Abstract
Background
The aim of this study was to investigate the immunological alterations that occur during the storage of erythrocyte suspensions which may lead to transfusion-related immunomodulation following allogeneic blood transfusion.
Materials and methods
One part of the erythrocyte suspensions obtained from donors was leucoreduced while the other part was not. The leucoreduced (LR) and non-leucoreduced (NL) erythrocyte suspensions were then further divided into three equal amounts which were stored for 0, 21 or 42 days prior to measurements, by enzyme-linked immunosorbent assays, of cytokine levels in their supernatants. T-helper (Th) lymphocyte subgroups and gene expression were analysed in the NL erythrocyte suspensions by flow cytometry and real-time polymerase chain reaction, respectively. Results were compared to those of storage day 0.
Results
By day 21, the number of Th2 cells had increased significantly and the numbers of Th1, Th22 and Treg cells had decreased significantly in the NL erythrocyte suspensions. On day 42 the numbers of Th2 and Treg cells in the NL suspensions were significantly increased while the number of Th1 cells was significantly decreased. The levels of transcription factors (TBX21, GATA3, and SPI.1) were significantly decreased on days 21 and 42, and AHR, FOXP3 and RORC2 levels were significantly increased on day 42 in NL erythrocyte suspensions. The decrease in interleukin-22 and increase in transforming growth factor-β levels found in NL erythrocyte suspensions on day 21 were statistically significant. Elevated levels of interleukin-17A were found in both LR and NL erythrocyte suspensions on day 42.
Discussion
Our results suggest that allogeneic leucocytes and cytokines may play significant roles in the development of transfusion-related immunomodulation.
Keywords: TRIM, immunomodulation, transfusion, Th cell subgroups, leucoreduction
Introduction
When performed at the right time and in the correct conditions, allogeneic blood transfusion is a lifesaving intervention. Although the benefit of such transfusions is obvious, they are associated with a real risk of serious and life-threatening complications. These complications can be classified as infectious, immunological and non-immunological. Transfusion-related immunomodulation (TRIM) is listed among the immunological complications, and can be defined as a consequence of changes within the immune system of the transfusion recipient, induced by allogeneic blood transfusion. It was first described in patients waiting for renal transplantation, based on the observation that allogeneic blood transfusions prolonged graft survival1. TRIM also seems to be associated with increases in cancer recurrence, graft survival, post-operative bacterial infection, and short-term mortality rates, decreases in Crohn’s disease recurrences and recurrent spontaneous abortion, and reactivation of certain latent infections such as those caused by cytomegalovirus and human immunodeficiency virus2–15. The role of allogeneic leucocytes16,17, microchimerism18,19, biological response modifiers (BRM)20, cytokines21–24, bioactive lipids25,26, erythrocyte suspension (ES) supernatant27,28, storage duration of blood components29,30, soluble Fas ligand21,31, soluble human leucocyte antigen (HLA) class I molecules21,32,33, and similar potential factors in the development of TRIM have been investigated, but the essential mechanism underlying TRIM has not yet been elucidated34. Major changes that occur in the recipient’s immune system can be summarised as a decrease in the number of T-helper cells (Th), a reduction in T-cell response, a decrease in natural killer cell function, insufficient antigen presentation, suppression of lymphocyte blastogenesis, an increased production of anti-idiotypic antibodies and anti-clonotypic antibodies35,36, a decrease in the CD4/CD8 ratio35–37, decreased late type hypersensitivity reactions35,36,38, decreases in cytokine production (interleukin-2 [IL-2], interferon-gamma [IFN-γ])35,36,39, and an impairment of monocyte/macrophage phagocytic function35,36,40. Based on these immunological changes, it has been postulated that the effect of TRIM may originate from three sources: mononuclear cells (MNC) within the blood component6,17,41, BRM-immunological mediators (BRM-IM) that accumulate within the blood component during storage6,20,28,42, and soluble HLA class I peptides within the allogeneic plasma6,33,42.
These factors are thought to cause TRIM via mechanisms such as clonal deletion43,44, immunosuppression16,28,42, 45–47, anergy48,49, microchimerism50,51, transition of the immune response from Th1 to Th239,49,51–59, apoptosis60–62. In addition to above-mentioned factors, duration of ES storage, number of transfusion products, erythrocytes and erythrocyte-derived microparticles have also been associated with TRIM63–83. In general, allogeneic MNC and related structures are considered to be the main cause of TRIM. It is, therefore, thought that the effects of TRIM can be eliminated by leucoreduction. However, several components such as free haemoglobin, lipids, cytokines and microparticles that have been shown to pass through the filter used for leucoreduction and accumulate during storage in leucoreduced (LR) ES and LR platelet suspensions limit the effect of leucoreduction27.
Cytokines constitute a group of BRM-IM which may also lead to the TRIM effect in the recipient. Cytokines are proteins produced by different cell types which mediate inflammatory and immune responses. They are the primary mediators that provide connections between the cells of the immune system. Alterations in cytokine levels during the storage of LR-ES and non-leucoreduced (NL) ES have been demonstrated in previous studies84,85. The cytokine content of NL-ES and LR-ES supernatants during storage may, therefore, provide valuable information regarding potential TRIM effects and the efficiency of leucoreduction.
In this study, we aimed to investigate the changes that occur in ES during the storage period and the relationship between leucoreduction and TRIM. To do this, MNC and BRM-IM that are considered the main cause of TRIM within the product were evaluated in order to detect the changes in Th subgroups and cytokine profiles which occur during storage of ES, their association with TRIM and the relationship between leucoreduction and TRIM.
Materials and methods
Donation and preparation of blood component samples
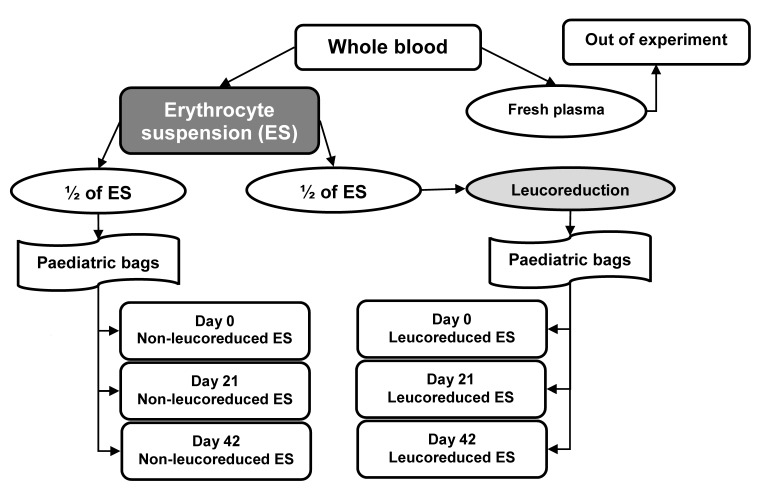
This study was approved by Uludag University School of Medicine Ethical Board (N. 2011–3/20). Ten units of whole blood were obtained from ten volunteers who met national blood donor selection criteria and were admitted to the “Dr. Raşit Durusoy” Blood Bank at the Uludag University School of Medicine. CPD/SAG-M quadruple paediatric component bags (Kansuk, Istanbul, Turkey) were used to store the blood donations. ES (with additive solution) and fresh plasma were obtained from the whole blood bags. The fresh plasma was not used in this study. NL-ES and LR-ES samples were prepared from ES for 0, 21 and 42 days of storage based on the algorithm shown in Figure 1. First, the ES was divided into two equal parts: one of these parts was then further divided, in three equal portions, into paediatric bags to be stored as the day 0, 21, and 42 NL-ES samples. The other part of the ES was connected under sterile conditions (Sterile Tube Connecting Device; Terumo, Lakewood, CO, USA) to a CPD/SAG-M quadruple paediatric component bag with whole blood filter (Kansuk). However, the main bag was disconnected from the new bag system before connection so that none of the solutions in this new bag system came into contact with the half unit of ES integrated into the system. After the connection procedure, the ES was filtered through the integrated 4 log leucocyte filter (Pall, Portsmouth, UK) and further divided into three equal portions as the LR-ES samples for 0, 21 and 42 days of storage. The NL-ES and LR-ES bags containing samples for storage for 21 and 42 days were stored in blood bank refrigerators (Nuve, Ankara, Turkey), while the day 0 samples were transferred to five test-tubes for laboratory analysis. All of the laboratory analyses were conducted on these samples. The same procedure was performed for laboratory analyses of the related samples stored for 21 and 42 days.
