Abstract
Drought is a major threat to plant growth and crop productivity. The phytohormone abscisic acid (ABA) plays a critical role in plant response to drought stress. Although ABA signaling-mediated drought tolerance has been widely investigated in Arabidopsis thaliana, the feedback mechanism and components negatively regulating this pathway are less well understood. Here we identified a member of Arabidopsis HD-ZIP transcription factors HAT1 which can interacts with and be phosphorylated by SnRK2s. hat1hat3, loss-of-function mutant of HAT1 and its homolog HAT3, was hypersensitive to ABA in primary root inhibition, ABA-responsive genes expression, and displayed enhanced drought tolerance, whereas HAT1 overexpressing lines were hyposensitive to ABA and less tolerant to drought stress, suggesting that HAT1 functions as a negative regulator in ABA signaling-mediated drought response. Furthermore, expression levels of ABA biosynthesis genes ABA3 and NCED3 were repressed by HAT1 directly binding to their promoters, resulting in the ABA level was increased in hat1hat3 and reduced in HAT1OX lines. Further evidence showed that both protein stability and binding activity of HAT1 was repressed by SnRK2.3 phosphorylation. Overexpressing SnRK2.3 in HAT1OX transgenic plant made a reduced HAT1 protein level and suppressed the HAT1OX phenotypes in ABA and drought response. Our results thus establish a new negative regulation mechanism of HAT1 which helps plants fine-tune their drought responses.
Author summary
Drought stress is a key environmental factor that severely reduces crop yield all over the world. The phytohormone abscisic acid (ABA) is known to mediate drought responses through regulating drought-responsive genes expression and stomatal closure, but the mechanisms that negatively regulate this process and prevent the adverse effects of excess drought responses on plant growth is less well studied. Here, we show that a HD-ZIP II transcription factor HAT1 negatively regulates ABA-mediated drought responses through suppressing ABA biosynthesis and signaling. The hat1hat3 mutant showed ABA-hypersensitive and drought-tolerant phenotypes, whereas the HAT1-overexpressing lines were insensitive to ABA and less tolerant to drought. Furthermore, we found SnRK2.3 kinase, a positive component of ABA signaling, interacts with and phosphorylates HAT1 to destabilize and suppress its binding activity. Overexpression of SnRK2.3 reduces HAT1 protein level and inhibits HAT1OX phenotypes in ABA and drought responses. Our results revealed a HAT1-mediated negative regulatory mechanism in attenuating the ABA signaling and drought response.
Introduction
As sessile organisms, plants need to respond and adapt to environmental stress to survive adverse conditions. Plants respond and adapt to stresses through a complex network of factors involved in stress hormone signaling and regulation of gene expression. The phytohormone abscisic acid (ABA) plays a key role in plant responses to biotic and abiotic stress, in particular drought and salinity [1–3].
Since the discovery of ABA receptors, PYRABACTINRESISTANCE1 (PYR1)/PYR1-LIKE (PYL)/REGULATORYCOMPONENTS OF ABA RECEPTORS (RCAR) [4,5], a core ABA signaling pathway has been proposed. In the absence of ABA, group A protein phosphatases type 2C (PP2Cs) interact with subclass III SNF1-related protein kinases (SnRK2.2, 2.3 and 2.6) which keeps the kinases inactive by blocking their catalytic cleft and by dephosphorylating the activation loop [6]. In the presence of ABA, ABA binds to the PYL receptors, forming a PYLs-ABA-PP2C complex and inhibiting phosphatase activity of PP2C [7,8]. This binding and inhibition of the PP2Cs releases the SnRK2s from PP2C-SnRK2 complexes, and the released SnRK2s are activated through autophosphorylation. The activated SnRK2s can then phosphorylate downstream effectors and activate ABA signaling [7,9,10]. Various transcription factors function in ABA signaling-mediated drought response [2,11]. The basic leucine zipper (bzip) family transcription factors including ABF1, ABF2 (AREB1), ABF3, and ABF4 (AREB2), which bind directly to ABREs of stress-responsive genes and stimulate their transcriptional activities, function in the ABA-dependent pathway and are major targets of SnRK2 protein kinases in the ABA core signaling pathway [12–14]. Additionally, some members of the MYB and MYC (bHLH) classes, the No Apical Meristem/Cup-Shaped Cotyledon (NAC), and WRKY families have also been shown to be induced by ABA or abiotic stress or to regulate stress responses, underscoring the importance of transcriptional regulation in plant stress responses [2,15,16]. Transcriptional regulation is one of the most essential mechanisms in the acquisition of stress tolerance [2,17].
However, in many cases, stress adaptation is exchanged for growth and productivity, therefore, it is necessary for plants to develop a resilient system to obtain the optimal trade-off for survival and growth. To this end, plants use elaborate mechanisms associated with posttranscriptional modulation [18] and posttranslational regulation [19,20], as well as transcriptional regulation. In particular, the appropriate control of transcription factors regulating plant growth and development genes is important, because these transcription factors negatively affect plant stress tolerance while being essential for increased productivity. The environmental conditions surrounding plants are constantly changing; thus, posttranslational regulation to control the protein levels of these transcription factors is considered an important mechanism to avoid adverse effects on plant survival. However, the negative components involved in regulation to efficiently coordinate ABA-dependent stress responses are less well known.
The homeodomain-leucine zipper protein (HD-ZIP) family constitute a large family of transcription factors that are unique to plants and is divided into four subfamilies (HD-ZIP I–IV) on the basis of the additional conserved domains, structures and physiological functions [21–23]. HD-ZIP proteins contain homeodomain (HD) that is responsible for specific DNA binding and the closely associated leucine zipper (LZ) domain which acts as a dimerization motif [24]. HD-ZIP proteins can bind to partially inverted repeats such as CAAT(A/T)ATTG (BS1 site), CAAT(C/G)ATTG (BS2 site) or as lightly modified version TAAT(C/T)ATTA for AtHB2/HAT4 [25]. Arabidopsis thaliana homeodomain-leucine zipper protein 1 (HAT1) and its close homologs belong to Class II HD-ZIP of transcription factors that mainly act as repressors by binding to their target genes promoters and play important roles in plant development and in response to the environment [25,26]. Previous works have shown that several members of the family, HAT1, HAT4/AtHB2 and AtHB4, are induced by shade avoidance and overexpression of HAT1 or HAT4 resulted in a similar effect in promoting cell elongation [23,25–27]. HAT2 expression is rapidly induced in response to auxin, and AtHB4 was also reported to modulate auxin, BRs and gibberellin responses [28,29]. It was recently reported that HAT1 is a substrate of BIN2 (BRASSINOSTEROID-INSENSITIVE 2) kinase and appears to function redundantly with other family members such as HAT3 to positively mediate BR responses [30]. HAT1 was also reported to participate in anti-CMV defense response in Arabidopsis and negatively regulates this process [31]. Collectively, these studies indicate that HAT1 is involved in the complex signaling and transcriptional networks coordinating plant growth and stress response. HAT1 promotes plant growth and development by BR signaling or other pathway.
In this study, we demonstrate that HAT1, which was previously reported as a critical regulator in BR-mediated plant growth and in viral defense response, is involved in ABA regulation of drought response by suppressing the ABA biosynthesis and signaling. We found that HAT1 and its homolog HAT3 act redundantly, as the expression of both HAT1 and HAT3 were repressed by ABA and drought, and the double mutant hat1hat3 displayed a reduced ABA sensitivity and enhanced drought tolerance phenotype that was stronger than the single mutants alone. HAT1-overexpressing transgenic plants exhibit a hyposensitive response to ABA and drought. Furthermore, we found that HAT1 physically interacts with and can be phosphorylated by SnRK2.3 in vitro and in vivo. SnRK2.3 phosphorylation of HAT1 decreased its protein stability and binding activity. Overexpressing SnRK2.3 in HAT1OX transgenic plant can suppress its phenotype in ABA and drought responses. Therefore we identified a new substrate of SnRK2.3 and established a novel negative regulation mechanism by which plants can efficiently coordinate drought responses.
Results
HAT1 acts as a negative factor in response to ABA signaling and in osmotic stress tolerance
From public data (http://bbc.botany.utoronto.ca/efp/cgi-bin/efpWeb.cgi), we found that HAT1 expression was reduced after ABA and osmotic stress treatment in seedlings, implying that HAT1 may be implicated in ABA and stress responses. To test the hypothesis, we examined the expression of HAT1 in different tissues and in seedlings treated with exogenous ABA or osmotic stress. Consistent with the public data, the expression level of HAT1 was highest in root, and lower in stem, leaf, and inflorescence (Fig 1A), and was significantly repressed by exogenous ABA and osmotic stress (Fig 1B). We further generated GUS reporter lines using HAT1 native promoter and examined the responsiveness of HAT1 expression in the presence of ABA and osmotic stress. As shown in S1A Fig, after ABA and osmotic stress treatments, GUS signals were reduced in cotyledons, leaves and roots as well as guard cells. To determine the subcellular localization of HAT1, we generated constructs that introduced the GFP sequence at the C-terminus of HAT1. The 35S:GFP and 35S:HAT1-GFP constructs were used to transfect Arabidopsis thaliana protoplasts. As shown in S1B Fig, 35S:GFP fluorescence was observed in the entire cell, while HAT1-GFP fusion protein localized in the nucleus. The expression of HAT3, its homolog, was similarly regulated, whereas the expression of HAT2 was not changed by ABA and osmotic stress (S3A and S3B Fig).
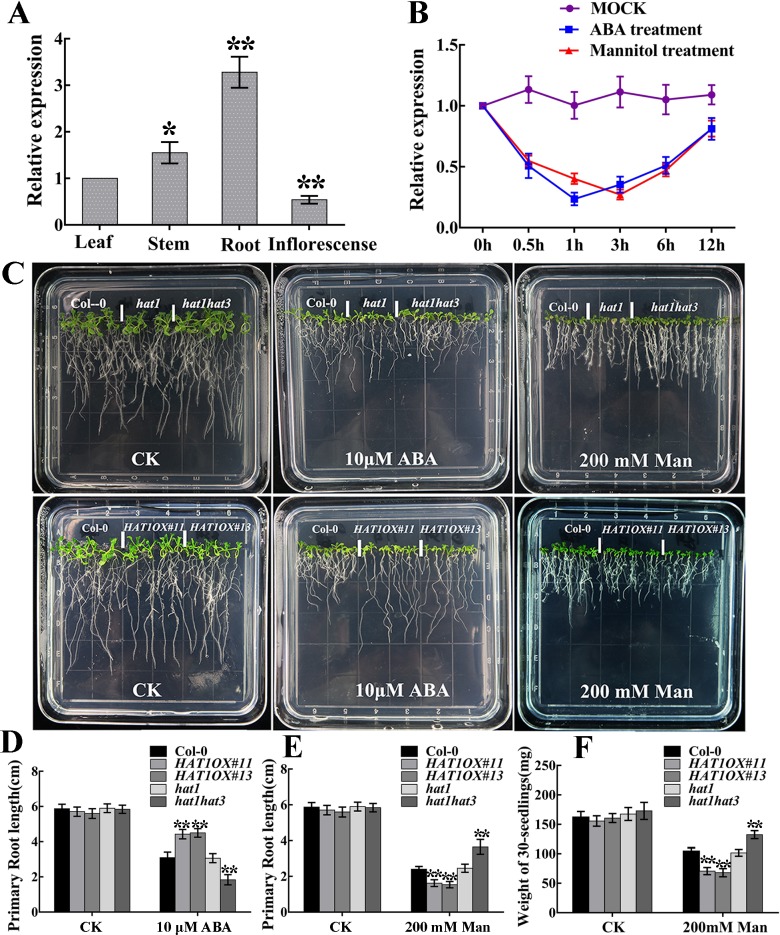
Fig 1. ABA sensitivity and osmotic stress tolerance of HAT1 mutants (hat1, hat1hat3) and HAT1-overexpressing lines.
