Abstract
Anopheles mosquitoes transmit at least 200 million annual malaria infections worldwide. Despite considerable genomic resources, mechanistic understanding of biological processes in Anopheles has been hampered by a lack of tools for reverse genetics. Here, we report successful application of the CRISPR/Cas9 system for highly efficient, site-specific mutagenesis in the diverse malaria vectors Anopheles albimanus, A. coluzzii, and A. funestus. When guide RNAs (gRNAs) and Cas9 protein are injected at high concentration, germline mutations are common and usually biallelic, allowing for the rapid creation of stable mutant lines for reverse genetic analysis. Our protocol should enable researchers to dissect the molecular and cellular basis of anopheline traits critical to successful disease transmission, potentially exposing new targets for malaria control.
Keywords: Anopheles, gene drive, reverse genetics, transgenics, CRISPR, Cas9
Anopheles mosquitoes are the exclusive vectors of mammalian malaria (White et al. 2011). Over the past decade, human malaria deaths have declined by nearly 50%, primarily due to increased use of insecticides that target the mosquito vector (Bhatt et al. 2015). However, emerging physiological and behavioral resistance in Anopheles populations threatens the sustainability of insecticidal control (David et al. 2005; Edi et al. 2014; Sougoufara et al. 2014; Ranson and Lissenden 2016). In order to maintain and extend the hard-won progress of the past decade, novel vector control strategies need to be developed and combined with traditional chemical control. The development of new tools in the fight against malaria mosquitoes is contingent upon improved mechanistic knowledge of myriad mosquito biological processing, including blood feeding, gametogenesis, gustation, immunity, olfaction, and metabolism, among many others.
In 2002, the African malaria mosquito Anopheles gambiae was the second arthropod to have its genome sequenced and, more recently, the genomes of 16 other anophelines were sequenced (Holt et al. 2002; Neafsey et al. 2013). Despite considerable genomic resources, progress in dissecting the molecular and cellular biology of malaria mosquitoes has been slow, primarily due to the difficulty in performing reverse genetic techniques that are routine in model organisms. Currently, the vast majority of Anopheles genes have no known function (Giraldo-Calderón et al. 2015), impeding the development of novel vector control strategies reliant upon understanding how individual genes contribute to the biology of the mosquito. Previously, genome editing in Anopheles relied on either transposon-based transgenesis with no control over where an insertion occurred (Grossman et al. 2001; Nolan et al. 2002; Perera et al. 2002; Meredith et al. 2011; Carballar-Lejarazú et al. 2013; Pondeville et al. 2014) or highly inefficient and expensive, site-specific genome editing technologies such as zinc finger nucleases or transcription activator-like effector nucleases (Windbichler et al. 2007; Smidler et al. 2013). Recently, the CRISPR/Cas9 genome editing technique has been successfully applied to a diversity of organisms (Bassett et al. 2013; Hsu et al. 2014; Port et al. 2014; Basu et al. 2015; Dong et al. 2015; Gantz et al. 2015; Hall et al. 2015; Kistler et al. 2015; Barrangou and Doudna 2016; Hammond et al. 2016; Li et al. 2017; Sharma et al. 2017; Staahl et al. 2017; Li et al. 2017a; Li et al. 2017b). With this technology, researchers can directly edit or modulate DNA sequences, allowing them to study the function of genes in vivo (Hsu et al. 2014). When used for site-directed mutagenesis, Cas9 protein and a small gRNA (sgRNA) that is complementary to a target sequence in the genome are delivered to germ cells. The Cas9 and sgRNA complex bind to the target sequence and cause a double-strand break, which will be repaired through nonhomologous end joining (NHEJ) or microhomology-mediated end joining (MMEJ) resulting in mismatches and indels relative to wild-type sequence (Bae et al. 2014; Basu et al. 2015; Maruyama et al. 2015). When exons are targeted, such mutagenesis will often result in premature stop codons or frameshifts that disrupt protein function. Despite high mutagenesis efficiency in other organisms, it is unclear if the CRISPR/Cas9 system will prove to be efficient in Anopheles, as egg injection alone often results in extremely high mortality and low transformation efficiencies, perhaps due to the inherent fragility of the eggs themselves. Here, we report successful development of an efficient site-specific mutagenesis protocol using the CRISPR/Cas9 system in various anophelines, facilitating reverse genetics in this important group of disease vectors.
