Abstract
The Hulunbuir short-tailed sheep (Ovis aries) is a breed native to China, in which the short-tail phenotype is the result of artificial and natural selection favoring a specific set of genetic mutations. Here, we analyzed the genetic differences between short-tail and normal-tail phenotypes at the genomic level. Selection signals were identified in genome-wide sequences. From 16 sheep, we identified 72,101,346 single nucleotide polymorphisms. Selection signals were detected based on the fixation index and heterozygosity. Seven genomic regions under putative selection were identified, and these regions contained nine genes. Among these genes, T was the strongest candidate as T is related to vertebral development. In T, a nonsynonymous mutation at c.G334T resulted in p.G112W substitution. We inferred that the c.G334T mutation in T leads to functional changes in Brachyury—encoded by this gene—resulting in the short-tail phenotype. Our findings provide a valuable insight into the development of the short-tail phenotype in sheep and other short-tailed animals.
Keywords: Hulunbuir short-tailed sheep, short-tail phenotype, whole genome sequencing, selective sweep, T/Brachyury gene, Genome Report
Sheep were among the earliest domesticated herbivores. Sheep domestication dates back to the end of the Mesolithic period, ∼11,000 yr ago (Chessa et al. 2009). Domestication and artificial selection have led to marked changes in sheep behavior, appearance, and other important traits (Megens et al. 2008). Most mammal tails are used for balance, communication, and attack. However, in sheep, tails store energy. The fat-tail phenotype is a trait necessary for survival in harsh environments (Pourlis 2011). It is exhibited by Hulunbuir short-tailed sheep, which have been bred for the past century by local herdsmen of the Hulunbuir grassland—a world-renowned highland pasture in arid and semi-arid regions of North China characterized by a short frost-free period, long and cold winter, and dry season constituting a considerable part of the year (Yang et al. 2012). The tails of Hulunbuir short-tailed sheep (Figure 1A) are of variable length, and are generally classified into two categories: extremely short, a tail exposing the anus (Figure 1C); and moderately short, a tail covering the anus (Figure 1D).
Figure 1.
Tail phenotypes of sheep. (A) Hulunbuir short-tailed sheep; (B) Barag sheep; (C) extremely short in Hulunbuir short-tailed sheep; (D) moderately short in Hulunbuir short-tailed sheep; (E) normal tail in Barag sheep.
Several candidate genes—including HES7 (Bessho et al. 2001), PAX1 (Wilm et al. 1998), T (Smith 1999), and WNT3A (Greco et al. 1996)—related to vertebral development in laboratory mice are reported to be related to the short-tail phenotype; however, the determinant genes related to the short-tail phenotype in sheep remain to be identified.
Recently, many genomic regions under putative selection—which may be related to domestication, adaptation, and other important traits—have been reported in chickens, cats, dogs, and pigs (Rubin et al. 2010; Axelsson et al. 2013; Moon et al. 2015; Xiao et al. 2016). However, detection of selection signals within a single species or population cannot determine whether the genomic region is under putative selection or related to genetic drift. Genomic regions under putative selection are identified using the fixation index (Fst), which is based on significant differences in allele frequencies between two populations. However, Fst does not identify the population wherein selection occurs; hence, it cannot be used to determine the direction of selection. Therefore, in the present study, we analyzed heterozygosity (Hp) to identify the specific genomic regions under putative selection. Axelsson et al. (2013) combined Fst and Hp to locate the genomic regions under putative selection, and the corresponding genes during dog domestication. Here, we applied the methodology of Axelsson et al. (2013) to locate the genomic regions related to the short-tail phenotype in Hulunbuir short-tailed sheep.
To verify the role of the determinant genes and identify novel genes regulating the short-tail phenotype, we selected sheep with extremely short tails from a population of Hulunbuir short-tailed sheep, and performed whole genome sequencing. The short-tail phenotype has previously been reported in Australian Merino sheep (James et al. 1991); however, to the best of our knowledge, we are the first to conduct molecular studies of the short-tail phenotype in sheep.
Materials and Methods
Ethics statement
All animal care and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Inner Mongolia Agricultural University.
