thus, each article can contribute to more than one category if it contains more than one type of experiment (although they are only counted once in the “all” columns). Interventions were divided into memory-impairing (-44.5 ± 18.6%, n = 71), memory-enhancing (75.9 ± 55%, n = 27) or non-effective (1.2 ± 21.8, n = 82). Additionally, the whole sample of articles is shown in grey (-6.1 ± 48% [-14.8 to 2.5], n = 122). Line and whiskers in the inset express median and interquartile interval. (B) Distributions of mean normalized effect sizes for articles, calculated as the percentage of the group with the highest mean. Interventions were divided into memory-impairing (46.2 ± 16.7%, n = 71), memory-enhancing (41.2 ± 12.2%, n = 27) or non-effective (13.6 ± 10.6, n = 82). Additionally, the whole sample of articles is shown in grey (31.5 ± 18.4% [28.2 to 34.8], n = 122). Values are expressed as mean ± SD [95% confidence interval].
(TIF)
(A) Mean freezing (%) in response to context during training and testing for control (left) and treated (right) groups (n = 25 experiments for which baseline freezing to context–i.e. freezing percentage before any footshocks were applied–was reported for the training session). (B) Mean freezing (%) in response to the first tone during training and in response to the average of all tones during testing, for control (left) and treated (right) animals (n = 10 experiments for which baseline freezing to tone–i.e. freezing percentage to the first tone before pairing with footshock–was reported for the training session). Mean freezing is higher in the test than in the training session in all 35 experiments, with p < 0.05 in 25 (78%) out of the 32 cases in which there was enough information (e.g. exact sample size) to perform an unpaired t test between sessions.
(TIF)
(A) Distribution of statistical power for memory-impairing interventions: based on each experiment’s variance and sample size, power varies according to the absolute difference to be detected for α = 0.05. Dashed lines show the three effect sizes used for point estimates of power in D, E and F. (B) Distribution of statistical power for memory-enhancing interventions. (C) Distribution of statistical power for non-effective interventions. (D) Distribution of statistical power to detect the upper-bound absolute effect size of 23.0% (i.e. mean of statistically significant experiments; right dashed line on A, B and C) for impairing (red), enhancing (blue), non-significant (yellow) and all (grey) experiments. Lines and whiskers express median and interquartile interval. (E) Distribution of statistical power to detect the intermediate absolute effect size of 17.1% (i.e. mean of significant experiments powered at 95% in the analysis described in D; middle dashed line on A, B and C). (F) Distribution of statistical power to detect the lower-bound absolute effect size of 14.5% (i.e. mean of all experiments; left dashed line on A, B and C).
(TIF)
(A) Distribution of statistical power calculated for Cohen’s d = 0.8 (i.e. large effects). for memory-impairing (red, 0.46 ± 0.15, n = 120), memory-enhancing (blue, 0.42 ± 0.10, n = 39), non-effective (yellow, 0.44 ± 0.12, n = 177) and all (grey, 0.44 ± 0.13, n = 336) experiments. (B) Distribution of statistical power calculated for Cohen’s d = 0.5. for memory-impairing (0.22 ± 0.07), memory-enhancing, (0.20 ± 0.05) non-effective (0.21 ± 0.07) and all experiments, (0.21 ± 0.07). (C) Distribution of statistical power calculated for Cohen’s d = 0.2 for memory-impairing (0.08 ± 0.01), memory-enhancing (0.07 ± 0.01), non-effective (0.07 ± 0.01) and all experiments, (0.08 ± 0.01). Lines and whiskers represent median and interquartile interval.