Materials and Methods
Mosquito strains
Four mosquito colonies were used in this study: A. coluzzii wild-type strain NGS, A. gambiae white-eyed mutant strain M2 (MRA-105), A. albimanus wild-type strain STECLA (MRA-126), and A. funestus wild-type strain FUMOZ (MRA-127). Strains with accession numbers were obtained from the Malaria Research and Reference Reagent Resource Center (MR4). Mosquitoes were maintained in insectaries at the University of California, Riverside (UCR), under standard conditions (White et al. 2011).
sgRNA design and generation
gRNAs were designed by searching both the sense and antisense strand of exon 2 of the white gene (ACOM037804, AALB006905, and AFUN003538) for the presence of protospacer-adjacent motifs with the sequence NGG using ZIFIT (http://zifit.partners.org/ZiFiT/ChoiceMenu.aspx) and CRISPR Design (http://crispr.mit.edu/) (Xie et al. 2014). Linear, double-stranded DNA templates for sgRNAs were generated by performing template-free PCR with Q5 high-fidelity DNA polymerase (NEB), the forward primer of each gRNA, and universal-sgRNAR. PCR conditions included an initial denaturation step of 98° for 30 sec, followed by 35 cycles of 98° for 10 sec, 58° for 10 sec, and 72° for 10 sec, followed by a final extension at 72° for 2 min. PCR products were purified with magnetic beads using standard protocols. gRNAs were generated by in vitro transcription (AM1334; Life Technologies) using 300 ng purified DNA as template in an overnight reaction incubated at 37°. MegaClear columns (AM1908; Life Technologies) were used to purify sgRNAs, which were then diluted to 1 μg/μl, aliquoted, and stored at 80° until use. Three possible off-target sites of each sgRNA in the different mosquito species were identified based on the CHOPCHOPv2 software (Labun et al. 2016) and local sgRNACas9 package (Xie et al. 2014), and analyzed by using a T7 endonuclease I (T7EI) assay, respectively. Briefly, genomic DNA was extracted from mosquitoes with the DNeasy blood & tissue kit (QIAGEN) following the manufacturer’s protocol. Target loci were amplified by PCR and PCR product was purified with a MinElute PCR purification Kit (QIAGEN). Next, 2 μl of NEB buffer 2, 200 ng of purified PCR product, and ddH2O (to a total volume of 19 μl) were mixed together and a hybridization reaction conducted in a PCR cycler with 5 min, 95°; ramp down to 85° at −2°/sec; ramp down to 25° at −0.1°/sec; and hold at 4°. Next, 1 μl (10 U) of T7EI was added and the reaction incubated at 37° for 15 min. The reaction was stopped by adding 2 μl of 0.25 M EDTA and loaded immediately on a 1.5% agarose gel. All primer sequences are listed in Supplemental Material, Table S1 in File S1. Recombinant Cas9 protein from Streptococcus pyogenes was purchased from PNA Bio (CP01) and diluted to 1 μg/μl in nuclease-free water with 20% glycerol, and stored in aliquots at −80°.
Microinjection
Mixed sex pupae were allowed to eclose into a single (L24.5 × W24.5 × H24.5 cm) cage. After allowing 5 d for mating, females were offered a bovine bloodmeal using the Hemotek (model# PS5) blood feeding system. A minimum of 60 hr was allowed for oogenesis, after which ovicups filled with ddH20 and lined with filter paper were introduced into cages, and females were allowed to oviposit in the dark. After ∼15 min, the ovicup was removed and unmelanized eggs were transferred onto a glass slide and rapidly aligned against a wet piece of filter paper. Aluminosilicate needles (AF100-64-10; Sutter) pulled on a Sutter P-1000 needle puller (heat 605, velocity 130, delay 80, pull 70, and pressure 500) and beveled using a Sutter BV-10 beveler were used for injections. An Eppendorf Femtojet was used to power injections, which were performed under a compound microscope at 100× magnification. Since eggs were injected prior to melanization, only 10–20 eggs were injected at a time, after which fresh eggs were obtained. After injection, eggs were floated in ddH20 and allowed to hatch spontaneously.
Mutation screens
The white-eye phenotype of G0 and G1 mosquitoes was assessed and photographed under a Leica M165 FC stereomicroscope. To molecularly characterize CRISPR/Cas9-induced mutations, genomic DNA was extracted from a single mosquito with a DNeasy blood & tissue kit (QIAGEN) and target loci were amplified by PCR. For T7EI assays, 1 μl of T7EI (NEB) was added to 19 μl of PCR product, digested for 15 min at 37°, and visualized on a 2% agarose electrophoresis gel stained with ethidium bromide. To characterize mutations introduced during NHEJ or MMEJ, PCR products containing the sgRNA target site were amplified, cloned into TOPO TA vectors (Life Technologies), purified, and Sanger sequenced at the UCR Genomics core.