Samples
Hulunbuir short-tailed sheep and Barag sheep were selected from the Autonomous County of Evenki in the Inner Mongolia Autonomous Region, China. Barag sheep (Figure 1B), which resemble Hulunbuir short-tailed sheep but have a normal tail morphology (Figure 1E), served as controls. We randomly selected 100 short-tailed sheep and 100 Barag sheep for tail-length measurement. Caudal vertebrae were collected from 10 short-tailed sheep and 10 Barag sheep. Blood samples were collected from the following sheep—eight unrelated 2-yr-old short-tailed sheep (four males and four females) with extremely short tails, and eight unrelated 2-yr-old Barag sheep (four males and four females) with normal tails were selected for whole genome sequencing; 120 short-tailed sheep and 110 Barag sheep were randomly selected to confirm the mutation site; ∼2 ml of blood was collected in EDTA-containing vacutainers and stored in liquid nitrogen (−196°). Genomic DNA was extracted from the whole blood samples using the DNeasy Blood and Tissue Kit (Qiagen, Duesseldorf, Germany).
X-ray analysis and specimen preparation
X-ray images of the caudal vertebrae were captured from the front using the ClearVet DR16 Imaging System. Deposits and muscle were carefully peeled off from the caudal vertebrae. The caudal vertebrae were fixed in ethanol for 5 d, and cleared by immersing in 0.5% NaOH for 2 d. To remove fat, oil, and adipose tissue, the caudal vertebrae were immersed in petrol for 3 d. Data were recorded and saved according to the order of X-ray images.
Sequencing and SNP calling
A sequencing library with an average insert size of 350 bp was constructed for each sample. Sequencing was performed on the Illumina Hisequation 2000 sequencer at Novogene Corporation to generate 150-bp paired-end reads; ∼13.1 Gb of high-quality data were generated, achieving an average fivefold genome coverage for each individual. The clean data were aligned to the sheep reference genome (Oar_v3.1 http://asia.ensembl.org/Ovis_aries/Info/Index) using Burrows-Wheeler Aligner 0.6.1 with parameters set as “mem -t 4 -k 32 -M” (Li and Durbin 2010); duplications were eliminated from the alignments using SAMtools with parameters set as “rmdup.” Single nucleotide polymorphism (SNPs) were detected using SAMtools with parameters set as “mpileup-m 2 -F 0.002 -d 1000” and annotated using ANNOVAR (Li et al. 2009; Wang et al. 2010). SNPs with low-quality scores (GQ < 20) and inter-SNP distance of <5 bp were filtered.
Selective sweep analysis
A sliding-window approach (100-kb windows sliding in 10-kb steps) was employed for quantifying Hp in the short-tailed sheep and Fst between the short-tailed and Barag sheep (Li et al. 2014). Hp was calculated using the formula where and are the sum of nMaj and nMin, respectively. We transformed the Hp values into Z-scores using the formula where is the overall average heterozygosity and is the SD for all windows within the group. Genetic differentiation between the short-tailed and Barag sheep was measured using fixation index (Fst) with the formula where and represent the frequencies of alleles A and a in populations of Hulunbuir short-tailed sheep and Barag sheep, respectively, and and represent the frequencies of alleles A and a, respectively, in the whole population (Clark and Hartl 2007). Fst values were Z-transformed using the formula for ZHp. The Hp and Fst data were calculated based on the R language package (http://www.r-project.org/). The selected regions were defined as genetic regions in overlapping windows with extremely low ZHp values (ZHp < −5) and extremely high ZFst values (ZFst > 4.5).
T sequencing
The primer pairs for the amplification of T exons were designed based on the sheep genome assembly (Oar_v3.1). The primer sequences are provided in Supplemental Material, Table S1. Sanger sequences of PCR duplicates were detected by Invitrogen Corporation, Shanghai, China.