Figure 1.
Algorithm for preparing the samples from whole blood.
Th lymphocyte subgroups, specific transcription factors and plasma cytokine profiles were investigated in NL-ES samples. Th subgroups and specific transcription factors were not measured in LR-ES samples because the number of leucocytes within the product following 4log leucoreduction is theoretically expected to be insufficient. Only plasma cytokine profiles were evaluated in these samples.
Evaluation of T-helper subgroups in non-leucoreduced erythrocyte suspensions
Th1, Th2, Th9, Th17, Th22 and Treg cells were determined by flow cytometry based on surface markers and intracellular cytokine expression in each NL-ES sample. The monoclonal antibodies used in our study were IgG1 FITC, IgG1 APC, IgG1 PE, IgG2A PE, IgG1 AF647/CD3 FITC/CD4 PE (BioLegend, San Diego, CA, USA), IgG1-PE Cy5 (eBioscience, Waltham, MA, USA), CD3 APC, CD4 FITC, IFN-γ PE, IL-4 APC, IL-5 PE, IL-9 PE, IL-21 APC, IL-22 PE, IL-17 AF647 / CD3 FITC / CD4 PE (BioLegend), IL-13 FITC, IL-21 APC, Foxp3 PE Cy5, CD4 FITC / CD25 PE, CD127 APC (eBioscience), CD3 PerCP and CD4 PE (BD Biosciences, San Jose, CA, USA). Stained cells were evaluated using flow cytometry (Navios; Beckman Coulter, Indianapolis, IN, USA).
Evaluation of transcription factors in non-leucoreduced erythrocyte suspensions by polymerase chain reaction
Each NL-ES sample was analysed for the expression of specific transcription factors TBX21, GATA3, PU.1, RORC2, AHR and FOXP3 of Th cell subgroups. This process consisted of MNC isolation, total RNA isolation, complementary DNA (cDNA) synthesis and then real-time polymerase chain reaction (RT-PCR).
MNC were isolated by density gradient using Ficoll (Histopaque-1077, Sigma-Aldrich, St. Louis, MO, USA). Total RNA was isolated from 5×106 cells using a commercially available kit (MO BIO Laboratories Inc., Carlsbad, CA, USA) and then used for cDNA synthesis with the commercial First Strand cDNA Synthesis Kit (New England BioLabs Inc, Ipswich, MA, USA). cDNA samples that were obtained were stored in a freezer at −20 °C until RT-PCR analysis.
Real-Time Ready (Roche, Mannheim, Germany) designed for this study were used to measure the expression of specific transcription factors by RT-PCR. Panels consisted of six genes related to the transcription factors (TBX21, GATA3, AHR, SPI.1, FOXP3, RORC2), five reference genes (HPRT1, RPL13A, ACTB, GAPDH, YWHAZ), positive and negative controls. The expression of specific transcription factors was measured using Light-Cycler 480 RT-PCR (Roche). Following the RT-PCR analysis, relative quantification of target gene expression was performed. All data were analysed using the ΔΔCt method.
Measurement of cytokine levels
For cytokine analysis, supernatants were obtained from NL-ES and LR-ES through centrifugation of test-tubes at 3,500 g for 10 minutes. The supernatants were then re-centrifuged at 15,000 g for 7 minutes to discard cell debris; plasma samples were transferred into clean test-tubes and stored at −80 °C in a freezer (Revco, St. Louis, MO, USA) until use. Interleukin (IL)-4, IL-6, IL-8, IL-9, IL-10, IL-13, IL-17, IL-22, tumour necrosis factor (TNF)-α, transforming-growth factor (TGF)-β and interferon (IFN)-γ levels were measured in these samples using commercial enzyme-linked immunosorbent assay (ELISA) kits (Bio-Legend) according to the manufacturer’s recommendations. Minimum detectable levels of the ELISA were as follows: 3.2 pg/mL for IL-4, 7.8 pg/mL for IL-6, 15.6 pg/mL for IL-8; 6 pg/mL for IL-9, 3.9 pg/mL for IL-10, 15.6 pg/mL for IL-13, 3.9 pg/mL for IL-17A, 62.5 pg/mL for IL-22, 15.6 pg/mL for TNF-α, 7.8 pg/mL for TGF-β, and 15.6 pg/mL for IFN-γ.
Statistical analysis
Continuous variables are presented as medians (range). Between-group comparisons were performed using the Mann-Whitney test, whereas within-group comparisons were performed using Wilcoxon’s ranked-sum test. Percent changes (change between last measurement and first measurement divided by first measurement multiplied by 100) in variables that were measured in dependent time periods were also calculated, and the between-group comparisons were performed using the Mann-Whitney test. Statistical analyses were conducted using SPSS v.21 (IBM Corp., New York, NY, USA), and p-values of <0.05 are considered statistically significant.
Results
T-helper cell subgroups
Alterations in Th cell subgroups during the storage of NL-ES are presented in Table I. At day 21, there were reductions in Th cells expressing IFN-γ (p=0.005), IL-22 (p=0.028) and FOXP3 (p=0.034) when compared to day 0. There were increases in the number of Th cells expressing IL-4 (p=0.005), IL-5 (p=0.005) and Th cells that do not express CD127 (p=0.005). At day 42, there were reductions in Th cells expressing IFN-γ (p=0.007) when compared to day 0. There were increases in the number of Th cells expressing IL-4 (p=0.005), IL-5 (p=0.005), IL-17A (p=0.028) and Th cells that do not express CD127 (p=0.005).
Table I.