Data availability
Genomic DNA from mosquito strains produced here will be made available upon request.
Results
In order to rapidly and easily detect successful CRISPR/Cas9 mutagenesis, we wanted to target a gene where knockout of only a single allele produces a visible phenotype. However, no dominant visible mutations for Anopheles have been previously reported. Thus, we chose to target the white gene, which codes a protein critical for eye pigment transport (Besansky et al. 1995). Knockout of the white gene results in a change from wild-type red eye color to white (unpigmented) eye color, a simple phenotype to score. Although the white allele is recessive, it is located on the X chromosome and thus hemizygous in male anophelines (XY sex determination system), meaning that successful knockout of a single allele in males will result in the white-eye phenotype.
Mutagenesis efficiency is concentration- and sgRNA-dependent
A. coluzzii belongs to the A. gambiae complex, which includes a number of major African malaria vectors. To determine the efficacy of CRISPR/Cas9 mutagenesis in this species complex, we designed two sgRNAs targeting exon 2 of the white protein gene (ACOM037804). First, we used AcsgRNA1 to test how different concentrations of both the sgRNA and Cas9 protein affected mutagenesis rates. We found that both embryo survival and mutagenesis rate were sgRNA and Cas9 concentration-dependent (Table 1). Greater than 50% of embryos survived control injections with only water; however, survival rates for embryos (37%) injected with even the lowest concentration of sgRNA and Cas9 decreased relative to control. With increasing concentrations of sgRNA and Cas9, embryo survival further decreased. Indeed, only 11% of embryos survived injections with the highest concentrations tested. Conversely, concentrations of sgRNA and Cas9 were positively correlated with mutagenesis rates; 46% of males injected with the lowest concentration had mosaic white eyes, while a remarkable 100% of males injected with the highest concentration had mosaic eyes (Figure 1 and Table 1). Importantly, at higher injection concentrations, a majority of injected females also had mosaic eyes. Since the white gene is recessive, the production of mosaic females demonstrates that the CRISPR/Cas9 system can mutate both copies of diploid Anopheles genes. Notably, we also observed G0-injected males and females with completely white eyes, suggesting that the vast majority of cells in the eyes were mutated. Based on the above results, we used an sgRNA concentration of 120 ng/μl and a Cas9 protein concentration of 300 ng/μl, which balances survival and mutagenesis efficiency, to further explore the CRISPR/Cas9 system in Anopheles. To determine if the sgRNA sequence had an effect on mutagenesis rate, we compared AcsgRNA1 from above against a second sgRNA (AcsgRNA2) targeting white. We found that AcsgRNA1 (93, 87%) produced mosaic G0 males and females at a much higher frequency than AcsgRNA2 (32, 25%), suggesting that the sgRNA sequence can have a large impact on mutagenesis efficiency (Table 2).
Table 1. Effect of sgRNA and Cas9 concentration on A. coluzzii survival and mutagenesis.
| AcsgRNA 1 | Cas9 | Number Injected | Survivors | Mosaic (%) | |||
|---|---|---|---|---|---|---|---|
| M | F | Total (%) | M (%) | F (%) | |||
| No injection | No injection | 300 | 137 | 118 | 255 (85) | 0 | 0 |
| Water | Water | 217 | 69 | 52 | 121 (56) | 0 | 0 |
| 30 (ng/μl) | 100 (ng/μl) | 185 | 31 | 38 | 69 (37) | 32 (46) | 0 |
| 60 | 200 | 251 | 48 | 33 | 81 (32) | 48 (59) | 0 |
| 120 | 300 | 219 | 31 | 16 | 47 (21) | 29 (94) | 12 (75) |
| 240 | 400 | 177 | 22 | 11 | 33 (19) | 20 (91) | 9 (82) |
| 480 | 500 | 228 | 12 | 12 | 24 (11) | 12 (100) | 10 (83) |
M, male; F, female.
