Abstract
Genetic improvement toward optimized and stable agronomic performance of soybean genotypes is desirable for food security. Understanding how genotypes perform in different environmental conditions helps breeders develop sustainable cultivars adapted to target regions. Complex traits of importance are known to be controlled by a large number of genomic regions with small effects whose magnitude and direction are modulated by environmental factors. Knowledge of the constraints and undesirable effects resulting from genotype by environmental interactions is a key objective in improving selection procedures in soybean breeding programs. In this study, the genetic basis of soybean grain yield responsiveness to environmental factors was examined in a large soybean nested association population. For this, a genome-wide association to performance stability estimates generated from a Finlay-Wilkinson analysis and the inclusion of the interaction between marker genotypes and environmental factors was implemented. Genomic footprints were investigated by analysis and meta-analysis using a recently published multiparent model. Results indicated that specific soybean genomic regions were associated with stability, and that multiplicative interactions were present between environments and genetic background. Seven genomic regions in six chromosomes were identified as being associated with genotype-by-environment interactions. This study provides insight into genomic assisted breeding aimed at achieving a more stable agronomic performance of soybean, and documented opportunities to exploit genomic regions that were specifically associated with interactions involving environments and subpopulations.
Keywords: Finlay-Wilkinson index, GGE, association mapping, meta-analysis, SoyNAM, Multiparent Advanced Generation Inter-Cross (MAGIC), multiparental populations, MPP
One of the objectives of plant breeders is to develop cultivars that are high yielding across extensive range of environmental conditions. However, the presence of genotype-by-environment interactions (GEI) (Crossa 1990; Kang 1997; Zobel et al. 1988) might complicate this labor. For example, the GEI of a cross-over type causes changes in ranking performance across environments, complicating the breeder’s task of selecting best candidate parents for next improvement cycle, and/or what to release as new cultivars for a given area or large region.
When significant, GEI has an important role in accounting for the phenotypic variation of quantitative traits and can be accommodated in statistical models designed for multi-environmental trials (Cooper et al. 1996). Stable genotypic performance is highly desirable in improved cultivars, which is important for food security and industrial uses (Boyer et al. 2013). Yield stability is defined as the ability of a genotype to avoid significant fluctuation in yield over a range of environmental conditions (Heinrich et al. 1983). However, responsiveness to advances in the agronomic improvement of a production environment is also an important aspect in breeding, which describes the cultivar’s ability to react to the change in the environmental conditions.
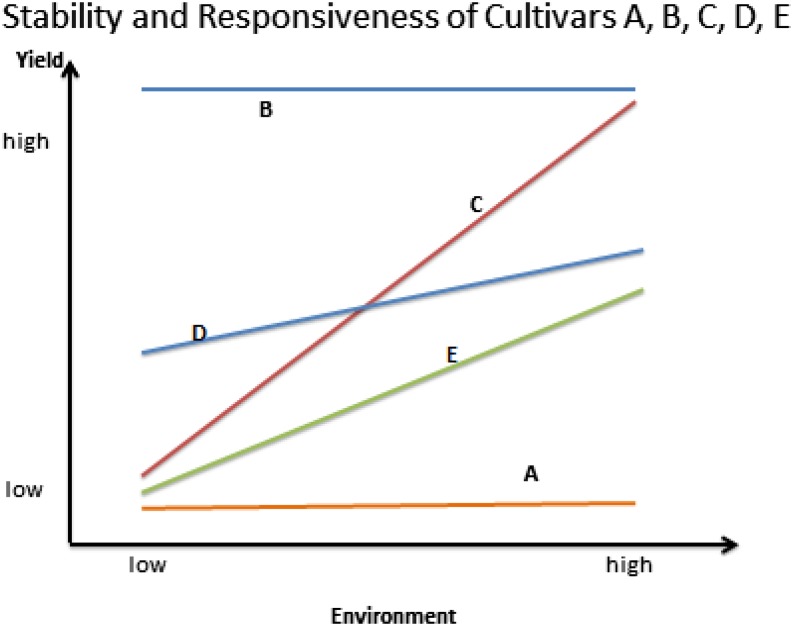
The difference between stability and responsiveness can be graphically conceptualized (Figure 1). Cultivar A is stable in environments of low and high agronomic productivity, but that stability comes at the cost of not responding to agronomic improvement. Cultivar B is also stable, and yields higher in both low and high environments than Cultivar A. Cultivar A and B represent an unrealistic situation in a context that involves consideration of genotypic yield response to a range of low-to-high yield environments because varieties do not perform exactly the same in terms of yield when the environment changes significantly. Cultivar C is responsive to improvement in the environmental productivity. With respect to just A and B, their GEI is a noncross-over type (i.e., B is always superior to A in both environments and thus rank per se selection will be successful). Cultivar D is higher yielding than either C or E when environmental productivity is low, but is lower yielding than C when productivity is high. In this instance, the greater stability of cultivar D (with less change in yield) vis-à-vis the greater responsivity of cultivar C results in a cross-over type of GEI (i.e., an inverted performance rank of C and D), thus making the choice of the best cultivar dependent upon the productivity of the targeted environment. Clearly, when cultivars C and D are compared, cultivar D is favored in environments of low productivity, but cultivar C is favored in environments of high productivity. When cultivars C and E are compared, they both respond to the environmental change but cultivar C is superior to cultivar E in both low and high environments.
Figure 1.
A graphical depiction of the difference between static and dynamic stability; showing the stability and responsiveness of Cultivars A, B, C, D, and E.
Becker and Leon (1988) classified stability as either static or dynamic. Static stability would be typified by a cultivar with a low yield variance (compared to other cultivars) over a range of low to high environmental productivities (i.e., cultivar A in Figure 1). Dynamic stability was characterized as a cultivar whose performance, when regressed across a low to high environmental productivity range, mirrors the overall mean regression performance of all cultivars in the same trial.
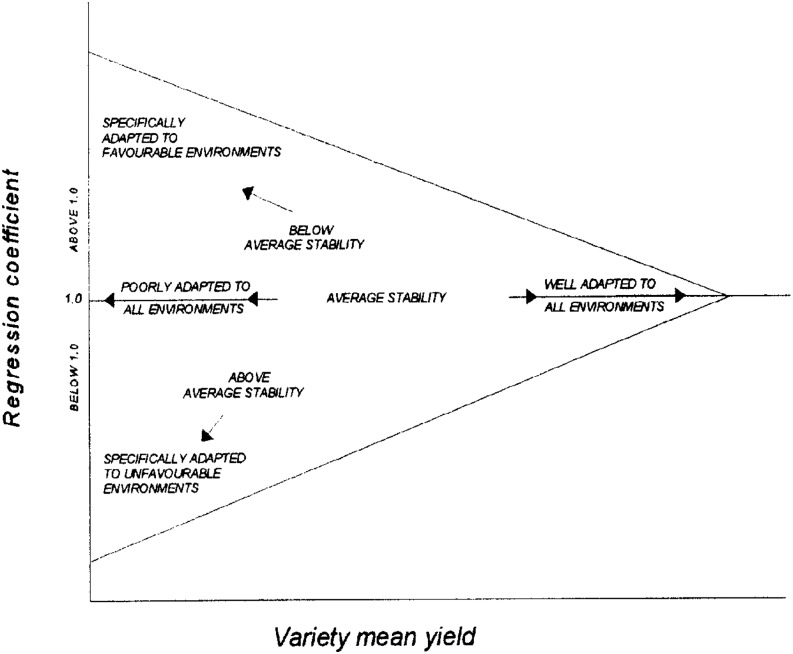
Many breeders have used the Finlay and Wilkinson (1963) approach of joint linear regression analysis to assess GEI, by plotting the individual genotypic regression coefficients (i.e., genotypic response to a linear array of environmental productivities) against the genotypic means over all environments to interpret the results (Figure 2). Genotypes with more “stability” have regression coefficients of less than unity, which is consistent with these genotypes performing well in low productivity environments, but also performing poorly in high productivity environments. Genotypes with regression coefficients >1 are more sensitive to environmental changes, thus the environmental conditions have a greater influence on their performance than the genotypes that have regression coefficients closer to zero.
Figure 2.
