Abstract
Thalamotomy is effective in treating refractory tremor in Parkinson's disease (PD). We herein report a PD patient who underwent left ventral intermediate nucleus and ventro oralis posterior nucleus thalamotomy using magnetic resonance imaging-guided focused ultrasound (MRgFUS). Right-side resting tremor and rigidity were abolished immediately following the ultrasound energy delivery. In addition, left-side resting tremor and rigidity also improved. No adverse events occurred during the procedure. We observed the exacerbation of bradykinesia, which might have been caused by edema around the target. This is the first report of thalamotomy using MRgFUS for PD patient from Japan. Further investigations concerning the efficacy and safety of this procedure are necessary.
Keywords: Parkinson's disease, MRI guided focused ultrasound, thalamotomy, ventral intermediate nucleus, ventro oralis posterior nucleus
Introduction
Parkinson's disease (PD) is a slowly progressive neurodegenerative disorder presenting with multiple motor and non-motor symptoms. Resting tremor is the most identifiable sign of PD, and it can occur in postural or kinetic forms, interfering with daily activities and being a source of social embarrassment. The primary therapeutic targets of current medications are bradykinesia, gait disturbance, and rigidity (1). Surgical intervention via deep brain stimulation (DBS) of the ventral intermediate nucleus (Vim), globus pallidus internus (Gpi), and subthalamic nucleus is a therapeutic option for PD, especially in advanced-stage patients or for medication-refractory patients. However, these treatments are invasive and extremely expensive. Magnetic resonance imaging (MRI)-guided focused ultrasound (MRgFUS) is a new technology that enables minimum intracranial focal ablation. Previous reports on the efficacy and safety of MRgFUS thalamotomy for essential tremor (ET) led to its Food and Drug Administration (FDA) approval (2). While MRgFUS thalamotomy has not yet been approved for use in treating PD, several reports concerning MRgFUS treatment for PD have been published, as shown in Table 1 (3-6).
Table 1.
Characteristics of PD Patients who Underwent MRgFUS Treatment.
| Reference | Patients (numbers) | Follow-up (months) | Main symptoms | Target | Outcomes | Adverse events | |
|---|---|---|---|---|---|---|---|
| UPDRS | Others | ||||||
| (3) | 13 | 3 | tremor akinesia | PTT | 45.7% reduction (part III) | none | |
| (4) | 7 | 3 to 12 | tremor | Vim | 49.7 % reduction (total) | Tremor disappeared in 4 patients during follow-up | DP: vertigo (n=4), headache (n=3), dizziness (n=2), lip paresthesia (n=1) AP: gait ataxia (n=1), unsteady feeling (n=1), hypogeusia (n=1) |
| (5) | 1 | 6 | LID | Gpi | 60.0% reduction (part III) (on medication) |
70% reduction of UdysRS | not described |
| (6) | 9* | 6 | tremor | Vim | 46.2% reduction (part II) | Tremor disappeared in 7 patients during follow-up | DP**: vertigo (n=14), headache (n=11), dizziness (n=4), nausea (n=3), burning scalp sensation (n=3), vomiting (n=2), lip paresthesia (n=2) AP**: gait ataxia (n=5), unsteady feeling (n=4), taste disturbances (n=4), asthenia (n=4), hand ataxia (n=3) |
LID: levodopa-induced dyskinesia, PTT: pallidothalamic tract, UdysRS: Unified Dyskinesia Rating Scale, DP: during the procedure, AP: after the procedure
* Seven of the nine patients in (6) were also reported in (4).
** Including the data of 18 ET patients and 3 ET-PD patients who underwent Vim thalamotomy
We herein report a 73-year-old Japanese PD patient who underwent left Vim and ventro oralis posterior nucleus (Vop) thalamotomy using MRgFUS.
Case Report
A right-handed 73-year-old man developed left hand resting tremor at 68 years of age. He was diagnosed with PD according to the UK Parkinson's Disease Society Brain Bank Criteria and started treatment with anti-Parkinsonian agents. At 73 years of age, we evaluated him as grade 2.5 on the Hoehn-Yahr scale and prescribed 300 mg of levodopa with benserazide. He had been annoyed by refractory tremor in the extremities and right-hand disability; however, we could not increase the dose of levodopa because of drug-induced hallucinations. We were unable to use dopamine agonists (pramipexole and ropinirole), monoamine oxidase (MAO)-B inhibitor, or catechol-O-methyltransferase (COMT) inhibitor because of daytime sleepiness and hallucination. Furthermore, he was concerned about the invasiveness of DBS and refused it. His Mini-Mental State Examination was 25/30, and he was not demented. The skull density ratio (the mean value for the ratio of Hounsfield units of marrow and cortical bone, which reflects the amount of ultrasound energy that can penetrate the skull effectively) (7) was 0.42 (exclusion criteria: ≤0.30).