(TIF)
(A) Distribution of mean sample size within articles. Gray bars show the distribution of mean sample size for the whole sample of articles (10.8 ± 4.2 [10.1–11.4], n = 149), while lines show the distribution of the mean sample size of memory-impairing (red, 11 ± 4.2, n = 60), memory-enhancing (blue, 10.1 ± 3.1, n = 19) and non-effective (yellow, 10.7 ± 4.4, n = 70) interventions within each article. Each article can contribute to more than one category if it contains more than one type of experiment, although they are counted only once in the full distribution. Values are expressed as mean ± SD [95% confidence interval]. (B) Frequency distribution of mean coefficients of variation for the whole sample of articles (0.44 ± 0.23 [0.41–0.48], n = 149) and for memory-impairing (0.49 ± 0.23, n = 60), memory-enhancing (0.38 ± 0.14, n = 19) and non-effective interventions (0.42 ± 0.23, n = 70) within each article. (C) Statistical power distribution across articles. Based on each experiment’s variance and sample size, mean power varies according to the difference to be detected for α = 0.05. C.i, C.ii and C.iii show the distribution of statistical power curves for memory-impairing, memory enhancing and non-effective interventions within articles, respectively. Vertical dotted lines mark the effect sizes estimates used for the power calculations in D, E and F. (D) Statistical power calculated based on the upper-bound effect size of 45.6% for the mean of memory-impairing, memory-enhancing and non-effective interventions (using the mean power for each type of experiment in each article) or for all experiments in each article. Line and whiskers express median and interquartile interval. Mean statistical power is 0.77 ± 0.21 [0.73–0.81] (n = 105) for the whole sample, 0.79 ± 0.21 (n = 60) for memory-impairing, 0.89 ± 0.12 (n = 19) for memory-enhancing and 0.74 ± 0.23 (n = 70) for non-effective interventions. (E) Same as D, but using the intermediate effect size of 37.2% for calculations. Mean statistical power is 0.67 ± 0.24 [0.63–0.72] (n = 105) for the whole sample, 0.68 ± 0.24 (n = 60) for memory-impairing, 0.79 ± 0.15 (n = 19) for memory-enhancing and 0.65 ± 0.26 (n = 70) for non-effective interventions. (F) Same as D and E, but using the lower-bound effect size estimate of 29.5%. Mean statistical power is 0.55 ± 0.24 [0.50–0.60], n = 105 for the whole sample, 0.54 ± 0.24 (n = 60) for memory-impairing, 0.64 ± 0.18 (n = 19) for memory-enhancing and 0.54 ± 0.28 (n = 70) for non-effective interventions.
(TIF)
(A) Correlation between absolute effect size and mean sample size. No significant correlation is found (r = -0.05, p = 0.34), although this is largely due to the presence of two outliers. (B) Correlation between absolute effect size and coefficient of variation. Correlation of the whole sample of experiments yields r = 0.02, p = 0.70 (n = 336). (C) Correlation between absolute effect size and statistical power based on upper-bound effect size of 23.0%. Correlation of the whole sample of experiments yields r = -0.24, p<0.0001*. (D) Correlation between absolute effect size and statistical power based on intermediate effect size of 17.1%; r = -0.27, p<0.0001*. (E) Correlation between absolute effect size and statistical power based on lower-bound effect size of 14.5%; r = -0.28, p<0.0001*. Asterisks indicate significant results according to Holm-Sidak correction for 28 experiment-level correlations.
(TIF)
(A) Correlation between effect size expressed in Cohen’s d and mean sample size. Correlation of the whole sample of experiments yields r = -0.15, p = 0.007, suggesting the presence of effect size inflation and/or publication bias. Grey line indicates the effect size needed to achieve p < 0.05 in a two-sample t test with equal numbers of animals in both groups for each sample size. Although significant results are invariably above the line, non-significant results can be observed on both sides due to the use of different statistical tests (e.g. post-hoc tests corrected for multiple comparisons) or different sample sizes between groups (B) Correlation between Cohen’s d and coefficient of variation. The negative correlation (r = -0.14, p = 0.01) is mathematically expected, as higher coefficients of variation will lead effect sizes to be smaller when expressed as Cohen’s d. (C) Correlation between Cohen’s d and statistical power to detect an effect size of d = 0.8. Correlation of the whole sample of experiments yields r = -0.16, p = 0.0025*. (D) Correlation between Cohen’s d and statistical power based on effect size of d = 0.5; r = -0.15, p = 0.0045. (E) Correlation between Cohen’s d and statistical power based on effect size of d = 0.2; r = -0.15, p = 0.0047. Asterisks indicate significant results according to Holm-Sidak correction for 28 experiment-level correlations.
(TIF)
Colors indicate memory-enhancing (red), memory-impairing (blue) or non-effective (yellow) experiments, all of which are pooled together in the analysis. Line and whiskers express median and interquartile interval. (A) Distribution of effect sizes across surgical (n = 22), pharmacological (n = 159), genetic (n = 188) and behavioral (n = 41) interventions. One-way ANOVA, p = 0.12. (B) Coefficients of variation across surgical (n = 18), pharmacological (n = 128), genetic (n = 158) and behavioral (n = 32) interventions. One-way ANOVA, p = 0.08. (C) Distribution of effect sizes across systemic (n = 194) and intracerebral (n = 216) interventions. Student’s t test, p = 0.45. (D) Coefficients of variation across systemic (n = 157) and intracerebral (n = 179) interventions. Student’s t test, p = 0.15. (E) Distribution of effect sizes across interventions applied pre- (n = 333) or post-training (n = 77). Student’s t test, p = 0.07. (F) Coefficients of variation across interventions applied pre- (n = 272) or post-training (n = 64). Student’s t test, p = 0.0015*. For all coefficient of variation analyses, 74 experiments were excluded due to lack of information on sample size for individual groups. Asterisks indicate significant results according to Holm-Sidak correction for 14 experiment-level comparisons.
