Abstract
A 60-year-old man developed pneumonia after undergoing autologous peripheral blood stem cell transplantation for diffuse large-B cell lymphoma. A urinary antigen test and sputum culture were both negative for Legionella pneumophila; however, a sputum sample that was examined by loop-mediated isothermal amplification (LAMP) was positive for Legionella spp. On admission, the results of blood culturing using a BACTEC system were negative for 7 days. However, L. pneumophila serogroup 5 was detected in a blood subculture using WYOα medium. The patient was successfully treated with a fluoroquinolone-based regimen. LAMP is useful for the diagnosis of Legionella spp.
Keywords: Legionella pneumonia, Legionella pneumophila serogroup 5, loop-mediated isothermal amplification, hematological malignancy, malignant lymphoma
Introduction
Legionella pneumophila, which can cause severe and fatal disease, was first reported at the American Legion Convention in Philadelphia in 1976 (1, 2). Because of the difficulties in diagnosing Legionella pneumonia, delays in the initiation of appropriate therapy, which have been associated with increased mortality, can occur (3). Recently, polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) methods have been gaining attention as diagnostic tools that allow for the early detection of the DNA of Legionella spp. (4, 5). LAMP is simple, easy to perform, cost-effective and amplifies DNA with high specificity and efficiency under isothermal conditions (60-65℃). Furthermore, the efficacy of LAMP is not affected by the co-presence of non-target DNA (6). In the present study, we reported a case of bacteremic pneumonia that was caused by L. pneumophila serogroup 5 and which was detected using LAMP before the blood culture results were obtained.
Case Report
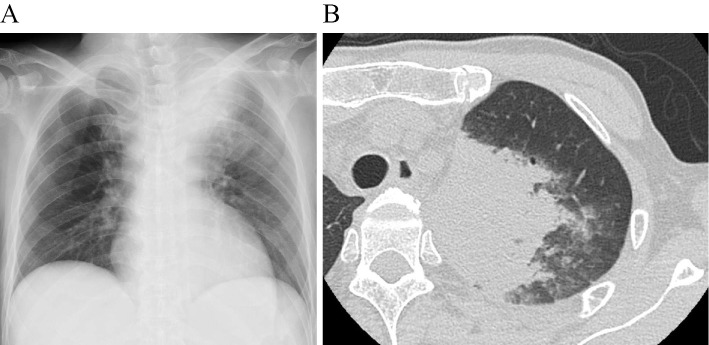
A 60-year-old man underwent high-dose chemotherapy followed by autologous peripheral blood stem cell transplantation (auto-PBSCT) for relapsed diffuse large B-cell lymphoma following rituximab-combined chemotherapy. He had no history of diabetes mellitus, chronic kidney disease, or chronic liver disease. In addition, he had never smoked. He was discharged from the hospital 48 days after auto-PBSCT, after recovering from myelosuppression after auto-PBSCT. However, he was readmitted 60 days after discharge due to fever, impaired consciousness, and low blood pressure. On admission, his temperature was 40.1℃, his blood pressure was 87/46 mmHg, his pulse was 144 beats per minute, his respiratory rate was 38 breaths per minute, his consciousness was impaired [Glasgow Coma Scale 12 (E3V3M6)], and he required 2 L/min of oxygen maintain an oxygen saturation of ≥90%. Coarse crackles were heard in the left upper lung field. The laboratory data revealed neutropenia (131 /μL), elevated of liver enzymes [aspartate aminotransferase (AST) 256 IU/L, alanine aminotransferase (ALT) 81 IU/L], lactate dehydrogenase (LDH) (784 IU/L), creatine kinase (5,985 IU/L), serum creatinine (2.31 mg/dL), serum urea nitrogen (39 mg/dL), C-reactive protein (34.2 mg/dL), and mild hyponatremia (134 mmol/L) (Table). Chest radiography revealed pneumonia in the left upper lung field (Figure A), and chest CT revealed consolidation and centrilobular nodules in the left upper lobe of the lung (Figure B). The clinical diagnosis on admission was pneumonia with septic shock and neutropenia. Empirical antimicrobial agents were administered immediately after obtaining two sets of blood cultures, as well as sputum and urine samples. Intravenous cefepime, ciprofloxacin, and amikacin were administered as an empirical treatment. The results from initial blood cultures, sputum Gram staining, and sputum cultures were all negative. Urine antigen tests for Streptococcus pneumoniae and L. pneumophila were also negative. Furthermore, the serum level of Aspergillus galactomannan antigen and β-D glucan were below the cut-off value.
