Abstract
Fibrosis is a prevalent pathological condition arising from the chronic activation of fibroblasts. This activation results from the extensive intercellular crosstalk mediated by both soluble factors and direct cell-cell connections. Prominent among these are the interactions of fibroblasts with immune cells, in which the fibroblast–mast cell connection, although acknowledged, is relatively unexplored. We have used a Tg mouse model of skin fibrosis, based on expression of the transcription factor Snail in the epidermis, to probe the mechanisms regulating mast cell activity and the contribution of these cells to this pathology. We have discovered that Snail-expressing keratinocytes secrete plasminogen activator inhibitor type 1 (PAI1), which functions as a chemotactic factor to increase mast cell infiltration into the skin. Moreover, we have determined that PAI1 upregulates intercellular adhesion molecule type 1 (ICAM1) expression on dermal fibroblasts, rendering them competent to bind to mast cells. This heterotypic cell-cell adhesion, also observed in the skin fibrotic disorder scleroderma, culminates in the reciprocal activation of both mast cells and fibroblasts, leading to the cascade of events that promote fibrogenesis. Thus, we have identified roles for PAI1 in the multifactorial program of fibrogenesis that expand its functional repertoire beyond its canonical role in plasmin-dependent processes.
Keywords: Cell Biology, Inflammation
Keywords: Fibrosis, Innate immunity, Skin
Introduction
Fibrosis is a pathological condition defined as the excessive deposition of extracellular matrix (ECM) components by activated fibroblasts (1). This activation is manifested in various forms including heightened fibroblast proliferation, collagen production, increased migration, and α–smooth muscle actin (α-SMA) expression. Consequently, fibrosis is often associated with cancer (2), scleroderma (3), and numerous other connective tissue disorders (4, 5). The prevalence of this pathology in numerous diseases and its association with a high mortality rate makes it imperative to understand the causal mechanisms of fibroblast activation in order to develop therapeutics to combat this state.
An important player in fibroblast activation is the innate immune system. Various cells including neutrophils (6), macrophages (7), and mast cells (8, 9) have been shown to be sources of a number of fibroblast-activating cytokines and/or chemokines. Though high numbers of mast cells have been consistently associated with an increasing severity of the fibrotic phenotype in numerous disorders (9–11), relatively little is known about the regulation of these cells in fibrosis. In recent years, it has also been observed that, apart from the mast cell secretome, which consists of fibroblast-activating substances (12, 13), a direct adhesion between these 2 cell types has also been found to be a prerequisite for fibroblast activation (14–16). However, the trigger for the increase in mast cell numbers in fibrotic tissue, as well as the mechanism(s) by which they adhere to fibroblasts and contribute to fibrogenesis, are questions that remain ill defined.
In order to answer this question, we have used a mouse model in which ectopic expression of Snail in the basal keratinocyte layer of the skin leads to the recapitulation of various aspects of fibrosis development seen in the fibrotic disorder scleroderma (17, 18). This is consistent with multiple reports of Snail upregulation in a variety of fibrotic tissues (4, 19–21). One of the factors secreted by these Tg cells is a serpin family protein, the plasminogen activator inhibitor type 1 (PAI1), often reported to be highly expressed in various fibrotic tissues (22, 23). The role of PAI1 in these cases has been credited to its antifibrinolytic function, in which the loss of plasmin and MMP activity leads to the accumulation of fibrin and other ECM components (22, 24, 25). Consistent with these observations, the removal of Pai1 has also been shown to rescue the disease phenotype (22, 26, 27). However, genetic ablation of fibrinogen or inhibition of PAI1 binding to the plasminogen activators did not rescue the fibrotic phenotype to the same extent as the deletion of Pai1 did (28, 29). Hence, the inability to recapitulate the effects of Pai1 deletion by inhibition of its downstream activity suggests that PAI1 has plasmin-independent functions in fibrogenesis. Although the antifibrinolytic functions of PAI1 have received most of the attention, more recent data demonstrate a correlation between heightened PAI1 expression and inflammation in numerous fibrotic conditions (22, 29, 30). Also important in fibrosis is the ability of PAI1 to regulate intracellular signaling in fibroblasts and other cell types through the urokinase/tissue plasminogen activator receptors and integrins on the cell surface (22, 31, 32). A recent study has shown that the upregulation of PAI1 in the epidermis in graft-versus-host disease and bleomycin-induced skin fibrosis is responsible for the disease pathology (29). Altogether, the literature points to novel roles of PAI1 in fibrosis that are yet to be discovered.
We have previously shown that other factors secreted from the Snail-Tg keratinocytes, such as fibulin 5 (17) contribute in different ways to the development of skin fibrosis. The existence of these simultaneously operating mechanisms makes it evident why inhibitors of IL-4 or TGF-β alone are yielding disappointing results as antifibrotic targets in clinical trials (33, 34). Thus, there is an imperative need to examine this disease condition as a complex of various interacting cell types and parallel signaling pathways. Among these, we identify PAI1 as another key player in fibrogenesis that might serve as a possible therapeutic target.
Results
PAI1 contributes to fibrogenesis in Snail-Tg skin.
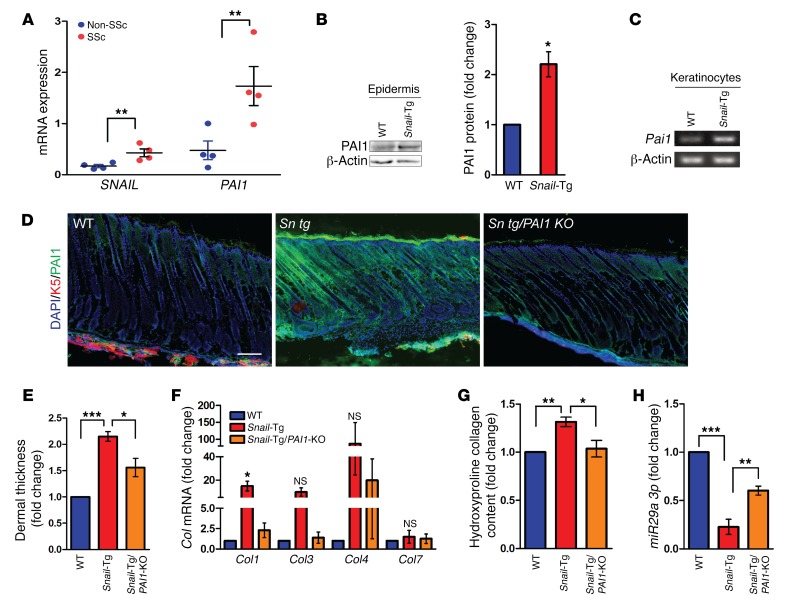
Consistent with the notion that SNAIL is a causal factor in cutaneous fibrosis, we previously reported (35) that the transcription factor Snail targeted to the basal layer of the epidermis via the keratin-14 promoter is sufficient to induce phenotypes that are hallmarks of fibrosis. The fibrosis-inducing activity of Snail was supported by the observation that the expression of this transcription factor was also significantly upregulated in skin samples from human scleroderma patients (Figure 1A). Interestingly, we observed that mRNA expression of PAI1 was also elevated in human scleroderma skin samples (Figure 1A). Furthermore, this correlation between Snail and Pai1 upregulation was extended to the Tsk2/+ mouse, an established skin fibrosis model (Supplemental Figure 1, A and B; supplemental material available online with this article; https://doi.org/10.1172/JCI99088DS1). Likewise, human samples of fibrotic lung, liver, and kidney also revealed a possible association between SNAIL upregulation and elevated PAI1 levels (Supplemental Figure 1, A and B). Although PAI1 has been implicated in a profibrotic role in all of these tissues, its function in skin pathology remains elusive (22, 23). In order to explore whether there is a link between PAI1 expression and SNAIL in epidermal keratinocytes, we first examined PAI1 expression in Snail-Tg skin. We observed that PAI1 protein levels increased in the Snail epidermis (Figure 1B). Consistent with our results, transcript levels of Pai1 also increased in the Snail-Tg keratinocytes (Figure 1C and Supplemental Figure 1C). Analysis of the Pai1 gene promoter revealed a canonical E box–binding site for the Snail1 transcription factor (data not shown), suggesting that SNAIL can directly regulate Pai1 expression in epidermal keratinocytes. The increase in total protein production in the epidermis was reflected in the amount of secreted PAI1, which was significantly higher in the neonatal Snail-Tg skin when compared with WT tissue (Figure 1D). Interestingly, the increase in PAI1 secretion did not persist in adult Snail-Tg mice (Supplemental Figure 1D), suggesting that PAI1 functions at the early stages of fibrogenesis. Since PAI1 belongs to the plasminogen activator inhibitor family along with PAI2 and PAI3 (22, 36), we examined whether SNAIL had an impact on their expression in epidermal keratinocytes. However, we found that the transcript levels of Pai2 and Pai3 did not change between the WT and Snail-Tg keratinocytes (Supplemental Figure 1C). Deletion of the Pai1 gene in the Snail-Tg mouse (Snail-Tg/Pai1-KO) was also not accompanied by a significant increase in PAI2 or PAI3 levels in either neonatal or adult tissues (Supplemental Figure 1E). Thus, our data suggest that SNAIL expression is sufficient to specifically induce PAI1 overexpression in epidermal keratinocytes in a cell-autonomous fashion.
Figure 1. PAI1 contributes to fibrosis in Snail-Tg skin.