(A, B) Expression patterns of HAT1. (A) qRT-PCR analysis of HAT1 expression in different tissues. (B) qRT-PCR analysis of HAT1 expression in response to exogenous ABA and osmotic stress. 12-day-old Col-0 seedlings were transferred to liquid 1/2 MS medium with or without 100 μM ABA or 200 mM mannitol and then the plants were harvested at the indicated time. Data are shown as mean SD of three independent experiments. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01). (C) Growth of different genotype seedlings on 1/2 MS medium with or without 10μM ABA or 200 mM mannitol. The 4-day-old seedlings were transferred to 1/2 MS or 1/2 MS medium supplemented 10μM ABA or 200 mM mannitol for 10 days, and then the photos were taken. (D-F) Quantification of primary root length and biomass in different genotypes after ABA treatment or mannitol treatment indicated in (C). CK, control check. Data represent mean ± SD of three independent replicates. asterisks indicate significant differences compared with Col-0 under the same treatment conditions. The significant difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
To investigate the role of HAT1 in the ABA response and in osmotic stress tolerance, we obtained T-DNA insertion mutants of HAT1, HAT2 and HAT3, hat1, hat2 and hat3, respectively. Then we created the double mutant hat1hat2, hat1hat3 and triple mutants hat1hat2hat3. The RT-PCR results showed that HAT1 expression was hardly detected in hat1, hat1hat2, hat1hat3 and hat1hat2hat3 mutants. Similarly, transcript of HAT2 was not observed in hat2, hat1hat2 and hat1hat2hat3 mutants, and HAT3 transcript was abolished in hat3, hat1hat3 or triple (hat1hat2hat3) mutants (S2A Fig). Western blotting using an anti-GFP antibody showed that HAT1-GFP accumulated in the two HAT1OX lines (S2B Fig). Next, we analyzed ABA sensitivity with regard to seedlings growth in Col-0, HAT1OX lines and knockout mutants. The 4-day-old seedlings grown on 1/2 MS medium were transferred to 10 μM ABA-containing medium for 10 days. As shown in Fig 1C and 1D and S3C and S3D Fig, root growth of double mutant hat1hat3 or triple mutant hat1hat2hat3 was dramatically retarded under ABA conditions compared with that of wide-type Columbia-0 (Col-0) and was similar among Col-0, hat1, hat2, hat3 and hat1hat2 with or without ABA treatment. To analyze the function of HAT1 in osmotic stress tolerance, 4-day-old Col-0 and knockout mutants were treated with mannitol, a stress treatment commonly used to mimic osmotic stress tolerance in the laboratory. The double mutant hat1hat3 or triple mutant hat1hat2hat3 displayed less inhibition on growth in the medium containing mannitol compared with Col-0, while the single mutant hat1, hat2, hat3 and double mutant hat1hat2 showed little difference after mannitol treatment in comparison with Col-0 (Fig 1C, 1E and 1F and S3C, S3E and S3F Fig). In contrast, the two HAT1-overexpressing lines (HAT1OX#11 and HAT1OX#13) showed significantly reduced ABA sensitivity and osmotic stress tolerance (Fig 1C bottom, Fig 1D, 1E and 1F). Together, these data indicate that HAT1 plays a negative role in ABA signaling and in osmotic stress tolerance, and it is functionally redundant with HAT3 in ABA and osmotic stress response.
HAT1 impairs ABA-induced stomatal closure and drought tolerance
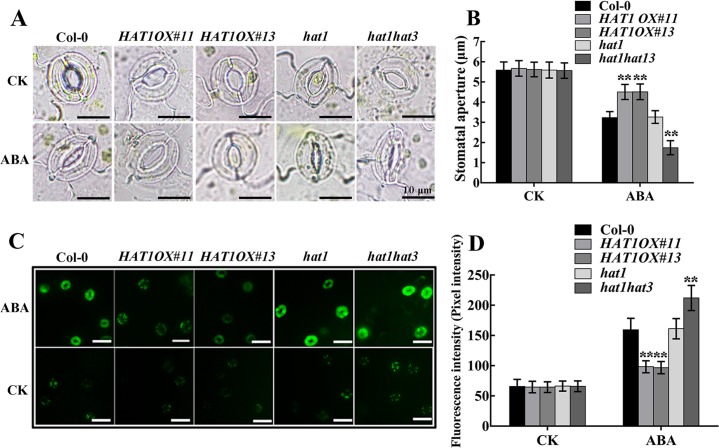
ABA regulation of stomatal movements is a well established model system for the study of plants response to drought stress. Thus, we measured the stomatal aperture from epidermal peels of Col-0, HAT1OX lines and knockout mutants. Overexpression of HAT1 suppressed ABA-mediated stomatal closure, while double mutant hat1hat3 and triple mutant hat1hat2hat3 exhibited an accelerated ABA sensitivity in stomatal closure and single mutants(hat1, hat2, hat3) or hat1hat2 showed little difference after ABA treatment in comparison with Col-0 (Fig 2A and 2B and S4A and S4B Fig), indicating that HAT1 and HAT3 function redundantly in regulating ABA-mediated stomatal closure. As H2O2 acts as an important signal molecular in ABA-induced stomatal closure, H2O2 accumulation in guard cells was measured by a fluorescence dye, 2,7-dichlorodihydro fluorescein diacetate (H2DCF-DA) [32,33]. As shown in Fig 2C and 2D, H2O2 accumulation in guard cells was less in HAT1OX lines, more in hat1hat3 double mutant, compared to Col-0 and hat1 after ABA treatment, suggesting that HAT1-impaired stomatal closure may be caused by changed H2O2 in guard cells. Next, we tested whether HAT1 plays a role in the drought stress response. When exposed to dehydration stress by withholding water for 10 days, HAT1OX lines displayed a withered phenotype, while the hat1hat3 double mutant largely remained turgid and single mutant hat1 showed little difference in comparison with Col-0 (Fig 3A). Measurement of leaves water loss showed that HAT1OX lines lost water much faster, while hat1hat3 displayed reduced water-loss rate than Col-0 and hat1 (Fig 3B). As a result, overexpression of HAT1 markly reduced plant survival under drought stress, whereas hat1hat3 showed enhanced survival compared to Col-0 and hat1 (S5A–S5C Fig).
Fig 2. HAT1 suppresses ABA-induced stomatal closure and H2O2 accumulation in guard cells.
(A) Epidermal peels of indicated genotypes were treated with or without ABA for 3 h after stomatal pre-opening under light for 2h, and the stomatal aperture was measured. Scale bars: 10 μm. (B) Stomatal apertures of different genotypes indicated in (A). (C, D) Fluorescence images (C) and pixel intensities (D) in guard cells preloaded with 50 μM H2DCFDA for 10 min in darkness. Each assay was repeated at least three times. The data are presented as means ± SD. (Student’s t-test: ** P<0.01). Scale bar: 25 μm.
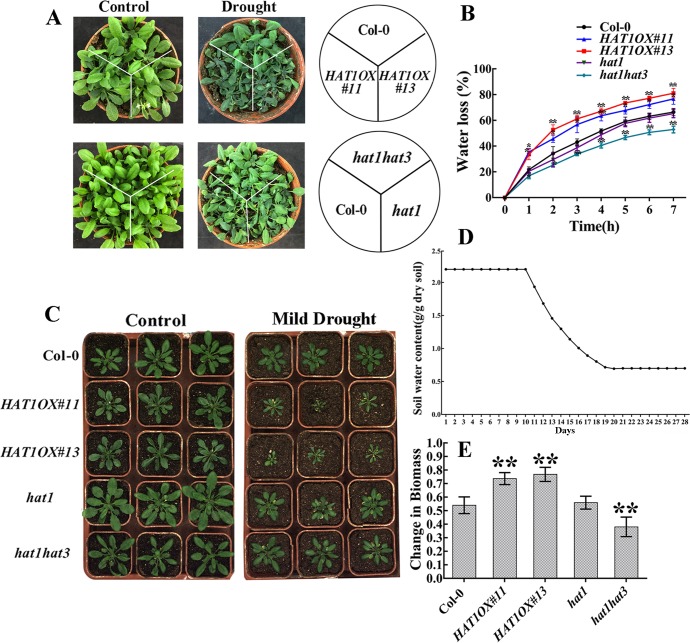
Fig 3. HAT1 negatively regulate drought stress responses in Arabidopsis thaliana.
(A) The phenotypes of Col-0, HAT1OX lines, hat mutants (hat1, hat1hat3) in response to progressive drought stress. Different genotype plants were grown in soil with sufficient water for 3 weeks (Watered), and then water was withheld for 10 d (Progressive drought), and the photos were taken. (B) Water loss from detached leaves of different genotype plants. Leaves at similar developmental stages were excised and weighed at the indicated time after detachment. The proportion of fresh weight losses was calculated on the basis of the initial weight of the leaves. Data are shown as mean SD of three independent experiments. The significance of difference was analyzed by Student’s t test (**P < 0.01). (C-E) Growth of Col-0, HAT1OX lines, hat1 mutants (hat1, hat1hat3) in response to mild drought stress. 3-week-old plants were subjected to mild drought treatment and the images of both drought-treated plants (right) and the well-watered plants (left) were taken (C). (D) Water loss from the peat pellets during the duration of the experiment. Control soil water content was maintained at a constant value of 2.2 g water g-1 dry soil (solid line) during the entire experiment. For the mild drought condition, soil water content was maintained at 0.7g H2O g-1 dry soil. (E) The change in biomass under mild drought among different genotypes compared to Col-0. After mild drought treatment, all the replications of the drought-treated and the well-watered control were harvested and the dry weights (biomass) were measured. Then calculate the reduction in biomass. Bars indicate SD calculated from three independent experiments. The significance of difference was analyzed by Student’s t test and Asterisks indicate significant difference from the wild type (*P < 0.05, **P < 0.01).
To study the responses of different genotypes to controlled soil water deficit drought, Col-0, HAT1OX lines and knock-out mutants (hat1, hat1hat3) were grown for 3 weeks under well-water condition (2.2g H2O/g dry soil) and then subjected to mild drought stress (Fig 3D). After grown under mild drought condition (0.7g H2O/g dry soil) for 9days, the biomass of both drought-treated and well-watered plants was measured and then the change in biomass was calculated. As shown in Fig 3C and 3E, HAT1OX lines showed more reduction in biomass compared to the Col-0 which is considered drought sensitive genotype, while the double mutant hat1hat3 displayed less reduction in biomass and hat1 showed similar reduction in biomass in comparison to Col-0. Altogether, these data demonstrate that HAT1 and HAT3 function redundantly and negatively to regulate ABA-mediated stomatal closure and drought response.