Table.
Laboratory Findings on Admission (Day1).
| Hematology | Biochemistry | Coagulation | ||||||
|---|---|---|---|---|---|---|---|---|
| WBC | 410 | /μL | TP | 5.7 | g/dL | APTT | 36.1 | second |
| Neu | 37 | % | Alb | 3.2 | g/dL | PT | 59.7 | % |
| Eos | 0 | % | BUN | 39 | mg/dL | FDP | 61.5 | µg/mL |
| Bas | 0 | % | Cre | 2.31 | mg/dL | |||
| Mono | 4 | % | AST | 256 | IU/L | |||
| Lym | 58 | % | ALT | 81 | IU/L | Arterial blood gas analysis O2: 2L/minute nasal cannula |
||
| Aty Lym | 1 | % | LDH | 784 | IU/L | |||
| RBC | 1.86×106 | /μL | ALP | 216 | IU/L | pH | 7.51 | |
| Hb | 6.2 | g/dL | γGT | 172 | IU/L | pCO2 | 19 | Torr |
| Plt | 6.000 | /μL | Na | 134 | mmol/L | pO2 | 95 | Torr |
| K | 5.0 | mmol/L | HCO3- | 16 | mmol/L | |||
| Cl | 97 | mmol/L | ||||||
| Ca | 8.4 | mg/dL | ||||||
| CK | 5,985 | IU/L | ||||||
| CRP | 34.2 | mg/dL | ||||||
WBC: white blood cell, RBC: red blood cell, Plt: platelet, TP: total protein, Alb: albumin, BUN: blood urea nitrogen, Cre: creatinine, CK: creatine phosphokinase
Figure.
Chest radiography revealed pneumonia in the left upper lung field (A), and chest CT revealed consolidation and centrilobular nodules in the left upper lobe of the lung (B).
On day 3, his impaired consciousness was found to have worsened. No white blood cells were found in a cerebrospinal fluid (CSF) examination. The glucose concentration, CSF protein levels, brain CT and MRI findings were normal. Legionella pneumonia was considered to be the most important differential diagnosis because the patient had headache, confusion, hyponatremia, and creatine kinase elevation, which are reported to be useful in the diagnosis of Legionella pneumonia. Thus, LAMP for the Legionella spp. (Eiken Chemical, Tokyo, Japan) was added to the stored sputum sample on day 3. The sputum sample was obtained on admission (day 1) and had been stored in the laboratory until day 1. However, it was difficult to distinguish whether impaired consciousness was a symptom of pneumonia or ciprofloxacin-associated encephalopathy. Ciprofloxacin was switched to azithromycin on the same day. The sputum sample was found to be positive for Legionella spp. by LAMP on day 8 (8 days after admission). As a result, combination therapy consisting of levofloxacin and azithromycin was initiated immediately after withdrawing cefepime on day 8. The BACTEK 9240 (Becton, Dickinson and Company, Sparks, USA) culture bottles were negative after 7 days of incubation. Subsequently, a subculture of the blood samples was initiated using Wadowsky-Yee-Okuda-α-ketoglutarate (WYOα) agar (Eiken Chemical). As a result, a Legionella strain with Gram-negative rod detected by Gram staining was cultured on the media but not on blood agar. The strain was identified as L. pneumophila serogroup 5 using monovalent immune sera (Denka Seiken, Tokyo, Japan). Levofloxacin and azithromycin were administered for 3 weeks and 7 days, respectively. After starting the treatment, the patient's consciousness impairment gradually improved, and the use of oxygen and vasopressors was stopped. Chest CT on day 23 (15 days after the diagnosis of L. pneumophila) revealed that the consolidation in the left upper lobe had decreased in size in comparison to the chest CT image that was obtained on admission. Although the pneumonia was treated successfully, the recurrence of lymphoma was discovered during admission.