(A) qPCR for SNAIL and PAI1 in skin samples from healthy individuals (Non-SSc) and scleroderma patients (SSc) (n = 4). (B) Western blot for PAI1 in WT and Snail-Tg epidermis and quantification. β-Actin was used as a loading control (n = 3). (C) Reverse transcriptase PCR of Pai1 in WT and Snail-Tg keratinocytes (n = 3). (D) WT, Snail-Tg (Sn tg), and Snail-Tg/Pai1-KO (Sn tg/PAI1 KO) neonatal skin analyzed for PAI1 secretion by immunostaining (n = 3). Scale bar: 50 μm. (E–H) WT, Snail-Tg, and Snail-Tg/Pai1-KO adult skin sections were analyzed for (E) dermal thickness by quantitation of H&E-stained sections (n = 4); (F) Col1, -3, -4, and -7 by qPCR (n = 3); (G) collagen protein levels by hydroxyproline assay (n = 5); and (H) miR29a 3p levels by qPCR (n = 3). Data represent the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, by Student’s t test (A and B) and 1-way ANOVA followed by Tukey’s post hoc analysis (E–H).
Though PAI1 expression was elevated in neonatal Snail-Tg skin, we hypothesized that the role of PAI1 in early fibrogenesis would be reflected in the final manifestation of the pathology. To analyze the functional outcome of the increased expression of PAI1 in Snail-Tg skin, we analyzed Snail-Tg/Pai1-KO mice. In brief, Snail-Tg/Pai1-KO mice were generated by mating Snail-Tg mice (35) with Pai1-KO mice (obtained from The Jackson Laboratory). A hallmark of fibrosis is the excessive deposition of ECM components (1), resulting in an increase in dermal thickness, which can be used as a proxy for measuring fibrotic progression. As we previously reported, the dermal thickness in Snail-Tg skin is significantly increased relative to its WT counterpart (17). The deletion of Pai1 in the Snail-Tg mouse was sufficient to cause a significant reduction in dermal thickness (Figure 1E and Supplemental Figure 1F). To probe the molecular basis of this PAI1-dependent increase in dermal thickness, we measured the levels of collagen, which is the major ECM protein in the skin. PAI1 is known to contribute to the accumulation of collagen in keloid fibroblasts and other fibrotic conditions (22, 24, 27), and likewise the transcript levels of collagens 1, 3, and 4 (Col1, -3, and -4), the major collagens in the dermis, increased in the Snail-Tg mouse but returned to WT levels when the Pai1 gene was removed (Figure 1F). However, this effect was not universal, as Col7 levels did not change in the Snail-Tg mouse. The transcriptional upregulation of collagen was reflected at the protein level, as evidenced by a hydroxyproline assay that showed an increase in total collagen content in Snail-Tg skin (Figure 1G). Again, this increase in total collagen protein levels was dependent on PAI1 expression. The PAI1-dependent change in collagen levels led us to explore the mechanisms of regulation of collagen. It has been reported that the microRNA miR29a regulates Col expression in fibrotic conditions such as systemic sclerosis (37). Consistent with this observation, miR29a expression was suppressed in Snail-Tg skin and was rescued in Snail-Tg/Pai1-KO tissue (Figure 1H). Though necessary for the upregulation of Col expression in Snail-Tg skin, we found that treatment of primary dermal fibroblasts with recombinant PAI1 was not sufficient to induce an increase in Col expression (Supplemental Figure 1G). Consistent with this, recombinant PAI1 did not directly regulate the expression of miR29a expression in fibroblasts (Supplemental Figure 1H). Altogether, these observations suggest an indirect role of PAI1 in fibroblast activation at an early stage in fibrogenesis.
PAI1 induces the development of a proinflammatory environment in the skin.
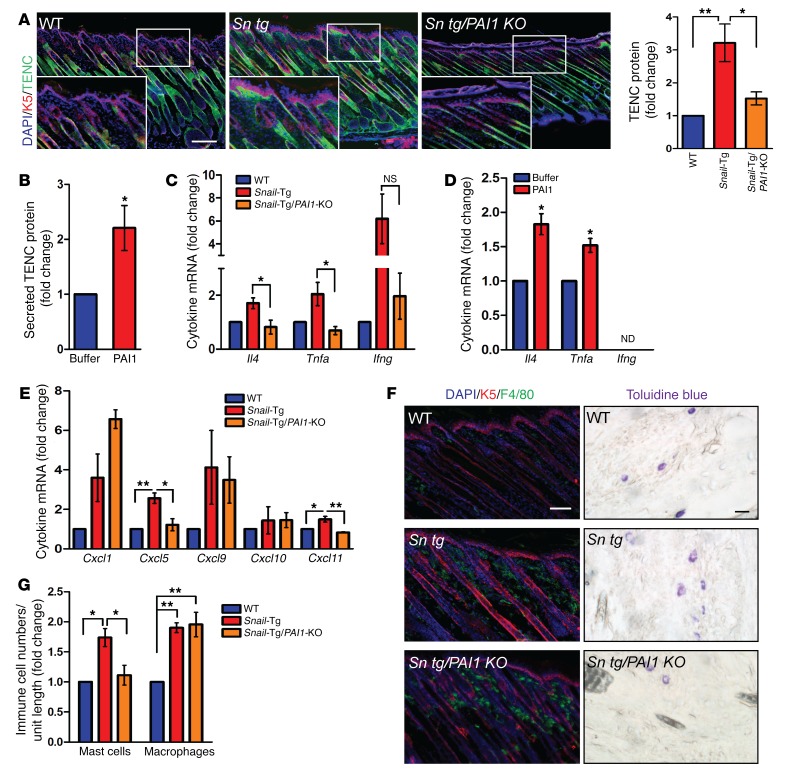
Interestingly, our analysis of phenotypic development revealed that PAI1 did not affect Col levels in neonatal skin or in recombinant PAI1–treated newborn fibroblasts (Supplemental Figure 2A). However, another ECM protein, tenascin C (TENC), was found to be upregulated in neonatal Snail-Tg skin near the epidermal-dermal junction and was substantially diminished when the Pai1 gene was deleted (Figure 2A and Supplemental Figure 2B). This effect was recapitulated in vitro, wherein treatment of newborn dermal fibroblasts with recombinant PAI1 resulted in a significant upregulation of TENC secretion (Figure 2B and Supplemental Figure 2C). Thus, our results suggest that PAI1 regulates TENC expression during the early stages of fibrogenesis in Snail-Tg skin.
Figure 2. PAI1 affects fibrogenesis during the inflammation phase in Snail-Tg skin.
(A) WT, Snail-Tg, and Snail-Tg/Pai1-KO pup skin sections were analyzed for TENC localization by immunostaining (n = 3; scale bar and original magnification: 50 μm) and total TENC protein levels by Western blotting (n = 3). (B) Quantification of secreted TENC in recombinant PAI1-treated fibroblast conditioned media (n = 3). qPCR for proinflammatory cytokines Tnfa, Il4, and Ifng in (C) neonatal WT, Snail-Tg, and Snail-Tg/Pai1-KO skin sections (n = 3) and (D) recombinant PAI1–treated fibroblasts (n = 3). (E and F) Neonatal WT, Snail-Tg, and Snail-Tg/Pai1-KO pup skin sections were analyzed for (E) the cytokines Cxcl1, Cxcl5, Cxcl9, Cxcl10, and Cxcl11 by qPCR (n = 3) and (F) macrophages by immunostaining (left; scale bar: 50 μm; n = 3) and mast cells by toluidine blue staining (right; scale bar: 10 μm; n = 4). (G) Quantification of cell numbers in F. Data represent the mean ± SEM. *P < 0.05 and **P < 0.01, by Student’s t test (B and D) and 1-way ANOVA followed by Tukey’s post hoc analysis (A, C, E, and G).
TENC plays a role in the development of the innate immune response in fibrotic conditions. It has been reported that TENC is upregulated in regions of immune cell infiltration and is associated with an upregulation of the cytokines IL-4, TNF-α, and IFN-γ (38–40). We observed that the levels of all 3 cytokines increased in neonatal Snail-Tg skin and that their expression was dependent on PAI1, as the expression levels decreased significantly when the Pai1 gene was deleted (Figure 2C). Furthermore, recombinant PAI1 treatment was sufficient to induce Il4 and Tnfa expression in primary dermal fibroblasts (Figure 2D). However, the levels of Ifng expressed in the fibroblasts was below detectable limits. We also found that the chemotactic cytokines Cxcl5 and Cxcl11 were increased in Snail-Tg skin and dependent on PAI1 (Figure 2E). However, though other chemokines such as Cxcl1 and Cxcl9 were increased in Snail-Tg skin, their expression did not appear to be dependent on PAI1. Our observations are consistent with reports that correlate elevated PAI1 levels with tissue inflammation in various fibrotic scenarios (22, 27, 41). We demonstrate that PAI1 is an important mediator of inflammatory cytokine production in dermal fibroblasts in neonatal Snail-Tg skin and that this mediation may be at least partly accomplished through the regulation of TENC expression.