HAT1 negatively regulates drought-responsive genes and positively regulates PP2Cs
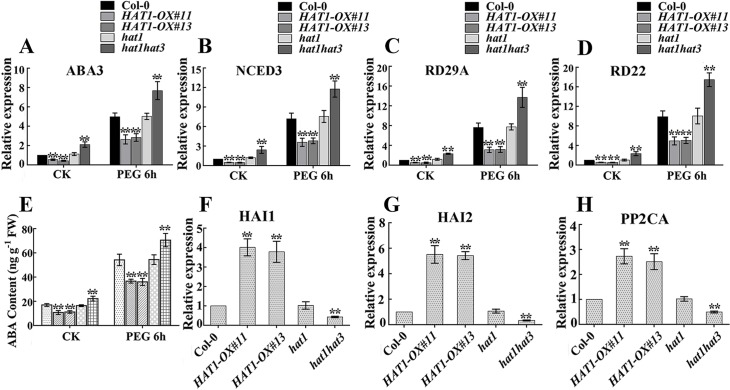
As HAT1 is a negative regulator of ABA signaling and drought response (Fig 1 and Fig 2), the expression of ABA or drought stress inducible marker genes were tested in different genotypes. We first determined the transcript levels of ABA response maker genes which were also ABA biosynthesis genes. These genes include ABA1 [34], AAO3 [35], ABA3 [36], and NCED3 [37]. Among the four genes, the expression of ABA3 and NCED3 were significantly reduced in HAT1OX lines and up-regulated in hat1hat3 double mutant under both control and osmotic stress conditions (Fig 4A and 4B). To determine whether or not ABA levels were affected, we quantified the ABA content in different genotypes. Under normal conditions, ABA level in HAT1OX seedlings was found to be lower than that in Col-0 and hat1 single mutants, whereas it was elevated in hat1hat3 double mutant. When exposed to 15% polyethylene glycol (PEG) 6000 that mimics a drought stress, HAT1OX lines had a reduced ABA level, and the hat1hat3 double mutants accumulated higher level of ABA compared with Col-0 and hat1 (Fig 4E). In addition, the induction of RD29A and RD22, which are well established drought-induced marker genes [16,38], was also tested in different genotypes. As expected, the expression of these two genes were reduced in HAT1OX lines and elevated in hat1hat3 double mutant compared with Col-0 and hat1 (Fig 4C and 4D). Furthermore, the expression of HAI1, HAI2 and PP2CA, which belong to PP2Cs, negative regulators of ABA signaling, was diminished by down-regulation of both HAT1 and HAT3 and enhanced by HAT1 overexpression (Fig 4F–4H). Taken together, these results indicate that HAT1 repressed drought-responsive genes and induced PP2C genes, which may account for the repressed drought tolerance in HAT1OX lines.
Fig 4. Expression profiles of ABA or drought stress-responsive genes.
(A-D) qRT-PCR analysis of ABA3 (A), NCED3 (B), RD29A (C), and RD22 (D) expression. 12-day-old seedlings were transferred to liquid MS medium with or without 15% PEG6000 and sampled after 6h treatment. Data are means of three replicates ± SD, asterisks indicate significant differences compared with Col-0 under the same treatment conditions. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01). (E) ABA content as determined by ELISA. Data are shown as mean SD of three independent experiments. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01). (F-H) qRT-PCR analysis of PP2Cs expression, HAI1 in (F), HAI2 in (G), PP2CA in (H). Three independent repeats were performed, the significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
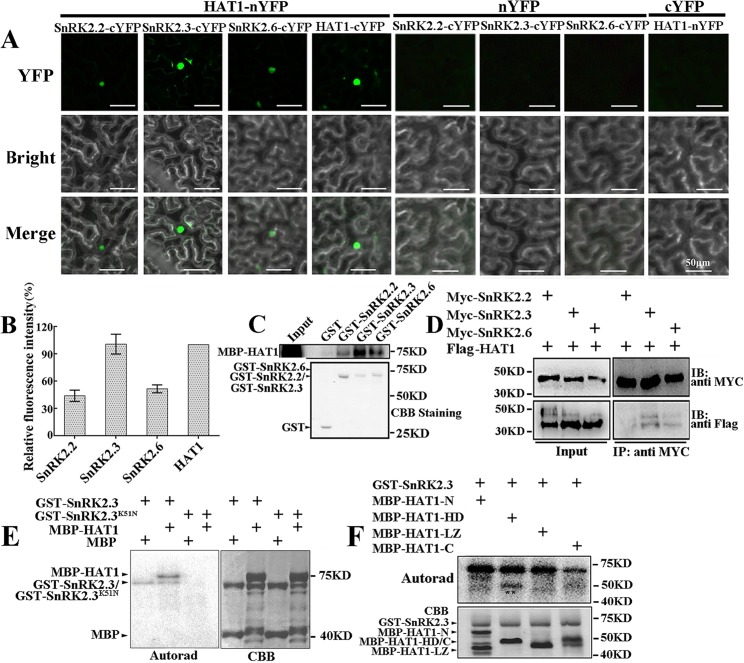
HAT1 physically interacts with SnRK2.2/2.3/2.6 and can be phosphorylated by SnRK2.3
The SnRK2 kinases are integral positive component of ABA signaling, and phosphorylate S/T residues in the RXXS/T motif in their substrates. There are 7 potential phosphorylation sites for SnRK2 kinases in the predicted HAT1 protein, which prompted us to test whether HAT1 was a substrate of SnRK2 kinases. First, we tested if subclass III SnRK2s could physically interact with HAT1. Bimolecular fluorescence complementation (BiFC) analysis was performed to examine the interaction of HAT1 with SnRK2.2, SnRK2.3, and SnRK2.6 in plants. We found that HAT1 interacts with all subgroup III SnRK2s in the nucleus and no fluorescence signal was detected in the negative controls (Fig 5A). Quantitative analyses of BiFC signals showed strong SnRK2.3-HAT1 interactions and weak signals for HAT1 interaction with other subgroup III SnRK2s (Fig 5B). GST pull-down experiment confirmed this interaction in vitro (Fig 5C). GST-SnRK2s, but not GST alone, pulled down a significant amount of MBP-HAT1 protein, demonstrating a direct interaction between SnRK2s and HAT1. Consistent with the result of BiFC assays, the interaction between SnRK2.3 and HAT1 is the strongest (Fig 5C). The in vivo interaction of SnRK2s with HAT1 were corroborated by co-immunoprecipitation (Co-IP) assay using Arabidopsis protoplasts co-expressing Myc-SnRK2s and HAT1-Flag fusion constructs (Fig 5D). We also generated a series of truncated HAT1 fragments (HAT1-1F (135–282), HAT1-2F (192–282), HAT1-3F (234–282)) which were fused with the C-terminal half of YFP and transformed them individually with SnRK2.3-nYFP into tobacco leaves. When deleted to amino acid 134 in HAT1, only a weak fluorescent signal was detected, while deletions to amino acid 191 and 233 in HAT1 totally abolished the interaction with SnRK2.3 (S6B Fig). Several truncated MBP-HAT1 (N-terminal region, HD, LZ, and C-terminal region of HAT1) were further used to map the specific domain of HAT1 required for the interaction with SnRK2.3. As shown in S6C Fig, HAT1 interacts with Snrk2.3 with its N-terminal region. Taken together, the N-terminal region in HAT1 mediates the interaction between HAT1 and SnRK2.3. Further, we conducted in vitro kinase assays to test whether SnRK2.3 can phosphorylate MBP-fusion HAT1 protein and found that SnRK2.3 can phosphorylate HAT1, but not MBP (Fig 5E). The kinase dead form of SnRK2.3 (SnRK2.3K51N) was used as a negative control and it totally abolished the phosphorylation of SnRK2.3 on HAT1 (Fig 5E). We further found that the homeodomain of HAT1 (MBP-HAT1-HD) can be phosphorylated by SnRK2.3 rather than the other regions (Fig 5F). In addition, the interaction of SnRK2.3 with HAT3 was also examined by BiFC analysis. As shown in S7A Fig, HAT3 interacts with SnRK2.3 in the nucleus, suggesting that SnRK2.3 may regulate HAT3 through a similar manner as HAT1.
Fig 5. SnRK2.3 interacts with and phosphorylates HAT1.
BiFC assays of interactions of HAT1 with the indicated SnRKs in N. benthamiana leaves (A) and quantification of relative fluorescence intensities (relative to that of HAT1-HAT1 interaction) in BiFC analyses (B). HAT1-nYFP/ HAT1-cYFP were used as positive control and nYFP/HAT1-cYFP and cYFP/SnRK2.2/ SnRK2.3/ SnRK2.6 were used as negative controls. Data represent mean ± SD of three independent replicates. Ten cells were analyzed in each replicate for each construct combination. Scale bars: 50 μm. Images were acquired using identical settings. (C) SnRK2s interacts with HAT1 in GST pull-down assay. GST, GST-SnRK2s and MBP-tagged HAT1 were used in this assay. HAT1 was detected by western blotting with anti-MBP antibody. (D) CoIP analysis of the interaction between SnRK2s and HAT1 in Arabidopsis leaf protoplasts. Myc-fused SnRKs (SnRK2.2/2.3/2.6) were immunoprecipitated using anti-Myc beads, and coimmunoprecipitated HAT1-Flag was then detected using an anti-Flag antibody. Experimental details are provided in Methods. (E, F) SnRK2.3 phosphorylates HAT1 in vitro. GST-SnRK2.3 was used to phosphorylate MBP or MBP-HAT1 (E) or truncated MBP-HAT1 (F).
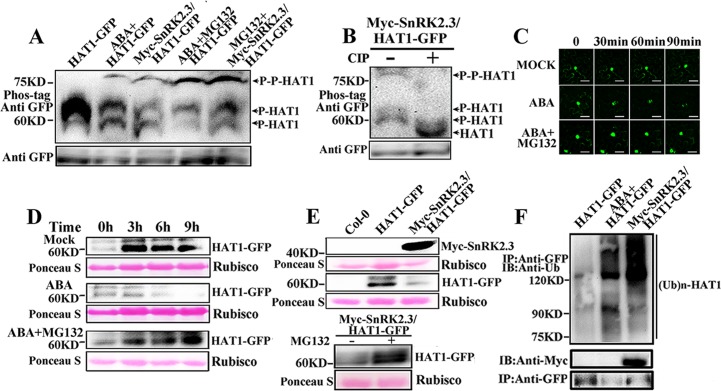
Proteasome-mediated HAT1 degradation is triggered by SnRK2.3 phosphorylation
To test whether phosphorylation of HAT1 by SnRK2.3 in vivo, the HAT1-GFP was immunoprecipitated from HAT1OX or SnRK2.3OX/HAT1OX transgenic seedlings treated with/without ABA or MG132 and detect the phosphorylation/dephosphorylation form using phos-tag gel blot analysis with an anti-GFP antibody (Fig 6A). Two faster-migrating bands can be detected in untreated plants. We found that ABA treatment or SnRK2.3 overexpression resulted in the appearance of a slower-migrating HAT1 in HATOX transgenic plants (Fig 6A). When subjected to phosphatase [calf-intestinal alkaline phosphatase (CIP)] treatment, all three bands disappeared and a new lower band which is likely the unphosphorylated form of HAT1 appeared, indicating that HAT1 exists mostly as phosphorylated forms in plants and an elevated phosphorylation of HAT1 is formed by ABA treatment or SnRK2.3 overexpression (Fig 6A and 6B). Furthermore, when treated with MG132, the phosphorylation level of HAT1 was significantly increased in ABA-treated HAT1OX or SnRK2.3OX/HAT1OX seedlings, indicating that super-phosphorylation form of HAT1 was instable (Fig 6A).
Fig 6. ABA treatment and SnRK2.3 phosphorylation destabilizes HAT1.
(A) SnRK2.3 phosphorylates HAT1 in vivo. HAT-GFP was prepared from HAT1OX transgenic plants treated with or without ABA or ABA plus MG132 or from SnRK2.3OX/HAT1OX transgenic plants treated with or without MG132, and then separated on SDS/PAGE gel containing Phos-tag reagent (NARD Institute). (B) Characterization of up-shifted band of HAT1 by dephosphorylation. HAT1 immunoprecipitated from SnRK2.3OX/HAT1OX transgenic plants with anti GFP beads were incubated with or without calf intestinal alkaline phosphatase (CIP) (sigma) and then separated on Phos-tag SDS-PAGE. The HAT1-GFP protein was detected by immunoblot analysis using anti-GFP antibody. (C) Time microscope images of a representative Nicotianabenthamiana leaf epidermal cell expressing HAT1-GFP exposed to 50 μM ABA. Results are representative microscope images from three separate experiments, and at least 5 independent cell images were captured per experiment. Scale bars: 50 μm. (D) ABA promotes HAT1 degradation through the proteasome. 12-day-old seedlings grown one half-strength MS plates were treated with (+) or without (-) 50μM ABA and/or 30μM MG132 for indicated time period. Total protein was extracted and analyzed by immunoblotting. Ponceau S staining was used to demonstrate equal loading. (E) SnRK2.3 promotes the degradation of HAT1 in vivo. Proteins extracts from 12-day-old HAT1OX seedlings or from SnRK2.3OX/HAT1OX seedlings treated with or without MG132 were subjected to immunoblot analysis using anti-GFP for HAT1 and anti-Myc antibody for SnRK2.3. (F) Ubiquitination level of HAT1 is enhanced by SnRK2.3 in vivo. HAT1-GFP protein was immunoprecipitated from 12-day-old HAT1OX seedlings treated with or without ABA and in SnRK2.3OX/HAT1OX seedlings. Immunoprecipitated proteins were detected by immunoblotting using anti-GFP or anti-Ubiquitin (Ub) antibody (Abcam) for HAT1 and anti-Myc antibody for SnRK2.3.
