Abstract
The subcutaneous implantable cardioverter defibrillators (SICD) is an alternative to the transvenous ICD for the prevention of sudden cardiac death (SCD). Multiple studies have shown that the SICD is safe and effective in treating ventricular arrhythmias. While earlier studies mainly enrolled younger patients with channelopathies, more recent reports included patients with “typical” indications for ICD therapy for the prevention of SCD. In this review we summarize the data available to date on the SICD while highlighting its pros and cons.
Keywords: Inappropriate shocks, Subcutaneous ICD, Sudden Cardiac death, Transvenous ICD
1. Introduction
Implantable cardioverter defibrillators (ICD), first successfully implanted in a human in 1980, have long been proven to improve mortality in a number of clinical conditions causative for sudden cardiac death. The first device weighed 250 g, requiring a thoracotomy for implantation and an epicardial defibrillator patch. Criteria for inclusion in the pilot study were strict, including only patients who had suffered at least two episodes of cardiac arrest with ventricular fibrillation documented at least once.[1] As with any new technology, there were early concerns about the safety and utility of an implantable defibrillator,[2] but the following four decades have seen a remarkable advancement in implantable defibrillator technology.
The indications for ICD have expanded greatly since Mirowski's initial pilot study, and ICDs are now a mainstay of therapy in the prevention of sudden cardiac death. The most recent guidelines for ICD implant include both primary and secondary prevention indications across a range of cardiac conditions.[3] Indications include survivors of cardiac arrest due to ventricular arrhythmias from an irreversible cause, heart failure with reduced left ventricular ejection fraction (LVEF) due to nonischemic or ischemic etiology, and high risk structural heart disease or cardiac channelopathies.[3] In 2009 over 130,000 ICDs were implanted in USA[4] and in 2013 over 85,000 devices were implanted in Europe.[5]
2. Methods
2.1. Problems with transvenous ICDs
While ICDs are certainly a life-saving technology, lead and device related complications are not insignificant. Complications of transvenous systems include both acute complications related to the implant procedure and late complications due to infection or lead malfunction. Acute complications include pneumothorax, traumatic pericardial effusion, lead dislodgement, hematoma and infection. While each of these complications is of a low event rate, one meta-analysis found an overall ICD complication rate of 9.1% over the first 16 months following implant.[6],[7]
Long-term complications are predominantly due to lead failure or device infection. Lead failure is significantly more common in defibrillator leads as compared to pacemaker leads, due to the complex, multicomponent engineering. Long-term studies have shown lead survival rates that drop quite abruptly after five years to only 60% at 8 years and with a 20% annual failure rate at 10 years.[8],[9] Some studies have shown that younger age is a risk factor for lead failure.[9] These numbers are significant as the rates of ICD implants continue to increase over time. Recent studies show that even patients with advanced heart failure with reduced LVEF and New York Heart Association (NYHA) class III symptoms have a close to 60% survival rate at five years with a mortality benefit related to ICD implant.[10]
Table 1. Pros and Cons of Transvenous ICD vs. Subcutaneous ICD.
| Transvenous system | Subcutaneous system | |
| Extraction | High risk | Low risk |
| Antitachycardia pacing | Available | Not available* |
| Backup pacing capabilities | Available | Not available* |
| Venous access requirements | Necessary | Not required |
| CRT capabilities | Available | Not available |
*Antitachycardia pacing and back up pacing could become available with the combined Subcutaneous ICD and leadless system. ICD: implantable cardioverter defibrillators.
Table 2. Summary of the Major SICD Studies.