Given the upregulation of inflammatory cytokines, we profiled the status of innate immune cells in Snail-Tg skin and their dependence on PAI1. As we have previously reported (17, 18), the numbers of macrophages and mast cells are significantly higher in neonatal Snail-Tg skin. Interestingly, we found that the number of mast cells was comparable to WT numbers in Snail-Tg/Pai1-KO skin, while the number of macrophages remained elevated (Figure 2, F and G). The increase in innate immune cell numbers correlates with the upregulation of the various cytokines in Snail-Tg skin, however, our data reveal that PAI1 expression primarily affected mast cell numbers in prefibrotic tissue. The failure of therapeutic modalities aimed at macrophages and neutrophils has led to an increasing interest in the role of mast cells in fibrosis (9, 42). This arises from the fact that mast cells have been associated with fibroblast activation by the release of fibroblast-activating substances as well as cell-cell adhesion (12, 43). An upregulation of mast cell numbers has been observed in numerous fibrotic disorders (8, 9, 11), but the mechanism by which their numbers are regulated within tissues remains elusive. We postulate that PAI1 can influence mast cell numbers in the skin, possibly via modulation of the immune environment.
Recruitment of mast cells in fibrosis and wound healing.
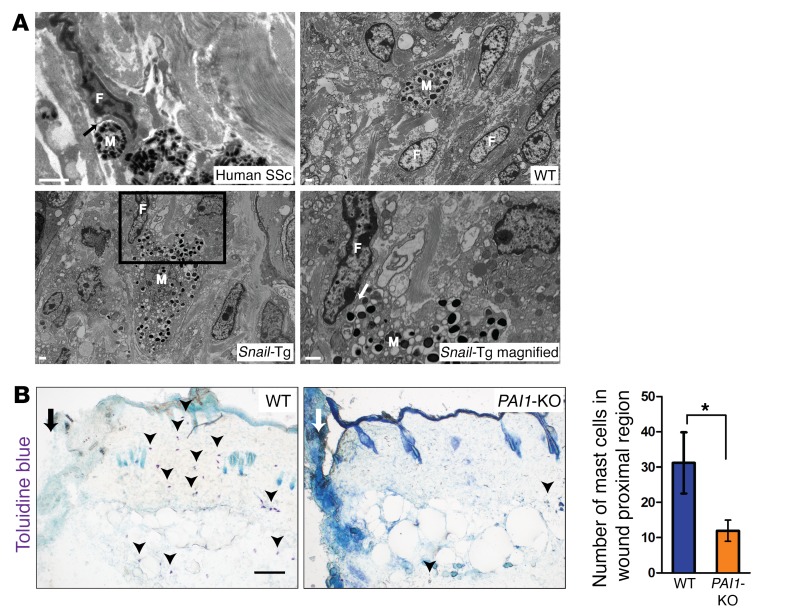
Although the upregulation of mast cell numbers has been reported in the skin of scleroderma patients (11, 44), the contribution of these cells to the fibrotic phenotype in human skin remains undetermined. As previously reported (42), we observed a direct mast cell–fibroblast interaction in human scleroderma patients, and this phenomenon was recapitulated in neonatal Snail-Tg skin (Figure 3A). In contrast to WT mouse skin, in which the few mast cells that are present are not in close apposition to other cells of the dermis, Snail-Tg skin had a majority of the increased number of mast cells adjacent to one or more fibroblasts. Further examination revealed that the mast cells in the Snail-Tg tissue shared multiple points of direct physical contact of its plasma membrane with the surface of the dermal fibroblasts (Figure 3A). Since tissue fibrosis is a pathological outcome of a normal wound-healing response, we hypothesized that PAI1 may play a similar role in mast cell infiltration into sites of tissue injury. Though mast cells are known to be involved in multiple phases of the wound-healing program and promote the inflammatory response, reepithelialization, angiogenesis, and scar formation, the mechanisms by which these activities are accomplished is unknown (45). Consistent with this hypothesis, it has been previously observed that PAI1 expression increases upon wounding as early as 1 day after injury (46). Intriguingly, we found that mast cell infiltration into the wound bed was dependent on the presence of PAI1 one day following cutaneous injury (Figure 3B), supporting the view that PAI1 may function in a role as a chemoattractant for mast cells.
Figure 3. Recruitment of mast cells and direct binding to fibroblasts.
(A) Scanning electron micrographs of mast cells (M) and fibroblasts (F) in skin samples from a human scleroderma patient (scale bar: 500 nm; black arrow indicates an area of direct mast cell–fibroblast interaction), and WT and Snail-Tg mice (scale bars: 1 μm). The magnified view of the boxed area shows direct fibroblast–mast cell interaction in Snail-Tg skin (scale bar: 1 μm; n = 2; white arrow denotes an area of direct fibroblast–mast cell interaction). (B) Toluidine blue staining for mast cells (arrowheads) in proximal wound region (marked by an arrow; scale bar: 100 μm) 1 day after injury in WT and Pai1-KO skin. Graph shows the quantification of mast cells in WT and Snail-Tg skin (n = 3). Data represent the mean ± SEM. *P < 0.05, by Student’s t test.
PAI1 acts as a chemoattractant for mast cells and mediates mast cell–fibroblast adhesion.
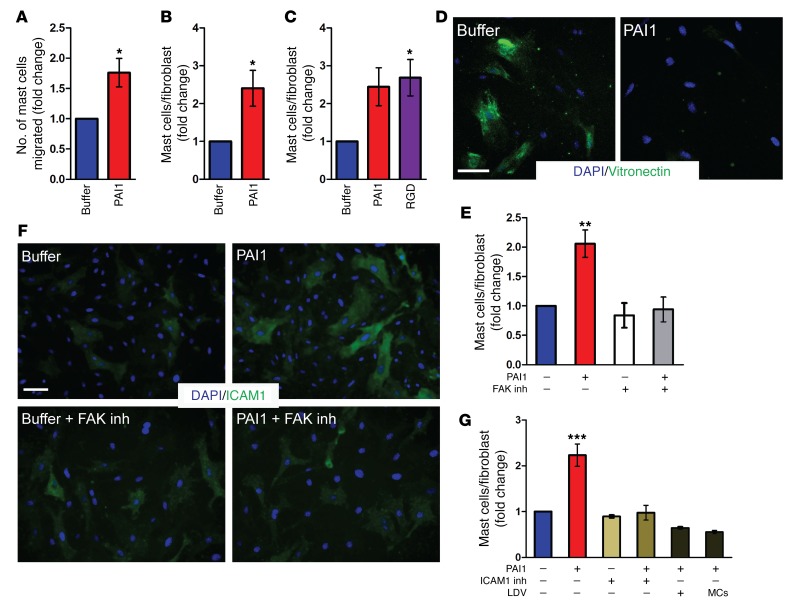
Mast cell homing into the prefibrotic tissue is a common feature of fibrosis, but the underlying mechanism remains unclear. The dependence of mast cells on PAI1 for infiltration into the injured site following wounding as well as in the prefibrotic skin of the Snail-Tg mouse led us to explore the possibility that PAI1 can directly serve as a chemotactic factor to induce an increase in mast cell numbers in the skin. Consistent with this prediction, we observed increased chemotaxis of murine MCP5 mast cells in the presence of recombinant PAI1 in a classical Transwell migration assay (Figure 4A). Increased mast cell numbers have been implicated in fibroblast activation via heterotypic cell-cell adhesion in many fibrotic tissues (16, 43). However, the identity of the regulatory agent that induces this adhesion is still unknown. We examined whether the effect of PAI1 extends beyond inducing chemotaxis of mast cells in fibrosis. Cocultures of mast cells and fibroblasts in the presence of recombinant PAI1 led to an increase in mast cell adhesions per fibroblast (Figure 4B and Supplemental Figure 3A), indicating that PAI1 is sufficient to stimulate fibroblast–mast cell attachment.
Figure 4. PAI1 mediates mast cell infiltration and increased fibroblast mast cell adhesion.
(A) Quantification of migrating mast cells into buffer or recombinant PAI1–containing media in a Transwell assay (n = 5). Quantification adherent mast cells on newborn dermal fibroblasts after (B) recombinant PAI1 treatment of fibroblast–mast cell cocultures (n = 4) and (C) pretreatment of fibroblasts with buffer, recombinant PAI1, and RGD peptide (n = 3). (D) Immunostaining for surface-bound vitronectin after treatment of fibroblasts with buffer or recombinant PAI1 (n = 3). Scale bar: 50 μm. (E) Quantification of adherent mast cells after pretreatment of fibroblasts with buffer or recombinant PAI1 in the absence or presence of FAK inhibitor (FAK inh) (n = 3). (F) Immunostaining of ICAM1 expression after recombinant PAI1 treatment of fibroblasts in the absence or presence of FAK inhibitor (n = 3). Scale bar: 50 μm. (G) Quantification of adherent mast cells after pretreatment of fibroblasts with buffer or recombinant PAI1 followed by incubation with ICAM1 inhibitor (ICAM1 inh) or LDV peptide, or with mast cells (MCs) preincubated with LDV peptide (n = 3). Data represent the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, by Student’s t test (A and B) and 1-way ANOVA followed by Tukey’s post hoc analysis (C, E, and G).