A generic depiction of FW genotype regression coefficients vs. genotypic means (from Finlay and Wilkinson 1963).
Easy access to molecular tools in the “omic” era has opened a new horizon to understand and exploit GEIs at the genomic level (Deshmukh et al. 2014; Cuevas et al. 2017). There is now a growing interest in investigating the genetic basis of GEI at a quantitative trait locus (QTL) level, allowing marker-assisted selection to be brought to the fore in terms of manipulating trait stability or responsiveness to environmental stimuli in breeding programs (Des Marais et al. 2013; Malosetti et al. 2013).
The behavior of cultivars in distinct environments is of special interest in breeding efforts targeting complex traits, such as grain yield, which are controlled by a large number of alleles, mostly presenting small effects, but which are very responsive to the environment (Des Marais et al. 2013; Xavier et al. 2016). The development of large experimental multiparental panels, known as next-generation populations (Morrell et al. 2012), has made studies of complex traits feasible, as it provides sufficient power and resolution to detect genomic associations for complex traits. Nested Association Mapping (NAM) populations are a special kind of multiparental panel, characterized by multiple biparental crosses that share a common parent.
The NAM structure enabled maize researchers to identify QTL for agriculturally important traits with high statistical power and mapping resolution (Yu et al. 2008). Such genetic resource, along with enough phenotypic data, can enable scientists to address questions about the genomic origins of genotype by environment interactions, and, in combination with environmental information yield stability, can be addressed for the genotypes. The first NAM population was developed in maize, and originated from 25 diverse maize lines mated to the inbred line B73, followed by selfing the F1 plants for six generations to generate 200 recombinant inbred lines (RILs) per mating (i.e., family) totaling to 5000 individuals in the maize NAM population, which then was used in multi-environmental scenarios for genomic prediction purposes (Guo et al. 2013) to identify GEI.
Another NAM population was created in soybean to take advantage of historic and more recent recombination in the examination of the genetic architecture of complex traits, and the influence of environmental variation with high statistical resolution power. In this study, the main objective was to perform a genome-wide association study (GWAS) involving the soybean NAM population to identify genomic regions that not only influenced grain yield across all environments, but also regions that influenced GEI.
Two standard analysis methods that were previously used to investigate connections between genetics and the environment in soybeans (Zhe et al. 2010) were herein adapted to conduct a whole-genome analysis: (a) a regression-based stability index (Malosetti et al. 2013), and (b) the principal components-based interaction (Malosetti et al. 2013). In the regression-based stability index, the regression coefficient of a linear regression model describes the genotype across different environments, and the deviation from the estimated regression describes the consistency of the genotype. When the regression coefficient is zero, the genotype is considered to be dynamically stable. The principal-component-based interactions can be evaluated via Genotype and Genotype-Environment (GGE) biplot analysis where the GEI can be accessed visually, and the stability of the genotypes and environments can be evaluated.
The stability testing and GEI testing were performed using the soybean NAM population. In this paper, we first describe the pedigree structure of the soybean NAM population, how the phenotypic data were collected in the multiple environments, and how the data were utilized for our analyses. Genome-wide associations were conducted using the Finlay-Wilkinson stability (FW) index (Finlay and Wilkinson 1963) to evaluate the dynamic stability. The GEI analysis was conducted via meta-analysis using genomic markers and phenotypic values from the multiple environments. Six QTL associated with responsiveness of grain yield to environmental factors and one QTL linked to grain yield stability were identified.
Materials and Methods
SoyNAM structure
This study was conducted using a soybean nested association mapping population named SoyNAM, which, as of now, is the largest published set of experimental plant population designed for genetic mapping, comprising 5600 recombinant inbred lines (RILs). The SoyNAM was developed by creating 40 biparental matings that involved one common high yielding parent (IA3023) mated to 40 unique parents (Table 1). Note that the 17 NAM parents were high-yielding cultivars or elite breeding lines nominated by eight state breeders, 15 NAM parents had diverse ancestry and originated from R. Nelson’s Agricultural Research Service-United States Department of Agriculture (ARS-USDA) program at University of Illinois, and 8 NAM parents were nominated by J. Specht at University of Nebraska-Lincoln, because of their high-yielding performance in drought conditions. For more descriptive information about the soybean NAM population, including midsummer and mature photos of the NAM parents, the reader can refer to https://www.soybase.org/SoyNAM/.
Table 1. The set of parental lines that were used to cross with the common parent, IA3023, and the corresponding SoyNAM family code.
| Elite Line | Family | Diverse Line | Family | PI Lines | Family |
|---|---|---|---|---|---|
| TN05-3027 | NAM 2 | LG03-2979 | NAM24 | PI-398881 | NAM40 |
| 4J105-3-4 | NAM 3 | LG03-3191 | NAM25 | PI-427136 | NAM41 |
| 5M20-2-5-2 | NAM 4 | LG04-4717 | NAM26 | PI-437169B | NAM42 |
| CL0J095-4-6 | NAM 5 | LG05-4292 | NAM27 | PI-507681B | NAM46 |
| CL0J173-6-8 | NAM 6 | LG05-4317 | NAM28 | PI-518751 | NAM48 |
| HS6-3976 | NAM 8 | LG05-4464 | NAM29 | PI-561370 | NAM50 |
| Prohio | NAM 9 | LG05-4832 | NAM30 | PI-404188A | NAM54 |
| LD00-3309 | NAM10 | LG90-2550 | NAM31 | PI-574486 | NAM64 |
| LD01-5907 | NAM11 | LG92-1255 | NAM32 | ||
| LD02-4485 | NAM12 | LG94-1128 | NAM33 | ||
| LD02-9050 | NAM13 | LG94-1906 | NAM34 | ||
| Magellan | NAM14 | LG97-7012 | NAM36 | ||
| Maverick | NAM15 | LG98-1605 | NAM37 | ||
| S06-13640 | NAM17 | LG00-3372 | NAM38 | ||
| NE3001 | NAM18 | LG04-6000 | NAM39 | ||
| Skylla | NAM22 | ||||
| U03-100612 | NAM23 |
The first two columns of the table represent the name and the family designation of the elite lines, the third and fourth columns are for the genetically diverse lines, and the last two columns are for the plant introduction lines.
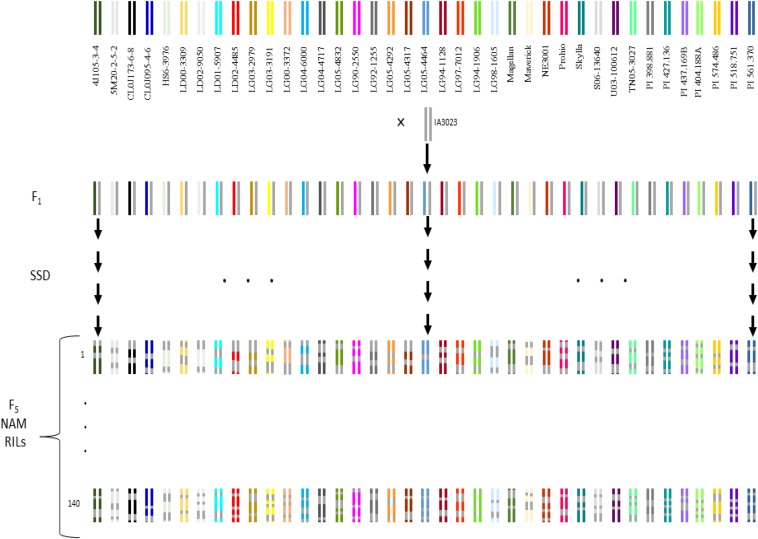
The development of the SoyNAM population (Song et al. 2017) was similar to the maize NAM population (Figure 3). Each of the 40 biparental matings resulted in 140 F5 derived RILs, derived from F2 plants via single-seed descent, and thus totaled to 5600 RILs. Some RILs had to be dropped due to mislabeling, and other generation advance errors, and one family was dropped because of uncertainty relative to the parental ancestry. The final NAM subset utilized for this investigation consisted of 39 families and 5143 RILs.
Figure 3.
Schematic diagram of the development of the SoyNAM.