In April 2017, we scheduled MRgFUS thalamotomy, which has been approved by the Review Board of Tokushukai Medical Alliance for PD patients suffering from medication-resistant tremor and who have refused other procedures involving DBS. The patient provided his written informed consent in accordance with the Declaration of Helsinki before treatment commenced.
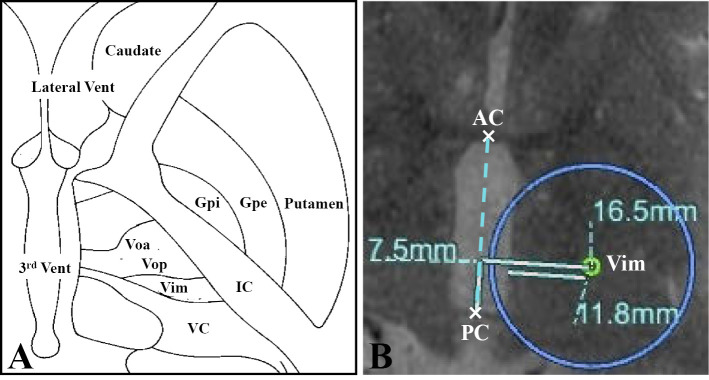
MRgFUS ultrasonic bursts (sonications) were performed using a 1.5-Tesla MRI (Signa HDx, GE Healthcare, Milwaukee, USA) and a focused ultrasound system (Exablate 4000Ⓡ, InSightec, Tirat Carmel, Israel), which received regulatory approved for ET in December 2016 in Japan. The day before thalamotomy, we performed T1- and T2-weighted MRI to plan the procedure. Based on the patient's preference, we selected the left Vim and Vop as the target. We chose the target for Vim at a point 7.5 mm anterior to the posterior commissure (PC) and 16.5 mm lateral from the midline, and 1.5 mm above the anterior commissure (AC)-PC line. The Vop is located immediately anterior to the Vim (Fig. 1).
Figure 1.
Anatomical structure of the thalamus and basal ganglia (A) and the MR image used for planning the procedure. Vent: ventricle, Voa: ventral oralis anterior nucleus, Vop: ventral oralis posteriornucleus, Vim: ventral intermediate nucleus, VC: ventral caudalis nucleus, IC: internal capsule, Gpi: globus pallidus internus, Gpe: globus pallidus externus
On the day of the procedure, we shaved his head completely and placed a stereotactic frame. We attached the head to a helmet with 1,024 ultrasonic transducers. A silicone membrane tightly sealed the space between his head and the transducer, enabling us to surround the head with chilled and degassed water and thereby preventing excessive scalp heating and minimizing acoustic reflection. We gradually increased the total energy of the sonications by either increasing the intensity or extending the sonication duration. We finished the sonications when an adequate reduction in both tremor and rigidity were observed. Right-side resting tremor and rigidity were abolished immediately following 10 sonications with an average maximum sonication time of 13.0 ± 3.4 seconds (range 10-17 seconds). The mean energy reached 5,382.7 ± 3,639.2 J (range 1,977-10,086 J) with a mean maximum temperature of 53.3 ± 6.9 °C (range 45-64 °C). In addition, his left-side resting tremor and rigidity also improved. No adverse events occurred during sonications. Bradykinesia was exacerbated after thalamotomy; however, we did not observe any right-sided weakness, pathological reflex, or ataxia.
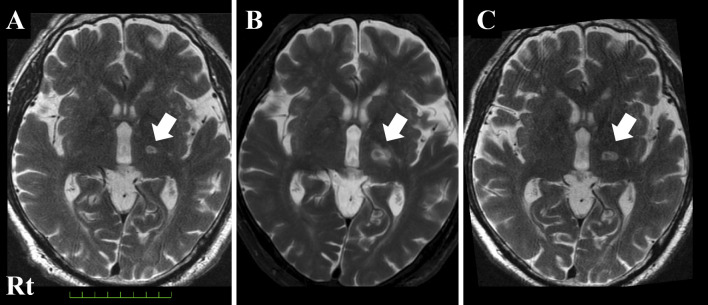
As we confirmed an edematous lesion around the target, we administered 30 mg of prednisolone for 5 days followed by 15 mg for 2 days, which improved his bradykinesia within 2 weeks from the procedure (Fig. 2). We administered the Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS)-Part III at baseline and 4 and 9 weeks after thalamotomy. The total score and the scores of items 3 (rigidity), 17 (rest tremor amplitude), and 18 (constancy of rest tremor) decreased from baseline. Neither tremor nor rigidity were exacerbated, despite reducing the dose of levodopa. However, the scores of items 5 (left hand movements) and 9 (arising from chair) increased after the procedure (Table 2).
Figure 2.