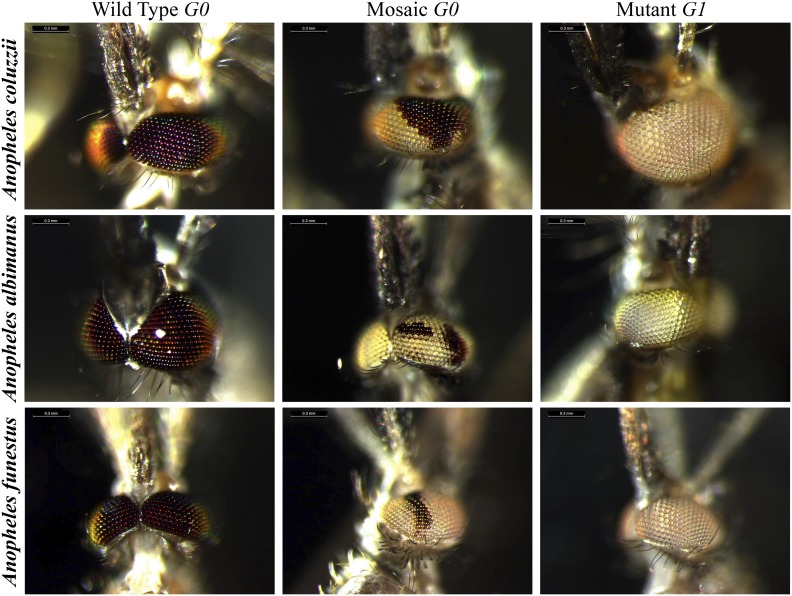
Figure 1.
CRISPR/Cas9 efficiently generates heritable, site-specific mutations in diverse Anopheles mosquitoes. On the left, representative images of wild-type anopheline eyes are shown for each species. In the center are representative G0 mosaic white-eyed mutant mosquitoes that were injected with sgRNA and Cas9 as embryos. On the right are representative homozygous white-eyed mutant G1 mosquitoes generated by crossing mosaic G0 male and female mosquitoes. CRISPR, clustered regularly interspaced short palindromic repeats; sgRNA, small guide RNA.
Table 2 .
G0 and G1 mutagenesis rates in three different Anopheles species.
| sgRNA | # Inj. | Survivors | Mosaic (%) | G0 M × White F | White M × G0 F | G0 M × G0 F | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | F | Total (%) | M (%) | F (%) | G1 Mutant M (%) | G1 Mutant F (%) | G1 Mutant M (%) | G1 Mutant F (%) | G1 Mutant M (%) | G1 Mutant F (%) | |||
| Anopheles coluzzii | AcsgRNA1 | 612 | 76 | 62 | 138 (23) | 71 (93) | 54 (87) | 991 (93) | 117 (91) | 851 (91) | 1232 (94) | 881 (86) | 939 (81) |
| AcsgRNA2 | 447 | 53 | 36 | 89 (20) | 17 (32) | 9 (25) | 1038 (88) | 1273 (84) | 751 (89) | 882 (91) | 751 (45) | 846 (49) | |
| Anopheles albimanus | AasgRNA1 | 573 | 81 | 58 | 139 (24) | 74 (91) | 43 (74) | N/A | N/A | 1317 (60) | 1577 (62) | ||
| AasgRNA2 | 511 | 79 | 68 | 147 (29) | 0 | 0 | N/A | N/A | N/A | N/A | |||
| Anopheles funestus | AfsgRNA1 | 237 | 15 | 11 | 26 (11) | 8 (53) | 7 (64) | N/A | N/A | 53 (65) | 92 (71) | ||
| AfsgRNA2 | 352 | 21 | 16 | 37 (11) | 5 (24) | 4 (25) | N/A | N/A | 37 (51) | 62 (61) | |||
Confirmation of germline mutations and site specificity
While mosaic G0 mosquitoes can be used for reverse genetics, the creation of stable, mutant lines permits more thorough investigation of gene function. Thus, we wanted to determine the proportion of G0 mosaic-eyed A. coluzzi that possessed germline mutations. To obtain the germline mutation rate, we crossed G0 mosaic-eyed males with females of an existing white-eye mutant line of A. coluzzii (M2) that was established > 20 yr ago (Figure S2A in File S1) (Mason 1967; Besansky et al. 1995; Benedict et al. 1996). Hemizygous male progeny of this cross will all have white eyes since they inherit a maternal mutated white gene; however, homozygous females will only have white eyes if they inherit a mutant allele from both parents. A remarkable 93% of G0 mosaic males injected with AcsgRNA1 produced G1 females with white eyes, while 88% of G0 AcsgRNA2 mosaic males passed on white-eye mutations to G1 female progeny (Table 2). To determine if female mosquitoes with mosaic eyes could also pass on the mutation, we performed a bulk cross of mosaic G0 males with mosaic G0 females and found that 83% (male 86% and female 81%) of the G1 progeny from AcsgRNA1-injected mosquitoes had fully white eyes, while 47% (male 45% and female 49%) of G1 progeny from AcsgRNA2-injected mosquitoes had fully white eyes (Table 2). We have now maintained multiple, white-eyed mutant lines in the laboratory for >15 generations, proving that the mutations introduced by CRISPR/Cas9 are highly stable. The combination of good G0 survival, biallelic mutation, and high germline transmission allows for the rapid creation of knockout A. coluzzii lines using CRISPR/Cas9.