(TIF)
(A) Percentage of significant results in articles with (n = 18) and without (n = 59) randomization. Line and whiskers express median and interquartile interval. Student’s t test, p = 0.13. (B) Mean normalized effect size of significant results in articles with (n = 14) and without (n = 47) randomization. Student’s t test, p = 0.79. (C) Mean statistical power (upper-bound estimate) in articles with (n = 16) and without (n = 49) randomization. Student’s t test, p = 0.33. (D) Percentage of significant results in articles with (n = 92) and without (n = 30) blinded/automated assessment of freezing. Student’s t test, p = 0.65. (E) Mean normalized effect size of significant results in articles with (n = 73) and without (n = 25) blinded/automated assessment. Student’s t test, p = 0.06. (F) Mean statistical power in articles with (n = 81) and without (n = 24) blind/automated assessment. Student’s t test, p = 0.17. (G) Percentage of significant results in articles with (n = 66) and without (n = 56) statement of conflict of interest. Student’s t test, p = 0.12. (H) Mean normalized effect size of significant results in articles with (n = 52) and without (n = 46) statement of conflict of interest. Student’s t test, p = 0.72. (I) Mean statistical power in articles with (n = 56) and without (n = 49) statement of conflict of interest. Student’s t test, p = 0.78. (J) Percentage of significant results per article with (n = 98) and without (n = 24) exact sample size description for fear conditioning experiments. Student’s t test, p = 0.63. (K) Mean normalized effect size of significant results for articles with (n = 76) and without (n = 22) sample size description. Student’s t test, p = 0.33. Sample size varies for each of the three variables, as not all papers have significant results or exact sample sizes allowing power calculations. On panels G-I, white circles indicate papers with a conflict of interest stated, while black circles indicate papers that stated no conflict of interest. According to Holm-Sidak correction for 17 article-level comparisons, none of the differences is significant.
(TIF)
The region of origin of articles were defined according to the affiliation of the corresponding author (or authors) of each article. Line and whiskers express median and interquartile interval. (A) Distribution of mean effect size for significant results across regions of origin. One-way ANOVA p = 0.49. (B) Percentage of significant results per paper across regions of origin. One-way ANOVA p = 0.34 (C) Distribution of mean statistical power (upper-bound estimate) across regions of origin. One-way ANOVA p = 0.98. (D) Distribution of study quality scores across regions of origin. One-way ANOVA p = 0.11. According to Holm-Sidak correction for 17 article-level comparisons, none of the differences is significant.
(TIF)
Impact factors were obtained from the 2013 Journal Citation Report. (A) Correlation between mean normalized effect size and impact factor. r = -0.05, p = 0.63 (n = 98). (B) Correlation between % of significant results per article and impact factor. r = -0.08, p = 0.37 (n = 121). (C) Correlation between mean statistical power (upper-bound estimate) and impact factor. r = 0.05, p = 0.62 (n = 104). (D) Correlation between study quality score and impact factor. r = 0.22, p = 0.01 (n = 121). According to Holm-Sidak correction for 8 article-level correlations, none of them are significant.
(TIF)
Volunteers were asked to judge each term on the left column as representing a strong, neutral or weak effect. Each term was given a score (strong, 2; neutral, 1; weak, 0) by each respondent and the mean score for each term (right column) was calculated based on an average of all 14 researchers. Single-measures intraclass correlation coefficient (reflecting agreement among researchers) was .234, while average-measures intraclass correlation coefficient (reflecting the aggregated reliability of the obtained means) was .839. Terms are ordered by score from weakest to strongest.
(DOCX)
Volunteers were asked to judge each term on the left column as representing similar means between both groups, a trend of difference or no information on the presence of a trend (neutral). Each term was given a score (trend, 2; neutral, 1; similar, 0) by each respondent and the mean score for each term (right column) was calculated based on an average of all 14 researchers. Single-measures intraclass correlation coefficient (reflecting agreement among researchers) was .597, while average-measures intraclass correlation coefficient (reflecting the aggregated reliability of the obtained means) was .962. Terms are ordered by score from most similar to most associated with a trend.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.