The RILs were planted at nine locations during the years of 2011, 2012, and 2013, but data were collected on only 18 unique environments (location–year) combinations. Phenotypic data were collected on grain yield (in kg/ha−1), days to maturity, plant height (in centimeter), lodging (score 1–5), seed size (mass of 100 seed in grams), plus seed composition (in % in the grain) of protein, oil, and fiber content. For this study, however, the focus was on grain yield adjusted to 13% moisture.
The entire set of RILs were grown at four locations in Nebraska, Iowa, Illinois, and Indiana in 2012 and 2013, but the 2013 Nebraska trial was unfortunately lost due to a hail storm. Only partial RIL sets were grown at other locations in one or more of the 3 yr. The number of RILs grown in the 18 environments was somewhat variable due to some missing plots (Table 2). The locations in Ohio were divided into two regions; the South Charleston area and the Wooster area. A considerable number of check cultivars (i.e., parents, high yield checks, and maturity checks) were grown in each trial environment to assess field variation (Table 3).
Table 2. Number of SoyNAM RILs with nonmissing plot data in each environment.
| Iowa | Illinois | Indiana | Kansas | Michigan | Missouri | Nebraska | Ohio1 | Ohio2 | |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | — | 2500 | — | — | — | — | 2500 | — | — |
| 2012 | 5111 | 5138 | 5041 | 3158 | 816 | 819 | 5127 | 1606 | 1626 |
| 2013 | 5100 | 5137 | 5136 | 3230 | — | 804 | — | 1619 | 1571 |
Table 3. Number of check cultivars in each environment.
| Iowa | Illinois | Indiana | Kansas | Michigan | Missouri | Nebraska | Ohio1 | Ohio2 | |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | — | 419 | — | — | — | — | 419 | — | — |
| 2012 | 825 | 825 | 825 | 525 | 125 | 125 | 825 | 250 | 253 |
| 2013 | 825 | 825 | 825 | 510 | — | 137 | — | 253 | 262 |
The experimental units were two-row plots (2.9 × 0.76 m), seeded at a density of ca. 36 plants/m2, arranged in a modified augmented design (Lin and Poushinsky 1983) without replication within each test environment. Yields were restricted by drought conditions during 2012 (Rippey 2015) although plots were always irrigated at Nebraska and Kansas.
The average grain yield of the RILs in the 18 environments ranged from a low of 2364 kg ha−1 in 2012 in Michigan to respective highs of 5057 and 5050 kg ha−1 in 2011 in Nebraska and 2013 in Indiana (Table 4).
Table 4. Average grain yield (kg ha−1) of SoyNAM RILs observed in each environment.
| Iowa | Illinois | Indiana | Kansas | Michigan | Missouri | Nebraska | Ohio1 | Ohio2 | |
|---|---|---|---|---|---|---|---|---|---|
| 2011 | — | 2780 | — | — | — | — | 5057 | — | — |
| 2012 | 2776 | 3386 | 4231 | 3871 | 2364 | 3414 | 4728 | 3394 | 2823 |
| 2013 | 2871 | 3115 | 5050 | 2747 | — | 4091 | — | 3629 | 4420 |
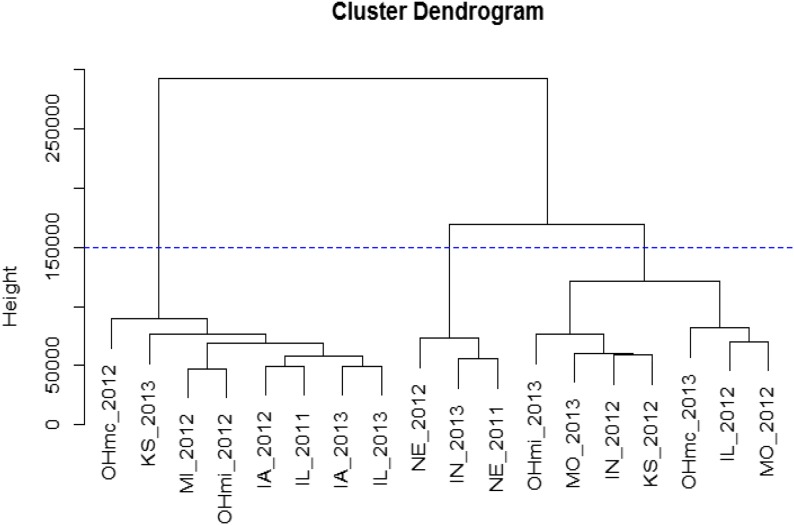
The yield data collected from the 18 environments were used to identify clusters of similarity among the environments (Figure 4). The cluster dendogram was created using a hierarchical clustering algorithm hclust implemented in R (R Core Team 2017) with the Ward-D agglomeration method using the Euclidean distance. The OHmc location represents the South Charleston, Ohio area, which was obtained by L.M., and the OHmi location represents the Wooster, Ohio area which was the responsibility of R.M. The eight site-year trials in the leftmost cluster were the distinctively lower yielding (i.e., <3400 kg ha−1) than the three rightmost clusters. Of the latter, the central cluster with the three site-year trials of NE 2011 and 2012 and Indiana 2013 was the highest yielding (i.e., >4700 kg ha−1), and was distinctly different from the other two clusters of four and three site trials (i.e., yield range of 3386–4420 kg ha−1).
Figure 4.
Hierarchical clustering of SoyNAM environments based on grain yield data based Ward D method and Euclidean distance.
Genotyping
Parental lines were sequenced to derive the SNP allele calls. A total of 5303 SNP loci were selected with the criterion of maximizing the number of families segregating for those loci. The SNPs were used to build the SoyNAM 6K BreadChip SNP array using the Illumina Infinium HD Assay platform (Illumina, Inc.) (Song et al. 2017). Among those SNPs, a subset of 4312 markers were selected by the SoyNAM group as quality-assured based on proportion of missing loci and correct segregation patterns. Both raw and quality assured genotypes are available in the R package SoyNAM. The missing genotypes were imputed using a random forest algorithm (Stekhoven and Bühlmann 2012).
The statistical model designed for association in multiple populations (Wei and Xu 2016) requires the markers to represent the parental sources, so we proceeded as follows: SNPs were recoded to express the allele of origin, where 0 represents homozygous for the founder parent, 1 as heterozygous, and 2 homozygous for the common parent IA3023. Using the allele and family information, the R package NAM (Xavier et al. 2015) recoded each locus into an incidence vector indicating the parental origin of the alleles, in other words, as the interaction marker-by-family, hence allowing markers to present different effects across families.
Association analysis
Genome-wide association analyses were based on the multiparental model proposed by Xavier et al. (2015) and implemented for NAM populations. A detailed theoretical description is presented by Wei and Xu (2016), who extended the methodology to multiparent advanced generation intercross-population (MAGIC) populations. The following mixed linear model describes how the association analyses were performed:
| (1) |
where y is the vector of phenotypes, X is the design matrix of fixed effects allocating the intercept and block effect estimated from checks, β is the fixed effect coefficients, Z is the incidence matrix of the marker data, α is the vector of regression coefficients associated with the haplotypes, ψ corresponds to the polygenic coefficients, and ε is the vector of residuals. The model assumes that α ∼ N(0, ), ∼ N(0, ) and ∼ N(0, ), where is the genetic variance associated with the haplotypes, is the genetic effect associated with the polygenic effects. The population structure is captured by the polygenic term with covariance K, which corresponds to the genomic relationship matrix that represents the genetic similarities among individuals through a linear additive kernel ( Xu 2016).
Statistical significance of single markers was evaluated through the likelihood ratio test (LRT) by comparing the log-likelihood of the model that includes the marker effect () with the log-likelihood of the model that does not (), i.e., the reduced model. represents the null hypothesis, and L1 represents the alternative hypothesis. The LRT (McCulloch and Searle 2001) for comparing the full model (L1) to the reduced model (L0) can be written as
| (2) |
In the random effects model, the LRT follows a mixture of chi-square and binomial distributions (Xavier et al. 2015), with p-values computed using a chi-square distribution with 0.5 degrees of freedom. Multiple test correction was performed via a Bonferroni threshold () to define which markers were significantly associated with the FW index. The Bonferroni threshold for 4312 SNPs is a −log (p-value) value of ∼5.