To confirm that the mosaic eye phenotype was caused by loss-of-function of the white gene, we performed a T7EI assay on five randomly chosen G0 AcsgRNA1 and AcsgRNA2 male mosquitoes with mosaic eyes. In the T7EI assay, T7EI will cut when NHEJ or MMEJ of the CRISPR-induced double strand break introduces a SNP or indel relative to the wild-type allele, whereas no digestion will occur in mosquitoes with two wild-type alleles. As expected, PCR fragments of the white gene from mosaic-eyed males were consistently cut into small bands by T7EI, while no activity was observed in nonmosaic male mosquitoes (Figure S4, A–E in File S1), confirming that the white-eye phenotype is caused by disruption of the white coding sequence. To sample the spectrum of mutations introduced by NHEJ or MMEJ, we performed Sanger sequencing of PCR products containing the two sgRNA target sites in G1 mosquitoes with white eyes. The sequencing results confirmed the presence of indels that were induced by NHEJ or MMEJ (Figure S3, A and B and Table S2 in File S1) in all mutant mosquitoes, which ranged in size from 2 to 54 bp. Finally, we screened for off-target activity of both sgRNAs by T7EI assay. Across three potential off-target loci for both sgRNAs, no evidence of mutagenesis was detected in G0 mosaic males, indicating high specificity of the sgRNAs (Figure S5 in File S1).
CRISPR/CAS9 activity in diverse Anopheles
To determine the applicability of the CRISPR/Cas9 system to diverse Anopheles species, we performed injections targeting white in A. albimanus (a minor vector of malaria on South America) and A. funestus (a major, understudied malaria vector in Africa) (Neafsey et al. 2013). For each species, we designed two sgRNAs targeting white and injected the individual sgRNAs (120 ng/μl) and Cas9 (300 ng/μl) directly into eggs.
As found in other studies, A. albimanus survived injections at a rate comparable to A. coluzzii (Perera et al. 2002). For AasgRNA1, we found that 91% of G0 males had mosaic white eyes, while 74% of G0 females were mosaics. Interestingly, injection of AasgRNA2 produced no mosquitoes with mosaic eyes, further reinforcing the impact of sgRNA choice on mutagenesis efficiency. Since no previously generated white-eyed line of A. albimanus was available, we bulk crossed AasgRNA1 G0 mosaic males and females to determine germline mutation rates (Table 2). Over 61% of the G1 progeny from the cross possessed fully white eyes, suggesting high germline mutagenesis efficiency in A. albimanus. As with A. coluzzii, T7EI assays consistently detected mutations in white in G0 mosaic males (Figure S4 in File S1) and sequencing showed indels in mosaic males ranging from 2 to 11 bp (Figure S3 in File S1). Additionally, no mutations were detected using T7EI assays at three potential off-target sites for AasgRNA1 (Figure S5 in File S1).
The survival rate of A. funestus embryos injected with either of two sgRNAs targeting the white gene (AfsgRNA1 and AfsgRNA2) was less than half that of A. coluzzii and A. albimanus. (Table 2). We attribute the lower survival rate to the unique morphology of A. funestus eggs at the poles (Figure 2), which makes injection challenging. Despite lower survival, a high proportion of G0 males (53 and 24%) and females (64 and 25%) for both sgRNAs displayed mosaic white eyes. Bulk crossing of G0 male and female mosaics (Figure S2B in File S1) produced 67% (AfsgRNA1) or 57% (AfsgRNA2) G1 progeny with fully white eyes, demonstrating high germline mutagenesis rates. As with previous species, the T7EI assay consistently identified mosaic males (Figure S4 in File S1) and Sanger sequencing revealed diverse indels (2–34 bp) in mutated males (Figure S3 in File S1). No mutagenic activity was detected for either sgRNA at the three most likely off-target genomic sites (Figure S5 in File S1).
Figure 2.
Morphology of eggs differs dramatically among anophelines. Eggs of the three species of Anopheles used in this study alongside an egg of the yellow fever mosquito Ae. aegypti for size comparison. Note the difference in pole shape between A. albimanus and A. funestus eggs, which likely contributes to differences in both survival and mutagenesis rates between these two species.