Given that PAI1 can orchestrate an increase in mast cell adhesion, we proceeded to investigate its mechanism of action. PAI1 is known to mediate intracellular signaling via the regulation of cell-surface integrins by unmasking the available RGD motif–binding site (47, 48). We hypothesized that this pathway should be activated merely by external addition of the RGD peptide (49, 50), which is the integrin-binding motif found on ECM proteins such as fibronectin. We found that a pulse of recombinant PAI1 treatment led to an increase in mast cell adhesion to fibroblasts (Figure 4C and Supplemental Figure 3B). Likewise, pretreatment of fibroblasts with the RGD peptide for a limited period of time was sufficient to replicate this effect of PAI1 (Figure 4C and Supplemental Figure 3B). It has previously been reported that PAI1 releases αV integrin from the soluble glycoprotein vitronectin (31, 51). In corroboration with these observations, a transient treatment of dermal fibroblasts with recombinant PAI1 caused a loss of surface-bound vitronectin on the cells (Figure 4D). Moreover, recombinant PAI1 treatment of fibroblasts also caused an upregulation of phosphorylated FAK (p-FAK) expression in these cells (Supplemental Figure 3C). This observation implies that the mast cell–fibroblast interaction is mediated through αVβ3 integrin and the downstream focal adhesion kinase (FAK) signaling pathway, which the RGD peptide can also activate. Consistent with the activation of FAK, treatment with the FAK inhibitor SC203950 resulted in the loss of mast cell–fibroblast adhesion induced by either recombinant PAI1 (Figure 4E and Supplemental Figure 3D) or the RGD peptide (Supplemental Figure 3E). Altogether, these data demonstrate that PAI1-mediated activation of FAK signaling on fibroblasts is responsible for the increase in mast cell adhesion to fibroblasts.
Next, we sought to determine the mechanism by which FAK signaling in dermal fibroblasts promotes their adhesion to mast cells. A number of cell-surface proteins on fibroblasts have been reported to mediate fibroblast mast cell interaction. Among these are membrane-bound stem cell factor (SCF) (15), hyaluronic acid receptors (52), fibrinogen (53), and gap junctions (54). However, pharmacological inhibition of these cell-surface proteins did not significantly alter the ability of recombinant PAI1 to induce fibroblast–mast cell attachment (Supplemental Figure 3F). Our results are further corroborated by earlier reports showing that neutralization of the β1 integrin chain on mast cells, which is known to mediate fibrinogen binding, and also of the c-Kit ligand on mast cells, which mediates binding to SCF (55), does not inhibit fibroblast mast cell adhesion. Thus, we studied the ability of other surface adhesion molecules regulated by integrin/FAK signaling that may render fibroblasts capable of adhering to mast cells in a PAI1-dependent manner. One of these is the intercellular adhesion molecule (ICAM) family, and in particular ICAM1, which has been reported to be upregulated through FAK activation (56). We observed that treatment with recombinant PAI1 was sufficient to cause an increase in the expression of the ICAM1 receptor on the surface of dermal fibroblasts (Figure 4F). Furthermore, this upregulation depended on FAK activation, indicating that PAI1 regulates ICAM1 expression on the surface of fibroblasts via the FAK pathway. Moreover, blocking the ICAM1 receptor with the neutralizing antibody YN1/1.7.4 abrogated the recombinant PAI1–induced adhesion of mast cells to fibroblasts (Figure 4G and Supplemental Figure 3G). Thus, we conclude that PAI1 mediates increased mast cell adhesion to fibroblasts through upregulation of the ICAM1 receptor on the cell surface of fibroblasts. Interestingly, the potential role of the ICAM1 receptor in mediating fibrogenesis is supported by data on fibrotic samples from human patients as well as from a mouse model of fibrosis (Tsk2/+), which revealed an upregulation of Icam1 mRNA in all of these conditions (Supplemental Figure 3H). Thus, we propose that the PAI1/FAK/ICAM1 receptor signaling pathway is conserved in a variety of fibrotic conditions.
To functionally test the role of ICAM1 receptors on fibroblasts in mediating the ability of fibroblasts to adhere to mast cells, we used an LDV peptide as a competitive inhibitor. ICAM receptors possess an LDV motif that mediates the binding to their ligand (57, 58). We hypothesized that this LDV motif is the main domain responsible for allowing fibroblasts to adhere to mast cells. In accordance with our hypothesis, competition of the LDV motif on the ICAM1 receptor with a soluble LDV peptide inhibited the ability of fibroblasts to adhere to mast cells (Figure 4G and Supplemental Figure 3I). Moreover, the loss of adhesion could not be recapitulated with the RGD peptide (Supplemental Figure 3J), suggesting that this ICAM1-mediated binding occurs specifically via its LDV motif.
We then investigated the cognate ligand on the mast cell that binds to the LDV domain of ICAM1 on dermal fibroblasts. Interestingly, the LDV peptides can be recognized by integrins (59). We therefore postulated that an integrin on the mast cell mediates its interaction with the LDV-containing ICAM1 receptor on fibroblasts. This would lead to a measurable activation of the FAK pathway in the adherent mast cells. Consistent with our prediction, we saw an upregulation of p-FAK expression in the adherent mast cells in the presence of recombinant PAI1 (Supplemental Figure 3K). This result suggests that PAI1-induced upregulation of the ICAM1 receptor on fibroblasts allows binding of the receptor to a resident integrin on the mast cell, leading to the cells’ adhesion and subsequent activation.
PAI1-mediated heterotypic adhesion leads to activation of both fibroblasts and mast cells.
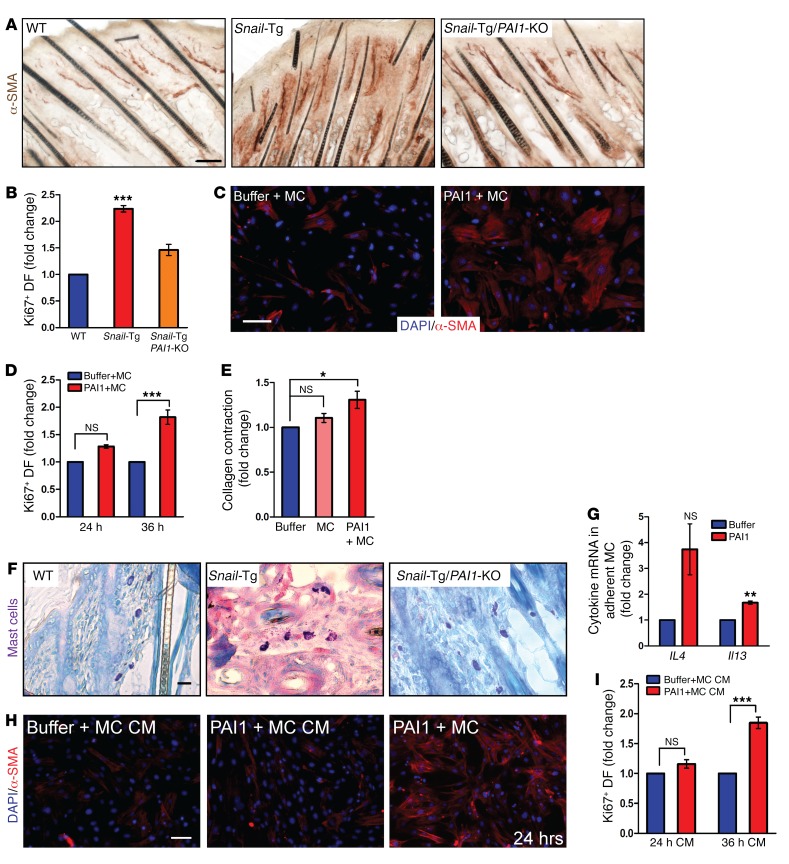
The role of PAI1 as an inducer of mast cell adhesion to fibroblasts led us to probe the effect of this heterotypic interaction on both cells. We first tested which characteristics of activated fibroblasts are impacted by PAI1 in vivo. We observed that the number of α-SMA–expressing dermal cells (Figure 5A) and proliferating fibroblasts marked by Ki67 and vimentin (Figure 5B and Supplemental Figure 4A) increased markedly in neonatal Snail-Tg skin. These phenotypes were absent upon deletion of Pai1 from the Snail-Tg background, indicating that PAI1 regulates fibroblast activation. However, recombinant PAI1 alone was insufficient to trigger α-SMA expression in fibroblasts (Supplemental Figure 4B) or increase fibroblast proliferation (Supplemental Figure 4, C and D) in vitro. However, when mast cells were cocultured with fibroblasts, the addition of recombinant PAI1 resulted in an increase in α-SMA expression after 24 hours (Figure 5C) and proliferation of fibroblasts after 36 hours (Figure 5D and Supplemental Figure 4, C and D). Activated fibroblasts have also been associated with an elevated contractile property. We found that collagen gel contraction was stimulated in fibroblast–mast cell cocultures treated with recombinant PAI1 (Figure 5E), to the same extent as that caused by treatment of fibroblasts with TGF-β, the common method used to activate fibroblasts (Supplemental Figure 4E). Although the phenomenon of mast cell–mediated fibroblast activation has previously been reported (15, 16, 43), the mechanism regulating this phenomenon is still unknown. These results demonstrate that the PAI1-dependent adhesion of fibroblasts to mast cells is sufficient to activate the fibroblasts.
Figure 5. PAI1-mediated fibroblast–mast cell adhesion leads to activation of both cell types.