Alterations of T-helper cell subgroups in stored erythrocyte suspensions (%).
| Day 0 | Day 21 | Day 42 | ||
|---|---|---|---|---|
| CD3+CD4+IFN-γ+ | median | 0.40 | 0.00 | 0.00 |
| (min:max) | (0.20:1.00) | (0.00:0.10) | (0.00:0.30) | |
|
| ||||
| CD3+CD4+IL-4+ | median | 0.25 | 2.00 | 3.70 |
| (min:max) | (0.10:0.70) | (0.50:5.90) | (1.70:11.80) | |
|
| ||||
| CD3+CD4+IL-5+ | median | 7.70 | 20.80 | 44.30 |
| (min:max) | (2.40:17.40) | (2.90:34.20) | (20.00:67.30) | |
|
| ||||
| CD3+CD4+IL-9+ | median | 0.65 | 0.50 | 0.30 |
| (min:max) | (0.20:1.60) | (0.00:1.40) | (0.00:1.20) | |
|
| ||||
| CD3+CD4+IL-13+ | median | 1.95 | 3.65 | 4.65 |
| (min:max) | (1.10:8.60) | (2.30:8.20) | (0.70:8.90) | |
|
| ||||
| CD3+CD4+IL-17A+ | median | 0.90 | 2.15 | 3.90 |
| (min:max) | (0.20:2.30) | (0.50:7.50) | (0.20:17.80) | |
|
| ||||
| CD3+CD4+IL-21+ | median | 6.65 | 6.40 | 6.15 |
| (min:max) | (1.20:9.00) | (2.50:21.70) | (0.40:20.30) | |
|
| ||||
| CD3+CD4+IL-22+ | median | 1.05 | 0.45 | 0.55 |
| (min:max) | (0.50:3.00) | (0.10:1.00) | (0.10:6.50) | |
|
| ||||
| CD4+CD25+highFoxp3+ | median | 2.70 | 0.00 | 29.40 |
| (min:max) | (0.00:24.30) | (0.00:6.90) | (0.00:64.80) | |
|
| ||||
| CD4+CD25+highCD127− | median | 0.30 | 1.70 | 3.20 |
| (min:max) | (0.20:1.60) | (0.80:5.80) | (0.40:33.60) | |
Results are presented as percentage rates, showing the median (minimum and maximum) values. p-values <0.05 are considered as statistically significant and significant changes are shown in italic.
Transcription factors
Changes in expression of specific transcription factors during the storage of NL-ES samples are presented in Table II. At day 21, there were reductions in the expression of TBX21 (p=0.005), GATA3 (p=0.005) and SPI.1 (p=0.007) when compared to day 0. At day 42, there were statistically significant reductions in TBX21 (p=0.005), GATA3 (p=0.005) and SPI.1 (p=0.005) gene expression and statistically significant increases in the expression of AHR (p=0.005), FOXP3 (p=0.005) and RORC2 (p=0.005) when compared to day 0.
Table II.
Alterations in T-cell subgroup-specific transcription factors in stored erythrocyte suspensions.
| ΔΔCt | Day 0 | Day 21 | Day 42 | |
|---|---|---|---|---|
| TBX21 | median | 0.00064 | 0.00000 | 0.00023 |
| (min:max) | (0.000:0.004) | (0.000:0.001) | (0.000:0.002) | |
|
| ||||
| GATA3 | median | 0.01750 | 0.00147 | 0.00000 |
| (min:max) | (0.006:0.027) | (0.000:0.005) | (0.000:0.000) | |
|
| ||||
| AHR | median | 0.02410 | 0.00560 | 0.38495 |
| (min:max) | (0.011:0.098) | (0.000:0.411) | (0.336:0.437) | |
|
| ||||
| SPI.1 | median | 0.25925 | 0.03665 | 0.00019 |
| (min:max) | (0.167:0.564) | (0.000:0.212) | (0.000:0.001) | |
|
| ||||
| FOXP3 | median | 0.00273 | 0.00040 | 0.05410 |
| (min:max) | (0.002:0.006) | (0.000:0.062) | (0.042:0.098) | |
|
| ||||
| RORC2 | median | 0.00131 | 0.00000 | 0.33805 |
| (min:max) | (0.000:0.006) | (0.000:0.458) | (0.232:0.492) | |
Results are presented as ΔΔCt values, showing the median (minimum and maximum) values. p values <0.05 are considered as statistically significant and significant changes are shown in italic.
Cytokine levels
IL-4, IL-6, IL-9, IL-10 and TNF-α could not be detected in any of the samples by ELISA. IL-22 and TGF-β levels were only detected in NL-ES plasma samples (Table III). There were no significant changes in IL-8, IL-13 and IFN-γ levels in either NL-ES or LR-ES at day 21 or day 42 when compared to day 0. IL-22 levels were decreased (p=0.043) and TGF-β levels were increased (p=0.008) in NL-ES samples at day 21, when compared to day 0. At day 42, TGF-β levels were increased in NL-ES samples when compared to the day 0 samples. The increase in TGF-β levels was very close to being statistically significant (p=0.051). There was an increase in IL-17A levels in LR-ES samples which was statistically significant (p=0.012). When percent changes in IL-8, IL-13, IL-17A and IFN-γ levels were compared between NL-ES and LR-ES groups, a greater increase in IL-17A levels was noted in LR-ES samples than in NL-ES samples at day 42 (p=0.050). IL-22 and TGF-β levels were excluded from this comparative analysis since these cytokines could not be detected in LR-ES samples.
Table III.
Alterations of cytokine levels in stored erythrocyte suspensions.
| (pg/mL) | IL-8 | IL-13 | |||
|---|---|---|---|---|---|
|
|
|
||||
| LR-ES | NL-ES | LR-ES | NL-ES | ||
| DAY 0 | median | 25.46 | 40.01 | 11.36 | 13.19 |
| (min:max) | (17.65:112.60) | (25.46:200.39) | (8.48:173.26) | (4.44:174.64) | |
|
| |||||
| DAY 21 | median | 34.56 | 63.50 | 16.05 | 16.38 |
| (min:max) | (12.45:102.84) | (27.41:1218.76) | (4.44:639.15) | (0.74:61.62) | |
|
| |||||
| DAY 42 | median | 28.71 | 36.51 | 23.28 | 21.43 |
| (min:max) | (20.26:495.62) | (27.41:2214.37) | (5.11:877.63) | (0.00:142.68) | |
|
| |||||
| IL-17A | IL-22 | ||||
|
|
|||||
| LR-ES | NL-ES | LR-ES | NL-ES | ||
|
| |||||
| DAY 0 | median | 5.58 | 4.24 | 0.00 | 3.67 |
| (min:max) | (2.55:9.78) | (2.93:14.41) | (0.00:0.00) | (0.00:650.46) | |
|
| |||||
| DAY 21 | median | 6.79 | 5.78 | 0.00 | 0.00 |
| (min:max) | (5.25:10.74) | (2.07:12.96) | (0.00:0.00) | (0.00:2.33) | |
|
| |||||
| DAY 42 | median | 9.20 | 3.66 | 0.00 | 0.00 |
| (min:max) | (6.30:13.93) | (1.87:14.70) | (0.00:0.00) | (0.00:454.40) | |
|
| |||||
| IFN-γ | TGF-β | ||||
|
|
|||||
| LR-ES | NL-ES | LR-ES | NL-ES | ||
|
| |||||
| DAY 0 | median | 22.88 | 23.34 | 0.00 | 1.39 |
| (min:max) | (0.01:161.48) | (0.01:481.48) | (0.00:0.00) | (0.00:62.72) | |
|
| |||||
| DAY 21 | median | 26.60 | 13.81 | 0.00 | 55.87 |
| (min:max) | (0.00:365.67) | (0.55:380.09) | (0.00:0.00) | (0.00:106.06) | |
|
| |||||
| DAY 42 | median | 12.65 | 19.39 | 0.00 | 36.84 |
| (min:max) | (0.00:304.74) | (1.48:537.30) | (0.00:0.00) | (0.00:162.61) | |
Results (pg/mL) are presented as the median (minimum and maximum) values. p-values <0.05 are considered as statistically significant and significant changes are shown in italic. LR-ES: leucoreduced erythrocyte suspension; NL-ES: non-leucoreduced erythrocyte suspension; IL-8: interleukin-8; IL-13: interleukin-13; IL-17A: interleukin-17A; IL-22: interleukin-22; IFN-γ: interferon-gamma; TGF-β; transforming growth factor-beta.
