Abstract
Bacillus sp. APB-6 harboring nitrile hydratase was used in the production of acrylamide from acrylonitrile. Bacillus sp. APB-6, for maximum production of Co++ containing nitrile hydratase, was cultured in the medium containing lactose (18.0 g l−1), peptone (1.0 g l−1), yeast extract (2.0 g l−1), MgSO4 (0. 5 g l−1), K2HPO4 (0.6 g l−1), urea (9.0 g l−1), and CoCl2 (0.01 g l−1), pH 7.0, and incubated at 35 °C for 24 h in an incubator shaker (160 rpm). Nitrile hydratase exhibited relatively high specificity for aliphatic nitriles. Free cells were immobilized using 2% (w/v) agar solution to enhance enzyme stability and reusability in repetitive cycles of acrylamide production. Under optimized conditions, nearly complete bioconversion of acrylonitrile was achieved with a fair recovery of 85% using free and immobilized cells equivalent to 500 mg dcw l−1. An efficient nitrile hydratase-mediated bioconversion of acrylonitrile to acrylamide at 1-l scale was achieved with time and space productivity of 426 g h−1 g−1 dcw using free cells.
Keywords: Nitrile hydratase, Bioconversion, Immobilization, Acrylonitrile, Acrylamide
Introduction
Application of microorganisms in the production of chemical commodities has been carried out for thousands of years. In the last few decades, the development of biocatalytic methods has emerged as valuable alternative to the conventional chemical methods. In the same pipeline of biocatalytic methods, bioconversions of nitriles provide an important option to customary chemical methods which involve high energy input, low yield of primary products, and high production rate of by-products. Nitrile degrading enzymes are of considerable importance in industrial bioconversion, being used as an alternative to chemical methods to afford a broad spectrum of useful carboxylic acids and amides along with their prominent application in the treatment of nitriles containing industrial effluents. Nitrile hydratase (Nhase; EC 4.2.1.84), a prominent enzyme of the lyase group, has drawn an increasing attention in the last few decades owing to catalytic role in potential conversions and production of amides (Egelkamp et al. 2017; Gilligan et al. 1993; Kobayashi and Shimizu 1998; Martinez et al. 2017; Prasad and Bhalla 2010; Pratush et al. 2017; Singh et al. 2016; Wang et al. 2017).
Acrylamide is used for a range of applications including synthesis of polyacrylamide. Polyacrylamide has found several applications as flocculants for wastewater treatment, soil conditioner to cut soil erosion, stock additives in paper and pulp industries, and in petroleum industry for enhanced oil recovery. Other uses of acrylamide include production of plastic, dye, adhesive, in food processing, cosmetic additive, and in research laboratories for protein separation through electrophoresis (Friedman 2003; Raj et al. 2006). The water management, petroleum, and paper industries account for about 77–84% of worldwide acrylamide consumption (Acrylamide-Chemical Economics Handbook). The global polyacrylamide market was estimated to grow at a compound annual growth rate of 6.3% during 2017–2025 and reach nearly $7.65 billion by 2025 (Polyacrylamides market).
Nitrile hydratase-mediated production of acrylamide is the first bioconversion process used successfully for the industrial production of commodity chemical (Yamada and Kobayashi 1996). Nhase from Rhodococcus sp. N774 and Pseudomonas chlororaphis B23 have been used as the first- and second-generation catalyst for commercial production of acrylamide. Nhase-mediated production of acrylamide is now performed by the third-generation catalyst from Rhodococcus rhodochrous J1 (Kobayashi and Shimizu 1998; Lan et al. 2017).
The culture conditions like production pH, temperature, and time have profound effect on the production of enzyme by microorganism. Majority of the Nhase producing microorganisms have been reported to grow well in the pH range 7.0–8.0 and most of them are mesophiles (Gilligan et al. 1993; Kobayashi et al. 1996; Lee et al. 1993; Nagasawa et al. 1986, 1988; Raj et al. 2006; Watanabe et al. 1987). Besides, thermophilic microbes with Nhase activity have also been reported (Prasad and Bhalla 2010; Yamaki et al. 1997). The time course of growth and Nhase production suggests that Nhase is produced from early exponential phase to stationary phase. Maximum production of Nhase has been observed at 48, 45, 76–80, and 24 h of incubation/growth, respectively, for Agrobacterium tumefaciens (Kobayashi et al. 1996), Brevibacterium sp. R 312 (Nagasawa et al. 1986), R. rhodochrous J1 (Nagasawa et al. 1988), and R. rhodochrous NHB-2 (Sankhian et al. 2003).