Skin sections from neonatal WT, Snail-Tg, and Snail-Tg/Pai1-KO animals were analyzed for (A) IHC of α-SMA expression (scale bar: 50 μm; n = 3) and (B) quantitation of Ki67 expression in vimentin+ fibroblasts (DF) (n = 3). (C and D) Buffer- or recombinant PAI1–treated fibroblast mast cell (MC) cocultures were analyzed for (C) α-SMA expression after 24 hours of treatment (scale bar: 50 μm; n = 3) and (D) Ki67 expression after 24 and 36 hours of treatment (n = 3). (E) Quantification of collagen contraction after 24 hours by fibroblasts, fibroblast–mast cell cocultures, and recombinant PAI1–treated fibroblast–mast cell cocultures (n = 4). (F) Giemsa staining of mast cells in neonatal WT, Snail-Tg, and Snail-Tg/Pai1-KO pup skin sections (scale bar: 10 μm; n = 4). (G) qPCR for Il4 and Il13 expression in fibroblast-adherent mast cells after 24 hours of treatment with recombinant PAI1 (n = 3). (H and I) Fibroblasts treated for 24 or 36 hours with conditioned media (CM) from fibroblast–mast cell cocultures in the presence or absence of recombinant PAI1 were analyzed for (H) α-SMA expression, with a 24-hour coculture as a positive control (scale bar: 50 μm; n = 3). (I) Quantitation of Ki67 expression (n = 3). Data represent the mean ± SEM. P values were calculated by Student’s t test (G) and 1-way ANOVA followed by Tukey’s post hoc analysis (B, D, E, and I) (*P < 0.05, **P < 0.01, and ***P < 0.001).
We next examined the status of the mast cells in fibrotic Snail-Tg skin. We observed that the mast cells in the Snail-Tg mouse showed an activated, degranulated phenotype when compared with WT mast cells (Figure 5F and Supplemental Figure 4F). PAI1 played a major role in this mast cell activation, as this phenotype was markedly lost in the Snail-Tg/Pai1-KO skin. Various cytokines including IL-4 and IL-13 are canonical markers for mast cell activation (60–62). Our analysis revealed that the transcript levels of Il4 and Il13 were significantly increased in the fibroblast-adherent mast cells following treatment with recombinant PAI1 for 24 hours (Figure 5G). Interestingly, treatment of nonadherent mast cells with recombinant PAI1 did not lead to their activation (Supplemental Figure 4G). Therefore, we conclude that adhesion of mast cells to fibroblasts in the presence of PAI1 enables activation of the mast cells, as evidenced by the upregulation of Il4 and Il13 in these cells
Our data indicate that PAI1 mediates the reciprocal activation of both mast cells and fibroblasts. However, the lag time in the upregulation of fibroblast proliferation led us to investigate whether the different types of fibroblast activation resulted directly from mast cell adhesion or from the release of soluble factors. We hypothesized that the conditioned media from the recombinant PAI1–treated cocultures would contain the factors released from activated mast cells and, therefore, that treatment of fibroblasts with the conditioned media would mimic the addition of the activating factors. We found that the treatment of fibroblasts with conditioned media from fibroblast–mast cell cocultures pretreated with PAI1 for 24 hours failed to cause upregulation of α-SMA levels (Figure 5H and Supplemental Figure 4H) or affect fibroblast proliferation (Figure 5I and Supplemental Figure 4I). This suggests that α-SMA expression is dependent on PAI1-mediated fibroblast–mast cell attachment. In contrast, the treatment of fibroblasts with conditioned media from fibroblast–mast cell cocultures treated with PAI1 for 36 hours was sufficient to activate fibroblast proliferation (Figure 5I and Supplemental Figure 4I). This suggests that the factors released after 24 hours of PAI1-treated fibroblast–mast cell coculture are particularly necessary for the activation of fibroblast proliferation.
In conclusion, we show that the PAI1-mediated heterotypic adhesion of fibroblasts to mast cells is sufficient for the activation of mast cells as well as the upregulation of α-SMA in fibroblasts. On the other hand, secreted factors released from the adhesion of fibroblasts and mast cells are capable of stimulating fibroblast proliferation. Thus, we have delineated the multiple avenues by which mast cells can activate fibroblasts in a PAI1-dependent manner.
Discussion
Altogether, our data reveal mechanistic roles for PAI1 in fibrogenesis that are independent of its canonical role in plasmin-dependent processes (Figure 6). We found that PAI1, secreted from Snail-Tg keratinocytes, was instrumental in the development of an inflammatory response via TENC upregulation. Additionally, it could function as a chemokine for mast cell migration into the prefibrotic tissue. Apart from its role in inflammation, PAI1 mediated the upregulation of the ICAM1 receptor on fibroblasts that drove their increased adhesion to mast cells. This heterotypic cell-cell interaction led to the activation of both mast cells and fibroblasts. Thus PAI1 plays multiple critical roles as a mediator of infiltration, adhesion, and activation of mast cells and fibroblasts in fibrogenesis.
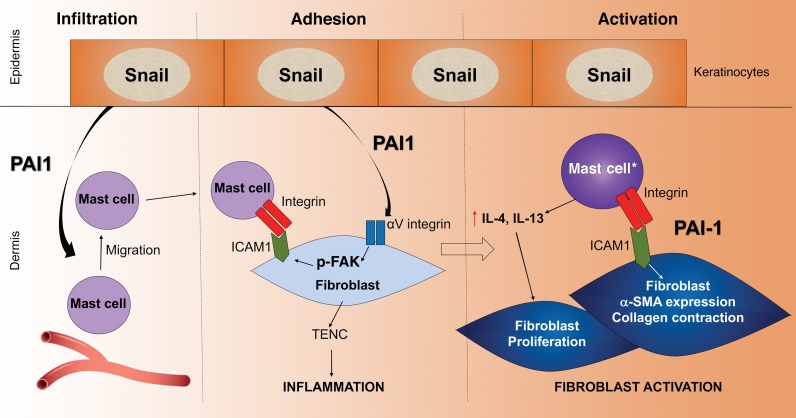
Figure 6. Model of the multiple roles of PAI1 in cutaneous fibrosis.
PAI1 secreted from the Snail-Tg keratinocytes functions in 3 potentially novel roles during fibrogenesis. 1: PAI1 acts as a chemoattractant for the infiltration of mast cells into the skin. 2: PAI1 stimulates integrin signaling in dermal fibroblasts that leads to the upregulation of tenacin C and the establishment of an inflammatory microenvironment, which is a key driver of fibrogenesis. In addition, this signaling pathway also leads to the upregulation of ICAM1 expression, which renders the fibroblast competent to adhere to the mast cell. 3: This adhesion culminates in the cellular activation phase of fibrosis development. In addition to the direct activation of a subset of fibroblasts (marked by α-SMA expression and increased contractile behavior), this physical interaction results in the activation of the adherent mast cells. The activated mast cells (mast cell*) then secrete cytokines such as IL-4 and IL-13 capable of promoting the proliferation of another subset of fibroblasts.
The elucidation of a chemotactic role for PAI1 may also be relevant in the context of numerous other scenarios. Interestingly, the secretome from Snail-expressing pancreatic ductal adenocarcinoma tumors has been implicated in the induction of mast cell infiltration (63). Although this secretome contains SCF, a well-known mast cell chemokine, the existence of other factors has not been explored. The loss of PAI1 expression has also been correlated with decreasing neutrophil and macrophage numbers in renal fibrosis (27, 64), indicating that its function as a chemokine might be extended to other immune cells. Apart from its direct role, PAI1 also mediates the upregulation of various cytokine signaling pathways in the skin. This includes the receptors for CXCL5 and CXCL11, which are expressed by human mast cells (65). These cytokines have also been implicated in neutrophil, NK cell, and T cell migration and activation (66). Interestingly PAI1 affects the T cell repertoire in Snail-Tg skin (data not shown), but the mechanism by which this is accomplished and its downstream effects remain to be explored.
The presence of mast cell–adherent fibroblasts in the skin of patients with scleroderma highlights the potential importance of this phenomenon in therapeutic development (42, 67, 68). We have discovered that PAI1 is capable of inducing the upregulation of ICAM1 on dermal fibroblasts that facilitates their adhesion to mast cells. Our findings are in line with earlier reports suggesting that during kidney injury, ablation of PAI1 expression or inhibition of its interaction with vitronectin reduces LPS-dependent ICAM1 expression (69). Moreover, targeting αV integrin, the mediator of PAI1-dependent adhesion, can abrogate fibrosis in several organs (70). Our results showing that the LDV motif of the ICAM1 receptor on dermal fibroblasts plays a key role in mast cell adhesion suggest that the integrins α4β1 or αIIββ3 may function as ligands on the mast cells (53, 58, 71).
The heterotypic cell-cell attachment between mast cells and fibroblasts culminates in the activation of both cell types in a temporal sequence. An acute response to this interaction is the increase in α-SMA expression in dermal fibroblast and a degranulated phenotype in mast cells. This is an important finding, as the trigger for mast cell degranulation in fibrotic tissues was not known (42). Degranulated mast cells are known reservoirs of various fibroblast activators such as histamine, tryptase, chymase, interleukins, and TGF-β (9, 72). A subsequent outcome of this heterotypic interaction is the production in activated mast cells of the profibrotic interleukins IL-4 and IL-13, which are likely candidates for the induction of fibroblast proliferation (73, 74). This is supported by the findings that targeting IL-4 receptor– and IL-13 receptor–expressing cells, including fibroblasts, can cause attenuation of lung fibrosis (75). The temporal delay in the induction of fibroblast proliferation may be the result of the requirement for the accumulation of IL-4 and IL-13 to achieve biologically active concentrations in the culture media. The different responses (α-SMA expression versus proliferation) of the fibroblasts hint at the possible heterogeneity in the fibroblast population. This is further reflected in the observation that primary dermal fibroblast cultures are not uniformly bound to surface vitronectin, nor do they express ICAM1 or bind to mast cells. This suggests the possibility that only certain subsets of fibroblasts are capable of being stimulated in a PAI1-dependent manner.