To investigate the function of the SnRK2.3 phosphorylation on HAT1 protein stability, we detect HAT1-GFP protein level in transgenic plants. First, we expressed HAT1-GFP fusion proteins in Nicotiana benthamiana epidermal cells and examined the effects of ABA and the proteasome inhibitor MG132 on GFP fluorescence. Time-course microscopic observation revealed that the HAT1-GFP fluorescence intensity was substantially reduced in leaves treated with ABA alone, whereas HAT1-GFP was more stable after application of ABA plus MG132 (Fig 6C). Similarly, HAT3-GFP fluorescence intensity was also rapidly reduced in response to ABA treatment and only slightly altered in response to the control stimulus (solvent used for ABA) and combined ABA and MG132 (S7B Fig). We then examined HAT1-GFP protein level in HAT1-GFP transgenic plants. As shown in Fig 6D, HAT1 protein increased in the liquid one-half MS (Murashige and Skoog) medium without ABA treatment (Fig 6D top panel). However, in the presence of ABA, HAT1-GFP protein clearly decreased in relation to the mock treatment after 3 h of treatment (Fig 6D middle panel). When we treated plants with ABA and MG132 together, the HAT1 protein level significantly increased as mock (Fig 6D bottom panel), suggesting that ABA triggers proteasome-mediated HAT1 degradation.
To investigate whether ABA-induced HAT1 degradation is mediated by SnRK2.3 phosphorylation in plant, we detected HAT1-GFP protein level in HAT1OX or SnRK2.3OX/HAT1OX transgenic seedlings. The transcriptional level of HAT1 was same in HAT1OX and SnRK2.3OX/HAT1OX (S8 Fig). As shown in Fig 6E, HAT1 protein was clearly degraded in SnRK2.3OX/HAT1OX transgenic plants, while this degradation was blocked by addition of MG132. We further examined the ubiquitination level of HAT1 in ABA-treated HAT1OX and in SnRK2.3OX/HAT1OX transgenic plants. As shown in Fig 6F, the ubiquitinated level of HAT1 was significantly increased in HAT1OX plants after treatment with ABA, or in SnRK2.3OX/HAT1OX transgenic plants. Taken together, these results indicated that SnRK2.3-mediated HAT1 phosphorylation facilitates the degradation of HAT1 via stimulating its ubiquitination.
SnRK2.3 represses the binding ability of HAT1 to HB site on promoter of ABA3 and NCED3
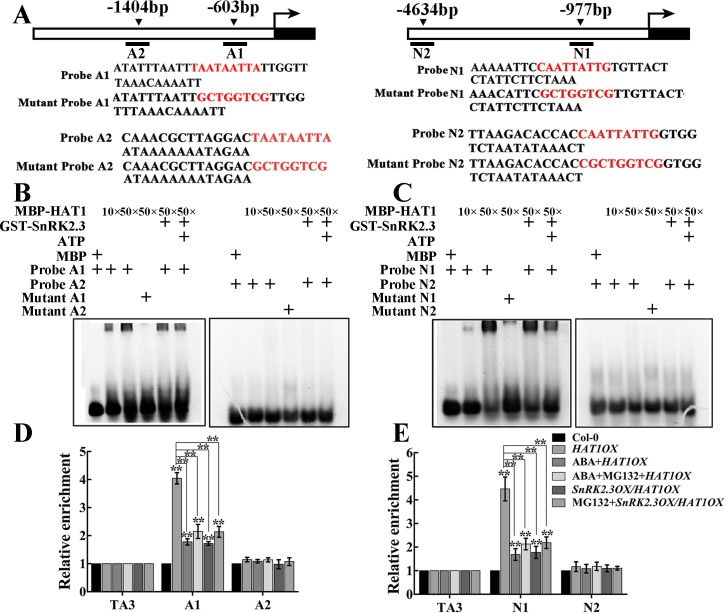
HAT1 acts as a regulator by binding to HB site within its target genes promoters. First, we analyzed promoter sequences of four ABA or drought-responsive genes (ABA3, NCED3, RD29A, RD22) and found that there were two HB-binding sites within the ABA3 and NCED3 promoter regions respectively (Fig 7A). To determine whether or not HAT1 bind to the ABA3 and NCED3 promoter, electrophoresis mobility shift assays (EMSAs) were conducted. The MBP-HAT1 fusion protein can bind to A1 fragment of ABA3 promoter and N1 fragment of NCED3 promoter, but this binding was abolished by mutation of HB sites in the probes (Fig 7B and 7C). The addition of GST-SnRK2.3 fusion protein was able to slightly inhibit the ability of HAT1 binding to the A1 fragment and N1 fragment (Fig 7B and 7C). When HAT1 was phosphorylated by SnRK2.3 in vitro, the binding affinity of phosphorylated HAT1 was dramatically reduced (Fig 7B and 7C). These data indicate that HAT1 protein can bind to the A1 fragment of ABA3 promoter and N1 fragment of NCED3 in vitro, and its binding ability is repressed by SnRK2.3 phosphorylation.
Fig 7. SnRK2.3 phosphorylation represses the binding ability of HAT1 to the promoters of ABA3 and NCED3.
(A) Schematic representation of ABA3 and NCED3 promoter. The upstream region and part of an exon of ABA3 and NCED3 are shown with a white box and black box, respectively. The arrowheads in the top indicate the sites containing HB sites in the ABA3 and NCED3 promoter. Black lines represent the DNA fragments amplified in the ChIP assay. Sequences of wild-type probe with HB sites and various mutant probes were shown. (B, C) Electrophoretic mobility shift assays (EMSAs) to examine HAT1 binding to the ABA3 (B) and NCED3 (C) promoter. MBP-HAT1 was immunoprecipitated with MBP agarose and incubated with purified GST-SnRK2.3 with kinase reaction buffer at 37°C for 30 min. The MBP, MBP-HAT1, phosphorylated MBP-HAT1 and GST-SnRK2.3 proteins were incubated with the WT or mutants probes. (D, E) ChIP-qPCR assay of HAT1 binding to ABA3 (D) and NCED3 (E) promoter in vivo. The 12-day-old HAT1OX were treated with 50 μM ABA or ABA plus MG132 for 3h, and the SnRK2.3OX/HAT1OX seedlings were treated with/without 30 μM MG132 alone for 3h, then the seedlings were harvested for ChIP-qPCR assay using anti-GFP antibody. Data are shown as mean SD of three independent experiments. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
To further test the effect of SnRK2.3 on the binding ability of HAT1 in vivo, we performed chromatin immunoprecipitation (ChIP) assays. We immunoprecipited HAT1-GFP protein from HAT1OX transgenic seedlings treated with/without ABA or ABA in combination with MG132 with anti-GFP antibody. TA3, a retrotransposable element, was used as the internal control [39]. ChIP-qPCR results indicated that HAT1 specifically bound to the A1 region of ABA3 and N1 region of NCED3, and other genomic fragments containing HB sites were not targeted by HAT1 (Fig 7D and 7E). The binding ability of HAT1 was reduced by both ABA treatment and ABA plus MG132 treatment (Fig 7D and 7E). Furthermore, HAT1 binding ability was significantly diminished by SnRK2.3 overexpression, and it cannot be recovered by addition of MG132 (Fig 7D and 7E). Altogether, these results support that SnRK2.3 represses the binding ability of HAT1 by phosphorylation.
SnRK2.3 Overexpression suppresses ABA-insensitivity and drought-hypersensitivity of HAT1OX plants
To confirm the regulation of HAT1 by SnRK2.3, we examined whether or not overexpression of SnRK2.3 can suppress HAT1OX phenotypes in ABA and drought responses. SnRK2.3OX/HAT1OX double overexpressing line displayed an enhanced ABA sensitivity in seedlings growth and was more tolerant to drought stress compared with HAT1OX, which was similar to Col-0 (Fig 8A–8D and S9 Fig). Moreover, SnRK2.3OX/HAT1OX showed less reduction in biomass under mild drought conditions compared to HAT1OX (Fig 8E and 8F). Then, we tested the influence of SnRK2.3 overexpression on HAT1 in the regulation of ABA or drought inducible marker genes expression. As shown in Fig 8G–8J, the expression of ABA3, NCED3, RD29A, and, RD22, were significantly up-regulated in SnRK2.3OX/HAT1OX, compared to HAT1OX, which reached to the expression level of Col-0. These data together with phenotype tests indicated that SnRK2.3 overexpression suppressed the ABA-insensitivity and drought-hypersensitivity of HAT1OX.
Fig 8. Overexpression of SnRK2.3 suppresses the ABA-insensitive and drought- hypersensitive phenotypes of HAT1-overexpressing line.
(A) Phenotypic comparison. 4-day-old Col-0, HAT1OX#13, and SnRK2.3OX/HAT1OX seedlings were transferred to 1/2 MS medium or 1/2 MS medium supplemented 10μM ABA or 200 mM mannitol for 10 days, and then the photos were taken. (B-D) Quantitication of primary root length and biomass in Col-0, HAT1OX#13, and SnRK2.3OX/HAT1OX seedlings after ABA treatment and mannitol treatment indicated in (A). Data represent mean ± SD of three independent replicates. asterisks indicate significant differences compared with Col-0 under the same treatment conditions. The significant difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01). (E, F) Growth of Col-0, HAT1OX#13, SnRK2.3OX/HAT1OX in response to mild drought stress. 3-week-old plants were subjected to mild drought treatment and the images of both drought-treated plants (right) and the well-watered plants (left) were taken (E) and the reduction in biomass of each genotype was measured (F). The average and SDs were from three independent experiments and At least 12 plants were measured for each genotype per replication. The significance of difference was analyzed by Student’s t test (**P<0.01). (G-J) Expression of ABA or drought-responsive genes in 12-day-old seedlings with or without 15% PEG 6000 treatment for 6h. Data are means of three replicates ± SD, asterisks indicate significant differences compared with Col-0 under same conditions (Student’s t-test: * P < 0.05, **P < 0.01).
Discussion
Currently, the most thoroughly understood in transcriptional regulation of ABA-mediated drought responses is AREB/ ABFs pathway, which activated the expression of drought-responsive genes in an ABA-dependent manner [40], however, the components involved in compromising drought response were less well studied. In this study, we identified SnRK2.3 interaction transcription factors HAT1 and HAT3 as important components to regulate ABA-mediated drought response. As negative regulators, HAT1 and HAT3 suppressed ABA sensitivity and drought tolerance. Furthermore, we found HAT1 was a substrate of SnRK2.3 and SnRK2.3 phosphorylation decreased HAT1 protein stability and binding activity. Our results identified a new negative component that regulates ABA signaling in Arabidopsis in response to drought and established a novel mechanism to attenuate stress response.