| SICD-IDE Study | Effortless Registry | SICD-PAS Study | |
| Number of Patients | 330 | 472 | 1637 |
| Average Age, yrs | 51.9 ± 15.5 | 49 ± 18 | 53.2 ± 15 |
| Mean EF, % | 36.1 ± 15.9 | 42 ± 19 | 32 ± 14.6 |
| Complications, % | 7.9% (180-day complication rate) | 3% and 6% (30-day and 1 year complication rate respectively) | 3.8% (30-day complication rate) |
| Acute Conversion Success of Induced VF | 100% | 99.7% | 98.7% |
| Spontaneous VT/VF Total Shock Efficacy | 97.1% | 100% | N/A* |
*The SICD-PAS was an acute complication study. Long-term Follow-up not published at this time. EF: ejection fraction; IDE: Investigational Device Exemption; PAS: post approval registry; SICD: subcutaneous implantable cardioverter defibrillator; VT/VF: ventricular tachycardia/ventricular fibrillation. This table is modified from Gold MR.[24]
Infection risk is another concern that persists throughout the life of a transvenous ICD. The time of generator exchange is a particular point of concern, as the infection risk is approximately double that of initial implant.[11] The REPLACE registry, a prospective multicenter evaluation of patients undergoing cardiac implantable electronic device (CIED) generator replacement, showed a 1.6% incidence of infection at time of replacement of ICD or cardiac resynchronization-defibrillator (CRT-D) generator.[12] A study of a contemporary cohort of patients with ICDs or CRT-Ds showed a median battery longevity of 5.9 years for single and dual chamber ICDs and 4.9 years for CRT-Ds.[13] As such, most patients with ICDs will undergo at least one generator exchange after initial implant, and some may undergo multiple, especially those with a positive response to CRT devices.
The primary concern with long-term complications of CIEDs is the need for lead extraction. This is certainly necessary in almost all cases of device infection, and while not absolutely necessary in situations of lead malfunction, it is frequently the preferred method of management. Device extraction of a chronic transvenous lead is a procedure with the potential for significant morbidity and mortality. While the absolute complication rates are low, the severity of potential complications, namely massive intrathoracic bleeding and death, is high. High volume centers describe a major procedural complication rate of 1.3%–1.9% and procedural mortality of 0.3%–0.65%.[14],[15] Furthermore, mortality following extraction is high, up to 10% at 12 months.[16]
2.2. The need for the S-ICD
The impetus for the development of a non-endovascular defibrillator system arose not only from the issues of managing complications as described above, but also for concern for specific patient populations including pediatric patients, those with difficult or absent venous access, and those at high risk for bacteremia such as dialysis patients.
In 2012 the Food and Drug Administration (FDA) approved the first entirely subcutaneous implantable defibrillator. While the basic components of the S-ICD are similar to that of the traditional transvenous device, i.e. a pulse generator and a defibrillator coil, there are significant differences, from implant technique to device capabilities, which will be described in detail below.
The S-ICD is comprised of two primary components: a pulse generator, implanted in a left lateral position in the midaxillary line at the level of the 5th–6th intercostal spaces, and a parasternal defibrillator coil (Figure 1). The entire system is implanted in the subadipose space, with the defibrillator coil tunneled from the pulse generator to the left parasternal line just below the xiphoid process and then superiorly along the parasternal line to just below the sternal notch (Figure 1). The procedure can be performed under general anesthesia, monitored anesthesia care (MAC) or moderate sedation, though registry data show the majority of implanters use general anesthesia.[17] Fluoroscopic guidance is not necessary for implantation, though can be used to help confirm anatomic landmarks. The S-ICD device can deliver a shock of up to 80 J.
Figure 1. Chest X-ray of patient with S-ICD.
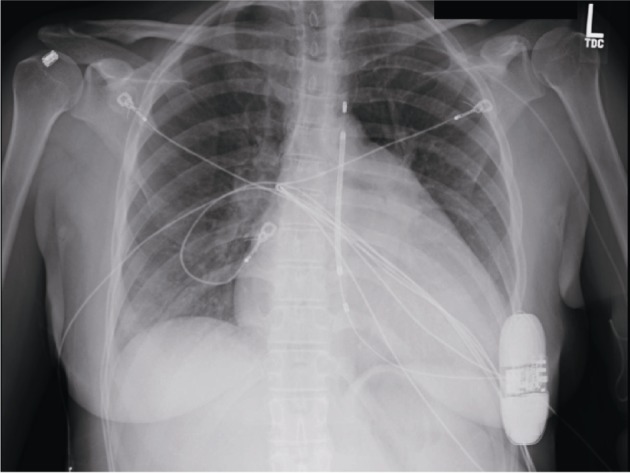
Pulse generator is located in left mid-axillary line at level of the 5th–6th intercostal spaces with defibrillator coil tunneled from the pulse generator to the left parasternal region.