Although there are numerous reports documenting an increase in mast cell numbers in various fibrotic scenarios (8, 11, 76, 77) including the skin fibrotic condition of scleroderma (11, 44, 78), there are nevertheless suggestions that these cells are not limiting factors in fibrosis development (79–81). These studies show that the reduction of mast cell numbers does not affect the dermal thickness or collagen bundling in bleomycin-induced skin fibrosis. A possible interpretation of these results is that the depletion of mast cells in these systems is not complete. Furthermore, the reduction of mast cells in these animals may have induced possible compensatory pathways that highlight the complexity and redundant nature of this disease. Our observations that Snail and Pai1 (Supplemental Figure 1) and Icam1 (Supplemental Figure 3) were upregulated not only in the skin but in other fibrotic conditions, along with independent observations of high mast cell numbers in the same cases, indicate that not only is this mechanism important, but it is also conserved in other tissues. Our work with the Snail-Tg mouse reinforces the opinion that fibrosis is a complex disorder with multiple parallel processes and provides a potential explanation for the lack of success of anti–TGF-β therapy so far (33, 82). Thus, we identify PAI1 as an important mediator of fibrosis development in the foundational stages of the disease.
Methods
Animal studies.
C57Bl6 (WT) and B6.129S2-Serpine1tm1/Mlg/J (Pai1-KO) mice were obtained from The Jackson Laboratory. The K14-Snail-Tg mouse was developed as described earlier (35). The K14-Snail tg/Pai1-KO mouse was developed after breeding the Pai1-KO and K14-Snail mice. All mice were maintained and bred at the animal facility at the NCBS under specific pathogen–free conditions. CD1 mice were mated with these mice and inbred for 8 to 10 generations to develop the above-mentioned strains, and mice of both backgrounds and sexes were used for all the experiments. Mice were sacrificed between P7 and P9 (neonatal) or at 8 weeks of age (adult), and skin samples were collected for DNA, RNA, and protein and for embedment in OCT or paraffin as required.
Gene expression.
Total RNA was extracted from skin biopsies or cell lysates using TRIzol Reagent (TaKaRa, Thermo Fisher Scientific). cDNA synthesis was done using Superscript III (Thermo Fisher Scientific), followed by quantitative PCR (qPCR) using Power SYBR Mix (Life Technologies, Thermo Fisher Scientific) in a Bio-Rad CFX384 machine. Actin, Tbp, or 18S expression was used as a reference for normalization. The primer sequences used are listed in Supplemental Table 1.
Sequencing data analysis was performed using data sets available from the NCBI’s Gene Expression Omnibus (GEO) database: bleomycin-induced lung fibrosis (GEO GSE25640); bile duct ligation–induced (chronic injury–induced) liver fibrosis (GEO GSE40041); Tsk2/+ mouse model of scleroderma (GEO GSE61728); and kidney fibrosis following unilateral ureteral obstruction (GEO GSE87212).
Human systemic scleroderma skin.
Skin-punch biopsies were taken from the arms of patients diagnosed with diffuse systemic sclerosis or nonsystemic sclerosis. RNA was isolated from skin samples and subjected to SNAIL and PAI1 gene expression qPCR analysis. The characteristics of the patients’ samples are provided in Supplemental Table 2.
Western blot analysis.
Protein lysates were made in Laemmli buffer from cells, frozen and pulverized skin biopsies, and conditioned media. The following primary antibodies were used at a dilution of 1:1,000: Pai1 (Abcam; ab66705); TENC (Abcam; ab108930); α-SMA (MilliporeSigma; A2547); vimentin (Abcam; ab92547); β-actin (Cell Signaling Technology; 4970S); β-tubulin (Cell Signaling Technology; 2146S); p-FAK (Cell Signaling Technology; 3283S); and FAK (CST 3285S). The HRP-labeled secondary antibodies (Jackson ImmunoResearch) were used at 1:3,000 dilution. Blots were developed on an ImageQuant LAS4000, and bands were quantified using Fiji software (ImageJ, NIH).
Immunostaining and histology.
Skin tissue pieces were fixed in Bouin’s solution, dehydrated, and embedded in paraffin or immediately embedded in OCT (Leica). For staining, 10-μm-thick sections or cells on coverslips were fixed in 4% paraformaldehyde (PFA). The primary following antibodies were used at a dilution of 1:200: K5 (generated in-house); Pai1 (Abcam; ab66705); TENC (MilliporeSigma; ab19013); F4/80 (eBioscience; 14-4801-81); vitronectin (R&D Systems; MAB38751); ICAM1 (Thermo Fisher Scientific; 14-0541-81); α-SMA (MilliporeSigma; A2547 and Abcam; ab5694); vimentin (Abcam; AB92547); Ki67 (Abcam; ab16667); p-FAK (Thermo Fisher Scientific; 44624G); and FAK (Cell Signaling Technology; 3285S). Alexa Fluor 488– or Alexa Fluor 568–labeled secondary antibodies (Jackson ImmunoResearch) were used at a dilution of 1:300. Hoescht stain was used to mark nuclei. For secreted protein staining, the use of detergent was completely avoided, and K5 staining was used as an internal control. A 3% H2O2 incubation after primary antibodies and HRP-labeled secondary antibodies (Jackson ImmunoResearch) was used for IHC. Development was done using DAB substrate (Vector Laboratories; SK4105). Dermal thickness was quantified from the average of multiple measurements made along the length of the H&E-stained sections. Toluidene blue staining was performed with 1% toluidine blue solution in 70% ethanol diluted at 1:10 in a 1% sodium chloride solution, pH 2, for 5 to 30 minutes, followed by extensive washing with water. Giemsa (MilliporeSigma) staining was done with solution diluted 1:50 in deionized water for 20 minutes, followed by extensive washing. Mast cell/macrophage quantification was done by counting the total number of cells as a ratio of the length of the section in pixels for multiple sections. Activated mast cells and Ki67+vimentin+ cells were counted as the total number over multiple sections. Ki67+ fibroblasts in culture were counted from multiple fields and represented as a ratio to the total number of fibroblasts in that field. Imaging was done on an Olympus IX73 microscope or FV1000 confocal microscope and analyzed on the Fiji software.
Hydroxyproline assay.
A hydroxyproline assay was performed as described earlier (17). In brief, skin biopsies were digested overnight in 6N HCl at 100°C. Supernatant was dried, dissolved in water, and incubated with chloramine T. Samples were developed with 7, 12-dimethylbenzanthracene (DMBA) (MilliporeSigma), and color was measured at 562 nm.
Hydrogel preparation.
Gel preparation and cell seeding were done as previously described (17).
Cell culture.
Primary fibroblast isolation was performed as described earlier (17). Fibroblasts were cultured in DMEM high glucose media with 10% FBS. The MCP5 mouse mast cell line was a gift of T. Kawakami (La Jolla Institute, San Diego, California, USA), and the cells were cultured in RPMI medium supplemented with 10% FBS and 1–5 ng recombinant IL-3 (Thermo Fisher Scientific). Fibroblasts were plated on 5-kPa polyacrylamide gels and treated 24 to 36 hours after plating for the required durations with 2-(N-morpholino) ethanesulfonic acid (MES) buffer or 60 ng recombinant human PAI1 (gift of Peter Andreasen, Aarhus University) in 10% FBS-containing media. For cocultures, MCP5 cells were added at a 3:1 ratio to fibroblasts. Treatment of conditioned media was done for 24 hours. Nonadherent MCP5 cells were collected by pelleting the culture medium, and PBS was sprayed to detach the adherent MCP5 cells, which were then collected by pelleting. Fibroblasts remained attached to the gels. All cells were processed as required for RNA and protein extraction or staining.
Adhesion assay.
Fibroblasts were plated on 5-kPa gels as stated above. Treatments with PAI1, RGD peptide, or buffer were done for 24 hours and then washed away, followed by incubation with the following inhibitors: RGD peptide (Santa Cruz Biotechnology; SC201176; 30 min) and FAK inhibitor (Santa Cruz Biotechnology; sc203950; 24 h), along with PAI1, buffer, and RGD and LDV peptides (Bi Biotech India; 5477/1; 30 min); fibrinogen (Thermo Fisher Scientific; F35200; 1 h); hyaluronidase (MilliporeSigma; H3506; 90 min); SCF-neutralizing antibody (R&D Systems; AB-455-NA; 90 min); and ICAM1-neutralizing antibody (Thermo Fisher Scientific; 14-0541-81; 90 min). MCP5 cells were added for 1 to 3 hours in the presence of inhibitors (except for preincubation with LDV), followed by fixation with 4% PFA. After washing, the gels were imaged on an Olympus CKX41 microscope. Counting of adherent mast cells was done with Fiji software. A GAP junction assay was performed by loading the mast cells with 10 μg/ml calcein AM (Thermo Fisher Scientific; C1430) for 30 minutes, followed by incubation with fibroblasts for 2 to 4 hours and confirmation of dye transfer by imaging.
Transwell migration.
MCP5 cells (1 × 105) were added to the upper chambers of 0.8-μm Transwell chambers in 3% FBS-containing media with the same media-containing buffer or 30 ng PAI1 in the lower chamber. Cells in the lower chamber were counted after 24 hours using a hemocytometer.
Collagen contraction.
Rat tail collagen plugs (MilliporeSigma; 08-115; 1 mg/ml) were made in 5% FBS-containing media with 100,000 fibroblasts ± 300,000 MCP5 cells. Each plug was kept in 500 μl media with buffer or 60 ng PAI1. TGF-β1 (R&D Systems; 240-B-002; 20 ng/ml) was used. Contraction was measured from the gel images after 24 hours.
