Abstract
The anatomy of the cords that form in Dupuytren's disease is complicated and a spiral cord is the most challenging variant to address. It displaces the neurovascular bundle toward or beyond the midline and closer to the skin. This article illustrates the surface anatomy of the neurovascular spiral to help surgeons identify this zone of danger that the authors term “the serpentine zone.” Careful dissection in this zone will help avoid iatrogenic digital neurovascular injury.
Keywords: Dupuytren's, spiral cords, surface anatomy
Introduction
Fasciectomy involving excision of a spiral cord can be a challenging procedure. Although the spiral itself is well documented, the surface markings have not been previously described. The authors propose the concept of “the serpentine zone” to indicate the area where the neurovascular bundle spirals, like a snake, around the cord. This zone extends from the distal palmar crease proximally to the proximal finger crease distally. It is in this zone that skin flap elevation and cord dissection should be performed with utmost care to avoid digital neurovascular damage.
The surface anatomy of one such cord is illustrated in a patient with Dupuytren's disease undergoing a limited fasciectomy, the mainstay of treatment for this fibroproliferative disease of the palmar and digital fascia. 1
In this disease process, normal fascial structures become thickened cords, type I collagen is replaced with type III, and myofibroblasts proliferate and contract. 2 Spiral cords, in particular, can distort the anatomy and increase the risk of neurovascular injury.
Clinical Case
In this case, a 69-year-old right-handed man presented with Dupuytren's contracture of the left ring finger with 70-degree fixed flexion of the proximal interphalangeal joint. Dissection revealed a radial spiral cord in the ring finger ( Fig. 1 ). The spiraling can be seen where the radial neurovascular bundle has been displaced beyond the midline toward the ulnar neurovascular bundle.
Fig. 1.
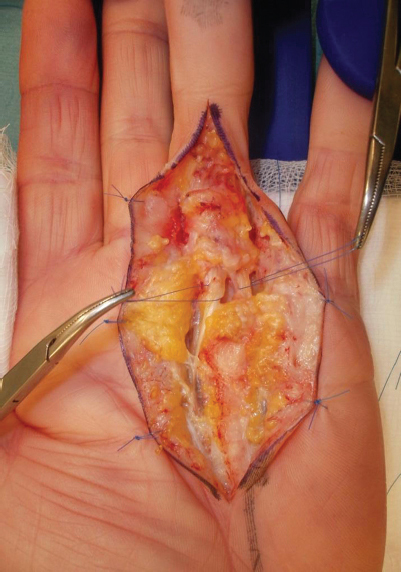
An intraoperative picture showing the radial spiral cord with a serpentine neurovascular bundle, deviated to the ulnar side, around which a suture loop has been passed for identification. The ulnar neurovascular bundle is also shown with a suture loop.
Following dissection and isolation of the digital neurovascular bundles, the skin flaps were replaced and the surface anatomy of the nerve spiraling toward the midline was carefully marked on the skin by a solid line for illustrative purposes ( Fig. 2 ). In this patient, the nerve is most vulnerable at the level noted by the black arrow. The hatched box indicates the proximal and distal limits of the serpentine zone, namely the distal palmar skin crease and the proximal finger crease, the area in which a spiral is commonly found. 3 The level of digital nerve spiraling can vary depending on the severity of the digital contracture but is usually limited within the limits of the serpentine zone.
Fig. 2.

Surface anatomy of the serpentine radial digital neurovascular bundle represented by the solid line from the radial side toward the midline incision. The arrow indicates the level at which the nerve was most deviated and superficial in this patient. The proximal extent of the serpentine zone is the distal palmar crease and the distal extent is the proximal finger crease.
For this patient, full correction of the finger was achieved following fasciectomy ( Fig. 3 ). Z-plasties were used for skin closure with absorbable 3–0 Vicryl Rapide sutures ( Fig. 4 ). Postoperative management was in the form of early hand therapy and night splintage.
Fig. 3.

Both neurovascular bundles have returned to their normal position and are parallel to each other following fasciectomy.
Fig. 4.

Wound closure using Z-plasties.
Review at 1 year found full extension and flexion of the digit, good healing of the scars, and normal sensation and perfusion ( Fig. 5A, B ).
Fig. 5.