Discussion
MNC within blood components and the supernatant are two major drivers of the TRIM phenomenon. Storage duration61,62,72–74,86 and number of transfused products7,71 are also thought to contribute to this effect. In a breakthrough study by Baumgartner et al., NL-ES and LR-ES supernatants and MNC were mixed in a culture medium and all supernatants were found to induce Treg induction28. It was reported that this induction was not associated with leucoreduction, storage duration or cytokines. We investigated the changes in MNC and supernatant within ES over a period of storage and examined the effectiveness of leucoreduction. Differently from Baumgartner et al., we aimed to investigate all Th subgroups and not only Treg cells. Analyses were performed directly on MNC in ES and supernatant rather than cell culture. Moreover, 4log leucocyte filters were used instead of 3log filters during the production of LR-ES.
Changes in T-helper cell profile
Previous studies reported contradictory results regarding the relationship between allogeneic MNC and the TRIM effect, some supporting7,71,87–89, some against this phenomenon90–97. CD4+ Th cells, being the primary cells mediating cellular or humoral immune responses, have been accused of being among those cells that are responsible. These cells develop from naïve Th cells in secondary lymphoid organs in response to specific antigen presentation and cytokine mediation98.
Effector Th cells are classified in subgroups such as Th1, Th2, Th9, Th17, Th22, and Treg according to their expression of intracellular cytokines under the influence of specific transcription factors. We investigated all these Th subgroups in our study, studying their surface markers, intracellular cytokine profiles and specific transcription factors.
Th1 cells differentiate from naïve Th cells under the influence of the specific transcription factor TBX21 (T-bet), which is expressed following IL-12 and IFN-γ stimulation, and produce high amounts of IFN-γ99–102. Significant reductions in TBX21 and CD3+CD4+IFN-γ+ levels in NL-ES noted on storage days 21 and 42 may suggest a reduction in the Th1 type response. This finding parallels those of previous studies demonstrating reductions in Th1 type cytokine levels in recipients following allogeneic blood transfusion39,51,56–59.
Th2 cells differentiate from naïve Th cells under the influence of the specific transcription factor GATA3, which is expressed following IL-4 stimulation, and produce IL-4, IL-5, IL-9, IL-10, and IL-1399,100,103,104. Increased numbers of CD3+CD4+IL-4+ and CD3+CD4+IL-5+ cells suggest that Th2 type cells within the product increased during storage. This result supports the theory that allogeneic blood transfusion leads to TRIM by transforming the recipient’s immune response from Th1 to Th239,49,51,54–59. This transformation might lead to TRIM in the recipient as a result of a process which begins within the product. Furthermore, reduction in co-stimulant expression due to an enhanced recipient Th2 response might also be a contributing factor to the TRIM phenomenon53,59. However, the inverse relationship between increased intracellular cytokine levels and decreased GATA3 levels that we noted in our study appears to be a paradox. Nevertheless, it is known that IL-4, which is endogenously synthesised as a result of GATA3 activation, is sufficient for the persistence of Th2 differentiation105. It is possible that GATA3 is depleted after inducing the IL-4 production necessary for Th2 differentiation, which might explain the above-mentioned paradox.
Th9 cells differentiate from naïve Th cells under the influence of TGF-β and IL-4 or from Th2 cells under the influence of TGF-β106. Their specific transcription factor is SPI.1107. They mostly produce IL-9108. Although Th9 cells appear to be the main source of IL-9, this cytokine is expressed by Th2 and Th17 cells as well109,110. The significant reduction in SPI.1 gene expression observed in this study, as well as some reduction in CD3+CD4+IL-9+ cell numbers at days 21 and 42 of storage, may indicate that Th9 activity in the product decreases. Considering the significant reduction noted in its transcription factor, the less than expected decrease in intracellular IL-9 might be due to additional expression of IL-9 from Th2 and Th17 cells109,110.
Th17 cells differentiate from naïve Th cells under the influence of the specific transcription factor RORC2, which is expressed following TGF-β and IL-6 stimulation, and produce IL-17A, IL-17F, and IL-21111,112. Significant increases in RORC2 and CD3+CD4+IL-17A+ levels at storage day 42 suggest an increase in Th17 activity within the product towards the end of the storage period. The increase in Th17 activity and decrease in Th1 activity might be due to the absolute antagonism between these two cell populations113–115. It has previously been reported that disturbance of the interaction between Th1 and Th17 cells and, especially, the absence of a Th1 response leads to a stronger autoimmune response in an experimental setting115. Reduced Th1 activity and increased Th17 activity may suggest a potential relationship between NL-ES that have approached the end of the storage period and autoimmunity.
Th22 cells differentiate from naïve Th cells under the influence of the specific transcription factor AHR, which is expressed following IL-6 and TNF-α stimulation, and produce IL-22104,116,117. An insignificant decrease in AHR level on storage day 21 and the significant increase in AHR level at day 42, the significant reduction in CD3+CD4+IL-22+ level at day 21 and insignificant reduction in CD3+CD4+IL-22+ level at day 42 all suggest a decrease in Th22 activity within the product during its period of storage.
Treg cells either develop naturally in the thymus (natural Treg; nTreg) or differentiate from naïve T cells in the presence of TGF-β (inducible Treg; iTreg)99,118. Treg cells specifically express a transcription factor called FOXP3, and intracellular Foxp3 expression is considered to be the most specific Treg marker99,118. However, activated effector T cells may also temporarily express low levels of intracellular FOXP3 under certain conditions118. In contrast to these cells, which express high levels of CD127, Treg cells express CD127 at either very low levels or not at all119–122. Unlike other Th cells, Treg cells suppress the immune response. Their main functions consist of self-tolerance and establishing and maintaining immune homeostasis. We found that the level of the transcription factor FOXP3 was increased at day 42, the CD4+CD25+highFOXP3+ level was decreased at day 21, and the CD4+CD25+high CD127− levels were increased at both days 21 and 42 of storage. These findings suggest that Treg cell activity tends to increase towards the end of the period of storage. There might be a relationship between transfusion of NL-ES approaching the end of its storage period and the immunosuppressive effect of transfusion. It has been demonstrated that the Th2 immune response inhibits Treg differentiation through binding of GATA3 to the FOXP3 promoter region, thus blocking its expression123. Our results suggest that, by day 21 of storage, GATA3 may have caused a reduction in Treg activity via inhibition of FOXP3. The increase in FOXP3 and CD4+CD25+highCD127− levels together with the disappearance of GATA3 observed at day 42 support this suggestion.
Cytokines in the supernatant and effectiveness of leucoreduction
Evidence suggests that that several BRM-IM21–24,27,81,124–128, soluble Fas ligand and soluble HLA class I molecules6,55,129,130 which accumulate in ES during storage may have a role in TRIM. One of the potential drivers of TRIM effects are cytokines21–24. Changes in cytokine levels in ES supernatants during storage and their effects have been previously demonstrated in various studies28,84,85,129,131,132. The effectiveness of leucoreduction in the prevention of TRIM is unclear. Contradictory results have been reported in several studies in favour7,71,89,133,134 and against6,135–137 its effectiveness. We, therefore, measured cytokine profiles in LR-ES and NL-ES supernatants in order to examine the relationship of these profiles with the TRIM effect.