Electron microscopy.
Skin samples from P7 WT and Snail-Tg pups were fixed in 2% glutaraldehyde, 4% formaldehyde in 0.05 M sodium cacodylate, and 2 mM calcium chloride and then embedded in EPON resin and processed. Slices were imaged using the MERLIN Compact VP Scanning Electron Microscope. Images of skin from patients with scleroderma were obtained from Rekha Samuel (CMC, Vellore, India).
Wounding studies.
Twelve-week-old WT and Pai1-KO mice were wounded with 5-mm punch biopsies, and wounded skin samples were embedded in OCT cryopreservation medium. Sections were stained with toluidine blue and imaged as mentioned above, and total mast cell numbers were quantified.
Statistics.
Comparisons of 2 groups were done using a 1-tailed, paired Student’s t test or a 1-tailed Mann-Whitney U test. One-way ANOVA followed by Tukey’s post hoc analysis was used for multiple group comparisons. GraphPad Prism 5.02 (GraphPad Software) was used for all statistical analyses. Data represent the mean ± SEM. P values of less than 0.05 were considered significant.
Study approval.
Animal work conducted at the NCBS/inStem Animal Care and Resource Centre was approved by the inStem Institutional Animal Ethics Committee following the norms specified by the Committee for the Purpose of Control and Supervision of Experiments on Animals (Government of India). Acquisition and processing of the human tissue were conducted according to the protocol approved by the IRB of the CMC. Informed consent was obtained from all patients for skin sample collection and experimentation. All experimental work was approved by the Institutional Biosafety Committee of inStem.
Author contributions
NP designed and performed experiments, evaluated and interpreted data, and wrote the manuscript. EYH and KB performed experiments. SPRB initiated the project and provided tissue samples. TM performed electron microscopy of mouse skin. RD performed bioinformatics analysis of fibrosis data sets. PA provided the recombinant PAI1 protein. TK provided the MCP5 mast cell line. RS provided the human scleroderma skin electron micrographs. RS, RG, DD, and PMJ provided the human scleroderma samples. CJ designed the experiments, provided guidance, and wrote the manuscript.
Supplementary Material
Acknowledgments
The authors would like to thank Apurva Sarin, Mitradas Panicker, Nicolai Sidenius, and members of the Jamora laboratory for their critical review of the work and insightful discussions. This manuscript is dedicated to the memory of our collaborator Peter Andreasen. This work was supported by grants from the Department of Biotechnology of the Government of India (BT/PR8738/AGR/36/770/2013); the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), NIH (5R01AR053185-03); and the American Cancer Society (15457-RSG-08-164-01-DDC); and by a Hellman Faculty Fellowship (to CJ). EYH is supported by funding from the Indian Council of Medical Research Senior Research Fellowship. The animal work was partially supported by the National Mouse Research Resource (NaMoR) (BT/PR5981/MED/31/181/2012;2013-2016) from the Department of Biotechnology. We thank the staff of the NCBS/inStem Animal Care Facility for assistance with animal husbandry, the NCBS Central Imaging and Flow Cytometry Facility (CIFF) for help with image acquisition, and the NCBS Electron Microscopy facility for the electron micrographs of skin tissues.
Version 1. 03/26/2018
Electronic publication
Version 2. 05/01/2018
Print issue publication
Funding Statement
Animal work was partially supported by the National Mouse Research Resource (NaMoR) grant (BT/PR5981/MED/31/181/2012;2013-2016) from the DBT. PI = Sumantra Chattarji
Senior Research Fellowship for Edries Yousaf Hajam
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information: J Clin Invest. 2018;128(5):1807–1819.https://doi.org/10.1172/JCI99088.
Contributor Information
Neha Pincha, Email: npincha@gmail.com.
Edries Yousaf Hajam, Email: yousafh@instem.res.in.
Krithika Badarinath, Email: krithikab@ncbs.res.in.
Surya Prakash Rao Batta, Email: battasurya@gmail.com.
Tafheem Masudi, Email: tafheem.masudi@outlook.com.
Rakesh Dey, Email: rakeshd@instem.res.in.
Peter Andreasen, Email: pa@mb.au.dk.
Toshiaki Kawakami, Email: toshi@lji.org.
Rekha Samuel, Email: samuelrekha@gmail.com.
Renu George, Email: renuegeorge@gmail.com.
Debashish Danda, Email: debashisdandacmc@hotmail.com.
Colin Jamora, Email: cjamora@ucsd.edu.
References
- 1.Zeisberg M, Kalluri R. Cellular Mechanisms of Tissue Fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;(304):C216–C225. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 3.Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol. 2010;37(1):11–25. doi: 10.1111/j.1346-8138.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 4.Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 2006;25(23):5603–5613. doi: 10.1038/sj.emboj.7601421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122(8):2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conese M, Copreni E, Di Gioia S, De Rinaldis P, Fumarulo R. Neutrophil recruitment and airway epithelial cell involvement in chronic cystic fibrosis lung disease. J Cyst Fibros. 2003;2(3):129–135. doi: 10.1016/S1569-1993(03)00063-8. [DOI] [PubMed] [Google Scholar]
- 7.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30(3):245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruber BL. Mast cells in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2003;5(2):147–153. doi: 10.1007/s11926-003-0043-3. [DOI] [PubMed] [Google Scholar]
- 9.Overed-Sayer C, Rapley L, Mustelin T, Clarke DL. Are mast cells instrumental for fibrotic diseases? Front Pharmacol. 2013;4:174. doi: 10.3389/fphar.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts IS, Brenchley PE. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol. 2000;53(11):858–862. doi: 10.1136/jcp.53.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claman HN. Mast cells and fibrosis. The relevance to scleroderma. Rheum Dis Clin North Am. 1990;16(1):141–151. [PubMed] [Google Scholar]
- 12.Artuc M, Steckelings UM, Henz BM. Mast cell-fibroblast interactions: human mast cells as source and inducers of fibroblast and epithelial growth factors. J Invest Dermatol. 2002;118(3):391–395. doi: 10.1046/j.0022-202x.2001.01705.x. [DOI] [PubMed] [Google Scholar]
- 13.Garbuzenko E, Berkman N, Puxeddu I, Kramer M, Nagler A, Levi-Schaffer F. Mast cells induce activation of human lung fibroblasts in vitro. Exp Lung Res. 2004;30(8):705–721. doi: 10.1080/01902140490517809. [DOI] [PubMed] [Google Scholar]
- 14.Koma Y, et al. Distinct role for c-kit receptor tyrosine kinase and SgIGSF adhesion molecule in attachment of mast cells to fibroblasts. Lab Invest. 2005;85(3):426–435. doi: 10.1038/labinvest.3700231. [DOI] [PubMed] [Google Scholar]
- 15.Hogaboam C, et al. Novel role of transmembrane SCF for mast cell activation and eotaxin production in mast cell-fibroblast interactions. J Immunol. 1998;160(12):6166–6171. [PubMed] [Google Scholar]
- 16.Trautmann A, Krohne G, Bröcker EB, Klein CE. Human mast cells augment fibroblast proliferation by heterotypic cell-cell adhesion and action of IL-4. J Immunol. 1998;160(10):5053–5057. [PubMed] [Google Scholar]
- 17.Nakasaki M, et al. The matrix protein Fibulin-5 is at the interface of tissue stiffness and inflammation in fibrosis. Nat Commun. 2015;6:8574. doi: 10.1038/ncomms9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du F, et al. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010;70(24):10080–10089. doi: 10.1158/0008-5472.CAN-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SW, et al. Snail as a potential target molecule in cardiac fibrosis: paracrine action of endothelial cells on fibroblasts through snail and CTGF axis. Mol Ther. 2013;21(9):1767–1777. doi: 10.1038/mt.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields MA, et al. Snail cooperates with KrasG12D to promote pancreatic fibrosis. Mol Cancer Res. 2013;11(9):1078–1087. doi: 10.1158/1541-7786.MCR-12-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe RG, et al. Hepatocyte-derived Snail1 propagates liver fibrosis progression. Mol Cell Biol. 2011;31(12):2392–2403. doi: 10.1128/MCB.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227(2):493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol. 2012;157(3):291–298. doi: 10.1111/j.1365-2141.2012.09074.x. [DOI] [PubMed] [Google Scholar]
- 24.Tuan TL, et al. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol. 2003;162(5):1579–1589. doi: 10.1016/S0002-9440(10)64292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flevaris P, Vaughan D. The role of plasminogen activator inhibitor type-1 in fibrosis. Semin Thromb Hemost. 2017;43(2):169–177. doi: 10.1055/s-0036-1586228. [DOI] [PubMed] [Google Scholar]
- 26.Cho S, et al. Association of elevated plasminogen activator inhibitor 1 levels with diminished lung function in patients with asthma. Ann Allergy Asthma Immunol. 2011;106(5):371–377. doi: 10.1016/j.anai.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oda T, et al. PAI-1 deficiency attenuates the fibrogenic response to ureteral obstruction. Kidney Int. 2001;60(2):587–596. doi: 10.1046/j.1523-1755.2001.030002587.x. [DOI] [PubMed] [Google Scholar]
- 28.Hattori N, et al. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106(11):1341–1350. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.et al. Resolution of skin fibrosis by neutralization of the antifibrinolytic function of plasminogen activator inhibitor 1. Arthritis Rheumatol. 2016;68(2):473–483. doi: 10.1002/art.39443. [DOI] [PubMed] [Google Scholar]