Data availability
The Illumina sequence reads were deposited in the NCBI Sequence Read Archive under the accession number SRP106953. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Measurements
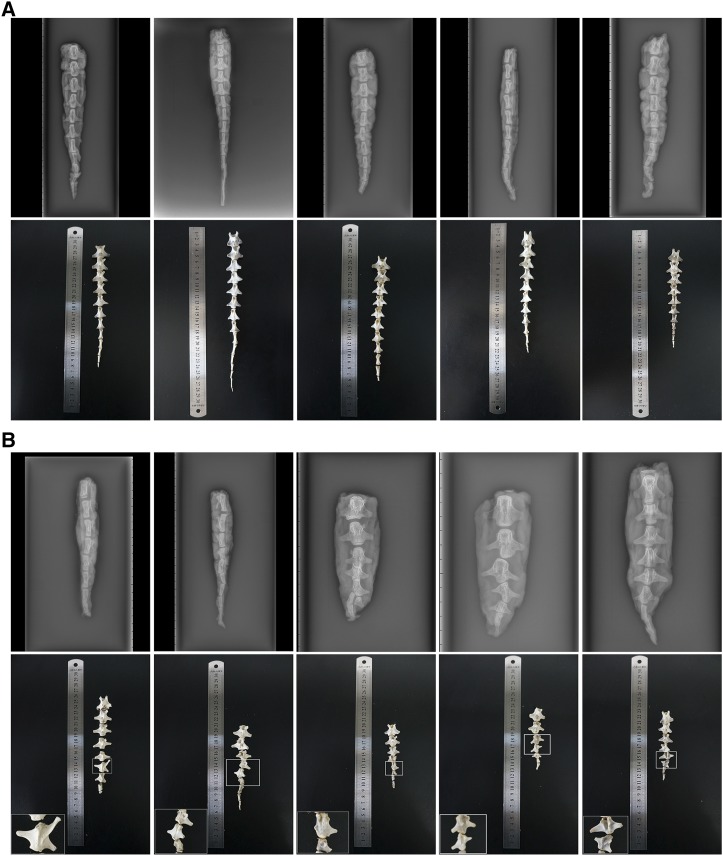
The average tail lengths of the Barag and Hulunbuir short-tailed sheep were 26.32 and 16.01 cm, respectively. The average lengths of the caudal vertebrae in the Barag and Hulunbuir short-tailed sheep were 21.12 and 13.86 cm, respectively. Deformed caudal vertebrae were observed in seven Hulunbuir short-tailed sheep (Figure 2).
Figure 2.
Phenotype of caudal vertebrae in the two populations. (A) Phenotype of the caudal vertebrae in Barag sheep; (B) phenotype of the caudal vertebrae in Hulunbuir short-tailed sheep. Top rows show X-rays and bottom rows show the caudal vertebrae that were placed based on the X-rays in (A and B).
Genome resequencing
Eight sheep with extremely short tails were selected from the short-tailed sheep, and eight Barag sheep with normal tails were selected. We performed genome sequencing for the 16 sheep and obtained 232.23 Gb of paired-end DNA reads. Of this, 231.07 Gb (99.50%) consisted of high-quality paired-end reads (Q20 ≥ 94.33% and Q30 ≥ 88.47%) that could be aligned to the sheep reference genome (Oar_v3.1) (Table S2). For individual sheep, the alignment rate was 98.13–98.75%. For the reference genome (excluding N’s), the average coverage depth was 4.59–5.27×. The reads with 1× coverage depth (covered by at least one base) accounted for >94.19% (Table S3). The read alignments were normal, and the reads were eligible for variation detection and other analyses.