Discussion
The CRISPR/Cas9 system offers the possibility of precise, efficient, and cost-effective mutagenesis in nonmodel organisms (Barrangou and Doudna 2016). While considerable genomic resources have been developed for malaria mosquitoes, no efficient tools for performing reverse genetics in these species exist, slowing the development of genetics-based vector control. Studies of the CRISPR/Cas9 system in the distantly related (diverged 145–200 million years ago) mosquito Aedes aegypti have demonstrated G0 mutagenesis rates between 3 and 50%, with high variability among injection operators and different sgRNAs (Basu et al. 2015; Kistler et al. 2015). While no systematic studies of CRISPR/Cas9 mutagenesis rates on any anopheline mosquitoes have been conducted, a few groups developing gene drive-related technologies (Adelman et al. 2017; Akbari et al. 2015; Champer et al. 2016; Marshall et al. 2015; Marshall et al. 2017) have recently reported high rates of mutagenesis when gRNAs and Cas9 were directly integrated into the genome of two Anopheles species (Gantz et al. 2015; Galizi et al. 2016; Hammond et al. 2016).
In summary, we report remarkably high rates of survival and mutagenesis in three different Anopheles species co-injected with Cas9 protein and sgRNAs targeting the white gene. Importantly, we describe the first, successful genetic engineering of A. funestus, demonstrating that the CRISPR/Cas9 system may even be useful in species where previous genome editing techniques proved too inefficient for practical use. Additionally, since high concentrations of sgRNA and Cas9 result in biallelic mutations, stable mutant lines can be rapidly generated, even when no visible marker is present. The following procedure can be used to generate such lines: (1) inject sgRNA and Cas9 into mixed sex eggs, (2) cross G0 survivors en masse, (3) isolate G0 females into individual ovicups, (4) screen five G1 larvae from each family for mutations (sequencing, T7EI, or an alternative assay), (5) conduct full sibling mating of families in which all G1 larvae are mutants, and (6) confirm stable generation of a mutant line by sequencing of G2 mosquitoes. The ability to rapidly and consistently create stable knockout lines should greatly accelerate mechanistic research into key cellular and molecular pathways in malaria mosquitoes.
We note that cleavage efficiency of the sgRNA/Cas9 complex is target site-dependent. In mammalian systems, it has been reported that the chromatin environment around the target site and certain features of the sgRNA sequence are major factors affecting the efficiency of DSB generation (Doench et al. 2014; Kuscu et al. 2014; Wang et al. 2015). Due to the limited number of sgRNAs we tested, we are unable to confirm whether these observations can be extended into Anopheles. However, the complete failure of AasgRNA2 to cause knockout is likely due to low thermodynamic stability of the sgRNA/Cas9 complex, or secondary structure at the target site preventing binding (Bassett and Liu 2014; Moreno-Mateos et al. 2015).
Having demonstrated the utility of the CRISPR/Cas9 system for site-specific mutagenesis of Anopheles, a logical next step is to systematically determine the efficiency of the system for integrating variously sized constructs into anopheline genomes via HDR. The ability to conduct efficient deletion and addition of known sequences at specific genomic positions will greatly speed progress toward genetic methods, such as gene drive, for the control of malaria vectors.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.1134/-/DC1.
Acknowledgments
We thank Timothy Lo for help with injections and the Malaria Research and Reference Reagent Resource Center (part of the Biodefense and Emerging Infections Research Resources Repository) for providing mosquito eggs to start colonies. Funding was provided by National Institutes of Health (NIH) grants 1R01 AI-113248 and 1R21 AI-115271 to B.J.W.; and NIH grants 5K22 AI-113060 and 1R21 AI-123937, and Defense Advanced Research Project Agency Safe Genes Program grant HR0011-17-2-0047 to O.S.A.
Author contributions: M.L. and B.J.W conceived, designed, and performed experiments. M.L., O.S.A., and B.J.W analyzed data and wrote the article.