We measured IL-4, IL-6, IL-8, IL-9, IL-10, IL-13, IL-17A, IL-22, IFN-γ, TNF-α, and TGF-β levels by ELISA in this study. Among the listed cytokines, IL-4, IL-6, IL-9, IL-10 and TNF-α were not detected in any of the samples. This might be due to their rapid degradation, the low sensitivity of the ELISA for their detection, their short half-life or their consumption within the product during the storage period. IL-22 and TGF-β levels were only measurable in NL-ES supernatants.
IL-8, IL-13, IL-17A, IFN-γ levels could be determined in LR-ES; among these only IL-17A was found to be significantly increased at storage day 42. Comparative analysis revealed that this increase tended to be greater than the increase in NL-ES samples which was close to statistical significance. It could be suggested that increased IL-17A in LR-ES might contribute to the TRIM effect by activating recipient Th17 cells following transfusion by weakening the Th1 response113–115 or by enhancing Th2 activity via transformation into Th17/Th2 cells138. If IL-17A does play a role in TRIM, this documented increase in IL-17A in LR-ES might be one of the reasons why leucoreduction is ineffective in preventing post-transfusion immunomodulation. The IL-8, IL-13 and IFN-γ levels together with IL-17A in samples not containing leucocytes were also interesting. It is possible that these cytokines originate either from sources within the product other than leucocytes, or leucocyte-related structures (exosomes, microparticles, etc.) that can permeate the leucocyte filter. However, it does not appear to be rational to expect these sources to produce higher levels of IL-17 in LR-ES samples than NL-ES supernatants containing leucocytes. Our results regarding IL-17A levels do, therefore, need questioning and further confirmation.
In NL-ES supernatants IL-8, IL-13, IL-17A, IL-22, IFN-γ, TGF-β levels could be measured; among these, only a decrease in IL-22 levels and an increase in TGF-β levels were found to be statistically significant at day 21 of storage. The increase in TGF-β levels in day 42 samples was close to statistical significance. This increase in TGF-β levels during the storage process supports previous studies suggesting a relationship between this cytokine and TRIM28,129. TGF-β, transferred to the recipient via allogeneic blood transfusion may reach high levels, especially in patients who receive multiple transfusions, and inhibit leucocyte activation or enhance the immunosuppressive features of TRIM by inducing differentiation of naive Th cells into Treg cells. Therefore, in contrast to the above-mentioned hypothesis, the change in TGF-β levels, which were increased in NL-ES but undetectable in LR-ES supernatants, may be an indicator of the benefit of leucoreduction in preventing TRIM.
In summary, IL-17A and TGF-β accumulation in supernatants may contribute to TRIM via the mechanisms suggested above. This leads to the hypothesis that the TRIM effect could occur not only via allogeneic blood components but also through autologous ones, which have approached the end of their storage period. On the other hand, our comparative analysis suggests that leucoreduction may be effective in the prevention of TRIM. While increased TGF-β levels in NL-ES support this suggestion, the increased level of IL-17A in LR-ES is difficult to interpret. In any case, as direct immunosuppressive effects of TGF-β overcome the potential proposed effects of IL-17A, leucoreduction might ultimately be considered as effective.
Conclusions
In conclusion, significant amounts of molecules and cells are transferred to the recipient via allogeneic blood transfusion. The persistence and activity of these transferred elements within the circulation may predispose to the development of TRIM. Our results suggest that allogeneic leucocytes and cytokines both play roles in the development of TRIM. It appears that leucoreduction may at least be effective in preventing cytokine-mediated TRIM. However, factors other than leucocytes will always limit this preventive effect. Finally, the TRIM phenomenon is not encountered following every transfusion, and it is possible that different mechanisms underlie the effects in different individuals. Numerous product- and recipient-related variables lead to these effects. It should not be forgotten that, in addition to leucocytes in the product and BRM-IM accumulated within the supernatant, erythrocytes, solutions within the bag systems, concomitant diseases and demographic characteristics of the patients receiving blood transfusion may also play roles in the TRIM effect. Valuable additional data could be provided by future studies designed to take all of these factors into account.
Footnotes
Funding and resources
This study was supported by a grant from Uludag University, Bursa, Turkey (grant number UAP[T]-2011/53).
Authorship contributions
SHB and HBO designed the study. SHB, YH and LTK provided the blood samples. SHB and FB performed the experiments. SHB, FB, FG, GG and HBO analysed and interpreted the data, and wrote the manuscript. FG, YH and HBO critically reviewed, edited and approved the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Opelz G, Sengar DP, Mickey MR, Terasaki PI. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc. 1973;5:253–9. [PubMed] [Google Scholar]
- 2.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery. Ann Surg. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 3.Velásquez JF, Cata JP. Transfusions of blood products and cancer outcomes. Rev Esp Anestesiol Reanim. 2015;62:461–7. doi: 10.1016/j.redar.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Bilgin YM, Brand A. Transfusion-related immunomodulation: a second hit in an inflammatory cascade? Vox Sang. 2008;95:261–71. doi: 10.1111/j.1423-0410.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg N, Heal JM. Effects of transfusion on immune function. Cancer recurrence and infection. Arch Pathol Lab Med. 1994;118:371–9. [PubMed] [Google Scholar]
- 6.Vamvakas EC, Blajchman MA. Transfusion-related immuno-modulation (TRIM): an update. Blood Rev. 2007;21:327–48. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 7.van de Watering LM, Hermans J, Houbiers JG, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562–8. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- 8.Sloand E, Kumar P, Klein HG, et al. Transfusion of blood components to persons infected with human immunodeficiency virus type 1: relationship to opportunistic infection. Transfusion. 1994;34:48–53. doi: 10.1046/j.1537-2995.1994.34194098603.x. [DOI] [PubMed] [Google Scholar]
- 9.Houbiers JG, van de Velde CJ, van de Watering LM, et al. Transfusion of red cells is associated with increased incidence of bacterial infection after colorectal surgery: a prospective study. Transfusion. 1997;37:126–34. doi: 10.1046/j.1537-2995.1997.37297203513.x. [DOI] [PubMed] [Google Scholar]
- 10.Chang H, Hall GA, Geerts WH, et al. Allogeneic red blood cell transfusion is an independent risk factor for the development of postoperative bacterial infection. Vox Sang. 2000;78:13–8. doi: 10.1159/000031143. [DOI] [PubMed] [Google Scholar]
- 11.Peters WR, Fry RD, Fleshman JW, Kodner IJ. Multiple blood transfusions reduce the recurrence rate of Crohn’s disease. Dis Colon Rectum. 1989;32:749–53. doi: 10.1007/BF02562122. [DOI] [PubMed] [Google Scholar]
- 12.Mowbray JF, Gibbings C, Liddell H, et al. Controlled trial of treatment of recurrent spontaneous abortion by immunisation with paternal cells. Lancet. 1985;1:941–3. doi: 10.1016/s0140-6736(85)91723-4. [DOI] [PubMed] [Google Scholar]
- 13.Heiss MM, Mempel W, Delanoff C, et al. Blood transfusion-modulated tumor recurrence: first results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994;12:1859–67. doi: 10.1200/JCO.1994.12.9.1859. [DOI] [PubMed] [Google Scholar]