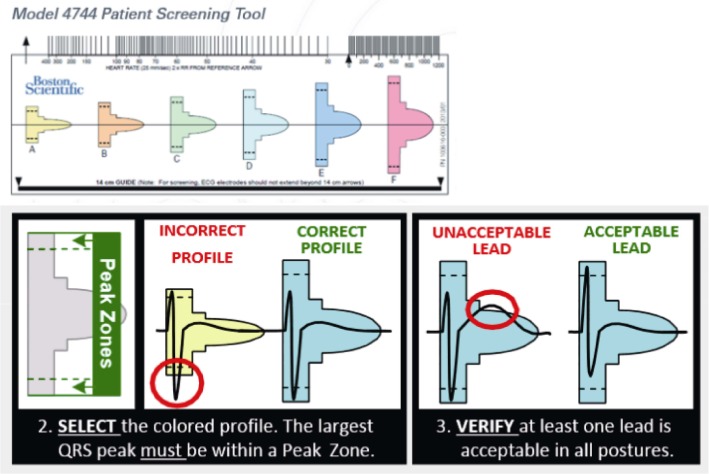
Because the S-ICD is not an endocardial device, there are two primary reasons a patient may not be a candidate for implantation. First, while the device has a programmable 30-second post-shock pacing capability, it is not otherwise a pacing device. Thus, for patients who have concomitant pacing needs, such as sinus node dysfunction, Atrio-ventricula (AV) block, or CRT indications, or who would benefit from anti-tachycardia pacing for rhythm termination, the S-ICD is not a functional option. Second, the detection algorithm that the device utilizes for detection of ventricular arrhythmias relies on a subcutaneous electrode. Patients must be screened to ensure adequate QRS and T wave sensing to avoid both undersensing of intrinsic QRS and T wave oversensing (TWOS), the latter of which is the predominant cause of inappropriate shocks in the S-ICD population.[18] The device has three sensing vectors; prior to implant patients are screened in both supine and either standing or sitting positions to ensure at least one vector has appropriate morphology sensing in both tested postures. The screening tool looks at the QRS amplitude as an absolute and in relation to the T wave amplitude (Figure 2).[19] Early outcomes on high rates of inappropriate shocks due to TWOS, especially during exercise, led some providers to add exercise testing to the pre-implant screening. Newer data shows that with current screening and detection algorithms, however, exercise screening does not improve upon discrimination of patients at risk for TWOS.[20]
Figure 2. The manual screening tool (courtesy of Boston Scientific).
The QRS need to fit in the rectangular space (correct profile or acceptable lead). In the acceptable profile the T wave need to be encased within the screening profile (acceptable vs. unacceptable profile).
2.3. The S-ICD Studies
The initial feasibility and early phase study for the S-ICD was published in 2010. The paper described both the initial evaluation of optimal configuration of generator and defibrillator coil, as well as outcomes on a total of sixty-one patients (6 patients in the initial pilot study and 55 patients in a follow up clinical trial).[21] The initial studies proved that the device could consistently and correctly detect and successful treat ventricular arrhythmias.
Following this, the pre-market Investigational Device Exemption (IDE) study was commenced. This was a prospective, nonrandomized multicenter trial that enrolled 330 patients between January 2010 and May 2011. The average age of the cohort was 51.9 ± 15.5 years and the average left ventricular ejection fraction was 36.1 ± 15.9 %. 79% of patients had a primary prevention indication for ICD and 41.4% had a prior myocardial infarction. The primary effectiveness endpoint, the acute induced Ventricular fibrillation (VF) conversion rate at time of implant, was reached in 100% of those who completed the full testing protocol. Furthermore, the study showed a 97.4% success rate in converting spontaneous Ventricular tachycardia (VT)/VF when occurring as discrete episodes, and no arrhythmic deaths even when VT/VF occurred in the setting of VT/VF storm. The primary safety end-point was the 180-day complication free rate, which was 92.1%. There were no cases of lead failure, endocarditis or bacteremia. As expected, given the subcutaneous nature of the device, there were no cases of cardiac perforation or tamponade, pneumothorax, or subclavian vein stenosis. The infection rate was 5.6%; the majority of these infections (14/18) were managed without system explantation. The inappropriate shock rate was 13.1%, and the majority of these cases were due to oversensing (either of T waves, broad QRS complexes, or external electrical noise).[22]