Footnotes
Communicating editor: R. Kulathinal
Literature Cited
- Adelman Z., Akbari O., Bauer J., Bier E., Bloss C., et al. , 2017. Rules of the road for insect gene drive research and testing. Nat. Biotechnol. 35: 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari O. S., Bellen H. J., Bier E., Bullock S. L., Burt A., et al. , 2015. Safeguarding gene drive experiments in the laboratory. Science 349: 927–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S., Kweon J., Kim H. S., Kim J. S., 2014. Microhomology-based choice of Cas9 nuclease target sites. Nat. Methods 11: 705–706. [DOI] [PubMed] [Google Scholar]
- Barrangou R., Doudna J. A., 2016. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 34: 933–941. [DOI] [PubMed] [Google Scholar]
- Bassett A. R., Liu J.-L., 2014. CRISPR/Cas9 and genome editing in Drosophila. J. Genet. Genomics 41: 7–19. [DOI] [PubMed] [Google Scholar]
- Bassett A. R., Tibbit C., Ponting C. P., Liu J.-L., 2013. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4: 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Aryan A., Overcash J. M., Samuel G. H., Anderson M. A. E., et al. , 2015. Silencing of end-joining repair for efficient site-specific gene insertion after TALEN/CRISPR mutagenesis in Aedes aegypti. Proc. Natl. Acad. Sci. USA 112: 4038–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict M. Q., Besansky N. J., Chang H., Mukabayire O., Collins F. H., 1996. Mutations in the Anopheles gambiae pink-eye and white genes define distinct, tightly linked eye-color loci. J. Hered. 87: 48–53. [Google Scholar]
- Besansky N. J., Bedell J. A., Benedict M. Q., Mukabayire O., Hilfiker D., et al. , 1995. Cloning and characterization of the white gene from Anopheles gambiae. Insect Mol. Biol. 4: 217–231. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Weiss D. J., Cameron E., Bisanzio D., Mappin B., et al. , 2015. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballar-Lejarazú R., Jasinskiene N., James A. A., 2013. Exogenous gypsy insulator sequences modulate transgene expression in the malaria vector mosquito, Anopheles stephensi. Proc. Natl. Acad. Sci. USA 110: 7176–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J., Buchman A., Akbari O. S., 2016. Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat. Rev. Genet. 17: 146–159. [DOI] [PubMed] [Google Scholar]
- David J.-P., Strode C., Vontas J., Nikou D., Vaughan A., et al. , 2005. The Anopheles gambiae detoxification chip: a highly specific microarray to study metabolic-based insecticide resistance in malaria vectors. Proc. Natl. Acad. Sci. USA 102: 4080–4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doench J. G., Hartenian E., Graham D. B., Tothova Z., Hegde M., et al. , 2014. Rational design of highly active sgRNAs for CRISPR-Cas9-mediated gene inactivation. Nat. Biotechnol. 32: 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S., Lin J., Held N. L., Clem R. J., Passarelli A. L., et al. , 2015. Heritable CRISPR/Cas9-mediated genome editing in the yellow fever mosquito, Aedes aegypti. PLoS One 10: e0122353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edi C. V., Djogbénou L., Jenkins A. M., Regna K., Muskavitch M. A. T., et al. , 2014. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 10: e1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizi R., Hammond A., Kyrou K., Taxiarchi C., Bernardini F., et al. , 2016. A CRISPR-Cas9 sex-ratio distortion system for genetic control. Sci. Rep. 6: 31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz V. M., Jasinskiene N., Tatarenkova O., Fazekas A., Macias V. M., et al. , 2015. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 112: E6736–E6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Calderón G. I., Emrich S. J., MacCallum R. M., Maslen G., Dialynas E., et al. , 2015. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 43: D707–D713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokcezade J., Sienski G., Duchek P., 2014. Efficient CRISPR/Cas9 plasmids for rapid and versatile genome editing in Drosophila. G3 Bethesda 4: 2279–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman G. L., Rafferty C. S., Clayton J. R., Stevens T. K., Mukabayire O., et al. , 2001. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Mol. Biol. 10: 597–604. [DOI] [PubMed] [Google Scholar]
- Hall A. B., Basu S., Jiang X., Qi Y., Timoshevskiy V. A., et al. , 2015. A male-determining factor in the mosquito Aedes aegypti. Science 348: 1268–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A., Galizi R., Kyrou K., Simoni A., Siniscalchi C., et al. , 2016. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34: 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. A., Subramanian G. M., Halpern A., Sutton G. G., Charlab R., et al. , 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298: 129–149. [DOI] [PubMed] [Google Scholar]
- Hsu P. D., Lander E. S., Zhang F., 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler K. E., Vosshall L. B., Matthews B. J., 2015. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 11: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuscu C., Arslan S., Singh R., Thorpe J., Adli M., 2014. Genome-wide analysis reveals characteristics of off-target sites bound by the Cas9 endonuclease. Nat. Biotechnol. 32: 677–683. [DOI] [PubMed] [Google Scholar]