SNP and selective sweep
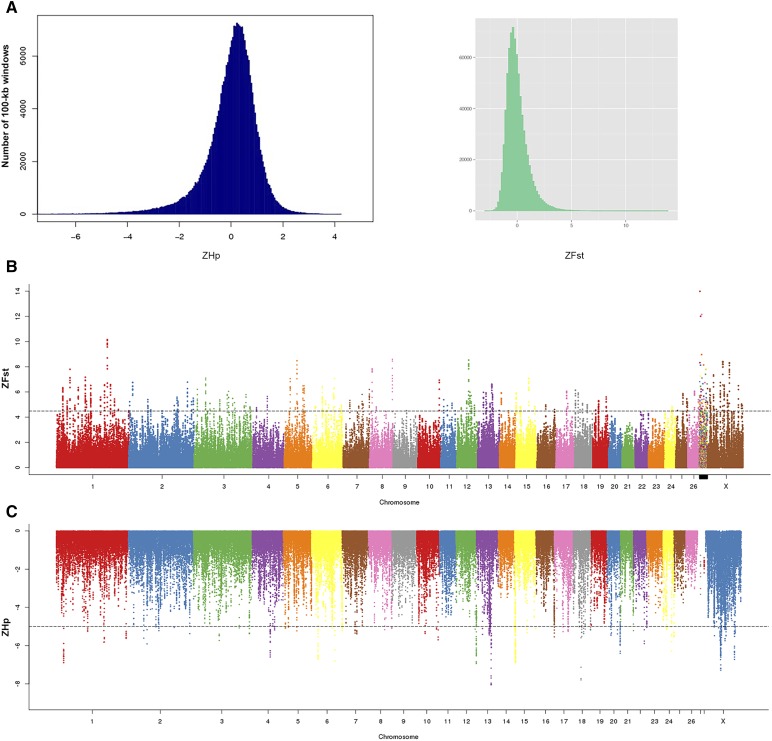
In the 16 sheep, we identified 72,101,346 SNPs, of which 492,307 SNPs were localized to coding regions, and responsible for 205,408 nonsynonymous nucleotide substitutions (202,338 missense, 2905 stop-gains, and 165 stop-losses) (Table S4). According to the SNP densities in the genome and reference genome assembly, the number of SNPs in the sliding window was <20 beginning from 100 kb; this trend persisted. Additionally, the regions containing <20 SNPs were stable. Therefore, the width of the sliding window was selected as 100 kb. Alignments were performed between the two populations, using a 50%-overlapping interval as the step length. For each window, Fst was calculated and Z-transformed into a ZFst value that obeyed standard normal distribution (Figure 3A). The larger the ZFst value, the greater the genetic differentiation between the populations. Hp was evaluated for the short-tailed sheep population with the same sliding-window parameters, and it was Z-transformed to standard normal distribution for the population (Figure 3A). Based on the Z value, we observed considerable genetic differentiation in many genomic regions between the populations, including those unrelated to tail morphology. To determine whether certain genetically different genomic regions were related to tail morphology, we overlapped the small interval of ZHp and large interval of ZFst. We set ZFst = 4.5 as the threshold for identifying the genomic regions under putative selection and selected windows with ZFst values greater than this threshold (Figure 3B). Moreover, we set ZHp = −5 as the threshold and selected windows with ZHp values less than this threshold (Figure 3C). The overlapping regions were identified and combined into seven candidate genomic regions (20–160 kb). These candidate regions covered a genomic length of 790 kb and contained nine genes (Table 1).
Figure 3.
Selective analysis of the sheep genome. (A) Distribution of Z-transformed average heterozygosity in the Hulunbuir short-tailed sheep and Z-transformed average fixation index (ZFst) for autosomal 100-kb windows. (B) Positive end of the (ZFst) distribution plotted along chromosomes (chromosomes are separated by color); horizontal dashed line indicates the cut-off (ZFst > 4.5) used for extracting outliers. (C) Negative end of the (ZHp) distribution plotted along chromosomes (chromosomes are separated by color); horizontal dashed line indicates the cut-off (ZHp < −5) used for extracting outliers.
Table 1. Overlapping genes identified using ZHp and ZFst.