GEI testing
Association analyses for grain yield were conducted for individual environments one at a time using the described model in (1). Meta-analysis, which combines data from multiple sources for analysis was utilized to infer markers that presented significant GEI from the estimated allele effects when using grain yield as response variable. Meta-analysis was performed with the following model:
| (3) |
where α is the vector of allele effects estimated from the association analysis for individual environments, W is the incidence matrix of parental source (where each column of refers to a parent, each element of is the marker effect of a family in a given environment), is the vector of regression coefficients representing the true marker effects, and is the vector of residuals. The environmental term is not added to the GGE model (Yan et al. 2007). A GGE model is a parametric, multivariate technique used to predict the adaptation and stability of a cultivar (Mortazavian et al. 2014), based on environment-centered principal components (Gauch 2006). Environment-centered principal components refer to the technique in which singular value decomposition (SVD) is applied to the residual matrix resulting from the environmental mean estimation.
The interaction term (γ) in model (4) is defined as a matrix with dimensions corresponding to the number of NAM parents by the number of environments whose entries are comprised with the first component from the single value decomposition of the residuals. The model is
| (4) |
where and are as defined above, γ represents the interaction term, and is the new vector of residuals, without the GE interactions captured by The multiplicative interaction of the family at the environment can be described as the product of the first set of eigenvectors (U and V) and the first eigenvalue (D) from the residual single value decomposition as follows:
| (5) |
Models (3) and (4) were fitted via weighted least squares, where the allele effects were weighted according to the number of individuals observed in each combinations of NAM family and environment. The statistical test is a likelihood ratio, as presented in (2). The model in (3) is assumed to represent the null hypothesis, whereas the model presented in (4) represent the alternative hypothesis. The LRT was assumed to have a chi-square distribution, where the number of degrees of freedom is a function of the number of environments in which the allele was observed and the number of parents segregating for that allele, which represents the genetic component (Gauch 1988). An empirical threshold of 50 [−log(p-value)] was chosen to define significant associations.
Data availability
All data are publically available in the R package SoyNAM. Access with the following command: data (soybase, package=“SoyNAM”). Data formatted for the analysis of the NAM package is available with the following command: SoyNAM::ENV().
Results
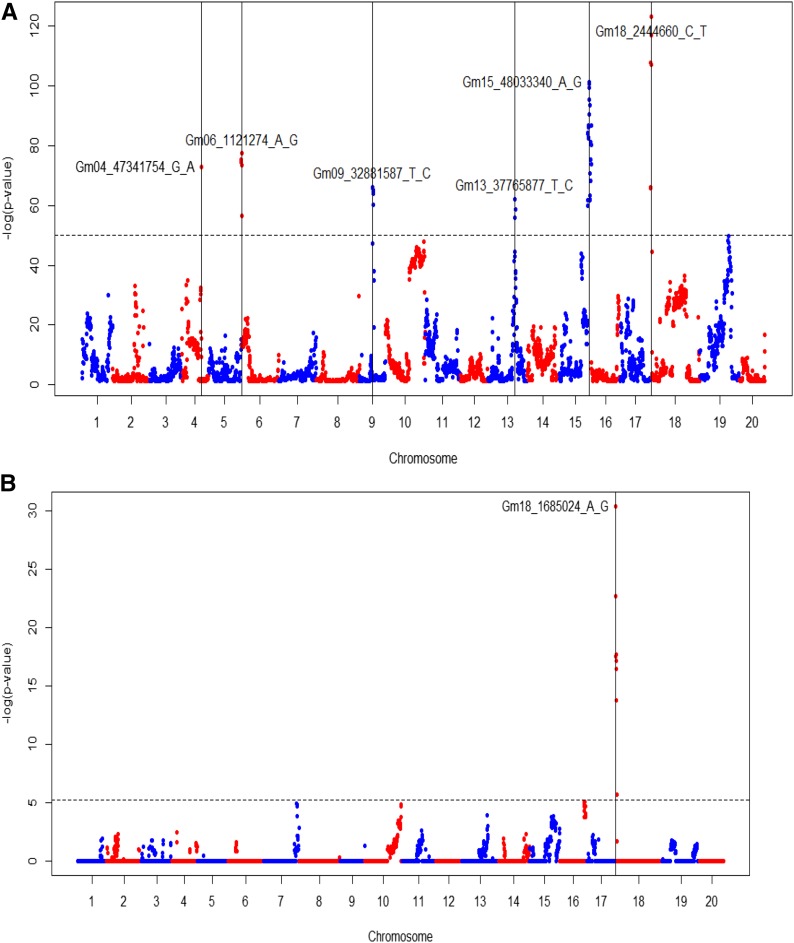
A meta-analysis of GEI identified six statistically significant peaks on chromosomes 4, 6, 9, 13, 15, and 18 (Figure 5A). Among these peaks, the QTL on chromosome 18 also appears to be significantly associated with grain yield stability, as measured with the FW index (Figure 5B).
Figure 5.
Genome-wide associations to environmental responsiveness. (A) Meta-analysis of the additive main effects and multiplicative interactions. (B) Associations with yield stability using the FW index with Bonferroni significance threshold.
The most significant SNPs from each QTL, and the annotations of the nearest gene from SNP physical position, are presented in Table 5. The annotation does not necessarily correspond to candidate gene due to the limitation of dataset, including the large linkage disequilibrium block (Hyten et al. 2006, 2007) and genotyping coverage in the SoyNAM population (Song et al. 2017). However, previous reports were traced in the soybean database “soybase” (Grant et al. 2010) supporting the involvement of these genes.
Table 5. Summary statistics of the significant QTL positions associated with GEI and stability in the SoyNAM data, and annotation from JBrowse Phytozome (Goodstein et al. 2012).
| Marker | Trait | Adj R2 | PC Load | -log(p) | Adj R2 | Nearest Gene | Annotationa |
|---|---|---|---|---|---|---|---|
| Gm04_47341754_G_A | GEI | 0.23 | 72.83 | Glyma.04g200700 | rRNA 2-O-Methyltransferase Fibrillarin | ||
| Gm06_1121274_A_G | GEI | — | 0.30 | 77.40 | — | Glyma.06g014900 | Protein cup-shaped cotyledon |
| Gm09_32881587_T_C | GEI | — | 0.22 | 66.19 | — | Glyma.09g132200 | Beta-carotene 3-Hydroxylase |
| Gm13_37765877_T_C | GEI | — | 0.20 | 62.11 | — | Glyma.13g276100 | VQ motif |
| Gm15_48033340_A_G | GEI | — | 0.24 | 101.38 | — | Glyma.15g252200 | Glutathione S-Transferase |
| Glyma.15g252100 | Gibberellin 2-Beta-Dioxygenase | ||||||
| Gm18_1685024_A_G | GEI | — | 0.35 | 123.12 | — | Glyma.18g127100 | Protein NRT1 |
| Gm18_2444660_C_T | Stability | 0.0126 | — | 30.35 | 0.0126 | Glyma.18g145700 | UDP-glucose 4-epimerase |
Annotation from JBrowse Phytozome (Goodstein et al. 2012).
The chromosome 18 QTL associated with stability explained 1% of the variance of the FW index (Table 5), and its effect on yield and stability is presented in the Appendix. Regarding the QTL peaks of GEI (Table 5), from 20 to 35% of the variation attributed to interactions between family and environment was captured in the first principal component.
Genes surrounding the peaks
The genes potentially linked to the regions associated with GEI, or yield stability, have been reported to be involved in environmental stress tolerance or in biosynthetic pathways that may underlie overtly measurable secondary traits such as yield stability and GEI. Details of each gene are presented below.
The rRNA 2-O-Methyltransferase Fibrillarin gene Glyma.04g200700, which corresponds to the SNP peak on chromosome 4 (Table 5), has been reported to be associated with responsiveness to flooding stress in soybeans (Komatsu et al. 2012; Won Oh et al. 2014). These regions also coincides with the flood stress resistance QTL previously reported by Githiri et al. (2006).
The protein giving rise to a cup-shaped cotyledon trait is an enzyme transcribed by the gene Glyma.06g014900, which has a position that corresponds to the peak on chromosome 6 (Table 5). This gene has been reported to be related to seed protein storage in soybeans (Han et al. 2009), and it was previously reported in the same region through QTL mapping (Ma et al. 2016). In another QTL study, Hwang et al. (2013) reported this region related to ureide content in soybeans, used as an indirect measure of drought resistance.