The EFFORTLESS S-ICD registry is an observational registry of patients from Europe and New Zealand implanted with the S-ICD since commercial availability of the system in 2009. Early outcomes were published in 2014.[17] The average age of the cohort was 49 ± 18 years and the average LVEF was 42 ± 19 %. 63% of patients had a primary prevention indication for ICD and 40% had ischemic cardiomyopathy. The mean duration of follow up was 558 days. This real world, post-PCT data showed very similar efficacy and safety outcomes to the IDE data. Of the patients who underwent Defibrillation threshold (DFT) testing at or shortly after implantation, 99.7% of patients were successfully converted with the S-ICD with a shock energy of ≤ 65 J in 95% of cases. When cases of spontaneous VT/VF were evaluated, the discrete VT/VF conversion efficacy was 100%, though 12% of these cases required more than one shock to convert. There were 6 VT/VF storm episodes in 4 patients; one of these patients died due to VF that was not successfully defibrillated. The overall infection rate was 4%, and the infection requiring explant rate was 2.2%. The inappropriate shock rate was 7%; 85% of these cases were due to oversensing. The overall patient complication event rate, as defined as events requiring an invasive procedure for correction, was 6.4%.[17]
A large-scale evaluation of S-ICD outcomes was published in 2015.[23] Combining the IDE and EFFORTLESS registries, the authors studied a total of 882 patients with a mean follow up of 651 ± 345 days. The average age of the cohort was 50 ± 17 years and the average LVEF was 39.4 ± 17.6%. 70% of patients had a primary prevention indication for ICD and 37.8 % had ischemic cardiomyopathy. 79.2% of patients were programmed with two therapy zones. The 30-day complication rate was 4.5%, and the complication rate over 3 years was 11.1%. The rate of acute complications was 2%; this included hematomas, sedation complications, and lead/generator malposition or displacement. 1.7% of patients developed an infection requiring removal or revision and 1.2% of patients developed device erosion. Of 111 discrete VT/VF episodes, 90.1% converted with the first shock and 98.2% converted with the 5 available shocks. There were no deaths due to unconverted episodes. Of 12 VT/VF storms, 10 converted with S-ICD shocks. 1 patient died due to VF that failed to convert with therapy and the other required external defibrillation. The time to therapy was 19.2 ± 5.3 seconds. There were 15 episodes of syncope reported by 15 patients; 3 of these were related to documented arrhythmias on day of syncope (2 patients with untreated VT/VF due to self-termination prior to shock delivery, and 1 patient with VF that terminated after 5 administered shocks). The rate of inappropriate therapy was 20.5% for patients with single zone programming, and 11.7% for patients with dual zone programming. 70% of inappropriate shocks were due to sensing issues; 39% due to TWOS, 21% due to oversensing of low amplitude signals, and 8% due to noncardiac oversensing. All-cause mortality was 2.9% over the study period.[23]
Recently, the SICD post approval registry (SICD-PAS), the largest registry of SICD patients in the U.S. was published.[24] This registry described the characteristics and acute outcomes of patients implanted with an SICD in a real world setting and outside the investigational study. This registry enrolled more than 1600 patients. The mean age of the cohort was 52 ± 15 years compatible with the trend to implant younger patients with an SICD. The mean LVEF was 32 ± 14.6% and 74% had congestive heart failure. In this registry, patients receiving the SICD had more traditional indications for an ICD in contrast to the earlier SICD registries from Europe, in which a larger proportion of young patients with channelopathies received the SICD.[25],[26] In the SICD-PAS, 98.7% of induced VT/VF were successfully converted.