HAT1 plays important roles in phytohormone-regulated developmental processes and stress response [23,25]. HAT1 interacts with BES1, a central regulator in BR signaling pathway, and functions as a BES1 co-repressor to inhibit BR-repressed genes and thus optimizes BR-regulated plant growth [30]. In addition, HAT1 acts as a repressor in plant defense response to CMV infection [31]. Thus, HAT1 may function as a transcriptional regulator to modulate plant growth and stress response. Several lines of evidence support the role of HAT1 as a negative regulator in ABA-mediated drought response. First, the expression of both HAT1 and its close homologs HAT3 is repressed by ABA and osmotic stress, indicating that these genes are ABA or stress-responsive factors. Second, HAT1 can bind to specific DNA sequences (HB binding sites) on promoter of NCED3 and ABA3, two key ABA biosynthesis genes, and represses these genes expression, leading to a reduction of ABA synthesis. In addition, drought-responsive genes like RD22 and RD29A, were also suppressed by HAT1. Third, consistent with the role of negative regulators for ABA signaling under stress conditions, HAT1OX displayed reduced sensitivity to ABA and less tolerance to drought stress, whereas the double knockout mutant hat1hat3 showed an enhanced ABA sensitivity and increased drought tolerance phenotypes. Finally, the modulation of HAT1 by SnRK2.3 kinase further suggests that HAT1 forms part of ABA signaling network to regulate ABA-dependent stress response.
Besides the repression by ABA at transcription level, HAT1 is regulated by ABA-activated SnRK2 kinases through a post-transcriptional modification mechanism. Post-translational modifications of transcription factors fine-tune their functions to effectively and precisely implement the stress response. SnRK2s-mediated phosphorylation of target proteins triggers most of the molecular actions of ABA signaling pathway [14,41,42]. In addition to the originally identified bZIP transcription factors AREBs (ABA-Responsive Element Binding factors) that function in ABA-responsive gene regulation, 58 putative substrates of ABA-activated SnRK2s were identified through mass spectrometry-based global phosphorylation profiling, which include components involved in flowering time regulation, RNA and DNA binding, miRNA and epigenetic regulation, signal transduction, chloroplast function, and many other cellular processes [41]. In this study, we identified an additional substrate for SnRK2.3 kinase. In contrast to bZIP transcription factors AREBs, which are stabilized by SnRK2s phosphorylation [43,44], SnRK2.3 phosphorylation promotes the degradation of HAT1. In addition to destabilizing HAT1 protein, we found that SnRK2.3 phosphorylated HAT1 on its homeodomain, which is responsible for specific DNA binding, leading to the reduction of its binding ability to the HB sites on the promoter of target genes. Our results thus suggest that SnRK2.3 phosphorylation of HAT1 can have different functional consequences, inhibiting both its DNA binding and protein accumulation. However, the mechanisms how phosphorylation by SnRKs mediates HAT1 degradation remain to be determined in future studies.
HAT1 belongs to Class II HD-ZIP transcription factors, which have been shown to regulate plant growth and development [45–47]. For example, ATHB4, ATHB2 and HAT3 are required for normal leaf development and blade growth [45]. ATHB4, a shade signaling component, acts redundantly to other members of the HD-Zip class-II subfamily to integrating shade perception and hormone-mediated growth [29]. HAT2 is an auxin inducible gene and modulates auxin-mediated morphogenesis [48]. In addition to the regulation of plant growth and development, several of the class II HD-ZIP transcription factors have been also reported to participate in plant responding to exogenous ABA and drought stress. ATHB17 has been characterized as a positive regulator of ABA response and multiple stress responses [46,49]. ABIG1/HAT22 is induced by ABA and drought stress, and relays ABA signaled growth inhibition and drought induced senescence [50]. HDG11 can promote main root elongation and lateral root formation in Arabidopsis and was able to confer drought tolerance in Arabidopsis, tobacco, rice, sweet potato, cotton and woody plant poplar (Populus tomentosa Carr.) [51–55]. It seems likely that a general role for HD-ZIP II proteins is to link environmental and developmental signals to growth control. As noted above, these class II HD-ZIP transcription factors share many similar characteristics though they have different expression patterns. Expression pattern of HAT1 and HAT3 in response to BR and ABA is analogous and functions in BR-mediated hypocotyl elongation and ABA-induced drought stress tolerance are redundant. So it proposed that HAT1 together with HAT3 played essential roles in balancing plant growth and stress responses. However whether ABA regulates HAT1 and HAT3 function and stability in a similar manner is unclear and further study will be needed.
Our results strongly indicate that HAT1 is an important part of mechanisms that functions to control basal ABA signaling and drought response. HAT1 can suppress ABA synthesis and signaling through down-regulating the expression of ABA3 and NCED3 via directly binding to their promoters, and ABA/drought-responsive genes, RD29A and RD22. In contrast, HAT1 promotes the expression of PP2Cs which negatively regulate the ABA response, enhancing the negative regulation of ABA signaling (Fig 9). When exposed to drought conditions, stress-induced ABA led to activation of SnRK2s, which in turn negatively regulates HAT1 functions by posttranslational regulation of its stability and binding ability. The suppression of HAT1 at both transcriptional and protein level appears to be an adaptive strategy of plant responses to water deficit, facilitating plants survival under drought conditions (Fig 9). When the environmental conditions are favorable, HAT1 and its homologous function to suppress drought response, prevent unnecessary activation of stress response, and ensure the normal growth of plants. HAT1 thus can be considered as a brake to fine tune ABA signaling and drought response (Fig 9).
Fig 9. Working model for HAT1 in the negative regulation of stress responses.
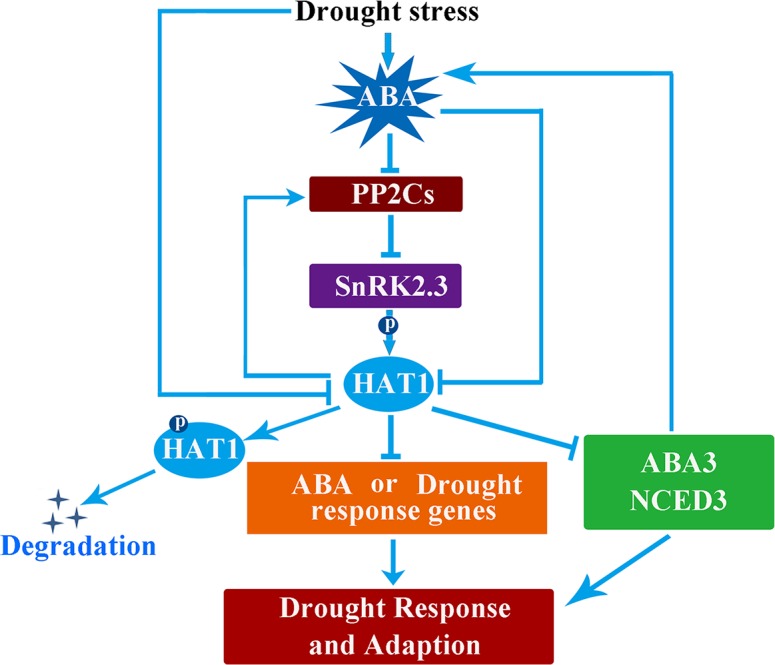
ABA, which is induced by drought stress, inhibits the activity of PP2Cs to release the kinase activity of SnRK2s kinase for further activation of downstream regulators through phosphorylation. HAT1 could directly bind to the promoters of ABA3 and NCED3 and negatively regulates the expression of these two genes, resulting in reduced ABA biosynthesis. Furthermore, HAT1 could positively regulate several PP2C genes expression, which in turn negatively regulates the ABA signaling. Thus, HAT1 acts as a repressor to keep the drought response silence during the environmental conditions are comfortable. Under stress conditions, stress-induced ABA accumulation could repress HAT1 at transcriptional and protein level, ensuring plants survive stress conditions. P, Phosphorylation.
In summary, this study revealed the mechanism of the negative regulatory function of HAT1 in ABA-mediated drought response (Fig 9). We found that ABA biosynthesis and signaling were repressed by HAT1. We also establish that HAT1 is phosphorylated by SnRK2.3 kinase and that SnRK2.3 phosphorylation promotes the proteasome-mediated HAT1 degradation and represses the binding ability of HAT1. The identification of negative regulators, like HAT1, and elucidation of the regulatory mechanism will lead to a better understanding of ABA signaling mechanism and drought response, which has potential in manipulating crop plants for drought tolerance.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used as the WT control. The HAT1-overexpressing lines (HAT1-OX#11 and HAT1-OX#13) were described previously [30]. T-DNA insertion mutants hat1, hat2 and hat3 were obtained from ABRC (Arabidopsis Biological Resource Center) [56], corresponding to line SALK_059835, SALK _091887 and SALK_056541. We performed cross to create the double mutant hat1hat2, hat1hat3 and triple mutant hat1hat2hat3. HAT1OX and mutants were identified (S2 Fig). All the plants were grown on half-strength MS plates and/or in soil under long-day conditions (16 h light/8 h dark) at 22°C.
Construction of plasmids and generation of transgenic plants
Gene-specific primers HAT1 were used to isolate HAT1, from a cDNA library by PCR. To generate the pZP211-HAT1-GFP, full-length HAT1 was amplified and cloned into the pZP211 vector with a GFP tag using the BamHI and SalI sites [57]. To generate the Myc-SnRK2.2/2.3/2.6, the coding regions of SnRK2.2/2.3/2.6 were cloned into pCAMBIA1307-63 Myc vector [58]. To generate HAT1-Promoter::GUS, 1.3-kb fragments upstream of HAT1 were amplified by PCR using primers HAT1p-F/R and inserted into the binary vector pBI121-GUS using HindIII and BamHI sites [59]. For BiFC assays, SnRK2.2/2.3/2.6 were cloned into the pXY103 vector fused to the C terminus of YFP, and HAT1 and its fragments were fused into the pXY104 vector fused to the N terminus of YFP [60]. For the recombinant protein and GST pull-down assay, the HAT1 coding region was amplified from Col-0 cDNA and various deletion constructs were incorporated into the pETMALc-H vector (MBP, BamHI/SalI) [61]. The coding regions of SnRK2s were inserted into the binary vector PGEX-6P-1(GST, BamHI/SalI). All primers are listed in S1 Table. The construct of HAT1-GFP driven by 35S promoter were transformed into Agrobacterium tumefaciens (strain GV3101), which were used to transform plants by the floral dip method. Transgenic lines were selected on half-strength MS medium that contained 50 μg ml-1 kanamycin. Transgene expression was analyzed by western blotting.
GUS staining and transient expression in protoplasts
Rosette leaves of 4-week-old A. thaliana plants grown under short day conditions were used for the isolation of protoplasts [62]. The relevant vectors 35S:HAT1–GFP, and 35S:GFP were used for protoplast transformation. A fluorescence microscope was used to observe GFP signals (Kim et al., 2001; Bae et al., 2008). For GUS staining, the transgenic plants with or without ABA and osmotic stress treatment were immersed in a staining solution (100 mM sodium-phosphate buffer, pH 7, 1 mM K4Fe(CN)6, 1 mM K3Fe(CN)6, 0.1% Triton X-100, 2 mM X-Gluc) overnight at 37°C in the dark followed by two times washes with 70% ethanol to remove chlorophyll. Samples were photographed using a stereoscope (Leica) equipped with a CCD camera. To test for GUS expression before and after ABA and osmotic stress, plants were treated with 100 μM ABA for 3 h and mannitol treatment for 6 h, respectively.
Phenotype analysis and drought stress treatment
For ABA sensitivity, different genotype seeds were grown vertically on 1/2 MS medium for 3–5 days and then transplanted to normal 1/2 MS medium or 1/2 MS medium containing 10μM ABA. The root growth was observed after about 10 days [63]. For the osmotic stress treatment, 4-day-old seedlings grown on half-strength MS medium (0.5% agar) were transferred to new agar plates containing 200 mM mannitol, and the primary root length and 30-seedlings fresh weight were measured after 10 days. The primary root lengths were measured with ImageJ (National Institutes of Health, Bethesda, MD, USA). Three independent experiments were performed.