( A , B ) Postoperative results showing full extension and flexion of the digit and good wound healing.
Discussion
In Dupuytren's disease, there are several cord types, but the spiral cord is one of the most challenging from the surgical point of view. Cords are formed by the transformation of the normal anatomical bands of the palmar and digital fascia. There are four main types of cords in the digits: central, lateral, retrovascular, and spiral. 4
These cords are diseased structures formed from normal fascial connections of the palmar fascial complex. The normal structures are the radial, ulnar, and central aponeurosis. These go on to connect with the palmodigital and then digital fascia. The central aponeurosis has horizontal, vertical, and longitudinal fibers. The longitudinal fibers split into three layers distally—the superficial inserting into the skin and the deep diving vertically to form the septae of Legueu and Juvara, which attach to the metacarpals and enclose the flexor tendons and neurovascular bundles. The middle of three layers arcs beneath the natatory ligament (the distal transverse fibers of the fascia) and then under the neurovascular bundles as the band of Gosset before returning in a palmar direction to insert into the lateral digital sheet and the Grayson's ligament (the ligament lying palmar to the neurovascular bundle that connects skin to the bone). Initially, these connections form a spiral around the digital nerve, but as the myofibroblasts in the cord contract, it straightens and moves toward the midline. As a result, the more pliable neurovascular bundle then spirals around the cord, thereby moving into a superficial position closer to the midline of the corresponding digit, and because of loss of subdermal fat, it can lie directly under the skin. 5 6 7
The formation of the spiral cord begins distal to the transversely orientated fibers of the ligament of Skoog at the level of the distal palmar crease with the spiral occurring from there anywhere up to the proximal finger crease. 5 7 Although much less commonly encountered, distal spirals have been reported. These were found either in isolation or in conjunction with a proximal spiral. It was postulated that further connections of the pretendinous and retrovascular bands are at fault. 8
During fasciectomy, dissection proximal to the distal palmar crease identifies the digital nerves before any spiraling, in a safe position on either side of the ray. The nerves can then be traced distally and protected. There is usually a small cuff of fatty tissue around the digital nerve at the zone of spiraling, which enables its identification and dissection.
This case illustrates the surface marking of the spiral neurovascular bundle. The area from the distal palmar skin crease to the proximal finger crease is the area where the neurovascular bundles can spiral abnormally. 9 The authors call this “the serpentine zone” due to its resemblance of the nerve spiral to a serpent, and as an aide memoir for the danger, it can pose during surgery. The serpentine zone serves as a cautious reminder to the surgeon, indicating the area where the greatest of care must be taken to identify and protect the neurovascular bundles.
Footnotes
Conflict of Interest None.
References
- 1.Henry M. Dupuytren's disease: current state of the art. Hand (NY) 2014;9(01):1–8. doi: 10.1007/s11552-013-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunton L M, Chhabra B. 7th ed. Philadelphia, PA: Elsevier; 2016. Hand, upper extremity and microvascular surgery; pp. 626–630. [Google Scholar]
- 3.Thomas R. The “Bowstring Arch Bridge” analogy to guide sequential portal placement during needle aponeurotomy in the treatment of Dupuytren contracture. J Hand Microsurg. 2016;8(03):185–186. doi: 10.1055/s-0036-1593391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McFarlane R M. Patterns of the diseased fascia in the fingers in Dupuytren's contracture. Displacement of the neurovascular bundle. Plast Reconstr Surg. 1974;54(01):31–44. doi: 10.1097/00006534-197407000-00004. [DOI] [PubMed] [Google Scholar]
- 5.McGrouther D A. Edinburgh, Scotland: Churchill Livingstone; 1990. Dupuytren's Disease; pp. 293–312. [Google Scholar]
- 6.Rayan G M. Dupuytren disease: anatomy, pathology, presentation, and treatment. J Bone Joint Surg Am. 2007;89(01):189–198. doi: 10.2106/00004623-200701000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Holland A J, McGrouther D A. Dupuytren's disease and the relationship between the transverse and longitudinal fibers of the palmar fascia: a dissection study. Clin Anat. 1997;10(02):97–103. doi: 10.1002/(SICI)1098-2353(1997)10:2<97::AID-CA5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Hettiaratchy S, Tonkin M A, Edmunds I A. Spiralling of the neurovascular bundle in Dupuytren's disease. J Hand Surg Eur Vol. 2010;35(02):103–108. doi: 10.1177/1753193409349855. [DOI] [PubMed] [Google Scholar]
- 9.Thomas R, O'Flaherty E. “Hills, Plains, and Valleys”: a topographical concept in the treatment of Dupuytren's contracture with needle aponeurotomy. Tech Hand Up Extrem Surg. 2017;21(02):55–59. doi: 10.1097/BTH.0000000000000154. [DOI] [PubMed] [Google Scholar]