T2-weighted MR image of the brain showing the coagulated lesion (white arrow, A: immediately after thalamotomy, B: 7 days after thalamotomy, C: 1 month after thalamotomy). The hypointense zone at the center was considered to indicate microbleeding. Surrounding edema was obvious at seven days after thalamotomy (B), but it improved after the administration of corticosteroid (C).
Table 2.
The Changes in the UPDRS Part III Score and Levodopa Dose.
| Baseline | After 4 weeks | After 9 weeks | ||
|---|---|---|---|---|
| MDS-UPDRS part III | ||||
| Total score | 39 | 19 | 20 | |
| 1 | Speech | 2 | 1 | 1 |
| 2 | Facial expression | 2 | 2 | 3 |
| 3 | Rigidity - neck | 1 | 0 | 0 |
| - RUE | 2 | 0 | 0 | |
| - RLE | 0 | 0 | 0 | |
| - LUE | 1 | 0 | 0 | |
| - LLE | 0 | 0 | 0 | |
| 4 | Finger tapping - Right | 1 | 0 | 1 |
| - Left | 1 | 1 | 0 | |
| 5 | Hand movements - Right | 1 | 1 | 1 |
| - Left | 1 | 2 | 2 | |
| 6 | Pronation-Supination movements of hands - Right | 1 | 1 | 1 |
| - Left | 2 | 2 | 1 | |
| 7 | Toe tapping - Right | 1 | 0 | 0 |
| - Left | 1 | 0 | 0 | |
| 8 | Leg agility - Right | 0 | 0 | 0 |
| - Left | 0 | 0 | 0 | |
| 9 | Arising from chair | 0 | 3 | 1 |
| 10 | Gait | 1 | 0 | 1 |
| 11 | Freezing of gait | 0 | 0 | 0 |
| 12 | Postural stability | 0 | 0 | 1 |
| 13 | Posture | 1 | 1 | 1 |
| 14 | Global spontaneity of movement | 1 | 1 | 2 |
| 15 | Posturaltremor of the hands - Right | 1 | 0 | 0 |
| - Left | 1 | 1 | 1 | |
| 16 | Kinetic tremor of the hands - Right | 2 | 0 | 0 |
| - Left | 2 | 1 | 1 | |
| 17 | Rest tremor amplitude - RUE | 3 | 0 | 0 |
| - RLE | 2 | 0 | 0 | |
| - LUE | 3 | 1 | 1 | |
| - LLE | 2 | 0 | 0 | |
| - Lip/Jaw | 0 | 0 | 0 | |
| 18 | Constancy of rest tremor | 3 | 1 | 1 |
| L-dopa | 300mg | 300mg | 250mg |
RUE: right upper extremity, RLE: right lower extremity, LUE: left upper extremity, LLE: left lower extremity
Discussion
The therapeutic use of focused ultrasound started in the 1950s. In those days, a craniotomy was essential to provide an acoustic window (8); however, we can now perform this procedure without any incisions or burr holes due to technical advances. Furthermore, we can monitor the location and the temperature of the targeted lesion in real time. Table 3 summarizes the features of the current radiological or neurosurgical approaches (9). MRgFUS thalamotomy is characterized by minimum invasiveness, no exposure to radiation, and an immediate therapeutic effect. The common sonication-related adverse events include vertigo or dizziness, headache, and nausea. Gait disturbance (including an unsteady feeling in walking) and paresthesia are typical adverse events reported to last after thalamotomy (2, 4, 6). These events have been reported to persist in 9% and 14% at 12 months, respectively (2). However, Zaaroor et al. found that no adverse event lasted beyond three months (6). Our patient experienced an exacerbation of bradykinesia, which led to a deterioration in the hand movements and ability to rise from a chair. Edema surrounding the target lesion might have resulted in the impairment of the decreased dopaminergic neurons from the substantia nigra to striatum. While an increasing trend in the exacerbation of bradykinesia was noted, whether it will be transient or permanent remains to be seen.
Table 3.
The Features of the Current Radiological and Neurosurgical Treatments in Functional Neurosurgery (9).