Beta-carotene 3-hydroxylase is the enzyme transcribed from the gene Glyma.09g132200, which corresponds to the peak on chromosome 9 (Table 5). This protein is associated with enhanced stress tolerance in Arabidopsis (Davison et al. 2002), by preventing photo-oxidative damage caused by excessive light. Genetic signals for resistance to ultraviolet β radiation were previous report in the soybeans chromosome 9 by Shim et al. (2015). β-Carotene hydroxylases have also been associated with nodulation in soybeans (Kim et al. 2013).
Glyma.13g276100, which corresponds to the SNP peak on chromosome 13 (Table 5), produces VQ motif, which has been reported to play an important role in abiotic stress, plant development, and nitrogen metabolism in soybeans (Wang et al. 2014).
The SNP Gm15_48033340_A_G has a genomic position close to two genes: Glyma.15g252200 and Glyma.15g252100 (Table 5). Glyma.15g252200 is transcribed into Glutathione S-Transferase (GST), which plays an important role in soybean development (McGonigle et al. 2000), and is associated with salt and drought tolerance in soybeans (Ji et al. 2010). GST is also related to mycorrhiza symbiosis (Hogekamp and Küster 2013), which may be involved in water and nutrient absorption. The second gene, Glyma.15g252100, is transcribed into Gibberellin 2-Beta-Dioxygenase, an enzyme involved in soybean nodulation (Zhu et al. 2013) and nodule activity (Méndez et al. 2014). The enzyme is also known to interfere with plant growth, root gravity, and salt stress tolerance (Shan et al. 2014).
The SNP Gm15_48033340_A_G also coincide with two QTL previously reported to be associated with resistance to root-knot nematode (Tamulonis et al. 1997) and soybean cyst nematode (Yue et al. 2001).
Two peaks were found on chromosome 18 (Table 5). Glyma.18g127100 (i.e., Nitrogen Transporter – 1, NRT1), which was the gene identified by SNP signal for stability, produces NRT1, which is responsible for the transport of nitrogen metabolites, and it is believed to play a role in the soybean nitrogen uptake (Yokoyama et al. 2001).
Several studies in Arabidopsis, maize and soybean have established correlation between grain yield with nitrogen remobilization and nitrogen storage capacity (Fan et al. 2009). In addition, soil nitrogen changes have been shown to produce improvements in grain yield and Nitrogen Use Efficiency (NUE) (Ma et al. 1999).
Nitrate Transporters such as NRT1 play key roles in nitrogen remobilization, with several members of NRT1 family performing specific roles in nitrogen uptake, efflux, and tissue-to-tissue nitrogen transport (Parker and Newstead 2014; Tsay et al. 1993; Lin et al. 2008). Given its plausible indirect effect on yield, the role of NRT1 in yield stability is not clear, as we do not have data on soil nitrogen level and other confounding factors in the different environments.
Glyma.18g145700, which is the nearest gene from the QTL identified on chromosome 18 for GEI, produces UDP-glucose 4-epimerase (BrUGE). BrUGE appears to be involved in the cell wall carbohydrate partitioning under nitrogen stress in rice (Guevara et al. 2014) and Arabidopsis (Barber et al. 2006). Epimerases have been found to be N-responsive, showing expression levels in N-dependent manner (Guevara et al. 2014). This further hints at the correlation of N-metabolism with yield stability.
Discussion
In yield trials, plant breeders evaluate genotypes in multiple environments to identify the superior ones, but the presence of GEI complicates the attainment of this objective. Ideally, genotypes that perform well across a broad range of environments are desired, though genotypes with specific superior yield responses in the lowest vs. highest ends of the range of environments may require selection of genotypes with specific adaptation (Figure 2). Still, it is important to partition environmental and genetic variability to determine how GEI impacts these variances, and how stable or responsive the selected cultivars are to environmental stimuli.
Abiotic stress
Soybean cultivars that display genetic tolerance to different stresses are more likely to endure suboptimal environmental conditions (Des Marais et al. 2013). The nearest genes colocalized with the QTL peaks were reported in literature to be related to the physiological response of soybeans and other species to various types of stresses. In summary, these genes were reported to play a role in soybean flood, drought and salt tolerance (Chr 4 and 15), tolerance to photo-oxidative stress (Chr 9), plant development, nodulation, and nitrogen metabolism under stress (Chr 9, 13, and 15), and water and nutrient uptake (Chr 15 and 18).
Tolerance to different stresses can frequently be inter-related. For instance, soybean nitrogen fixation is favored under optimal environmental conditions, such that minor abiotic stresses can disrupt the process (Salvagiotti et al. 2008). Water-related stresses inhibit nodulation by restricting photosynthate supply to nodules (Gil-Quintana et al. 2013). However, genotypes with enhanced enzymatic degradation of ureide are drought tolerant by being able to perform nitrogen fixation under stress (Purcell et al. 2000; Sinclair et al. 2007; Ray et al. 2015).
Associations in multiple environments
Genome-wide analysis including interactions between markers and environment has been scarce in plant due to the lack of statistical power (El-Soda et al. 2014). Attempts to identify GEI through genome-wide scans have been performed in barley (Malosetti et al. 2004) and maize (Boer et al. 2007) in a multi-step approach based on regressing the QTL effects on environmental factors to detect interactions. These studies also found few regions significant for GEI, which indicates an oligogenic architecture for interactions where only some genomic regions were responsive to environmental stimuli.
Quantitative nature of secondary traits
Stress-related genes are important when the crop is exposed to harsh environments. Genomic regions that contain these genes are unlikely to show significant association to yield when the phenotypic data were collected from optimal environmental conditions, because the phenotypic variation attributed to these genes is hidden under stress-free seasons (Schlichting and Smith 2002). However, the inverse may also be true, in that productivity-improving genes may not be readily detectable in low productivity environments.
In this context, GEI and stability are secondary traits used to investigate variation of a primary trait of interest—grain yield. Figure 5 indicates that a number of genomic regions were responsive to environmental stimuli. The environment-dependent expression of these regions modulates the phenotype under unfavorable conditions (Smith 1990), resulting in the stress-related genes to play a major role to the stability of grain yield. On another hand, De Bruin and Pedersen (2009) reported that cultivars resistant to soybean cyst nematode were able to maintain good performance standards in low yielding environments, being more stable than their susceptible counterparts.
GEI, stability, and breeding
Breeding soybeans for high yield can be translated into the development of cultivar able to not only thrive under adverse farming conditions, but also have the ability to be responsive to optimal management practices (Board and Kahlon 2011; Ramachandra et al. 2015). In other words, breeders desire to increase the genetic yield baseline along with yield potential. For instance, modern soybean cultivars outperform older cultivars while presenting stability index >1 (Rincker et al. 2014). The challenge is breeding for both higher yield performance and stability often presents negative genetic correlation (Rosielle and Hamblin 1981).
In order to achieve an ideotype that is both high yielding and stress tolerant the target environments, it is important to be aware of biotic and abiotic stresses in that region, and which “omic” tools provide the necessary information to address the issue (Mir et al. 2012). Those may include phenomic sensors, genome-wide selection, or the introgression of QTL that confer stability or yield advantage to the specific target environment (van Eeuwijk et al. 2010). In addition, if the genetic progress toward regional ideotypes is slow through conventional methods, or if the exiting genetic resources are scarce, the knowledge of the genes involved in GEI and stability can still provide target regions for genetic engineering (Manavalan et al. 2009).
Interactions between the environment and genetics occur in the genomic regions that respond to the environmental stimuli (Smith 1990). We show here that genomic regions exist with strong GEI signaling (Figure 5). The knowledge of the genes that are possibly involved, their functionality and location allow us to strategize the best ways of making good use of these regions.
Role of NAM
The identification of QTL that modulate grain yield under complex interactions between genomic regions and environmental stimuli can be improved by using multi-parental families with large progeny numbers, which are generated in a NAM project. Aside from the greater statistical power associated with the large number of individuals, the multi-parental model (Wei and Xu 2016) allows for the estimation of SNP effects from each parent–environment combination. Such information is particularly valuable by informing which NAM parent provided the most favorable haplotype in each environment or set of environments, similar to which-who-where analysis (Yan 2016). Such variability of the allele effects across families and environments is particularly important for QTL introgression.