To study the promotion of stomatal closure by ABA, fully expanded young leaves of 4-week-old Arabidopsis plants were harvested and incubated in MES-KCl buffer (50 mM KCl, 10 mM MES-KOH, pH 6.15), at 22°C and exposed to light for 2 h. Once the stomata were fully open, leaves were incubated in MES-KCl buffer alone or containing 50 μM ABA. Control treatments involved the addition of DMSO, an appropriate solvent with ABA. After treatment for 3h under light conditions, the epidermal strips were immediately peeled carefully from the abaxial surface of leaves, and stomatal apertures were measured with an optical microscope (Nikon, Optiphot-2) fitted with a camera lucida and a digitizing table linked to a personal computer [64]. The stomatal aperture sizes were analysed by the software image J. To avoid any potential rhythmic effects on stomatal aperture, experiments were always started at the same time of the day. Blinded stomatal aperture experiments were conducted by another group in the laboratory who are not aware of any information about the control group (WT) and test group (mutants and transgenic plants) (S2 Data Blinded experiments). For the ROS accumulation assay in guard cells, prepared epidermal peels with or without ABA treatment were loaded with 50 μM 2,7-dichlorofluorescin diacetate for 10 min (H2DCF-DA; Sigma-Aldrich) in dark, as described previously [65]. Fluorescence emission of guard cells was analyzed using image J. Three independent experiments were performed.
To measure leaf water loss, rosette leaves of similar developmental stages from 4-week-old plants were excised from their roots, placed in open Petri dishes, and kept on the lab bench for the indicated time, and then their fresh weights were monitored, with three replicates per time-point [66]. Water loss was expressed as a percentage of weight loss at the indicated time versus initial fresh weight.
For the progressive drought treatment experiment, 10-day-old plants were transferred from 1/2 MS medium to water-saturated soil and the plants were grown in the same glasshouse with 120 μmol m-2 s-1 under a 16 h: 8 h, light: dark photoperiod (23°C) for 2 weeks, then the plants were deprived of water for 14 days and the survival rates of plants were determined 5 d after re-watering (rehydration) [67]. Relative electrolyte leakage rates were measured as described by Julieta V. Cabello et al. [68]. Three independent experiments were performed.
The mild drought treatment was conducted as previously described [69,70], with a slight modification. Briefly, 12-day-old Arabidopsis seedlings of different genotypes grown on 1/2 MS medium were transferred to pots. Before transfer, the relative water content of the pots was set at 2.2 g water g-1 dry soil. The plants were kept to grow for 10 days. During this growth period, the water content of the soil was kept constant until 10 days, after which it was lowered daily to target 0.7 g water g-1 dry soil and mild drought stress treatment began. Control soil water content (well water) was maintained at a constant value of 2.2 g water g-1 dry soil during the entire experiment. Fig 3D showed the water loss from the peat pellets during the duration of the experiment. After mild drought treatment for 9 days, images of each genotype were taken. To quantify the biomass change of each genotype, the dry weights of detached rosettes of both the drought-treated and the well-watered control were measured. The reduction in biomass was calculated using the following equation:
Reduction in Biomass (RB) = (Biomass of Well Watered Control–Biomass of Drought Treated) / (Biomass of Well Watered Control)
PEG treatment and ABA content determination
Polyethylene glycol (PEG) 6000 was used to mimic drought stress [66]. Arabidopsis seedlings grown on 1/2 MS medium plates were transferred to 1/2 MS liquid medium (CK) and 1/2MS liquid medium containing 15% PEG (drought stress treatment) for indicated time, and then the seedlings were harvested for gene expression analysis or ABA content assay.
For ABA content assay, 0.5g 12-day-old seedlings with or without 15% PEG treatment were homogenized in 2 mL of 80% methanol, and incubated with additional 3 mL of 80% methanol overnight at 4°C. After centrifugation (4000 r/min for 10 min, 4°C), the supernatant was passed through a C18-SepPak classic cartridge (Waters, Milford, USA) [71]. ABA content measurement was performed by using a Plant hormone abscisic acid (ABA) ELISA Kit (BIOSAMITE, CK-E90047). Three independent experiments with different biological repeats were done.
RNA extraction and reverse-transcription PCR
12-day-old seedlings grown under long-day conditions were used for qRT-PCR analysis of ABA or drought stress-responsive genes. Total RNA extraction, cDNA synthesis and qRT-PCR were performed as described by Zhang et al. (2010) [72]. Briefly, total RNAs were extracted using RNAprep pure Plant Kit (from Transgene Biotech Co. Ltd. of Qiagen, Beijing) according to the manufactures’ protocols. Total RNAs treated with DNase I (Transgene Biotech Co. Ltd. of Qiagen, Beijing) were converted into cDNAs using M-MLV Reverse Transcriptase Kit (Invitrogen, USA). Real-time qPCR analysis was carried out using the SYBR® Premix Ex TaqTM II (TAKARA) on a BIO-RAD CFX ConnectTM Real-Time System, following the manufacturer’s instruction. Three independent experiments were performed, and three technical replicates of each experiment were performed. Actin2 genes was used as an internal control for normalization of transcript levels [73]. All primers used for gene expression analysis are shown in S1 Table.
Protein interaction assay
For GST pull-down assay, HAT1 and HAT1 fragments fused with MBP were purified with amylose resin (NEB). SnRK2.3 fused with GST was purified with glutathione beads (Sigma, G4510). GST pull-down assays were performed as described Yin et al. [74]. The assays were repeated three times with similar results.
For the BiFC assay, SnRK2s were cloned into the pXY103 vector and fused to the C terminus of YFP, and HAT1 and its fragments were fused into the pXY104 vector and fused to the N terminus of YFP. The resulting plasmids were introduced into Agrobacterium tumefaciens (strain GV3101), and then infiltrated into young leaves of Nicotiana benthamiana. Infected leaves were analyzed 48h after infiltration. YFP fluorescence was observed under a fluorescence microscope (Leica).
For the Co-IP assays in the Arabidopsis protoplasts, full-length coding sequences of HAT1 and SnRK2.3 were individually cloned into tagging plasmids behind Flag or Myc tag sequences in the sense orientation behind the cauliflower mosaic virus 35S promoter. Flag-fused HAT1 and Myc-fused SnRK2s were then transformed into Arabidopsis protoplasts. After overnight incubation at 23°C, the protoplasts were lysed, sonicated, and centrifuged. Co-IP assays were performed using transiently expressed proteins as described previously [75]. Briefly, the protein extracts were mixed with Myc agarose beads (Sigma-Aldrich) and then incubated at 4°C for 2 h. After being was hed at least five times, the agarose beads were recovered and mixed with the SDS sample buffer. The samples were detected by immunoblotusing anti-Myc antibody, and the coimmunoprecipitated protein was then detected using an anti-Flag antibody.
In vitro kinase assay and detection of in vivo HAT1 phosphorylation
The in vitro kinase assay was performed as previously described as Yin et al. [74]. MBP, MBP-HAT1, and truncated MBP-HAT1 were incubated with GST-SnRK2.3 kinase in 20 μL of kinase buffer [20 mM Tris (pH 7.5), 100 mM NaCl, and 12 mM MgCl2] and 10 μCi 32P ATP. After incubation at 37°C for 60 min, the reactions were stopped by adding 20 μL of 2×sodium dodecyl sulfate (SDS) buffer and boiling for 5 min. Proteins were resolved by polyacrylamide gel electrophoresis (PAGE) and phosphorylation was detected by exposing to a phosphor screen, and signals were obtained by a Typhoon 9410 phosphor imager. The in vivo phosphorylated HAT1 was examined by Phostag reagent (NARD Institute) with or without CIP treatment as described Guan et al [76].
Protein extraction and immunoblot analysis
Total protein was extracted from Arabidopsis using extraction buffer as described previously [77]. Briefly, plant material was ground in the Eppendorf tube using 2×sodium dodecyl sulfate (SDS) sample buffer, centrifuged at 13,000g for 10 min, and the supernatant was saved. For immunoblot analysis, total protein was separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to PVDF membranes. The membrane was blocked for 1 h in TBST buffer (10 mM Tris, pH 7.6, 150 mM NaCl, 1.0% Tween20) with 5% skim milk powder at room temperature and then incubated with specific primary antibodies in TBST buffer for 1 h. After the membrane washed by TBST buffer for several times, the blot was incubated with horseradish peroxide-conjugated secondary antibody (goat anti-rabbit IgG, Thermo fisher) at a dilution of 1/10000 for detection by the enhanced chemilumine scence assay.
The EMSA and ChIP assays
EMSA was performed using an Electrophoretic Mobility-Shift Assay (EMSA) Kit *with SYBR Green and SYPRO Ruby EMSA stains* (MolcularprobesTM, E33075). The binding reactions were carried out in 20 μL binding buffer [25 mM HEPES-KOH pH 8.0, 50 mM KCl, 1 mM dithiothreitol (DTT) and 10% glycerol] with approximately 1 ng probe (10000 cpm) and recombinant proteins purified from E. coli. After 30 min incubation on ice, the reactions were resolved by 5% native polyacrylamide gels with 1×TGE buffer (6.6 g L-1Tris, 28.6 g L-1 glycine, 0.78 g L-1EDTA, pH 8.7). The assays were repeated three times with similar results.
ChIP was performed as previously described [78]. Briefly, 14-day-old seedlings of HAT1OX and SnRK2.3OX/HAT1OX seedlings were treated as above described. 1.5 g of the samples were cross-linked with formaldehyde and nuclei were isolated using sucrose gradients. Chromatin was sonicated to generate fragments with the average size of 300 bp and precipitated using anti-GFP antibody. Immunocomplexes were harvested by protein A beads, washed and reverse cross-linked by boiling in the presence of Chelex resin (Bio-Rad, http://www.bio-rad.com/). The level of precipitated DNA fragments was quantified by RT-qPCR using specific primer sets (S1 Table). Col-0 was the negative control and the values in control plants were set to 1 after normalization against TA3 for qPCR analysis. Three biological replicates were carried out through the whole process.
Supporting information
(A) GUS staining for expression patterns of HAT1. Transgenic plants expressing HAT1-Promoter::GUS at seedlings (left) and leaves of adult plants (right) were stained with 5-bromo-4-chloro-3-indolyl β-D-glucuronide (X-Gluc). GUS expression was examined in cotyledons, roots and guard cells before and after 100 μM abscisic acid (ABA) treatment for 3 h and mannitol treatment for 6 h. (B) Subcellular localizations of HAT1-GFP. Protoplasts from wild-type (WT) plants were transformed with 35S:HAT1-GFP or 35S:GFP. The signals were observed under a fluorescence microscope. GFP, green fluorescent protein. Cell images were also taken under bright field as a control. Bars, 20 μm.
(TIF)
(A) Reverse transcription-PCR was employed to estimate the transcription levels of HAT1, HAT2 and HAT3 in T-DNA insertion mutants. (B) HAT1 protein was detected by western blotting with anti-GFP antibody. Similar HAT1 protein levels in HAT1OX#11 and HAT1#13 line.
(TIF)
(A, B) Expression patterns of HAT2 and HAT3 in response to ABA and osmotic stress. 12-day-old Col-0 seedlings were transferred to liquid MS medium containing 100 μM ABA and 200 mM mannitol and then the plants were harvested at the indicated time. Actin2 was used as the internal control. Data are shown as mean ± SD of three independent experiments. (C) Growth of different genotype seedlings on 1/2 MS medium with/without 10μM ABA or 200 mM mannitol. The 4-day-old seedlings were transferred to 1/2 MS or 1/2 MS medium supplemented 10μM ABA or 200 mM mannitol for 10 days, and then the photos were taken. (D-F) Quantification of primary root length and biomass in different genotypes after ABA treatment or mannitol treatment indicated in (C). The average and SDs were from three replications, asterisks indicate significant differences compared with Col-0 under the same treatment conditions. The significant difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
(TIF)
(A) Epidermal peels of indicated genotypes were treated with or without ABA for 2 h after stomatal pre-opening under light for 3h, and the stomatal aperture was measured by microscope. Scale bars: 10μm. (B) Stomatal apertures of different genotypes indicated in (A). Bars indicate SD calculated from three replications and at least 20 stomatals were measured for each genotype per replication. The significance of difference was analyzed by Student’s t test (**P < 0.01).