- 14.Gantt CL. Red blood cells for cancer patients. Lancet. 1981;2:363. doi: 10.1016/s0140-6736(81)90673-5. [DOI] [PubMed] [Google Scholar]
- 15.Amato A, Pescatori M. Perioperative blood transfusions and recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;1:CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark DA, Gorczynski RM, Blajchman MA. Transfusion-related immunomodulation due to peripheral blood dendritic cells expressing the CD200 tolerance signaling molecule and alloantigen. Transfusion. 2008;48:814–21. doi: 10.1111/j.1537-2995.2008.01654.x. [DOI] [PubMed] [Google Scholar]
- 17.Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–21. [PubMed] [Google Scholar]
- 18.Dzik WH. Mononuclear cell microchimerism and the immuno-modulatory effect of transfusion. Transfusion. 1994;34:1007–12. doi: 10.1046/j.1537-2995.1994.341195065024.x. [DOI] [PubMed] [Google Scholar]
- 19.Beko KR, Tran HO, Hewitt CW, et al. Mechanisms of prior blood transfusion-cyclosporine-induced tolerance: a potential role for immune-cellular chimerism. Transplant Proc. 1991;23:147–8. [PubMed] [Google Scholar]
- 20.Nielsen HJ, Reimert CM, Pedersen AN, et al. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion. 1996;36:960–5. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 21.Ghio M, Contini P, Negrini S, et al. Down regulation of human natural killer cell-mediated cytolysis induced by blood transfusion: role of transforming growth factor-β(1), soluble Fas ligand, and soluble class I human leukocyte antigen. Transfusion. 2011;51:1567–73. doi: 10.1111/j.1537-2995.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- 22.Kristiansson M, Soop M, Shanwell A, Sundqvist KG. Prestorage versus bedside white blood cell filtration of red blood cell concentrates: effects on the content of cytokines and soluble tumor necrosis factor receptors. J Trauma. 1996;40:379–83. doi: 10.1097/00005373-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Hodge G, Markus C, Nairn J, Hodge S. Effect of blood storage conditions on leucocyte intracellular cytokine production. Cytokine. 2005;32:7–11. doi: 10.1016/j.cyto.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Ghio M, Ottonello L, Contini P, et al. Transforming growth factor-1 in supernatants from stored red blood cells inhibits neutrophil locomotion. Blood. 2003;102:1100–7. doi: 10.1182/blood.V102.3.1100. [DOI] [PubMed] [Google Scholar]
- 25.Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. 2011;51:2549–54. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–67. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimshaw K, Sahler J, Spinelli SL, et al. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion. 2011;51:874–80. doi: 10.1111/j.1537-2995.2011.03095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumgartner JM, Silliman CC, Moore EE, et al. Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg. 2009;208:110–9. doi: 10.1016/j.jamcollsurg.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adamson JW. New blood, old blood, or no blood? N Engl J Med. 2008;358:1295–6. doi: 10.1056/NEJMe0800520. [DOI] [PubMed] [Google Scholar]
- 30.Mynster T, Dybkjoer E, Kronborg G, Nielsen HJ. Immunomodulating effect of blood transfusion: is storage time important? Vox Sang. 1998;74:176–81. [PubMed] [Google Scholar]
- 31.Puppo F, Contini P, Ghio M, et al. Soluble human MHC class I molecules induce soluble Fas ligand secretion and trigger apoptosis in activated CD8(+) Fas (CD95)(+) T lymphocytes. Int Immunol. 2000;12:195–203. doi: 10.1093/intimm/12.2.195. [DOI] [PubMed] [Google Scholar]
- 32.Magee CC, Sayegh MH. Peptide-mediated immuno-suppression. Curr Opin Immunol. 1997;9:669–75. doi: 10.1016/s0952-7915(97)80047-7. [DOI] [PubMed] [Google Scholar]
- 33.Ghio M, Contini P, Ubezio G, et al. Blood transfusions with high levels of contaminating soluble HLA-I correlate with levels of soluble CD8 in recipients’ plasma; a new control factor in soluble HLA-I-mediated transfusion-modulated immunomodulation? Blood Transfus. 2014;12(Suppl 1):105–8. doi: 10.2450/2012.0199-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–95. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 35.Brunson ME, Alexander JW. Mechanisms of transfusion-induced immunosuppression. Transfusion. 1990;30:651–8. doi: 10.1046/j.1537-2995.1990.30790385527.x. [DOI] [PubMed] [Google Scholar]
- 36.Blajchman MA, Bordin JO. Mechanisms of transfusion-associated immunosuppression. Curr Opin Hematol. 1994;1:457–61. [PubMed] [Google Scholar]
- 37.Kaplan J, Sarnaik S, Gitlin J, Lusher J. Diminished helper/suppressor lymphocyte ratios and natural killer activity in recipients of repeated blood transfusions. Blood. 1984;64:308–10. [PubMed] [Google Scholar]
- 38.Nielsen HJ, Hammer JH, Moesgaard F, Kehlet H. Comparison of the effects of SAG-M and whole-blood transfusions on postoperative suppression of delayed hypersensitivity. Can J Surg. 1991;34:146–50. [PubMed] [Google Scholar]
- 39.Wood ML, Gottschalk R, Monaco AP. Effect of blood transfusion on IL-2 production. Transplantation. 1988;45:930–5. doi: 10.1097/00007890-198805000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Waymack JP, Gallon L, Barcelli U, Alexander JW. Effect of blood transfusions on macrophage function in a burned animal model. Curr Surg. 1986;43:305–7. [PubMed] [Google Scholar]
- 41.Blajchman MA, Bardossy L, Carmen R, et al. Allogeneic blood transfusion-induced enhancement of tumor growth: two animal models showing amelioration by leukodepletion and passive transfer using spleen cells. Blood. 1993;81:1880–2. [PubMed] [Google Scholar]
- 42.Ghio M, Contini P, Mazzei C, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93:1770–7. [PubMed] [Google Scholar]
- 43.Terasaki PI. The beneficial transfusion effect on kidney graft survival attributed to clonal deletion. Transplantation. 1984;37:119–25. doi: 10.1097/00007890-198402000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Naji A. Induction of tolerance by intrathymic inoculation of alloantigen. Curr Opin Immunol. 1996;8:704–9. doi: 10.1016/s0952-7915(96)80089-6. [DOI] [PubMed] [Google Scholar]
- 45.Puppo F, Ghio M, Contini P, et al. Fas, Fas ligand and transfusion immunomodulation. Transfusion. 2001;41:416–8. doi: 10.1046/j.1537-2995.2001.41030416.x. [DOI] [PubMed] [Google Scholar]
- 46.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 47.Singal DP, Leber B, Harnish DG, et al. Molecular genetic basis for the antiidiotypic antibody response associated with successful renal allograft survival in humans. Transplant Proc. 1991;23:1059–61. [PubMed] [Google Scholar]
- 48.Mincheff MS, Meryman HT, Kapoor V, et al. Blood transfusion and immunomodulation: a possible mechanism. Vox Sang. 1993;65:18–24. doi: 10.1111/j.1423-0410.1993.tb04519.x. [DOI] [PubMed] [Google Scholar]
- 49.Kirkley SA. Proposed mechanisms of transfusion-induced immunomodulation. Clin Diagn Lab Immunol. 1999;6:652–7. doi: 10.1128/cdli.6.5.652-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu A, Li Q, Shi H, et al. Donor-derived bone marrow transfusion produces mixed chimerism and promotes a Th2 shift in Th1/Th2 balance in rat heterotopic small bowel transplantation. Dig Liver Dis. 2012;44:988–94. doi: 10.1016/j.dld.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Gafter U, Kalechman Y, Sredni B. Blood transfusion enhances production of T-helper-2 cytokines and transforming growth factor beta in humans. Clin Sci. 1996;91:519–23. doi: 10.1042/cs0910519. [DOI] [PubMed] [Google Scholar]
- 52.Betz M, Fox BS. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J Immunol. 1991;146:108–13. [PubMed] [Google Scholar]