Outcomes in unique patient populations have been studied and the S-ICD performance has been shown to be similar to transvenous systems. These include:
(1) Patients with concurrent transvenous pacing: small case series have described the safety and feasibility of S-ICDs to appropriately sense and treat VT/VF even in the case of concomitant ventricular pacing.[27]
(2) End stage renal disease (ESRD): patients with ESRD requiring dialysis can be safely implanted with the S-ICD with no increase in implant related complications or inappropriate shocks.[28]
(3) Hypertrophic cardiomyopathy: patients with hypertrophic cardiomyopathy were a population for which there was concern for a high inappropriate shock rate due to high voltage T waves, as well as high DFTs due to left ventricular hypertrophy. Pooled data from the EFFORTLESS and IDE cohorts show no significant difference in successful defibrillation at implant testing, one-year complication free rates, or inappropriate shocks. The event rate for spontaneous VT/VF was low for the Hypertrophic cardiomyopathy (HCM) population, but all events successfully converted with a single shock.[29]
(4) Congenital heart disease (CHD): within larger registries of S-ICD implants, small cohorts of patients with congenital heart disease have been identified. The data supports that S-ICDs can be implanted safely and with low rates of complications. The devices successfully identify and treat ventricular arrhythmias (mostly based on implant testing due to low rates of clinical ventricular arrhythmias in follow up).[30],[31] Rates of inappropriate shocks in one case series was significant at 21% over a median follow up of 14 months,[31] though similar rates (25%) have been reported in patients with CHD with transvenous devices.[32]
The outcomes as described above show similar performance between transvenous ICDs and subcutaneous ICDs in efficacy, safety of implantation and long-term outcomes. A 2017 meta-analysis of studies directly comparing clinical outcomes between the two technologies supports this conclusion. Rates of infection [0.34% vs. 0.31%, S-ICD vs. Transvenous (TV)], system failure (0.32% vs. 0.24%) and total inappropriate therapy (8.3% vs. 9.46%) were similar without statistical significant in the minor differences. Lead complications were significantly lower in subcutaneous systems [0.14% vs. 1.02%, odds ratios (OR): 0.13].[33] Furthermore, first shock efficacy in terminating spontaneous VT/VF is similar between the SICD and transvenous ICD. For instance, in the pooled IDE and EFFORLESS analysis the first shock efficacy for terminating VT/VF was 90.1%, and 98.2% of all spontaneous episodes were successfully treated by the SICD. Two trials evaluating transvenous ICDs, the SCD-HeFT and MADIT-CRT trials, showed first shock efficacy in terminating clinical VT/VF of 83% and 90%, respectively.[34],[35]
The concerns about inappropriate shocks are not insignificant, not only as related to patient comfort and the anxiety caused by inappropriate shocks, but also because of signals that inappropriate ICD shocks are associated with increased all-cause mortality.[36] However, it must be pointed out that with changes in the initial ECG screening process, adjustments to detection algorithms and dual zone programming, inappropriate shock rates are comparable for subcutaneous and transvenous devices. Within the EFFORTLESS registry, dual zone programming had an overall inappropriate shock rate of 6.4% vs. 12% for single zone programming.[17] Furthermore, the mechanism for inappropriate shocks differs significantly between the two ICD technologies. Inappropriate shocks in transvenous systems are most frequently due to atrial fibrillation, which itself is independently associated with increased mortality in heart failure patients.[37] This is in comparison to S-ICDs, where inappropriate shocks are mostly due to TWOS.[33] There is evidence that in a transvenous ICD population, patients who receive inappropriate shocks attributable to non-AF/AFL causes do not have a significant difference in survival compared with patients who did not receive any ICD shocks.[38] Whether or not inappropriate shocks will carry any mortality association in S-ICD populations will have to be determined in longer term trials.
2.4. Future application
Subcutaneous ICD technology in its present form is nearing a decade. Future applications are already in development. A multi-component system comprised of a leadless pacemaker with Anti-tachycardia pacing (ATP) capabilities and a subcutaneous ICD, the two components of which can wirelessly communicate with one another, has been successfully studied in multiple animal models.[39] The potential for further reducing the size of the generator will surely be explored. The role of DFT testing is likely to evolve, similar to its course in transvenous system. Preliminary nonrandomized data shows a strategy that omits implant DFT testing does not lead to clinically different outcomes in device efficacy.
3. Conclusions
The S-ICD has been shown across patient populations to be a safe and effective device for appropriately sensing malignant ventricular arrhythmias and delivering successful rescue therapy. The higher rates of inappropriate shocks seen in early studies have been partially ameliorated with dual zone programming and improvement in screening and detection algorithms. While patients with pacing indications are not candidates for the S-ICD, the technology is one that can arguably be recommended for all other patients, and particularly those with challenging venous anatomy, younger age, increased risk for blood stream infection, and prior infection related to transvenous device.
Footnotes
Disclosure: Mikhael El-Chami is a consultant for Boston Scientific and Medtronic.