(TIF)
(A) Phenotypes of different genotypes in response to progressive drought stress. 4-week-old plants were subjected to drought stress by withholding watering (drought) for 14 days (when the lethal effect was observed in the Col-0), followed by rehydration for 5days. (B) Membrane stability status of different genotypes subjected to drought stress for 7 days and 14 days. Data is presented as a percentage of electrolyte leakage. Data are shown as mean SD of three independent experiments. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01). (C) Percentage of plants that survived the treatment mentioned in (A). Survival rate was recorded 5 days after rewatering. Bars indicate SD calculated from three replicated experiments. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
(TIF)
(A) Schematic representation of a series of truncation mutations of HAT1. HD, homeodomain; LZ, leucine zipper domain. (B) BiFc assay for the interaction of SnRK2.3 with HAT1 fragments. The truncated HAT1 fragments were fused with n-YFP and co-expressed with SnRK2.3-cYFP, respectively. (C) SnRK2.3 interacts with N-terminal region of HAT1 in GST pull-down assay. GST, GST-SnRK2.3 and MBP-tagged different domains of HAT1 were used in this assay. MBP-tagged domains of HAT1 were detected by western blotting with anti-MBP antibody.
(TIF)
(A) BiFC analysis of SnRK2.3 and HAT3 interactions with fusions to N- and C-terminal fragments of YFP, respectively. The constructs were expressed in tobacco leaves and the reconstitution of YFP is determined. Scale bars: 50 μm. (B) Time microscope images of Nicotianabenthamiana leaf epidermal cells expressing HAT3-GFP exposed to 50 μM ABA. The experiment was repeated three times with similar results and representative photos were displayed. Scale bar: 50 μm.
(TIF)
The expression of HAT1 (A) and SnRK2.3 (B) was tested by qRT-PCR in Col-0, HAT1OX#13 and SnRK2.3OX/HAT1OX. Data are shown as mean ± SD and Three independent experiments were done (Student’s t-test: ** P<0.01).
(TIF)
(A-C) Drought phenotypes (A), membrane stability status (B) and survival rates (C) of Col-0, HAT1OX#13, and SnRK2.3OX/HAT1OX plants subjected to progressive drought stress. In F, data is presented as a percentage of electrolyte leakage. The average and SDs were from three biological repeats in A. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
(TIF)
(XLSX)
(XLSX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files. The underlying numerical data for all graphs have been uploaded as additional supplementary files.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (http://www.nsfc.gov.cn/) [31570237 and 3140021 to DZ; 31670235 to HL]; the National Basic Research Program of China (http://program.most.gov.cn/) [973 Program (2015CB150100) to HL]; the Development Project of Transgenic Crops of China (http://program.most.gov.cn/) (2016ZX08009-003-002 to HL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679. doi: 10.1146/annurev-arplant-042809-112122 [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi-Shinozaki K, Shinozaki K (2006) Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57: 781–803. doi: 10.1146/annurev.arplant.57.032905.105444 [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein R (2013) Abscisic acid synthesis and response. The Arabidopsis Book 11: e0166 doi: 10.1199/tab.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071. doi: 10.1126/science.1173041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, et al. (2009) Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 324: 1064 doi: 10.1126/science.1172408 [DOI] [PubMed] [Google Scholar]
- 6.Soon F-F, Ng L-M, Zhou XE, West GM, Kovach A, et al. (2012) Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 335: 85–88. doi: 10.1126/science.1215106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melcher K, Ng L-M, Zhou XE, Soon F-F, Xu Y, et al. (2009) A gate–latch–lock mechanism for hormone signalling by abscisic acid receptors. Nature 462: 602–608. doi: 10.1038/nature08613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. science 324: 1068–1071. doi: 10.1126/science.1173041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, et al. (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068. doi: 10.1126/science.1172408 [DOI] [PubMed] [Google Scholar]
- 10.Miyazono K-i, Miyakawa T, Sawano Y, Kubota K, Kang H-J, et al. (2009) Structural basis of abscisic acid signalling. Nature 462: 609–614. doi: 10.1038/nature08583 [DOI] [PubMed] [Google Scholar]
- 11.Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annual review of plant biology 53: 247 doi: 10.1146/annurev.arplant.53.091401.143329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi H-i, Hong J-h, Ha J-o, Kang J-y, Kim SY(2000) ABFs, a family of ABA-responsive element binding factors. Journal of Biological Chemistry 275: 1723–1730. [DOI] [PubMed] [Google Scholar]
- 13.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, et al. (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences 97: 11632–11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii H, Zhu J-K (2009) Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proceedings of the National Academy of Sciences 106: 8380–8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushton DL, Tripathi P, Rabara RC, Lin J, Ringler P, et al. (2012) WRKY transcription factors: key components in abscisic acid signaling. Plant Biotechnology Journal 10: 2–11. doi: 10.1111/j.1467-7652.2011.00634.x [DOI] [PubMed] [Google Scholar]
- 16.Abe H, Urao T, Ito T, Seki M, Shinozaki K, et al. (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) Function as Transcriptional Activators in Abscisic Acid Signaling. The Plant Cell 15: 63–78. doi: 10.1105/tpc.006130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohama N, Sato H, Shinozaki K, Yamaguchishinozaki K (2017) Transcriptional Regulatory Network of Plant Heat Stress Response. Trends in Plant Science 22: 53–65. doi: 10.1016/j.tplants.2016.08.015 [DOI] [PubMed] [Google Scholar]
- 18.Reyes JL (2010) Post-transcriptional gene regulation of salinity and drought responses by plant microRNAs. Plant Cell & Environment 33: 481–489. [DOI] [PubMed] [Google Scholar]
- 19.Miura K, Hasegawa PM (2010) Sumoylation and other ubiquitin-like post-translational modifications in plants. Trends in Cell Biology 20: 223 doi: 10.1016/j.tcb.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 20.Lyzenga WJ, Stone SL (2012) Abiotic stress tolerance mediated by protein ubiquitination. Journal of Experimental Botany 63: 599 doi: 10.1093/jxb/err310 [DOI] [PubMed] [Google Scholar]
- 21.Schena M, Davis RW (1994) Structure of homeobox-leucine zipper genes suggests a model for the evolution of gene families. Proceedings of the National Academy of Sciences of the United States of America 91: 8393–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henriksson E, Olsson AS, Johannesson H, Johansson H, Hanson J, et al. (2005) Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiology 139: 509–518. doi: 10.1104/pp.105.063461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends in plant science 12: 419–426. doi: 10.1016/j.tplants.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 24.Johannesson H, Wang Y, Engström P (2001) DNA-binding and dimerization preferences of Arabidopsis homeodomain-leucine zipper transcription factors in vitro. Plant molecular biology 45: 63–73. [DOI] [PubMed] [Google Scholar]
- 25.Harris JC, Hrmova M, Lopato S, Langridge P (2011) Modulation of plant growth by HD‐Zip class I and II transcription factors in response to environmental stimuli. New Phytologist 190: 823–837. doi: 10.1111/j.1469-8137.2011.03733.x [DOI] [PubMed] [Google Scholar]
- 26.Ciarbelli AR, Ciolfi A, Salvucci S, Ruzza V, Possenti M, et al. (2008) The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant molecular biology 68: 465–478. doi: 10.1007/s11103-008-9383-8 [DOI] [PubMed] [Google Scholar]
- 27.Ohgishi M, Oka A, Morelli G, Ruberti I, Aoyama T (2001) Negative autoregulation of the Arabidopsis homeobox gene ATHB‐2. The plant journal 25: 389–398. [DOI] [PubMed] [Google Scholar]
- 28.Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, et al. (2002) The HAT2 gene, a member of the HD‐zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. The Plant Journal 32: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 29.Sorin C, Salla-Martret M, Bou-Torrent J, Roig-Villanova I, Martínez-García JF (2009) ATHB4, a regulator of shade avoidance, modulates hormone response in Arabidopsis seedlings. Plant Journal 59: 266 doi: 10.1111/j.1365-313X.2009.03866.x [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Ye H, Guo H, Johnson A, Zhang M, et al. (2014) Transcription factor HAT1 is phosphorylated by BIN2 kinase and mediates brassinosteroid repressed gene expression in Arabidopsis. The Plant Journal 77: 59–70. doi: 10.1111/tpj.12368 [DOI] [PubMed] [Google Scholar]
- 31.Zou LJ, Deng XG, Han XY, Tan WR, Zhu LJ, et al. (2016) Role of Transcription Factor HAT1 in Modulating Arabidopsis thaliana Response to Cucumber Mosaic Virus. Plant and Cell Physiology 57. [DOI] [PubMed] [Google Scholar]
- 32.Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, et al. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734. doi: 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- 33.Allan AC, Fluhr R (1997) Two Distinct Sources of Elicited Reactive Oxygen Species in Tobacco Epidermal Cells. Plant Cell 9: 1559 doi: 10.1105/tpc.9.9.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, et al. (1996) MOLECULAR IDENTIFICATION OF ZEAXANTHIN EPOXIDASE OF NICOTIANA PLUMBAGINIFOLIA, A GENE INVOLVED IN ABSCISIC ACID BIOSYNTHESIS AND CORRESPONDING TO THE ABA LOCUS OF ARABIDOPSIS THALIANA. Embo Journal 15: 2331–2342. [PMC free article] [PubMed] [Google Scholar]
- 35.Seo M, Peeters AJM, Koiwai H, Oritani T, Marionpoll A, et al. (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proceedings of the National Academy of Sciences 97: 12908–12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liming Xiong MI, Lee Hojoung, Zhu Jian-Kang(2001) The Arabidopsis LOS5/ABA3 Locus Encodes a Molybdenum Cofactor Sulfurase and Modulates Cold Stress and Osmotic StressResponsive Gene Expression. Plant Cell 13: 2063–2083. doi: 10.1105/TPC.010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chernys JT, Zeevaart JAD (2000) Characterization of the 9-cis-epoxycarotenoid dioxygenase gene family and the regulation of abscisic acid biosynthesis in avocado. Plant physiology 124: 343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi-Shinozaki K, Shinozaki K (1993) Characterization of the expression of a desiccation-responsive rd29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Molecular & General Genetics Mgg 236: 331–340. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Yu X, Thompson A, Guo M, Yoshida S, et al. (2009) Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant Journal for Cell & Molecular Biology 58: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida T, Mogami J, Yamaguchishinozaki K (2014) ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Current Opinion in Plant Biology 21: 133–139. doi: 10.1016/j.pbi.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 41.Wang P, Xue L, Batelli G, Lee S, Hou YJ, et al. (2013) Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proceedings of the National Academy of Sciences of the United States of America 110: 11205–11210. doi: 10.1073/pnas.1308974110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, et al. (2009) In vitro reconstitution of an abscisic acid signalling pathway. Nature 462: 660 doi: 10.1038/nature08599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uno Y, Furihata T, Abe H, Yoshida R, Shinozaki K, et al. (2000) Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proceedings of the National Academy of Sciences of the United States of America 97: 11632 doi: 10.1073/pnas.190309197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furihata T, Maruyama K, Fujita Y, Umezawa T, Yoshida R, et al. (2006) Abscisic acid-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proceedings of the National Academy of Sciences of the United States of America 103: 1988–1993. doi: 10.1073/pnas.0505667103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turchi L, Carabelli M, Ruzza V, Possenti M, Sassi M, et al. (2013) Arabidopsis HD-Zip II transcription factors control apical embryo development and meristem function. Development 140: 2118–2129. doi: 10.1242/dev.092833 [DOI] [PubMed] [Google Scholar]