- 53.Perez RV, Swanson C, Morgan M, et al. Portal venous transfusion up-regulates Kupffer cell cyclooxygenase activity: a mechanism of immunosuppression in organ transplantation. Transplantation. 1997;64:135–9. doi: 10.1097/00007890-199707150-00023. [DOI] [PubMed] [Google Scholar]
- 54.Blumberg N, Heal JM. The transfusion immunomodulation theory: the Th1/Th2 paradigm and an analogy with pregnancy as a unifying mechanism. Semin Hematol. 1996;33:329–40. [PubMed] [Google Scholar]
- 55.Lannan KL, Sahler J, Spinelli SL, et al. Transfusion immunomodulation--the case for leukoreduced and (perhaps) washed transfusions. Blood Cells Mol Dis. 2013;50:61–8. doi: 10.1016/j.bcmd.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pandey P, Chaudhary R, Aggarwal A, et al. Transfusion-associated immunomodulation: Quantitative changes in cytokines as a measure of immune responsiveness after one time blood transfusion in neurosurgery patients. Asian J Transfus Sci. 2010;4:78–85. doi: 10.4103/0973-6247.67021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu X, Yu B, You P, et al. Ubiquitin released in the plasma of whole blood during storage promotes mRNA expression of Th2 cytokines and Th2-inducing transcription factors. Transfus Apher Sci. 2012;47:305–11. doi: 10.1016/j.transci.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Guo J-R, Xu F, Jin X-J, et al. Impact of allogenic and autologous transfusion on immune function in patients with tumors. Asian Pac J Cancer Prev. 2014;15:467–74. doi: 10.7314/apjcp.2014.15.1.467. [DOI] [PubMed] [Google Scholar]
- 59.Babcock GF, Alexander JW. The effects of blood transfusion on cytokine production by TH1 and TH2 lymphocytes in the mouse. Transplantation. 1996;61:465–8. doi: 10.1097/00007890-199602150-00026. [DOI] [PubMed] [Google Scholar]
- 60.Feng JF, Chen F, Liu H, Liu J. Induction of immune tolerance by pre-infusion of apoptotic lymphocytes derived from peripheral blood of donor rats before liver transplantation. Minerva Chir. 2013;68:183–9. [PubMed] [Google Scholar]
- 61.Cheng J, Zhou L, Qin YS, et al. Donor apoptotic lymphocyte transfusion-induced liver allograft tolerance by up-regulation of CD4(+)CD25(+) regulatory T cells in peripheral blood. Transplant Proc. 2009;41:3893–7. doi: 10.1016/j.transproceed.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 62.Dzik WH. Apoptosis, TGF beta and transfusion-related immunosuppression: Biologic versus clinical effects. Transfus Apher Sci. 2003;29:127–9. doi: 10.1016/S1473-0502(03)00115-0. [DOI] [PubMed] [Google Scholar]
- 63.Long K, Meier C, Bernard A, et al. T-cell suppression by red blood cells is dependent on intact cells and is a consequence of blood bank processing. Transfusion. 2014;54:1340–7. doi: 10.1111/trf.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cholette JM, Pietropaoli AP, Henrichs KF, et al. Longer RBC storage duration is associated with increased postoperative infections in pediatric cardiac surgery. Pediatr Crit Care Med. 2015;16:227–35. doi: 10.1097/PCC.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127:124–31. doi: 10.1016/j.amjmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Holst LB, Petersen MW, Haase N, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woods BI, Rosario BL, Chen A, et al. The association between perioperative allogeneic transfusion volume and postoperative infection in patients following lumbar spine surgery. J Bone Joint Surg Am. 2013;95:2105–10. doi: 10.2106/JBJS.L.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kriebardis A, Antonelou M, Stamoulis K, Papassideri I. Cell-derived microparticles in stored blood products: innocent-bystanders or effective mediators of post-transfusion reactions? Blood Transfus. 2012;10(Suppl 2):25–38. doi: 10.2450/2012.006S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008;84:1316–25. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 70.Lion N, Crettaz D, Rubin O, Tissot J-D. Stored red blood cells: a changing universe waiting for its map(s) J Proteomics. 2010;73:374–85. doi: 10.1016/j.jprot.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 71.Bilgin YM, van de Watering LMG, Eijsman L, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation. 2004;109:2755–60. doi: 10.1161/01.CIR.0000130162.11925.21. [DOI] [PubMed] [Google Scholar]
- 72.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Can J Anaesth. 1997;44:1256–61. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 73.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–2. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 74.Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 75.Hendrickson JE, Hod EA, Spitalnik SL, et al. Storage of murine red blood cells enhances alloantibody responses to an erythroid-specific model antigen. Transfusion. 2010;50:642–8. doi: 10.1111/j.1537-2995.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hendrickson JE, Hod EA, Hudson KE, et al. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion. 2011;51:2695–702. doi: 10.1111/j.1537-2995.2011.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yazdanbakhsh K, Bao W, Zhong H. Immunoregulatory effects of stored red blood cells. Hematol Am Soc Hematol Educ Progr. 2011;2011:466–9. doi: 10.1182/asheducation-2011.1.466. [DOI] [PubMed] [Google Scholar]
- 78.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 79.Brusko TM, Wasserfall CH, Agarwal A, et al. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–6. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 80.Chauveau C, Rémy S, Royer PJ, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 81.Cairo G, Pietrangelo A. Iron regulatory proteins in pathobiology. Biochem J. 2000;352:241–50. [PMC free article] [PubMed] [Google Scholar]
- 82.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atzil S, Arad M, Glasner A, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008;109:989–97. doi: 10.1097/ALN.0b013e31818ddb72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weisbach V, Wanke C, Zingsem J, et al. Cytokine generation in whole blood, leukocyte-depleted and temporarily warmed red blood cell concentrates. Vox Sang. 1999;76:100–6. [PubMed] [Google Scholar]
- 85.Stack G, Baril L, Napychank P, Snyder EL. Cytokine generation in stored, white cell-reduced, and bacterially contaminated units of red cells. Transfusion. 1995;35:199–203. doi: 10.1046/j.1537-2995.1995.35395184274.x. [DOI] [PubMed] [Google Scholar]
- 86.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 87.Opelz G, Vanrenterghem Y, Kirste G, et al. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–7. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 88.Wallis JP, Chapman CE, Orr KE, et al. Effect of WBC reduction of transfused RBCs on postoperative infection rates in cardiac surgery. Transfusion. 2002;42:1127–34. doi: 10.1046/j.1537-2995.2002.00181.x. [DOI] [PubMed] [Google Scholar]
- 89.Blumberg N, Zhao H, Wang H, et al. The intention-to-treat principle in clinical trials and meta-analyses of leukoreduced blood transfusions in surgical patients. Transfusion. 2007;47:573–81. doi: 10.1111/j.1537-2995.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 90.Collier AC, Kalish LA, Busch MP, et al. Leukocyte-reduced red blood cell transfusions in patients with anemia and human immunodeficiency virus infection: the Viral Activation Transfusion Study: a randomized controlled trial. JAMA. 2001;285:1592–601. doi: 10.1001/jama.285.12.1592. [DOI] [PubMed] [Google Scholar]
- 91.Hiesse C, Busson M, Buisson C, et al. Multicenter trial of one HLA-DR-matched or mismatched blood transfusion prior to cadaveric renal transplantation. Kidney Int. 2001;60:341–9. doi: 10.1046/j.1523-1755.2001.00805.x. [DOI] [PubMed] [Google Scholar]