References
- 1.Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303:322–324. doi: 10.1056/NEJM198008073030607. [DOI] [PubMed] [Google Scholar]
- 2.Lown B, Axelrod P. Implanted standby defibrillators. Circulation. 1972;46:637–639. doi: 10.1161/01.cir.46.4.637. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary. Heart Rhythm. 2008;5:934–955. doi: 10.1016/j.hrthm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34:1013–1027. doi: 10.1111/j.1540-8159.2011.03150.x. [DOI] [PubMed] [Google Scholar]
- 5.Raatikainen MJ, Arnar DO, Zeppenfeld K, et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace. 2015;1:i1–75. doi: 10.1093/europace/euu300. [DOI] [PubMed] [Google Scholar]
- 6.Ezzat VA, Lee V, Ahsan S, et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our 'real-world' data an underestimation? Open Heart. 2015;2:e000198. doi: 10.1136/openhrt-2014-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezzat VA, Lee V, Ahsan S, et al. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. 2011;58:995–1000. doi: 10.1016/j.jacc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Dorwarth U, Frey B, Dugas M, et al. Transvenous defibrillation leads: high incidence of failure during long-term follow-up. J Cardiovasc Electrophysiol. 2003;14:38–43. doi: 10.1046/j.1540-8167.2003.02305.x. [DOI] [PubMed] [Google Scholar]
- 9.Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of > 10 years. Circulation. 2007;115:2474–2480. doi: 10.1161/CIRCULATIONAHA.106.663807. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DJ, Al-Khatib SM, Zeitler EP, et al. New York Heart Association class and the survival benefit from primary prevention implantable cardioverter defibrillators: a pooled analysis of 4 randomized controlled trials. Am Heart J. 2017;191:21–29. doi: 10.1016/j.ahj.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchant FM, Quest T, Leon AR, et al. Implantable cardioverter-defibrillators at end of battery life: opportunities for risk (re)-stratification in ICD recipients. J Am Coll Cardiol. 2016;67:435–444. doi: 10.1016/j.jacc.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Uslan DZ, Gleva MJ, Warren DK, et al. Cardiovascular implantable electronic device replacement infections and prevention: results from the REPLACE Registry. Pacing Clin Electrophysiol. 2012;35:81–87. doi: 10.1111/j.1540-8159.2011.03257.x. [DOI] [PubMed] [Google Scholar]
- 13.Zanon F, Martignani C, Ammendola E, et al. Device longevity in a contemporary cohort of ICD/CRT-D patients undergoing device replacement. J Cardiovasc Electrophysiol. 2016;27:840–845. doi: 10.1111/jce.12990. [DOI] [PubMed] [Google Scholar]
- 14.El-Chami MF, Merchant FM, Levy M, et al. Outcomes of Sprint Fidelis and Riata lead extraction: Data from 2 high-volume centers. Heart Rhythm. 2015;12:1216–1220. doi: 10.1016/j.hrthm.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 15.Fu HX, Huang XM, Zhong LI, et al. Outcomes and complications of lead removal: can we establish a risk stratification schema for a collaborative and effective approach? Pacing Clin Electrophysiol. 2015;38:1439–1447. doi: 10.1111/pace.12736. [DOI] [PubMed] [Google Scholar]
- 16.Gomes S, Cranney G, Bennett M, et al. Long-term outcomes following transvenous lead extraction. Pacing Clin Electrophysiol. 2016;39:345–351. doi: 10.1111/pace.12812. [DOI] [PubMed] [Google Scholar]
- 17.Lambiase PD, Barr C, Theuns DA, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–1665. doi: 10.1093/eurheartj/ehu112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olde Nordkamp LR, Brouwer TF, Barr C, et al. Inappropriate shocks in the subcutaneous ICD: Incidence, predictors and management. Int J Cardiol. 2015;195:126–133. doi: 10.1016/j.ijcard.2015.05.135. [DOI] [PubMed] [Google Scholar]
- 19.S-ICD Screen Guide. KDG Web site. 2018. [Accessed Jan 5, 2018]. http://www.kdg.com/BSC/SICD/story_content/external_files/CRM-223202-AA_S-ICD_screen_guide_Final.pdf.