- 46.Min YP, Kim SA, Lee SJ, Kim SY (2013) ATHB17 is a positive regulator of abscisic acid response during early seedling growth. Molecules & Cells 35: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice EA, Khandelwal A, Creelman RA, Griffith C, Ahrens JE, et al. (2014) Expression of a Truncated ATHB17 Protein in Maize Increases Ear Weight at Silking. Plos One 9: e94238 doi: 10.1371/journal.pone.0094238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, et al. (2002) The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant Journal 32: 1011 [DOI] [PubMed] [Google Scholar]
- 49.Ping Z, Rong C, Ping X, Jie W, Mao JL, et al. (2017) ATHB17 enhances stress tolerance by coordinating photosynthesis associated nuclear gene and ATSIG5 expression in response to abiotic stress. Scientific Reports 7: doi: 10.1038/srep45492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu T, Longhurst AD, Talavera-Rauh F, Hokin SA, Barton MK (2016) TheArabidopsistranscription factor ABIG1 relays ABA signaled growth inhibition and drought induced senescence. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu P, Cai XT, Wang Y, Xing L, Chen Q, et al. (2014) HDG11 upregulates cell-wall-loosening protein genes to promote root elongation in Arabidopsis. Journal of Experimental Botany 65: 4285–4295. doi: 10.1093/jxb/eru202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai XT, Xu P, Wang Y, Xiang CB (2015) Activated expression of AtEDT1/HDG11 promotes lateral root formation in Arabidopsis mutant edt1 by upregulating jasmonate biosynthesis. Journal of Integrative Plant Biology 57: 1017–1030. doi: 10.1111/jipb.12347 [DOI] [PubMed] [Google Scholar]
- 53.Yu L, Chen X, Wang Z, Wang S, Wang Y, et al. (2013) Arabidopsis Enhanced Drought Tolerance1/HOMEODOMAIN GLABROUS11 Confers Drought Tolerance in Transgenic Rice without Yield Penalty. Plant Physiology 162: 1378–1391. doi: 10.1104/pp.113.217596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu LH, Wu SJ, Peng YS, Liu RN, Chen X, et al. (2016) Arabidopsis EDT1/HDG11 improves drought and salt tolerance in cotton and poplar and increases cotton yield in the field. Plant Biotechnology Journal 14: 72–84. doi: 10.1111/pbi.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu H, Chen X, Hong YY, Wang Y, Xu P, et al. (2008) Activated Expression of an Arabidopsis HD-START Protein Confers Drought Tolerance with Improved Root System and Reduced Stomatal Density. Plant Cell 20: 1134–1151. doi: 10.1105/tpc.108.058263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alonso JM (2003) Arabidopsis thaliana Genome-Wide Insertional Mutagenesis of. Science 301: págs. 653–657. [DOI] [PubMed] [Google Scholar]
- 57.Hajdukiewicz P, Svab Z, Maliga P (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Molecular Biology 25: 989–994. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Liu J, Wang G, Cha JY, Li G, et al. (2015) A chaperone function of NO CATALASE ACTIVITY1 is required to maintain catalase activity and for multiple stress responses in Arabidopsis. Plant Cell 27: 908 doi: 10.1105/tpc.114.135095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin X, Zhang J, Shao C, Lin S, Jiang L, et al. (2009) Isolation and Characterization of a Curcin Promoter from Jatropha curcas L. and Its Regulation of Gene Expression in Transgenic Tobacco Plants. Plant Molecular Biology Reporter 27: 275–281. [Google Scholar]
- 60.Yu X, Li L, Li L, Guo M, Chory J, et al. (2008) Modulation of brassinosteroid-regulated gene expression by jumonji domain-containing proteins ELF6 and REF6 in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 105: 7618–7623. doi: 10.1073/pnas.0802254105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pryor KD, Leiting B (1997) High-level expression of soluble protein in Escherichia coli using a His6-tag and maltose-binding-protein double-affinity fusion system. Protein Expression & Purification 10: 309–319. [DOI] [PubMed] [Google Scholar]
- 62.Yoo SD, Cho YH, Sheen J (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572. doi: 10.1038/nprot.2007.199 [DOI] [PubMed] [Google Scholar]
- 63.Fujii H, Verslues PE, Zhu JK (2007) Identification of Two Protein Kinases Required for Abscisic Acid Regulation of Seed Germination, Root Growth, and Gene Expression in Arabidopsis. Plant Cell 19: 485–494. doi: 10.1105/tpc.106.048538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Desikan R, Last K, Harrett‐Williams R, Tagliavia C, Harter K, et al. (2006) Ethylene‐induced stomatal closure in Arabidopsis occurs via AtrbohF‐mediated hydrogen peroxide synthesis. Plant Journal 47: 907–916. doi: 10.1111/j.1365-313X.2006.02842.x [DOI] [PubMed] [Google Scholar]
- 65.Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, et al. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731 doi: 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- 66.Wang ZY, Xiong L, Li W, Zhu JK, Zhu J (2011) The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 23: 1971–1984. doi: 10.1105/tpc.110.081943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang J, Choi H, Im M, Kim SY (2002) Arabidopsis Basic Leucine Zipper Proteins That Mediate Stress-Responsive Abscisic Acid Signaling. Plant Cell 14: 343–357. doi: 10.1105/tpc.010362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cabello JV, Chan RL (2012) The homologous homeodomain-leucine zipper transcription factors HaHB1 and AtHB13 confer tolerance to drought and salinity stresses via the induction of proteins that stabilize membranes. Plant Biotechnology Journal 10: 815–825. doi: 10.1111/j.1467-7652.2012.00701.x [DOI] [PubMed] [Google Scholar]
- 69.Harb A, Pereira A (2011) Screening Arabidopsis Genotypes for Drought Stress Resistance. Methods in Molecular Biology 678: 191 doi: 10.1007/978-1-60761-682-5_14 [DOI] [PubMed] [Google Scholar]
- 70.Clauw P, Coppens F, De BK, Dhondt S, Van DT, et al. (2015) Leaf responses to mild drought stress in natural variants of Arabidopsis. Plant Physiology 167: 800–816. doi: 10.1104/pp.114.254284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001) Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiology 127: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang DW, Fei X, Zhang ZW, Chen YE, Du JB, et al. (2010) Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell & Environment 33: 2121–2131. [DOI] [PubMed] [Google Scholar]
- 73.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Journal of Biological Chemistry 139: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, et al. (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. 109: 181–191. [DOI] [PubMed] [Google Scholar]
- 75.Zhou H, Zhao J, Yang Y, Chen C, Liu Y, et al. (2012) Ubiquitin-specific protease16 modulates salt tolerance in Arabidopsis by regulating Na(+)/H(+) antiport activity and serine hydroxymethyltransferase stability. Plant Cell 24: 5106 doi: 10.1105/tpc.112.106393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan Q, Zhu J (2014) The Protein Phosphatase RCF2 and Its Interacting Partner NAC019 Are Critical for Heat Stress-Responsive Gene Regulation and Thermotolerance in Arabidopsis. Plant Cell 26: 438–453. doi: 10.1105/tpc.113.118927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martínez-García JF, Monte E, Quail PH (1999) A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant Journal 20: 251–257. [DOI] [PubMed] [Google Scholar]
- 78.Yu X, Li L, Zola J, Aluru M, Ye H, et al. (2011) A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. Plant Journal 65: 634 doi: 10.1111/j.1365-313X.2010.04449.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) GUS staining for expression patterns of HAT1. Transgenic plants expressing HAT1-Promoter::GUS at seedlings (left) and leaves of adult plants (right) were stained with 5-bromo-4-chloro-3-indolyl β-D-glucuronide (X-Gluc). GUS expression was examined in cotyledons, roots and guard cells before and after 100 μM abscisic acid (ABA) treatment for 3 h and mannitol treatment for 6 h. (B) Subcellular localizations of HAT1-GFP. Protoplasts from wild-type (WT) plants were transformed with 35S:HAT1-GFP or 35S:GFP. The signals were observed under a fluorescence microscope. GFP, green fluorescent protein. Cell images were also taken under bright field as a control. Bars, 20 μm.
(TIF)
(A) Reverse transcription-PCR was employed to estimate the transcription levels of HAT1, HAT2 and HAT3 in T-DNA insertion mutants. (B) HAT1 protein was detected by western blotting with anti-GFP antibody. Similar HAT1 protein levels in HAT1OX#11 and HAT1#13 line.
(TIF)
(A, B) Expression patterns of HAT2 and HAT3 in response to ABA and osmotic stress. 12-day-old Col-0 seedlings were transferred to liquid MS medium containing 100 μM ABA and 200 mM mannitol and then the plants were harvested at the indicated time. Actin2 was used as the internal control. Data are shown as mean ± SD of three independent experiments. (C) Growth of different genotype seedlings on 1/2 MS medium with/without 10μM ABA or 200 mM mannitol. The 4-day-old seedlings were transferred to 1/2 MS or 1/2 MS medium supplemented 10μM ABA or 200 mM mannitol for 10 days, and then the photos were taken. (D-F) Quantification of primary root length and biomass in different genotypes after ABA treatment or mannitol treatment indicated in (C). The average and SDs were from three replications, asterisks indicate significant differences compared with Col-0 under the same treatment conditions. The significant difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
(TIF)
(A) Epidermal peels of indicated genotypes were treated with or without ABA for 2 h after stomatal pre-opening under light for 3h, and the stomatal aperture was measured by microscope. Scale bars: 10μm. (B) Stomatal apertures of different genotypes indicated in (A). Bars indicate SD calculated from three replications and at least 20 stomatals were measured for each genotype per replication. The significance of difference was analyzed by Student’s t test (**P < 0.01).
(TIF)
(A) Phenotypes of different genotypes in response to progressive drought stress. 4-week-old plants were subjected to drought stress by withholding watering (drought) for 14 days (when the lethal effect was observed in the Col-0), followed by rehydration for 5days. (B) Membrane stability status of different genotypes subjected to drought stress for 7 days and 14 days. Data is presented as a percentage of electrolyte leakage. Data are shown as mean SD of three independent experiments. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01). (C) Percentage of plants that survived the treatment mentioned in (A). Survival rate was recorded 5 days after rewatering. Bars indicate SD calculated from three replicated experiments. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
(TIF)
(A) Schematic representation of a series of truncation mutations of HAT1. HD, homeodomain; LZ, leucine zipper domain. (B) BiFc assay for the interaction of SnRK2.3 with HAT1 fragments. The truncated HAT1 fragments were fused with n-YFP and co-expressed with SnRK2.3-cYFP, respectively. (C) SnRK2.3 interacts with N-terminal region of HAT1 in GST pull-down assay. GST, GST-SnRK2.3 and MBP-tagged different domains of HAT1 were used in this assay. MBP-tagged domains of HAT1 were detected by western blotting with anti-MBP antibody.
(TIF)
(A) BiFC analysis of SnRK2.3 and HAT3 interactions with fusions to N- and C-terminal fragments of YFP, respectively. The constructs were expressed in tobacco leaves and the reconstitution of YFP is determined. Scale bars: 50 μm. (B) Time microscope images of Nicotianabenthamiana leaf epidermal cells expressing HAT3-GFP exposed to 50 μM ABA. The experiment was repeated three times with similar results and representative photos were displayed. Scale bar: 50 μm.
(TIF)
The expression of HAT1 (A) and SnRK2.3 (B) was tested by qRT-PCR in Col-0, HAT1OX#13 and SnRK2.3OX/HAT1OX. Data are shown as mean ± SD and Three independent experiments were done (Student’s t-test: ** P<0.01).
(TIF)
(A-C) Drought phenotypes (A), membrane stability status (B) and survival rates (C) of Col-0, HAT1OX#13, and SnRK2.3OX/HAT1OX plants subjected to progressive drought stress. In F, data is presented as a percentage of electrolyte leakage. The average and SDs were from three biological repeats in A. The significance of difference was analyzed by Student’s t test (*P < 0.05, **P < 0.01).
(TIF)
(XLSX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. The underlying numerical data for all graphs have been uploaded as additional supplementary files.