- Labun K., Montague T. G., Gagnon J. A., Thyme S. B., Valen E., 2016. CHOPCHOP v2: a web tool for the next generation of CRISPR genome engineering. Nucleic Acids Res. 44: W272–W276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Bui M., Akbari O., 2017a. Embryo Microinjection and Transplantation Technique for Nasonia vitripennis Genome Manipulation. J. Vis. Exp. DOI:10.3791/56990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Bui M., Yang T., Bowman C. S., White B.J., et al. , 2017b. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, Aedes aegypti. Proceedings of the National Academy of Sciences 114. (49):E10540–E10549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Au L. Y. C., Douglah D., Chong A., White B. J., et al. , 2017. Generation of heritable germline mutations in the jewel wasp Nasonia vitripennis using CRISPR/Cas9. Sci. Rep. 7: 901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Dougan S. K., Truttmann M. C., Bilate A. M., Ingram J. R., et al. , 2015. Increasing the efficiency of precise genome editing with CRISPR-Cas9 by inhibition of nonhomologous end joining. Nat. Biotechnol. 33: 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason G. F., 1967. Genetic studies on mutations in species A and B of the Anopheles gambiae complex. Genet. Res. 10: 205–217. [DOI] [PubMed] [Google Scholar]
- Marshall J. M., Buchman A., Akbari O. S., 2017. Overcoming evolved resistance to population-suppressing homing-based gene drives. Scientific reports 7: 3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Akbari O. S., 2015. Gene drive strategies for population replacement, pp. 169–200 in Genetic Control of Malaria and Dengue Academic Press. [Google Scholar]
- Meredith J. M., Basu S., Nimmo D. D., Larget-Thiery I., Warr E. L., et al. , 2011. Site-specific integration and expression of an anti-malarial gene in transgenic Anopheles gambiae significantly reduces Plasmodium infections. PLoS One 6: e14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Mateos M. A., Vejnar C. E., Beaudoin J.-D., Fernandez J. P., Mis E. K., et al. , 2015. CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 12: 982–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey D. E., Christophides G. K., Collins F. H., Emrich S. J., Fontaine M. C., et al. , 2013. The evolution of the Anopheles 16 genomes project. G3 (Bethesda) 3: 1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan T., Bower T. M., Brown A. E., Crisanti A., Catteruccia F., 2002. piggyBac-mediated germline transformation of the malaria mosquito Anopheles stephensi using the red fluorescent protein dsRED as a selectable marker. J. Biol. Chem. 277: 8759–8762. [DOI] [PubMed] [Google Scholar]
- Perera O. P., Harrell R. A., II, Handler A. M., 2002. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol. Biol. 11: 291–297. [DOI] [PubMed] [Google Scholar]
- Pondeville E., Puchot N., Meredith J. M., Lynd A., Vernick K. D., et al. , 2014. Efficient ΦC31 integrase–mediated site-specific germline transformation of Anopheles gambiae. Nat. Protoc. 9: 1698–1712. [DOI] [PubMed] [Google Scholar]
- Port F., Chen H.-M., Lee T., Bullock S. L., 2014. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc. Natl. Acad. Sci. USA 111: E2967–E2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H., Lissenden N., 2016. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32: 187–196. [DOI] [PubMed] [Google Scholar]
- Sharma A., Heinze S. D., Wu Y., Kohlbrenner T., Morilla I., et al. , 2017. Male sex in houseflies is determined by Mdmd, a paralog of the generic splice factor gene CWC22. Science 356: 642–645. [DOI] [PubMed] [Google Scholar]
- Smidler A. L., Terenzi O., Soichot J., Levashina E. A., Marois E., 2013. Targeted mutagenesis in the malaria mosquito using TALE nucleases. PLoS One 8: e74511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sougoufara S., Diédhiou S. M., Doucouré S., Diagne N., Sembène P. M., et al. , 2014. Biting by Anopheles funestus in broad daylight after use of long-lasting insecticidal nets: a new challenge to malaria elimination. Malar. J. 13: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staahl B. T., Benekareddy M., Coulon-Bainier C., Banfal A. A., Floor S. N., et al. , 2017. Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat. Biotechnol. 35: 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang Z.-T., Seo S.-O., Choi K., Lu T., et al. , 2015. Markerless chromosomal gene deletion in Clostridium beijerinckii using CRISPR/Cas9 system. J. Biotechnol. 200: 1–5. [DOI] [PubMed] [Google Scholar]
- White B. J., Collins F. H., Besansky N. J., 2011. Evolution of Anopheles gambiae in relation to humans and malaria. Annu. Rev. Ecol. Evol. Syst. 42: 111–132. [Google Scholar]
- Windbichler N., Papathanos P. A., Catteruccia F., Ranson H., Burt A., et al. , 2007. Homing endonuclease mediated gene targeting in Anopheles gambiae cells and embryos. Nucleic Acids Res. 35: 5922–5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Shen B., Zhang C., Huang X., Zhang Y., 2014. sgRNAcas9: a software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PLoS One 9: e100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genomic DNA from mosquito strains produced here will be made available upon request.