- 20.Afzal MR, Evenson C, Badin A, et al. Role of exercise electrocardiogram to screen for T-wave oversensing after implantation of subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2017;14:1436–1439. doi: 10.1016/j.hrthm.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 22.Weiss R, Knight BP, Gold MR, et al. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 23.Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615. doi: 10.1016/j.jacc.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 24.Gold MR, Aasbo JD, El-Chami MF, et al. Subcutaneous implantable cardioverter-defibrillator post-approval study: clinical characteristics and perioperative results. Heart Rhythm. 2017;14:1456–1463. doi: 10.1016/j.hrthm.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Olde Nordkamp LR, Dabiri Abkenari L, Boersma LV, et al. The entirely subcutaneous implantable cardioverter-defibrillator: initial clinical experience in a large Dutch cohort. J Am Coll Cardiol. 2012;60:1933–1939. doi: 10.1016/j.jacc.2012.06.053. [DOI] [PubMed] [Google Scholar]
- 26.Jarman JW, Todd DM. United Kingdom national experience of entirely subcutaneous implantable cardioverter-defibrillator technology: important lessons to learn. Europace. 2013;15:1158–1165. doi: 10.1093/europace/eut016. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Patton KK, Prutkin JM, et al. Concomitant use of the subcutaneous implantable cardioverter defibrillator and a permanent pacemaker. Pacing Clin Electrophysiol. 2016;39:1240–1245. doi: 10.1111/pace.12955. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Patton KK, Prutkin JM, et al. Outcome of subcutaneous implantable cardioverter defibrillator implantation in patients with end-stage renal disease on dialysis. J Cardiovasc Electrophysiol. 2015;26:900–904. doi: 10.1111/jce.12705. [DOI] [PubMed] [Google Scholar]
- 29.Lambiase PD, Gold MR, Hood M, et al. Evaluation of subcutaneous ICD early performance in hypertrophic cardiomyopathy from the pooled EFFORTLESS and IDE cohorts. Heart Rhythm. 2016;13:1066–1074. doi: 10.1016/j.hrthm.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 30.D'Souza BA EA, Garcia FC, et al. Outcomes in patients with congenital heart disease receiving the subcutaneous implantable-cardioverter defibrillator: results from a pooled analysis from the IDE study and EFFORTLESS S-ICD Registry. JACC Clin Electrophysiol. 2016;2:615–622. doi: 10.1016/j.jacep.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Moore JP, Mondésert B, Lloyd MS, et al. Clinical experience with the subcutaneous implantable cardioverter-defibrillator in adults with congenital heart disease. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004338. [DOI] [PubMed] [Google Scholar]
- 32.Vehmeijer JT, Brouwer TF, Limpens J, et al. Implantable cardioverter-defibrillators in adults with congenital heart disease: a systematic review and meta-analysis. Eur Heart J. 2016;37:1439–1448. doi: 10.1093/eurheartj/ehv735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu-Ray I, Liu J, Jia XM, et al. Subcutaneous versus transvenous implantable defibrillator therapy a Meta-analysis of case-control studies. JACC: Clinical Electrophysiology. 2017;3:1475–1483. doi: 10.1016/j.jacep.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 34.Blatt JA, Poole JE, Johnson GW, et al. No benefit from defibrillation threshold testing in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) J Am Coll Cardiol. 2008;52:551–556. doi: 10.1016/j.jacc.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 35.Kutyifa V, Huth Ruwald AC, Aktas MK, et al. Clinical impact, safety, and efficacy of single-versus dual-coil ICD leads in MADIT-CRT. J Cardiovasc Electrophysiol. 2013;24:1246–1252. doi: 10.1111/jce.12219. [DOI] [PubMed] [Google Scholar]
- 36.Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 37.Li A, Kaura A, Sunderland N, et al. The significance of shocks in implantable cardioverter defibrillator recipients. Arrhythm Electrophysiol Rev. 2016;5:110–116. doi: 10.15420/AER.2016.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell BD, Saxon LA, Boehmer JP, et al. Survival after shock therapy in implantable cardioverter-defibrillator and cardiac resynchronization therapy-defibrillator recipients according to rhythm shocked. The ALTITUDE survival by rhythm study. J Am Coll Cardiol. 2013;62:1674–1679. doi: 10.1016/j.jacc.2013.04.083. [DOI] [PubMed] [Google Scholar]
- 39.Tjong FV, Reddy VY. Permanent leadless cardiac pacemaker therapy: a comprehensive review. Circulation. 2017;135:1458–1470. doi: 10.1161/CIRCULATIONAHA.116.025037. [DOI] [PubMed] [Google Scholar]