| Chr | Interval Location | ZFst | ZHp | Candidate Gene | Annotation | Gene Location |
|---|---|---|---|---|---|---|
| 1 | 69800001–69950000 | 4.9462 | −5.4862 | FNBP1L | Formin-binding protein 1-like | 69805549–69915406 |
| 1 | 69800001–69950000 | 4.7863 | −5.9525 | BCAR3 | Breast cancer anti-estrogen resistance 3 | 69930328–70015474 |
| 6 | 37440001–37580000 | 4.9422 | −5.0057 | LCORL | Ligand-dependent nuclear receptor corepressor-like | 37365236–37452332 |
| 8 | 87770001–87890000 | 8.4551 | −5.1642 | T | T, Brachybury homolog | 87796143–87805552 |
| 15 | 16940001–17060000 | 4.7804 | −6.5384 | SLC35F2 | Solute carrier family 35, member F2 | 16854039–16994650 |
| 15 | 16940001–17060000 | 4.6566 | −5.9519 | RAB39A | RAB39A, member RAS oncogene family | 166995339–17027728 |
| 15 | 17090001–17100000 | 4.7602 | −6.0915 | CUL5 | Cullin 5 | 17088942–17210813 |
| X | 77440001–77470000 | 4.8223 | −5.1215 | IRAK1 | Interleukin-1 receptor-associated kinase 1 | 77447119–77450460 |
| X | 77440001–77470000 | 4.8801 | −5.0186 | MECP2 | Methyl-CpG-binding protein 2 | 77461651–77467940 |
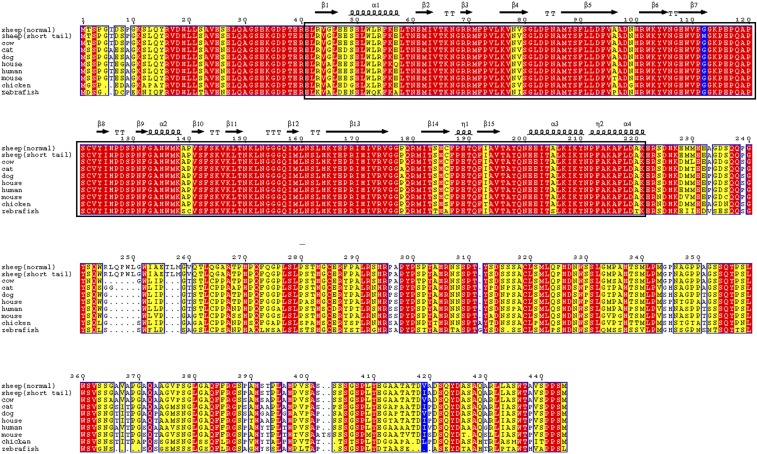
Of these nine annotated genes, T, which is a gene related to development of the spine in mice (Beddington et al. 1992), harbored 10 mutations, including eight synonymous and two nonsynonymous mutations. The two nonsynonymous substitutions in T were considered putative mutations, including G-to-T transversion in exon two (c.G334T) and G-to-A transition in exon nine (c.G1255A), which corresponded to glycine-to-tryptophan substitution at amino acid residue 112 (p.G112W) and valine-to-isoleucine substitution at amino acid residue 419 (p.V419I), respectively (Figure 4).
Figure 4.
Alignment of amino acid sequences of Brachyury among animals. Brachyury is a protein encoded by T. Amino acid residues where p.G112W and p.V419I were located are indicated in blue. Identical amino acids, conserved substitutions, and semiconserved substitutions are indicated in red, yellow, and white respectively. Dots represent gaps in the alignment.
Validation using Sanger sequencing
The identified nonsynonymous nucleotide substitutions in T were verified using Sanger sequencing with a large sample size in 120 short-tailed and 110 Barag sheep. Variation data were deposited in GenBank under the accession number MF996360. We detected the heterozygous form of c.G334T in the short-tailed sheep, but not in the Barag sheep. However, we detected c.G1255A in both populations (Table 2). We inferred that the c.G334T mutation in T is the primary cause of the short-tail phenotype. However, this does not exclude the possibility that other genes control the short-tail phenotype.
Table 2. Summary of genotypic data in the two populations.