- 92.van Hilten JA, van de Watering LM, van Bockel JH, et al. Effects of transfusion with red cells filtered to remove leucocytes: randomised controlled trial in patients undergoing major surgery. BMJ. 2004;328:1281. doi: 10.1136/bmj.38103.735266.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nathens AB, Nester TA, Rubenfeld GD, et al. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–7. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 94.Vamvakas EC. White-blood-cell-containing allogeneic blood transfusion and postoperative infection or mortality: an updated meta-analysis. Vox Sang. 2007;92:224–32. doi: 10.1111/j.1423-0410.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- 95.Lange MM, van Hilten JA, van de Watering LM, et al. Leucocyte depletion of perioperative blood transfusion does not affect long-term survival and recurrence in patients with gastrointestinal cancer. Br J Surg. 2009;96:734–40. doi: 10.1002/bjs.6636. [DOI] [PubMed] [Google Scholar]
- 96.van de Watering LM, Brand A, Houbiers JG, et al. Perioperative blood transfusions, with or without allogeneic leucocytes, relate to survival, not to cancer recurrence. Br J Surg. 2001;88:267–72. doi: 10.1046/j.1365-2168.2001.01674.x. [DOI] [PubMed] [Google Scholar]
- 97.Skånberg J, Lundholm K, Haglind E. Effects of blood transfusion with leucocyte depletion on length of hospital stay, respiratory assistance and survival after curative surgery for colorectal cancer. Acta Oncol. 2007;46:1123–30. doi: 10.1080/02841860701441830. [DOI] [PubMed] [Google Scholar]
- 98.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jäger A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–84. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wan YY. Multi-tasking of helper T cells. Immunology. 2010;130:166–71. doi: 10.1111/j.1365-2567.2010.03289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yamane H, Paul WE. Memory CD4+ T cells: fate determination, positive feedback and plasticity. Cell Mol Life Sci. 2012;69:1577–83. doi: 10.1007/s00018-012-0966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 103.Parronchi P, De Carli M, Manetti R, et al. IL-4 and IFN (alpha and gamma) exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 human T cell clones. J Immunol. 1992;149:2977–83. [PubMed] [Google Scholar]
- 104.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–7. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schmitt E, Germann T, Goedert S, et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–96. [PubMed] [Google Scholar]
- 107.Chang H-C, Sehra S, Goswami R, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cosmi L, Maggi L, Santarlasci V, et al. T helper cells plasticity in inflammation. Cytometry A. 2014;85:36–42. doi: 10.1002/cyto.a.22348. [DOI] [PubMed] [Google Scholar]
- 109.Elyaman W, Bradshaw EM, Uyttenhove C, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–90. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nowak EC, Weaver CT, Turner H, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–60. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 112.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–68. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 114.Tanaka K, Ichiyama K, Hashimoto M, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol. 2008;180:3746–56. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- 115.Peck A, Mellins ED. Plasticity of T-cell phenotype and function: the T helper type 17 example. Immunology. 2010;129:147–53. doi: 10.1111/j.1365-2567.2009.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duhen T, Geiger R, Jarrossay D, et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–63. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 117.Eyerich S, Eyerich K, Pennino D, et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009;119:3573–85. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fritzsching E, Kunz P, Maurer B, et al. Regulatory T cells and tolerance induction. Clin Transplant. 2009;23:10–4. doi: 10.1111/j.1399-0012.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 119.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–4. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 121.Kang SM, Tang Q, Bluestone JA. CD4+ CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–63. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 122.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mantel P-Y, Kuipers H, Boyman O, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Theurl I, Fritsche G, Ludwiczek S, et al. The macrophage: a cellular factory at the interphase between iron and immunity for the control of infections. Biometals. 2005;18:359–67. doi: 10.1007/s10534-005-3710-1. [DOI] [PubMed] [Google Scholar]
- 125.Schaible UE, Kaufmann SHE. Iron and microbial infection. Nat Rev Microbiol. 2004;2:946–53. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 126.Walker EM, Walker SM. Effects of iron overload on the immune system. Ann Clin Lab Sci. 2000;30:354–65. [PubMed] [Google Scholar]
- 127.Jacobi KE, Wanke C, Jacobi A, et al. Determination of eicosanoid and cytokine production in salvaged blood, stored red blood cell concentrates, and whole blood. J Clin Anesth. 2000;12:94–9. doi: 10.1016/s0952-8180(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 128.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–94. [PMC free article] [PubMed] [Google Scholar]
- 129.Hodge GL, Hodge SJ, Nairn J, et al. Poststorage leuko-depleted plasma inhibits T-cell proliferation and Th1 response in vitro: characterization of TGFbeta-1 as an important immunomodulatory component in stored blood. Transplantation. 2005;80:95–101. doi: 10.1097/01.tp.0000163866.43866.44. [DOI] [PubMed] [Google Scholar]
- 130.Cata JP, Wang H, Gottumukkala V, et al. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690–701. doi: 10.1093/bja/aet068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shanwell A, Kristiansson M, Remberger M, Ringdén O. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997;37:678–84. doi: 10.1046/j.1537-2995.1997.37797369441.x. [DOI] [PubMed] [Google Scholar]
- 132.Karam O, Tucci M, Toledano BJ, et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion. 2009;49:2326–34. doi: 10.1111/j.1537-2995.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 133.Friese RS, Sperry JL, Phelan HA, Gentilello LM. The use of leukoreduced red blood cell products is associated with fewer infectious complications in trauma patients. Am J Surg. 2008;196:56–61. doi: 10.1016/j.amjsurg.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 134.Hébert PC, Fergusson D, Blajchman MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003;289:1941–9. doi: 10.1001/jama.289.15.1941. [DOI] [PubMed] [Google Scholar]
- 135.Vamvakas EC. Meta-analysis of randomized controlled trials investigating the risk of postoperative infection in association with white blood cell-containing allogeneic blood transfusion: the effects of the type of transfused red blood cell product and surgical setting. Transfus Med Rev. 2002;4:304–14. doi: 10.1053/tmrv.2002.35209. [DOI] [PubMed] [Google Scholar]
- 136.Vamvakas EC, Blajchman MA. Prestorage versus poststorage white cell reduction for the prevention of the deleterious immunomodulatory effects of allogeneic blood transfusion. Transfus Med Rev. 2000;14:23–33. doi: 10.1016/s0887-7963(00)80113-3. [DOI] [PubMed] [Google Scholar]
- 137.Phelan HA, Sperry JL, Friese RS. Leukoreduction before red blood cell transfusion has no impact on mortality in trauma patients. J Surg Res. 2007;138:32–6. doi: 10.1016/j.jss.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 138.Cosmi L, Maggi L, Santarlasci V, et al. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222–30e1–4. doi: 10.1016/j.jaci.2009.10.012. [DOI] [PubMed] [Google Scholar]