Multi-parental association mapping informs which parental lines display the most optimal haplotype that should be used as QTL donors (Xavier et al. 2015; Wei and Xu 2016). An example is shown in Table 6 and Figure 6 by demonstrating the FW index for 19 different NAM parents. Estimated allele effect of SNP Gm18_1685024_A_G on grain yield is shown in the second column of Table 6 for the 19 NAM parents, and the FW index is shown in the third column of Table 6. The index is a deviation from a regression coefficient showing the contribution of GEI. Since the FW index is low for all of the NAM parents it shows that the effect of this QTL is influenced by the environment, thus showing GEI. This statistical procedure for associations acknowledges the possibility that the same allele may have different effects in different families and environments.
Table 6. Stability peak on chromosome 18 for the NAM parents segregating for the SNP.
| Allele Donor | Yield (kg ha–1) | FW Index |
|---|---|---|
| Intercept | 3663.52 | 0.899 |
| NAM3 | 58.44 | −0.253 |
| NAM4 | −6.96 | 0.236 |
| NAM5 | 20.91 | −0.262 |
| NAM10 | 44.05 | −0.306 |
| NAM11 | 21.54 | −0.042 |
| NAM12 | 8.82 | −0.172 |
| NAM13 | 13.69 | −0.035 |
| NAM14 | −23.89 | 0.057 |
| NAM15 | 2.94 | −0.289 |
| NAM18 | −5.21 | 0.240 |
| NAM27 | 72.91 | −0.267 |
| NAM28 | −15.95 | 0.233 |
| NAM33 | −42.27 | 0.045 |
| NAM36 | −11.05 | 0.185 |
| NAM39 | −28.09 | 0.133 |
| NAM48 | −27.23 | 0.132 |
| NAM50 | −42.17 | 0.076 |
| NAM64 | −44.43 | 0.064 |
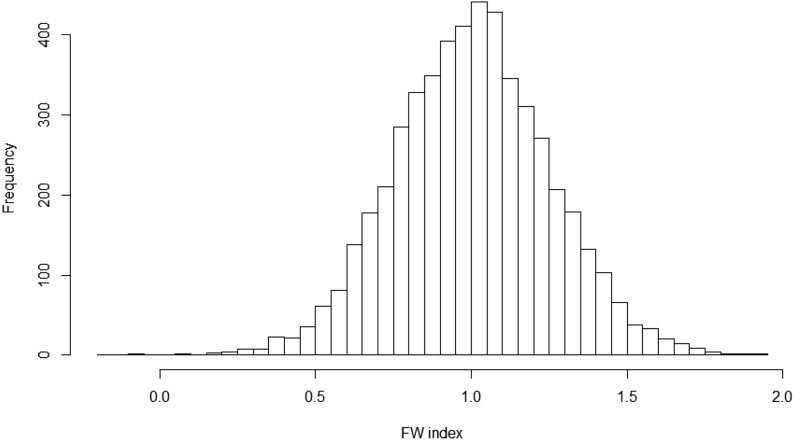
The allele effect of Gm18_1685024_A_G on grain yield and upon stability expressed by the FW index.
Figure 6.
Histogram of FW index of grain yield stability.
The distribution of the FW index of grain yield stability is normal with the center ∼1 (shown in Figure 6) demonstrating that most of the positions have no GEI effect on the grain yield. However, the tails of the distribution confirm the existence of GEI in the 19 NAM parents for grain yield stability.
The interaction between family and QTL can be observed when, for instance, the linkage phase between the SNP marker and the QTL is in coupling in some families and repulsion in others (Lynch and Walsh 1998). Another possibility is the interaction between the QTL and the genetic background (Liao et al. 2001), which may have implications in the direction and magnitude of allele effects in different families. These scenarios can be allied to an additional order of interactions: between QTL and environment (Wang et al. 1999; Xing et al. 2002; van Eeuwijk et al. 2010).
On another hand, if the panel has a sufficiently dense coverage, noninteractive association techniques can be applied in NAM populations to search for “universal” QTL (Palomeque et al. 2010) and causative quantitative trait nucleotides, thus alleles with stable performance across families or environments. Tian et al. (2011) pinpointed a few genes associated with leaf architecture in maize using a NAM population with high-density genotyping. For the SoyNAM, such approach can be enabled by the imputation or projection of the current SNP panel of 4323 SNPs into a higher-density platform (Howie et al. 2011).
Conclusion
Stability analysis can aid plant breeders in the selection procedure, and give cultivar recommendations. Identifying the chromosome regions that influence stability can further enhance the selection process. In this study we evaluated the multi-parental population referred to as the SoyNAM population, and conducted genome-wide stability analysis with the FW index as the phenotype, and genome-wide meta-analysis based on the AMMI analysis estimates of the GEI component. We identified six chromosomal regions that were responsible for the GEI, and we found that one of these chromosomal regions was also associated with yield stability.
The success of stability analysis, and the proportion of the phenotypic variability explained by GEI, can be influenced by genotypes and the population structure. In this study, we evaluated a family structure in soybean consisting one common parent crossed with 39 different parents. Stability analysis was performed using a nested family structure in multiple environments, which led to the identification of chromosome segments that are associated with yield stability and GEI.
We envision future direction of this research as the investigation of grain yield stability conditional to different environmental representation: genome-wide prediction studies of yield performance is needed along with enviro-typing (Xu 2016), targeting the soybean genomic assisted breeding research through transdisciplinary investigations involving genetics and precision agriculture. The representation of management and environmental parameters in continuous scale (Lee et al. 2016) could represent a potential emerging area for genomic studies of GEI and stability in soybeans.
Acknowledgments
We thank Ben Hall for providing Figure 3. We also thank the United Soybean Board for funding the SoyNAM project.
Author contributions: A.X., D.J., and R.H. prepared the manuscript. A.X. wrote the article with significant contributions from R.H., V.R., and D.J. A.X. ran the analysis. B.W.D., R.N., and J.E.S. selected the parental germplasm. J.E.S. and B.W.D. developed the populations. Q.S. and P.B.C. genotyped the lines. W.D.B. developed the experimental design and contributed to the analysis. A.X., W.M.M., and S.X. developed the genome-wide association study analysis of stability and meta-analysis of interactions. J.E.S., G.L.G., B.W.D., R.N., R.M., J.G.S., L.M., D.W., W.S., and K.M.R. collected the phenotypic data. D.J., R.H., and A.J.L. reviewed the statistical analysis. K.M.R., B.W.D., J.E.S., and W.D.B. led the article development.