- 30.Rabieian R, Boshtam M, Zareei M, Kouhpayeh S, Masoudifar A, Mirzaei H. Plasminogen activator inhibitor type-1 as a regulator of fibrosis. J Cell Biochem. 2018;119(1):17–27. doi: 10.1002/jcb.26146. [DOI] [PubMed] [Google Scholar]
- 31.Vial D, McKeown-Longo PJ. PAI1 stimulates assembly of the fibronectin matrix in osteosarcoma cells through crosstalk between the αvβ5 and α5β1 integrins. J Cell Sci. 2008;121(pt 10):1661–1670. doi: 10.1242/jcs.020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160(5):781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta. 2013;1832(7):1049–1060. doi: 10.1016/j.bbadis.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamora C, et al. A signaling pathway involving tgf-β2 and snail in hair follicle morphogenesis. PLoS Biol. 2005;3(1):e11. doi: 10.1371/journal.pbio.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binder BR, et al. Plasminogen activator inhibitor 1: physiological and pathophysiological roles. News Physiol Sci. 2002;17:56–61. doi: 10.1152/nips.01369.2001. [DOI] [PubMed] [Google Scholar]
- 37.Maurer B, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62(6):1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 38.El-Karef A, et al. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol. 2007;211(1):86–94. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
- 39.Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal. 2009;3(3–4):287–310. doi: 10.1007/s12079-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midwood K, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 41.Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106(12):1441–1443. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hügle T. Beyond allergy: the role of mast cells in fibrosis. Swiss Med Wkly. 2014;144:w13999. doi: 10.4414/smw.2014.13999. [DOI] [PubMed] [Google Scholar]
- 43.Wygrecka M, et al. Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: role of transmembrane SCF and the PAR-2/PKC-α/Raf-1/p44/42 signaling pathway. Am J Pathol. 2013;182(6):2094–2108. doi: 10.1016/j.ajpath.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Nishioka K, Kobayashi Y, Katayama I, Takijiri C. Mast cell numbers in diffuse scleroderma. Arch Dermatol. 1987;123(2):205–208. [PubMed] [Google Scholar]
- 45.Wulff BC, Wilgus TA. Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol. 2013;22(8):507–510. doi: 10.1111/exd.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7(3):154–165. doi: 10.1046/j.1524-475X.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- 47.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature. 1996;383(6599):441–443. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- 48.Pedroja BS, Kang LE, Imas AO, Carmeliet P, Bernstein AM. Plasminogen activator inhibitor-1 regulates integrin αvβ3 expression and autocrine transforming growth factor β signaling. J Biol Chem. 2009;284(31):20708–20717. doi: 10.1074/jbc.M109.018804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu CH, Law JB, Suryana M, Low HY, Sheetz MP. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc Natl Acad Sci U S A. 2011;108(51):20585–20590. doi: 10.1073/pnas.1109485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo B-H, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006;18(5):579–586. doi: 10.1016/j.ceb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stefansson S, et al. The contributions of integrin affinity and integrin-cytoskeletal engagement in endothelial and smooth muscle cell adhesion to vitronectin. J Biol Chem. 2007;282(21):15679–15689. doi: 10.1074/jbc.M702125200. [DOI] [PubMed] [Google Scholar]
- 52.Termei R, Laschinger C, Lee W, McCulloch CA. Intercellular interactions between mast cells and fibroblasts promote pro-inflammatory signaling. Exp Cell Res. 2013;319(12):1839–1851. doi: 10.1016/j.yexcr.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 53.Oki T, et al. Integrin αIIbβ3 induces the adhesion and activation of mast cells through interaction with fibrinogen. J Immunol. 2006;176(1):52–60. doi: 10.4049/jimmunol.176.1.52. [DOI] [PubMed] [Google Scholar]
- 54.Au SR, Au K, Saggers GC, Karne N, Ehrlich HP. Rat mast cells communicate with fibroblasts via gap junction intercellular communications. J Cell Biochem. 2007;100(5):1170–1177. doi: 10.1002/jcb.21107. [DOI] [PubMed] [Google Scholar]
- 55.Trautmann A, Feuerstein B, Ernst N, Bröcker EB, Klein CE. Heterotypic cell-cell adhesion of human mast cells to fibroblasts. Arch Dermatol Res. 1997;289(4):194–203. doi: 10.1007/s004030050180. [DOI] [PubMed] [Google Scholar]
- 56.Nakayamada S, Okada Y, Saito K, Tamura M, Tanaka Y. Beta1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor kappaB ligand on osteoblasts and osteoclast maturation. J Biol Chem. 2003;278(46):45368–45374. doi: 10.1074/jbc.M308786200. [DOI] [PubMed] [Google Scholar]
- 57.Humphries MJ. Insights into integrin-ligand binding and activation from the first crystal structure. Arthritis Res. 2002;4(suppl 3):S69–S78. doi: 10.1186/ar563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hermand P, Gane P, Callebaut I, Kieffer N, Cartron JP, Bailly P. Integrin receptor specificity for human red cell ICAM-4 ligand. Critical residues for αIIβ3 binding. Eur J Biochem. 2004;271(18):3729–3740. doi: 10.1111/j.1432-1033.2004.04313.x. [DOI] [PubMed] [Google Scholar]
- 59.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 60.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J Exp Med. 1995;181(4):1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLeod JJ, Baker B, Ryan JJ. Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75(1):57–61. doi: 10.1016/j.cyto.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174(2):1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 63.et al. Snail cooperates with KrasG12D in vivo to increase stem cell factor and enhance mast cell infiltration. Mol Cancer Res. 2014;12(10):1440–1448. doi: 10.1158/1541-7786.MCR-14-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roelofs JJ, et al. Plasminogen activator inhibitor-1 regulates neutrophil influx during acute pyelonephritis. Kidney Int. 2009;75(1):52–59. doi: 10.1038/ki.2008.454. [DOI] [PubMed] [Google Scholar]
- 65. Juremalm M, Nilsson G. Chemokine receptor expression by mast cells. In: Saito H, Okayama Y, eds. Mast Cells in Allergic Diseases. Vol. 87. Basel, Switzerland: Karger; 2005:130–144. [DOI] [PubMed] [Google Scholar]
- 66.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 67.Hügle T, White K, van Laar JM. Cell-to-cell contact of activated mast cells with fibroblasts and lymphocytes in systemic sclerosis. Ann Rheum Dis. 2012;71(9):1582. doi: 10.1136/annrheumdis-2011-200809. [DOI] [PubMed] [Google Scholar]
- 68.Manetti M, et al. Evidence for progressive reduction and loss of telocytes in the dermal cellular network of systemic sclerosis. J Cell Mol Med. 2013;17(4):482–496. doi: 10.1111/jcmm.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta KK, Donahue DL, Sandoval-Cooper MJ, Castellino FJ, Ploplis VA. Abrogation of plasminogen activator inhibitor-1-vitronectin interaction ameliorates acute kidney injury in murine endotoxemia. PLoS One. 2015;10(3):e0120728. doi: 10.1371/journal.pone.0120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henderson NC, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19(12):1617–1624. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grodzki AC, Pástor MV, Sousa JF, Oliver C, Jamur MC. Differential expression of integrin subunits on adherent and nonadherent mast cells. Braz J Med Biol Res. 2003;36(8):1101–1109. doi: 10.1590/S0100-879X2003000800017. [DOI] [PubMed] [Google Scholar]
- 72.Theoharides TC, et al. Mast cells and inflammation. Biochim Biophys Acta. 2012;1822(1):21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol. 2001;107(6):1001–1008. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- 74.Kaviratne M, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J Immunol. 2004;173(6):4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 75.Jakubzick C, et al. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J Immunol. 2003;171(5):2684–2693. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- 76.Everett ET, Pablos JL, Harley RA, LeRoy EC, Norris JS. The role of mast cells in the development of skin fibrosis in tight-skin mutant mice. Comp Biochem Physiol A Physiol. 1995;110(2):159–165. doi: 10.1016/0300-9629(94)00127-F. [DOI] [PubMed] [Google Scholar]
- 77.Garbuzenko E, et al. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy. 2002;32(2):237–246. doi: 10.1046/j.1365-2222.2002.01293.x. [DOI] [PubMed] [Google Scholar]
- 78.Shiota N, Kakizoe E, Shimoura K, Tanaka T, Okunishi H. Effect of mast cell chymase inhibitor on the development of scleroderma in tight-skin mice. Br J Pharmacol. 2005;145(4):424–431. doi: 10.1038/sj.bjp.0706209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miyazawa S, Hotta O, Doi N, Natori Y, Nishikawa K, Natori Y. Role of mast cells in the development of renal fibrosis: use of mast cell-deficient rats. Kidney Int. 2004;65(6):2228–2237. doi: 10.1111/j.1523-1755.2004.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Willenborg S, et al. Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin-induced fibrosis. J Invest Dermatol. 2014;134(7):2005–2015. doi: 10.1038/jid.2014.12. [DOI] [PubMed] [Google Scholar]
- 81.Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl. 2010;119(119):S22–S26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varga J, Pasche B. Antitransforming growth factor-β therapy in fibrosis: recent progress and implications for systemic sclerosis. Curr Opin Rheumatol. 2008;20(6):720–728. doi: 10.1097/BOR.0b013e32830e48e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.