In most of the biocatalytic processes for nitrile hydratase--mediated synthesis of amides, free/unimmobilized cells have been used widely. To improve operational stability and reusability of free cells, the immobilization of cells turn out to be very advantageous. In addition, immobilization has also been reported to catalyze a broader range of nitrile substrate than free cells. Different immobilization methods, such as, adsorption, entrapment, cross-linking, and membrane immobilization have been used for nitrile degrading processes utilizing free cells and enzymes (Velankar et al. 2010). Agar, alginate, di-ethylaminoethyl cellulose, polyacrylamide, and sol–gel matrix (Martinez et al. 2014; Mersinger et al. 2005; Raj et al. 2008, 2010) have been used previously for immobilization of biocatalyst containing cells to develop an enzymatic process for bioconversion of acrylonitrile to acrylamide.
Bacillus sp. APB-6, isolated in our laboratory (Pandey et al. 2011), was investigated and proved as a potential source of Nhase activity. In the present communication, optimization of culture conditions for production of Nhase by Bacillus sp. APB-6 and bench scale production of acrylamide using free and immobilized cells are being reported.
Materials and methods
Chemicals
The nitriles and amides were purchased from Alfa Aesar, A Johnson Matthey Company (earlier Lancaster Synthesis). Media components were procured from HiMedia. For high--performance liquid chromatography, HPLC grade solvents were procured from Merck. All other chemicals and inorganic salts were of analytical grade and procured from different sources.
Microorganism
Bacillus sp. APB-6 was used as a source for Nhase production. The organism was grown aerobically in 250 ml Erlenmeyer flasks containing 50 ml medium containing beef extract (3.0 g l−1), peptone (5.0 g l−1), glucose (10.0 g l−1), and yeast extract (1.0 g l−1) (pH 7.5), and incubated at 30 °C, 160 rpm in an incubator shaker for 24 h to prepare preculture for the production of Nhase. Preculture was inoculated into growth medium and incubated at 30 °C and 160 rpm for 24 h. The biomass was harvested by centrifugation at 8000g for 5 min at 4 °C. The cells were washed twice and suspended in potassium phosphate buffer (100 mM, pH 7.0) and stored at 4 °C until further use.
To determine the dry cell weight, the culture broth was appropriately diluted in pre-weighed eppendorf tubes and the absorbances were measured at 600 nm. The culture in each tube was centrifuged at 10,000g for 15 min, supernatant decanted, and the pellet was dried at 80 °C until constant weight was reached. A graph was plotted between dry weight of cells and their absorbance at 600 nm.
Immobilization
The free cells of Bacillus sp. APB-6 were immobilized by the method of Kierstan and Coughlan (1985). 2% (w/v) agar solution was prepared in potassium phosphate buffer (100 mM, pH 7.0) by vigorous shaking at 100 °C and then cooled down to 50 °C in water bath. The cell suspension containing appropriate amount of dry cells was added to melted agar and mixed thoroughly. This mixture was immediately poured into clean petri dish and kept for 30 min at 4 °C for solidification. The enzyme containing solidified agar gel was cut into small beads of equal size using a metallic cork borer and washed with potassium phosphate buffer (100 mM, pH 7.0) to remove untrapped or loosely trapped enzyme. The immobilized cells were stored at 4 °C till further use.
Enzyme assay
Nhase activity of Bacillus sp. APB-6 was assayed using the method developed by Hjort et al. (1990). The assay reaction mixture contained 100 mM potassium phosphate buffer (pH 7.0), appropriate amount of resting cells’ suspension, and acrylonitrile. The reaction was carried out at 55 °C for 20 min and quenched by adding an equal volume of 0.1 N HCl. The amount of acrylamide produced in the reaction mixture was determined by measuring the absorbance at 235 nm. One unit of nitrile hydratase activity was defined as the amount of enzyme that catalyzed the formation of 1 µmol of acrylamide per min under standard assay conditions. The specific activity of the enzyme was expressed as units per milligram of dry cell weight (U mg−1 dcw). All the experiments were performed independently in triplicate. The values were presented as mean ± SD.
Analytical method
High-performance liquid chromatography (HPLC) was performed using Series 200 Ic pump (Perkin Elmer) equipped with Oyster ODS3 column (150 × 4.6 mm, 5 μm particle size; Merck) and 785A Programmable Absorbance Detector (Applied Biosystem). The absorbance was monitored using NetWin Software (Netel Chromatographs, India). The reaction mixture was centrifuged at 10,000g for 5 min and the analysis of acrylamide and acrylic acid was done at a flow rate of 1.0 ml per min at ambient temperature using acetonitrile with 5 mM orthophosphoric acid (5:95 v/v) as mobile phase at 235 nm.