Footnotes
Communicating editor: D. J. de Koning
Literature Cited
- Barber C., Rösti J., Rawat A., Findlay K., Roberts K., et al. , 2006. Distinct properties of the five UDP-D-glucose/UDP-D-galactose 4-epimerase isoforms of Arabidopsis thaliana. J. Biol. Chem. 281: 17276–17285. [DOI] [PubMed] [Google Scholar]
- Becker H. C., Leon J., 1988. Stability analysis in plant breeding. Plant Breed. 101: 1–23. [Google Scholar]
- Board J. E., Kahlon C. S., 2011. Soybean yield formation: what controls it and how it can be improved, in Soybean Physiology and Biochemistry. INTECH Open Access Publisher, Rijeka, Croatia. [Google Scholar]
- Boer M. P., Wright D., Feng L., Podlich D. W., Luo L., et al. , 2007. A mixed-model quantitative trait loci (QTL) analysis for multiple-environment trial data using environmental covariables for QTL-by-environment interactions, with an example in maize. Genetics 177: 1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Byrne P., Cassman K. G., Cooper M., Delmer D., et al. , 2013. The US drought of 2012 in perspective: a call to action. Glob. Food Secur. 2: 139–143. [Google Scholar]
- Cooper M., Brennan P. S., Sheppard J. A., 1996. Plant Adaptation and Crop Improvement: A Strategy for Yield Improvement of Wheat Which Accommodates Large Genotype by Environment Interactions. CAB International, Wallingford, Oxfordshire. [Google Scholar]
- Crossa J., 1990. Statistical analyses of multi-location trials. Adv. Agron. 44: 55–86. [Google Scholar]
- Cuevas J., Crossa J., Montesinos-López O. A., Burgueño J., Pérez-Rodríguez P., et al. , 2017. Bayesian genomic prediction with genotype × environment interaction kernel models. G3 7: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison P. A., Hunter C. N., Horton P., 2002. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 418: 203–206. [DOI] [PubMed] [Google Scholar]
- De Bruin J. L., Pedersen P., 2009. Growth, yield, and yield component changes among old and new soybean cultivars. Agron. J. 101: 124–130. [Google Scholar]
- Deshmukh R., Sonah H., Patil G., Chen W., Prince S., et al. , 2014. Integrating omic approaches for abiotic stress tolerance in soybean. Front. Plant Sci. 5: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais D. L., Hernandez K. M., Juenger T. E., 2013. Genotype-by-environment interaction and plasticity: exploring genomic responses of plants to the abiotic environment. Annu. Rev. Ecol. Evol. Syst. 44: 5–29. [Google Scholar]
- El-Soda M., Malosetti M., Zwaan B. J., Koornneef M., Aarts M. G., 2014. Genotype× environment interaction QTL mapping in plants: lessons from Arabidopsis. Trends Plant Sci. 19: 390–398. [DOI] [PubMed] [Google Scholar]
- Fan S.-C., Lin C.-S., Hsu P.-K., Lin S.-H., Tsay Y.-F., 2009. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell 21: 2750–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay K. W., Wilkinson G. N., 1963. The analysis of adaptation in a plant-breeding program. Crop Pasture Sci. 14: 742–754. [Google Scholar]
- Gauch H. G., Jr., 1988. Model selection and validation for yield trials with interaction. Biometrics 44: 705–715. [Google Scholar]
- Gauch H. G., Jr., 2006. Statistical analysis of yield trials by AMMI and GGE. Crop Sci. 46: 1488–1500. [Google Scholar]
- Gil-Quintana E., Larrainzar E., Seminario A., Díaz-Leal J. L., Alamillo J. M., et al. , 2013. Local inhibition of nitrogen fixation and nodule metabolism in drought-stressed soybean. J. Exp. Bot. 64: 2171–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githiri S. M., Watanabe S., Harada K., Takahashi R., 2006. QTL analysis of flooding tolerance in soybean at an early vegetative growth stage. Plant Breed. 125: 613–618. [Google Scholar]
- Goodstein D. M., Shu S., Howson R., Neupane R., Hayes R. D., et al. , 2012. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 40: 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D., Nelson R. T., Cannon S. B., Shoemaker R. C., 2010. SoyBase, the USDA-ARS soybean genetics and genomics database. Nucleic Acids Res. 38: D843–D846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara D. R., El-Kereamy A., Yaish M. W., Mei-Bi Y., Rothstein S. J., 2014. Functional characterization of the rice UDP-glucose 4-epimerase 1, OsUGE1: a potential role in cell wall carbohydrate partitioning during limiting nitrogen conditions. PLoS One 9: e96158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Tucker D. M., Wang D., Basten C. J., Ersoz E., et al. , 2013. Accuracy of across-environment genome-wide prediction in maize nested association mapping populations. G3 Bethesda 3: 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Fan R., Zhao T., Yu J., Yu D., 2009. Seed storage protein components are associated with curled cotyledon phenotype in soybean. Afr. J. Biotechnol. 8: 6063–6067. [Google Scholar]
- Heinrich G. M., Francis C. A., Eastin J. D., 1983. Stability of grain sorghum yield components across diverse environments. Crop Sci. 23: 209–212. [Google Scholar]
- Hogekamp C., Küster H., 2013. A roadmap of cell-type specific gene expression during sequential stages of the arbuscular mycorrhiza symbiosis. BMC Genomics 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B., Marchini J., Stephens M., 2011. Genotype imputation with thousands of genomes. G3 1: 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S., King C. A., Davies M. K., Ray J. D., Cregan P. B., et al. , 2013. QTL analysis of shoot ureide and nitrogen concentrations in soybean [Glycine max (L.) Merr.] Crop Sci. 53: 2421–2433. [Google Scholar]
- Hyten D. L., Song Q., Zhu Y., Choi I. Y., Nelson R. L., et al. , 2006. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 103: 16666–16671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyten D. L., Choi I. Y., Song Q., Shoemaker R. C., Nelson R. L., et al. , 2007. Highly variable patterns of linkage disequilibrium in multiple soybean populations. Genetics 175: 1937–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W., Zhu Y., Li Y., Yang L., Zhao X., et al. , 2010. Over-expression of a glutathione S-transferase gene, GsGST, from wild soybean (Glycine soja) enhances drought and salt tolerance in transgenic tobacco. Biotechnol. Lett. 32: 1173–1179. [DOI] [PubMed] [Google Scholar]
- Kang M. S., 1997. Using genotype-by-environment interaction for crop cultivar development. Adv. Agron. 62: 199–252. [Google Scholar]
- Kim Y. K., Kim S., Um J. H., Kim K., Choi S. K., et al. , 2013. Functional implication of β-carotene hydroxylases in soybean nodulation. Plant Physiol. 162: 1420–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S., Kuji R., Nanjo Y., Hiraga S., Furukawa K., 2012. Comprehensive analysis of endoplasmic reticulum-enriched fraction in root tips of soybean under flooding stress using proteomics techniques. J. Proteomics 77: 531–560. [DOI] [PubMed] [Google Scholar]
- Lee E. A., Deen W., Hooyer M. E., Chambers A., Parkin G., et al. , 2016. Involvement of year-to-year variation in thermal time, solar radiation and soil available moisture in genotype-by-environment effects in maize. Crop Sci. 56: 2180–2192. [Google Scholar]
- Liao C. Y., Wu P., Hu B., Yi K. K., 2001. Effects of genetic background and environment on QTLs and epistasis for rice (Oryza sativa L.) panicle number. Theor. Appl. Genet. 103: 104–111. [Google Scholar]
- Lin C. S., Poushinsky G., 1983. A modified augmented design for an early stage of plant selection involving a large number of test lines without replication. Biometrics 39: 553–561. [Google Scholar]
- Lin S. H., Kuo H. F., Canivenc G., Lin C. S., Lepetit M., et al. , 2008. Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20: 2514–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M., Walsh B., 1998. Genetics and Analysis of Quantitative Traits, Vol. 1 Sinauer, Sunderland, MA. [Google Scholar]
- Ma B., Dwyer L., Gregorich E., 1999. Soil nitrogen amendment effects on nitrogen uptake and grain yield of maize. Agron. J. 91: 650–656. [Google Scholar]
- Ma Y., Kan G., Zhang X., Wang Y., Zhang W., et al. , 2016. Quantitative trait loci (QTL) mapping for glycinin and β-conglycinin contents in soybean (Glycine max L. Merr.). J. Agric. Food Chem. 64: 3473–3483. [DOI] [PubMed] [Google Scholar]
- Malosetti M., Voltas J., Romagosa I., Ullrich S. E., van Eeuwijk F. A., 2004. Mixed models including environmental covariables for studying QTL by environment interaction. Euphytica 137: 139–145. [Google Scholar]