| Breed | c.G334T | c.G1255A | |||||
|---|---|---|---|---|---|---|---|
| N | (G/G) | (G/T) | (T/T) | (G/G) | (G/A) | (A/A) | |
| Hulunbuir short-tailed sheep | 120 | 17 | 103 | 0 | 81 | 0 | 39 |
| Barag sheep | 110 | 110 | 0 | 0 | 97 | 0 | 13 |
Discussion
Hulunbuir short-tailed sheep are fat-tailed sheep, with large quantities of adipose deposited in their tail regions. Most previous research studies on sheep tails have focused on adipose deposition (Moradi et al. 2012; Wang et al. 2014; Yuan et al. 2017). Liu et al. (2015) suggested that tail length in short-tailed sheep is related to adipose deposition. However, we believe that tail length is related to the length of the caudal vertebrae. This prediction was supported by our observation of deformed vertebrae in the tails of seven Hulunbuir short-tailed sheep. The specific molecular mechanism functioning in the short-tail phenotype remains to be elucidated; however, we believe that the mutated T gene plays an important role in regulating this phenotype. Of the nine investigated genes, BCAR3 (Agthoven et al. 1998), FNBP1L (Huett et al. 2009), IRAK1 (Li et al. 2015), CUL5 (Byrd et al. 1997), and RAB39A (Seto et al. 2013) are related to the immune system; MECP2 (Tao et al. 2009) and SLC35F2 (Sonuga-Barke 2013) are related to neurodevelopment; and LCORL (Signer-Hasler et al. 2012) is related to skeletal frame size. Only the T gene is related to development of the spine, and this gene seems to be associated with a short-tail phenotype in mice (Beddington et al. 1992).
We identified seven candidate genomic regions that included T. This gene was further verified using Sanger sequencing. T encodes a transcription factor named Brachyury, which is the key regulator of mesoderm formation during early development. The Brachyury protein is an important functional transcription factor, in which ∼180 amino acid residues located near the N-terminus display DNA-binding activity; this is the T domain. This region can specifically bind to a 5-bp functional domain in DNA (TCACA) (Kispert and Herrmann 1993; Palena et al. 2007; Fernando et al. 2010). Brachyury is specifically expressed in the notochord of the mesoderm during gastrulation, and it regulates growth and development of the embryonic notochord. However, Brachyury is not expressed during mid-to-late pregnancy (Sangoi et al. 2011).
As early as 1927, Dobrovolskaïa-Zavadskaïa described the phenotype of a Brachyury mutation in mice (Kavka and Green 1997). Mice heterozygous for the Brachyury mutation had short and slightly curved tails. Homozygous or compound heterozygous mice died in utero after ∼10 d of the embryonic period because of failure to form the dorsal cord and allantois (Showell et al. 2004). The short-tail phenotype in mice was first discovered in 1927, but it was not until 1990 that the T gene was cloned (Herrmann et al. 1990). Pennimpede et al. (2012) characterized an inducible miRNA-based on an in vivo knockdown mouse model of T, which exhibited skeletal defects.
We identified two loci of nonsynonymous mutations in T using genome sequencing in short-tailed sheep, and we localized the c.G334T mutation in the T domain of the Brachyury gene. Specific regions in the T domain are highly sensitive to structural changes caused by mutations, and these mutations may result in conformational changes in the protein. By combining X-ray diffraction and analysis of the DNA-binding domain in Xenopus, Muller observed high similarity between amino acids in this region and those in contact with DNA (Müller and Herrmann 1997). In the present study, we inferred that the p.G112W mutation affects the binding ability of the T domain to DNA.
We did not detect the c.T334T mutation in the short-tailed sheep population, consistent with the theory that homozygotes or compound heterozygotes result in embryonic lethality (Buckingham et al. 2013). The c.G334G mutation was detected in individuals with normal tails. Wu et al. (2010) observed that, when mice with moderately long tails were mated with short-tailed mice, the offspring showed short-tail and normal-tail phenotypes; however, the genotypes of the offspring were not analyzed. This may explain the phenomenon of the c.G334G mutation in individuals with normal tails in the short-tailed sheep population. Herdsmen continue to breed this group and eliminate individuals with nonideal tail types and other individuals exhibiting poor phenotypes; we propose that this explains the nonconformity of genotypic frequencies with genetic theory.
In conclusion, the results of our present study suggest that the c.G334T mutation in T directly results in the short-tail phenotype in sheep. The candidate genes identified in our study provide the basis for understanding the molecular mechanism of the short-tail phenotype in sheep and other short-tailed animals.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.117.300307/-/DC1.
Acknowledgments
We thank Wang Yu of the Bureau of Animal Husbandry of Evenki for sample collection. This work was supported by the Technology Innovation Project of the Inner Mongolia Agricultural University (NDPYTD 2010-6) and the Key Program of the Department of Science and Technology, Inner Mongolia Autonomous Region (201602057).