Discussion
We successfully treated a patient who was diagnosed with bacteremic healthcare-associated pneumonia (HCAP) caused by L. pneumophila serogroup 5, which was detected using LAMP and a blood subculture. Legionella pneumonia is often severe in immunocompromised patients (1, 2), such as the present case. Monotherapy with azithromycin or fluoroquinolone is typically administered as the standard therapy for Legionella pneumonia (7). However, some experts have suggested that combination therapy with macrolide and fluoroquinolone is an important therapeutic option especially for patients with severe Legionella pneumonia (8). This was why we administered the combination of levofloxacin and azithromycin after obtaining the definite diagnosis.
Legionella pneumonia accounts for 2 - 9% of community-acquired pneumonia (CAP) cases (9). L. pneumophila was identified as the causative agent in more than 80% of Legionella pneumonia cases, and approximately 50% of the cases of Legionella pneumonia are caused by L. pneumophila serogroup 1. Among the serogroups of L. pneumophila, L. pneumophila serogroup 5 is reported to cause 0.6% and 0.7% of the cases of nosocomial and community-acquired Legionnaires' disease, respectively (10). L. pneumophila is less common in patients with HCAP than in patients with CAP. The prevalence of L. pneumophila is reportedly 2.4% and 8.8% in patients with HCAP and CAP, respectively (11). In addition, to the best of our knowledge, only a few cases of bacteremic pneumonia caused by L. pneumophila have been reported (12).
In the present case, the diagnosis of HCAP caused by Legionella pneumophila serogroup 5 was difficult, because the results of the blood sample cultured for 7 days using the BACTEC system, sputum culturing, and a urinary antigen test were negative. However, we strongly suspected Legionella pneumoniabased on the patient's clinical characteristics, which included headache, confusion, hyponatremia, and elevated creatine kinase; these findings are reported to be useful in the diagnosis of Legionella pneumonia (13). Thus, LAMP for Legionella spp. (Eiken Chemical) was initially performed using the stored sputum sample (14). After obtaining a positive LAMP result, blood subculturing was performed on WYOα agar. This approach allowed for the isolation L. pneumophila serogroup 5 from the blood.
The methods used to detect Legionella infection include antibody titers, indirect immunofluorescence assays, enzyme-linked immunosorbent assays, sputum culture and blood culturing, urinary antigen tests, PCR, and LAMP (5). Blood and sputum cultures show 100% specificity and are considered to be the gold standard methods for the diagnosis of Legionella pneumonia (5). However, a culture diagnosis requires special media (which is buffered by activated charcoal and WYOα media), adequate samples and a suitable technique. Furthermore, positive results are obtained after several days (5, 15). The detection of a 4-fold increase in the antibody titers by serological assays, including indirect immunofluorescence assays and enzyme-linked immunosorbent assays, takes 3 to 4 weeks (5). The urinary antigen test is a rapid diagnostic test that can be performed within an hour; the sensitivity and specificity are approximately 80% and 99%, respectively (16). However, this test is only available for detection of the L. pneumophila serogroup 1 (17).
To the best of our knowledge, no well-designed studies have determined the sensitivity and specificity of a PCR of samples from the lower respiratory tract in the detection of Legionella among pneumonia patients. However, following the introduction of the routine PCR testing of respiratory specimens (instead of the routine culturing of Legionella spp. from the specimens) in Christchurch, New Zealand, the burden of Legionnaires' diseases was recognized to be much greater than previously thought (18). This implies that the PCR may have higher sensitivity than the culture methods.
LAMP was developed in Japan (6), which it has been approved for the diagnosis of Legionella pneumonia since October 2010. The detection limit of LAMP is comparable to that of a PCR (6). The sensitivity and specificity of LAMP in the detection of reference Legionella spp. strains were found to be high in a previous study (19). In this context, LAMP may enable clinicians to identify cases of Legionella pneumonia, that are difficult to diagnose using other conventional methods, as was seen in the present case and in a previous report, wherein routine PCR was indicated (18).