| RF-thermo coagulation | γ-knife | DBS | MRgFUS | |
|---|---|---|---|---|
| Hair shaving | slight | none | partial | complete |
| Incision / Burr hole | must | none | must | none |
| Device implantation | no | no | yes | no |
| Radiation | low (X-ray, CT) | high | low (X-ray, CT) | low (screening CT) |
| General anesthesia | no | no | yes* | no |
| Therapeutic duration | 1hr | 1hr | 2~4hrs | 4hrs |
| Efficacy onset | immediate | delayed up to 1 year | adjustable | immediate |
| Bilateral procedure | impossible | impossible | possible | possible |
| post-treatment MRI | possible | possible | impossible | possible |
| Hemorrhagic complications | possible | almost none | possible | possible |
| Infection | possible | none | possible | none |
| Long-term data | yes | yes | yes | unknown |
* For implantation of pulse generator
Resting tremor is a major symptom of PD, and it usually improves with dopaminergic therapy. However, the responsiveness of resting tremor to medication is often of a lesser extent than bradykinesia and rigidity (1). There have been several reports on the clinical efficacy and safety of Vim thalamotomy using MRgFUS for medication refractory tremor in ET and PD (2, 4, 6). The Vim has been regarded as a generator of tremor because of its rhythmic neural activity related to contralateral tremor (10). In addition, connections with the Vim, primary motor cortex, and dentate nucleus were suggested to correlate with tremor (11). We therefore selected the Vim as the therapeutic target for tremor. In addition, we added the Vop because rigidity was considered to be associated with the pallidal-Vo complex, the combination of the ventral oralis anterior nucleus (Voa) plus Vop (10).
Our patient also showed improvement in left-side resting tremor and rigidity. Anecdotal evidence exists concerning the therapeutic effects of unilateral Vim thalamotomy on ipsilateral tremor in PD patients (12, 13); however, the reason for this effect has yet to be clearly explained. This is the first experience of Vim plus Vop thalamotomy for a PD patient using MRgFUS in Japan. Although the reason for the effect is unclear, not only contralateral but also ipsilateral tremor and rigidity improved. The long-term efficacy and safety of MRgFUS thalamotomy for PD patients is still unclear, and we must investigate the exacerbation of bradykinesia after the procedure. In addition, we must explore the adverse events, including gait and sensory disturbances. However, these findings overall suggest that MRgFUS thalamotomy may become a viable therapeutic option for PD patients with medication-resistant symptoms who refuse other neuroradiological or neurosurgical procedures.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Mr. Yoshiaki Murayama (Clinical Research Center, Shonan Fujisawa Tokushukai Hospital, Japan) for his great contributions to the MRgFUS treatment.
References
- 1.Sung YH, Chung SJ, Kim SR, Lee MC. Factors predicting response to dopaminergic treatment for resting tremor of Parkinson's disease. Mov Disord 23: 137-140, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Elias WJ, Lipsman N, Ondo WG, et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 375: 730-739, 2016. [DOI] [PubMed] [Google Scholar]
- 3.Magara A, Bühler R, Moser D, Kowalski M, Pourtehrani P, Jeanmonod D. First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease. J Ther Ultrasound 2: 11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlesinger I, Eran A, Sinai A, et al. MRI guided focused ultrasound thalamotomy for moderate-to-severe tremor in Parkinson's disease. Parkinsons Dis 2015: 219149, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Na YC, Chang WS, Jung HH, Kweon EJ, Chang JW. Unilateral magnetic resonance-guided focused ultrasound pallidotomy for Parkinson disease. Neurology 85: 549-551, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Zaaroor M, Sinai A, Goldsher D, Eran A, Nassar M, Schlesinger I. Magnetic resonance-guided focused ultrasound thalamotomy for tremor: a report of 30 Parkinson's disease and essential tremor cases. J Neurosurg 128: 202-210, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Chang WS, Jung HH, Zadicario E, et al. Factors associated with successful magnetic resonance-guided focused ultrasound treatment: efficiency of acoustic energy delivery through the skull. J Neurosurg 124: 411-416, 2016. [DOI] [PubMed] [Google Scholar]
- 8.Meyers R, Fry WJ, Fry FJ, Dreyer LL, Schultz DF, Noyes RF. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J Neurosurg 16: 32-54, 1959. [DOI] [PubMed] [Google Scholar]
- 9.Rohani M, Fasano A. Focused ultrasound for essential tremor: review of the evidence and discussion of current hurdles. Tremor and other hyperkinetic movements. Tremor Other Hyperkinet Mov (NY) 7: 462, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narabayashi H, Yokochi F, Nakajima Y. Levodopa-induced dyskinesia and thalamotomy. J Neurol Neurosurg Psychiatry 47: 831-839, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang JR, Feng T, Hou YN, Chan P, Wu T. Functional connectivity of Vim nucleus in tremor- and akinetic-/rigid-dominant Parkinson's disease. CNS Neurosci Ther 22: 378-386, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diederich N, Goetz CG, Stebbins GT, et al. Blinded evaluation confirms long-term asymmetric effect of unilateral thalamotomy or subthalamotomy on tremor in Parkinson's disease. Neurology 42: 1311-1314, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Franzini A, Marchetti M, Brait L, et al. Deep brain stimulation and frameless stereotactic radiosurgery in the treatment of bilateral parkinsonian tremor: target selection and case report of two patients. Acta Neurochir (Wien) 153: 1069-1075, 2011. [DOI] [PubMed] [Google Scholar]