Footnotes
Communicating editor: D. J. de Koning
Literature Cited
- Agthoven T. V., Agthoven T. L. A. V., Dekker A., Spek P. J. V. D., Vreede L., et al. , 1998. Identification of BCAR 3 by a random search for genes involved in antiestrogen resistance of human breast cancer cells. EMBO J. 17: 2799–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson E., Ratnakumar A., Arendt M. L., Maqbool K., Webster M. T., et al. , 2013. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495: 360. [DOI] [PubMed] [Google Scholar]
- Beddington R. S., Rashbass P., Wilson V., 1992. Brachyury—a gene affecting mouse gastrulation and early organogenesis. Dev. Suppl. 114: 157. [PubMed] [Google Scholar]
- Bessho Y., Sakata R., Komatsu S., Shiota K., Yamada S., et al. , 2001. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 15: 2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham K. J., McMillin M. J., Brassil M. M., Shively K. M., Magnaye K. M., et al. , 2013. Multiple mutant T alleles cause haploinsufficiency of Brachyury and short tails in Manx cats. Mamm. Genome 24: 400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd P. T., McConville C. M., Smith A. D., Cooper P. R., Taylor A. M., 1997. Identification and analysis of expression of human VACM-1, a cullin gene family member located on chromosome 11q22–23. Genome Res. 7: 71–75. [DOI] [PubMed] [Google Scholar]
- Chessa B., Pereira F., Arnaud F., Amorim A., Goyache F., et al. , 2009. Revealing the history of sheep domestication using retrovirus integrations. Science 324: 532–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando R. I., Litzinger M., Trono P., Hamilton D. H., Schlom J., et al. , 2010. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J. Clin. Invest. 120: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T. L., Takada S., Newhouse M. M., McMahon J. A., McMahon A. P., et al. , 1996. Analysis of the vestigial tail mutation demonstrates that Wnt-3a gene dosage regulates mouse axial development. Genes Dev. 10: 313. [DOI] [PubMed] [Google Scholar]
- Hartl D. L., Clark A. G., 2007. Principles of Population Genetics, Ed. 4. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Herrmann B. G., Labeit S., Poustka A., King T. R., Lehrach H., 1990. Cloning of the T gene required in mesoderm formation in the mouse. Nature 343: 617. [DOI] [PubMed] [Google Scholar]
- Huett A., Ng A., Cao Z., Kuballa P., Komatsu M., et al. , 2009. A Novel hybrid yeast-human network analysis reveals an essential role for FNBP1L in antibacterial autophagy. J. Immunol. 182: 4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P. J., Ponzoni R. W., Gare D. R., Cockrum K. S., 1991. Inheritance of short tailedness in South Australian Merinos. Proceedings of the Australian Association of Animal Breeding and Genetics, Vol. 9, pp. 404–407. [Google Scholar]
- Kavka A. I., Green J. B. A., 1997. Tales of tails: Brachyury and the T-box genes. Biochim. Biophys. Acta 1333: 73–84. [DOI] [PubMed] [Google Scholar]
- Kispert A., Herrmann B. G., 1993. The Brachyury gene encodes a novel DNA binding protein. EMBO J. 12: 4898–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Huang S., Mo S., Zhang N., Zhou L., et al. , 2015. Susceptibility of autoimmune diseases in three polymorphisms of infection-associated gene IRAK1. J. Infect. Dev. Ctries. 9: 614–623. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R., 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Tian S., Yeung C. K. L., Meng X., Tang Q., et al. , 2014. Whole-genome sequencing of Berkshire (European native pig) provides insights into its origin and domestication. Sci. Rep. 4: 4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang H., Liu R., Mingming W. U., Zhang S., et al. , 2015. Genome-wide detection of selection signatures of distinct tail types in sheep populations. Acta Vet. Zootech. Sin. 46: 1721–1732. [Google Scholar]