LAMP is simple and easy to perform and only requires a laboratory water bath or a heat block for the reactions (6). Thus, LAMP may be useful, particularly in geographical areas with limited medical resources, such as developing countries. In this context, the introduction of LAMP may add new insights into the epidemiology of pneumonia through the detection of hidden Legionnaires'disease, particularly in developing countries.
In conclusion, to our knowledge, this is the first English-language report to provide a detailed description of the diagnosis of a case of Legionella pneumonia using LAMP. Few cases of bacteremic pneumonia caused by L. pneumophila serogroup 5 have been reported to date. LAMP is a useful diagnostic tool for detecting Legionella pneumonia, which is difficult to diagnose using other conventional methods.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Fraster DW, Tsai TR, Orenstein W, et al. Legionnaires' disease: description of an epidemic of pneumonia. N Engl J Med 97: 1189-1197, 1977. [DOI] [PubMed] [Google Scholar]
- 2.Dominguez A, Alvarez J, Sabria M, et al. Factors infl uencing the case-fatality rate of Legionnaires' disease. Int J Tuberc Lung Dis 13: 407-412, 2009. [PubMed] [Google Scholar]
- 3.Heath CH, Grove DI, Looke DF. Delay in appropriate therapy of Legionella-pneumonia associated with increased mortality. Eur J Clin Microbiol Infect Dis 15: 286-290, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Lu X, Mo ZY, Zhao HB, Yan H, Shi L. LAMP-based method for a rapid identification of Legionella spp. and Legionella pneumophila. Appl Microbiol Biotechnol 92: 179-187, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Diederen BM. Legionella spp. and Legionnaires' disease. J Infec 56: 1-12, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28: E63, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gershengorn HB, Keene A, Dzierba AL, Wunsch H. The association of antibiotic treatment regimen and hospital mortality in patients with Legionella pneumonia. Clin Infect Dis 60: e66-e79, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S, Yanagihara K, Izumikawa K, et al. The clinical efficacy of fluoroquinolone and macrolide combination therapy compared with single-agent therapy against community-acquired pneumonia caused by Legionella pneumophila. J Infect 59: 222-224, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Stout JE, Yu VL. Legionellosis. N Engl J Med 337: 682-687, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch Intern Med 154: 2417-2422, 1994. [PubMed] [Google Scholar]
- 11.Carratalà J, Mykietiuk A, Fernández-Sabé N, et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med 167: 1393-1399, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Lai CC, Tan CK, Chou CH, et al. Hospital-acquired pneumonia and bacteremia caused by Legionella pneumophila in an immunocompromised patient. Infection 38: 135-137, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Pedro-Botet ML, Sabria M. Legionellosis. Semin Respir Crit Care Med 26: 625e34, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Annaka T, Yoshino M, Momoda T, et al. Rapid and simple detection of Legionella species by LAMP, a new DNA amplification method. Nihon Rinsho Biseibutsugaku Zasshi 13: 19-25, 2003(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 15.den Boer JW, Yzerman EP. Diagnosis of Legionella infection in Legionnaires' disease. Eur J Clin Microbiol Infect Dis 23: 871-878, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires' disease. J Clin Microbiol 41: 838-840, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helbig JH, Uldum SA, Lück PC, Harrison TG. Detection of Legionella pneumophila antigen in urine samples by the BinaxNOW immunochromatographic assay and comparison with both Binax Legionella Urinary Enzyme Immunoassay (EIA) and Biotest Legionella Urin Antigen EIA. J Med Microbiol 50: 509-516, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch DR, Podmore RG, Anderson TP, et al. Impact of routine systematic polymerase chain reaction testing on case finding for Legionnaires' disease: a pre-post comparison study. Clin Infect Dis 57: 1275-1281, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Mo ZY, Zhao HB, et al. LAMP-based method for a rapid identification of Legionella spp. and Legionella pneumophila. Appl Microbial Biotechnol 92: 179-187, 2011. [DOI] [PubMed] [Google Scholar]