- Malosetti M., Ribaut J. M., van Eeuwijk F. A., 2013. The statistical analysis of multi-environment data: modelling genotype-by-environment interaction and its genetic basis. Front. Physiol. 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavalan L. P., Guttikonda S. K., Tran L. S. P., Nguyen H. T., 2009. Physiological and molecular approaches to improve drought resistance in soybean. Plant Cell Physiol. 50: 1260–1276. [DOI] [PubMed] [Google Scholar]
- McCulloch C. E., Searle S. R., 2001. Generalized, Linear and Mixed Models. John Wiley, New York. [Google Scholar]
- McGonigle B., Keeler S. J., Lau S. M. C., Koeppe M. K., O’Keefe D. P., 2000. A genomics approach to the comprehensive analysis of the glutathione S-transferase gene family in soybean and maize. Plant Physiol. 124: 1105–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez C., Baginsky C., Hedden P., Gong F., Carú M., et al. , 2014. Gibberellin oxidase activities in Bradyrhizobium japonicum bacteroids. Phytochemistry 98: 101–109. [DOI] [PubMed] [Google Scholar]
- Mir R. R., Zaman-Allah M., Sreenivasulu N., Trethowan R., Varshney R. K., 2012. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 125: 625–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell P. L., Buckler E. S., Ross-Ibarra J., 2012. Crop genomics: advances and applications. Nat. Rev. Genet. 13: 85–96. [DOI] [PubMed] [Google Scholar]
- Mortazavian S. M. M., Nikkhah H. R., Hassani F. A., Sharif-al-Hosseini M., Taheri M., et al. , 2014. GGE biplot and AMMI analysis of yield performance of barley genotypes across different environments in Iran. J. Agric. Sci. Technol. 16: 609–622. [Google Scholar]
- Palomeque L., Liu L. J., Li W., Hedges B. R., Cober E. R., et al. , 2010. Validation of mega-environment universal and specific QTL associated with seed yield and agronomic traits in soybeans. Theor. Appl. Genet. 120: 997–1003. [DOI] [PubMed] [Google Scholar]
- Parker J., Newstead S., 2014. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 507: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell L. C., King C. A., Ball R. A., 2000. Soybean cultivar differences in ureides and the relationship to drought tolerant nitrogen fixation and manganese nutrition. Crop Sci. 40: 1062–1070. [Google Scholar]
- Ramachandra D., Madappa S., Phillips J., Loida P., Karunanandaa B., 2015. Breeding and biotech approaches towards improving yield in soybean, pp. 131–192 in Recent Advancements in Gene Expression and Enabling Technologies in Crop Plants. Springer, New York. [Google Scholar]
- Ray J. D., Dhanapal A. P., Singh S. K., Hoyos-Villegas V., Smith J. R., et al. , 2015. Genome-wide association study of ureide concentration in diverse maturity Group IV soybean [Glycine max (L.) Merr.] accessions. G3 Bethesda 5: 2391–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2017. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna: Available at: https://www.R-project.org/. [Google Scholar]
- Rincker K., Nelson R., Specht J., Sleper D., Cary T., et al. , 2014. Genetic improvement of US soybean in maturity groups II, III, and IV. Crop Sci. 54: 1419–1432. [Google Scholar]
- Rippey B. R., 2015. The US drought of 2012. Weather Clim. Extrem. 10: 57–64. [Google Scholar]
- Rosielle A. A., Hamblin J., 1981. Theoretical aspects of selection for yield in stress and non-stress environment. Crop Sci. 21: 943–946. [Google Scholar]
- Salvagiotti F., Cassman K. G., Specht J. E., Walters D. T., Weiss A., et al. , 2008. Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crops Res. 108: 1–13. [Google Scholar]
- Schlichting C. D., Smith H., 2002. Phenotypic plasticity: linking molecular mechanisms with evolutionary outcomes. Evol. Ecol. 16: 189–211. [Google Scholar]
- Shan C., Mei Z., Duan J., Chen H., Feng H., et al. , 2014. OsGA2ox5, a gibberellin metabolism enzyme, is involved in plant growth, the root gravity response and salt stress. PLoS One 9: e87110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim H. C., Ha B. K., Yoo M., Kang S. T., 2015. Detection of quantitative trait loci controlling UV-B resistance in soybean. Euphytica 202: 109–118. [Google Scholar]
- Sinclair T. R., Purcell L. C., King C. A., Sneller C. H., Chen P., et al. , 2007. Drought tolerance and yield increase of soybean resulting from improved symbiotic N 2 fixation. Field Crops Res. 101: 68–71. [Google Scholar]
- Smith H., 1990. Signal perception, differential expression within multigene families and the molecular basis of phenotypic plasticity. Plant Cell Environ. 13: 585–594. [Google Scholar]
- Song Q., Yan L., Quigley C., Jordan B. D., Fickus E., et al. , 2017. Development and genetic characterization of the soybean nested association mapping (NAM) population. Plant Genome 10: 1–14. [DOI] [PubMed] [Google Scholar]
- Stekhoven D. J., Bühlmann P., 2012. MissForest: non-parametric missing value imputation for mixed-type data. Bioinformatics 28: 112–118. [DOI] [PubMed] [Google Scholar]
- Tamulonis J. P., Luzzi B. M., Hussey R. S., Parrott W. A., Boerma H. R., 1997. DNA marker analysis of loci conferring resistance to peanut root-knot nematode in soybean. Theor. Appl. Genet. 95: 664–670. [Google Scholar]
- Tian F., Bradbury P. J., Brown P. J., Hung H., Sun Q., et al. , 2011. Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat. Genet. 43: 159–162. [DOI] [PubMed] [Google Scholar]
- Tsay Y. F., Schroeder J. I., Feldmann K. A., Crawford N. M., 1993. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72: 705–713. [DOI] [PubMed] [Google Scholar]
- van Eeuwijk F. A., Bink M. C., Chenu K., Chapman S. C., 2010. Detection and use of QTL for complex traits in multiple environments. Curr. Opin. Plant Biol. 13: 193–205. [DOI] [PubMed] [Google Scholar]
- Wang D. L., Zhu J., Li Z. K. L., Paterson A. H., 1999. Mapping QTLs with epistatic effects and QTL× environment interactions by mixed linear model approaches. Theor. Appl. Genet. 99: 1255–1264. [Google Scholar]
- Wang X., Zhang H., Sun G., Jin Y., Qiu L., 2014. Identification of active VQ motif-containing genes and the expression patterns under low nitrogen treatment in soybean. Gene 543: 237–243. [DOI] [PubMed] [Google Scholar]
- Wei J., Xu S., 2016. A random-model approach to QTL mapping in multiparent advanced generation intercross (MAGIC) populations. Genetics 2: 471–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won Oh M., Nanjo Y., Komatsu S., 2014. Identification of nuclear proteins in soybean under flooding stress using proteomic technique. Protein Pept. Lett. 21: 458–467. [DOI] [PubMed] [Google Scholar]
- Xavier A., Xu S., Muir W. M., Rainey K. M., 2015. NAM: association studies in multiple populations. Bioinformatics 31: 3862–3864. [DOI] [PubMed] [Google Scholar]
- Xavier A., Muir W. M., Craig B., Rainey K. M., 2016. Walking through the statistical black boxes of plant breeding. Theor. Appl. Genet. 129: 1933–1949. [DOI] [PubMed] [Google Scholar]
- Xing Y., Tan Y., Hua J. P., Sun X., Xu C., et al. , 2002. Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theor. Appl. Genet. 105: 248–257. [DOI] [PubMed] [Google Scholar]
- Xu Y., 2016. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 129: 653–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., 2016. Analysis and handling of G× E in a practical breeding program. Crop Sci. 56: 2106–2118. [Google Scholar]
- Yan W., Kang M. S., Ma B., Woods S., Cornelius P. L., 2007. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 47: 643–653. [Google Scholar]
- Yokoyama T., Kodama N., Aoshima H., Izu H., Matsushita K., et al. , 2001. Cloning of a cDNA for a constitutive NRT1 transporter from soybean and comparison of gene expression of soybean NRT1 transporters. Biochim. Biophys. Acta 1518: 79–86. [DOI] [PubMed] [Google Scholar]
- Yu J., Holland J. B., McMullen M. D., Buckler E. S., 2008. Genetic design and statistical power of nested association mapping in maize. Genetics 178: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue P., Sleper D. A., Arelli P. R., 2001. Mapping resistance to multiple races of in soybean PI 89772. Crop Sci. 41: 1589–1595. [Google Scholar]
- Zhe Y., Lauer J. G., Borges R., De Leon N., 2010. Effects of genotype× environment interaction on agronomic traits in soybean. Crop Sci. 50: 696–702. [Google Scholar]
- Zhu M., Dahmen J. L., Stacey G., Cheng J., 2013. Predicting gene regulatory networks of soybean nodulation from RNA-Seq transcriptome data. BMC Bioinformatics 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel R. W., Wright M. J., Gauch H. G., 1988. Statistical analysis of a yield trial. Agron. J. 80: 388–393. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are publically available in the R package SoyNAM. Access with the following command: data (soybase, package=“SoyNAM”). Data formatted for the analysis of the NAM package is available with the following command: SoyNAM::ENV().