- Megens H. J., Crooijmans R. P., San C. M., Hui X., Li N., et al. , 2008. Biodiversity of pig breeds from China and Europe estimated from pooled DNA samples: differences in microsatellite variation between two areas of domestication. Genet. Sel. Evol. 40: 103–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S., Kim T. H., Lee K. T., Kwak W., Lee T., et al. , 2015. A genome-wide scan for signatures of directional selection in domesticated pigs. BMC Genomics 16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M. H., Nejati-Javaremi A., Moradi-Shahrbabak M., Dodds K. G., McEwan J. C., 2012. Genomic scan of selective sweeps in thin and fat tail sheep breeds for identifying of candidate regions associated with fat deposition. BMC Genet. 13: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller C. W., Herrmann B. G., 1997. Crystallographic structure of T domain-DNA complex of the Brachyury transcription factor. Nature 389: 884–888. [DOI] [PubMed] [Google Scholar]
- Palena C., Polev D. E., Tsang K. Y., Fernando R. I., Litzinger M., et al. , 2007. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin. Cancer Res. 13: 2471–2478. [DOI] [PubMed] [Google Scholar]
- Pennimpede T., Proske J., König A., Vidigal J. A., Morkel M., et al. , 2012. In vivo knockdown of Brachyury results in skeletal defects and urorectal malformations resembling caudal regression syndrome. Dev. Biol. 372: 55–67. [DOI] [PubMed] [Google Scholar]
- Pourlis A. F., 2011. A review of morphological characteristics relating to the production and reproduction of fat-tailed sheep breeds. Trop. Anim. Health Prod. 43: 1267. [DOI] [PubMed] [Google Scholar]
- Rubin C. J., Zody M. C., Eriksson J., Meadows J. R., Sherwood E., et al. , 2010. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464: 587. [DOI] [PubMed] [Google Scholar]
- Sangoi A. R., Karamchandani J., Lane B., Higgins J. P., Rouse R. V., et al. , 2011. Specificity of brachyury in the distinction of chordoma from clear cell renal cell carcinoma and germ cell tumors: a study of 305 cases. Mod. Pathol. 24: 425–429. [DOI] [PubMed] [Google Scholar]
- Seto S., Sugaya K., Tsujimura K., Nagata T., Horii T., et al. , 2013. Rab39a interacts with phosphatidylinositol 3-kinase and negatively regulates autophagy induced by lipopolysaccharide stimulation in macrophages. PLoS One 8: e83324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell C., Binder O., Conlon F. L., 2004. T-box genes in early embryogenesis. Dev. Dyn. 229: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signer-Hasler H., Flury C., Haase B., Burger D., Simianer H., et al. , 2012. A genome-wide association study reveals loci influencing height and other conformation traits in horses. PLoS One 7: e37282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J., 1999. T-box genes: what they do and how they do it. Trends Genet. 15: 154–158. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. J. S., 2013. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381: 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Hu K., Chang Q., Wu H., Sherman N. E., et al. , 2009. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc. Natl. Acad. Sci. USA 106: 4882–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Li M., Hakonarson H., 2010. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhou G., Xu X., Geng R., Zhou J., et al. , 2014. Transcriptome profile analysis of adipose tissues from fat and short-tailed sheep. Gene 549: 252–257. [DOI] [PubMed] [Google Scholar]
- Wilm B., Dahl E., Peters H., Balling R., Imai K., 1998. Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency. Proc. Natl. Acad. Sci. USA 95: 8692–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Shao Y., Chen B., Liu C., Xue Z., et al. , 2010. Identification of a novel mouse brachyury (T) allele causing a short tail mutation in mice. Cell Biochem. Biophys. 58: 129–135. [DOI] [PubMed] [Google Scholar]
- Xiao X., Xin S., Hu X. S., Yan Z., Liu Y. C., et al. , 2016. Whole genome sequencing identifies a missense mutation in HES7 associated with short tails in Asian domestic cats. Sci. Rep. 6: 31583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Qin Z., Li W., Xu B., 2012. Temporal and spatial variations of vegetation cover in Hulun Buir grassland of inner Mongolia, China. Arid Land Res. Manage. 26: 328–343. [Google Scholar]
- Yuan Z., Liu E., Liu Z., Kijas J. W., Zhu C., et al. , 2017. Selection signature analysis reveals genes associated with tail type in Chinese indigenous sheep. Anim. Genet. 48: 55–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Illumina sequence reads were deposited in the NCBI Sequence Read Archive under the accession number SRP106953. The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.