Optimization of culture conditions for Nhase production
Bacillus sp. APB-6 was grown in 13 media (pH 7.5) at 30 °C, and the medium with optimal Nhase production was selected for subsequent experiments. The composition of different media is shown in Table 1. To determine optimal medium components and culture conditions for the production of Nhase of Bacillus sp. APB-6, medium constituents and culture conditions were studied by changing one factor at a time. The parameters explored were selection of carbon source (dextrose, fructose, galactose, sorbitol, rhamnose, xylose, arabinose, sucrose, maltose, lactose, and melibiose; each at a constant concentration of 55 mM); optimum concentration of carbon source (2.0–30.0 g l−1), peptone (1.0–10.0 g l−1), yeast extract (0.5–5.0 g l−1), Urea (1.5–15.0 g l−1), mineral elements [K2HPO4 (0.1–1.0 g l−1), MgSO4·7H2O (0.1–1.0 g l−1)]; and physical conditions [production pH (pH 6.0–10), production temperature (25–60 °C), inoculum size (2–16% v/v), and inoculum age (8–36 h)]. All factors were optimized sequentially using one factor at a time approach and the optimized value for each factor was used as control for subsequent experiment.
Table 1.
Composition of media used for production of Nhase of Bacillus sp. APB-6
| Medium | Composition (g l−1) | References |
|---|---|---|
| 1 | Glycerol (10.0), KH2PO4 (0.5), K2HPO4 (0.5), MgSO4·7H2O (0.1), yeast extract (1.0), peptone (5.0) | Kobayashi et al. (1993) |
| 2 | Glucose (10.0), yeast extract (1.0), peptone (3.0), K2HPO4 (0.5), MgSO4·7H2O (0.5), urea (7.5), CoCl2·6H2O (0.01) | Nagasawa et al. (1991) |
| 3 | Na2HPO4·2H2O (2.5), KH2PO4 (2.0), MgSO4·7H2O (0.5), FeSO4·7H2O (0.03), CoCl2·6H2O (0.06), yeast extract (0.1) | Bhalla et al. (1992) |
| 4 | Glucose (10.0), peptone (3.0), yeast extract (1.0), K2HPO4 (0.5), KH2PO4 (0.5), MgSO4·7H2O (0.005), yeast extract (1.0), FeSO4·7H2O (0.005), CoCl2·6H2O (0.005) | Kobayashi et al. (1996) |
| 5 | (NH4)2HPO4 (5.0), peptone (5.0), yeast extract (3.0), K2HPO4 (5.0), MgSO4·7H2O (0.2), FeSO4·7H2O (0.02) | Bhalla et al. (1992) |
| 6 | Tryptone (30.0), yeast extract (15.0), NaCl (5.0) | Piotraschke et al. (1994) |
| 7 | Peptone (20.0), NaCl (5.0), glucose (2.0), beef extract (3.0), yeast extract (3.0) | Robas et al. (1993) |
| 8 | Glycerol (10 ml l−1), peptone (5.0), malt extract (3.0), yeast extract (3.0), CoCl2·6H2O (0.01), acetonitrile (0.2% v/v) | Modified MY medium |
| 9 | Glucose (10.0), peptone (5.0), malt extract (3.0), yeast extract (3.0), FeSO4·7H2O (0.01) | Modified MY medium |
| 10 | Glycerol (10.0 ml l−1), peptone (5.0), malt extract (3.0), yeast extract (3.0), CoCl2·6H2O (0.01) | Modified MY medium |
| 11 | Glucose (10.0), MgSO4·7H2O (0.5), urea (7.5), CoCl2·6H2O (0.01) | Self-formulated |
| 12 | Glucose (10.0), urea (7.5), CoCl2·6H2O (0.01) | Self-formulated |
| 13 | Glucose (10.0), MgSO4·7H2O (0.5), urea (7.5), FeSO4·7H2O (0.01) | Self-formulated |
Reactions parameters for bioconversion
The reaction parameters, such as time course of enzyme reaction, substrate concentration, and enzyme–substrate concentration, for higher bench scale production of acrylamide were optimized. Reusability potential of enzyme entrapped in agar discs was also assessed by recycling the discs.
Bioconversion of acrylonitrile
The Nhase-mediated bench scale conversion of acrylonitrile was performed at 1 l scale in BIOFLO C-32 Fermenter (New Brunswick Scientific, USA). The reaction mixture consisted of acrylonitrile, Tris buffer, and appropriate amount of free/immobilized cells.
Results and discussion
The optimal production of enzyme depends on the culture conditions, whereas the development of a bioprocess to produce commodity chemical is essentially a shared result of optimal reaction parameters. Different parameters of culture conditions and medium composition were optimized using one factor at a time approach. The following are the results of the experiments performed for nitrile hydratase production and bench scale bioconversion of acrylonitrile to acrylamide using free and immobilized cells of Bacillus sp. APB-6.
Optimization of culture conditions
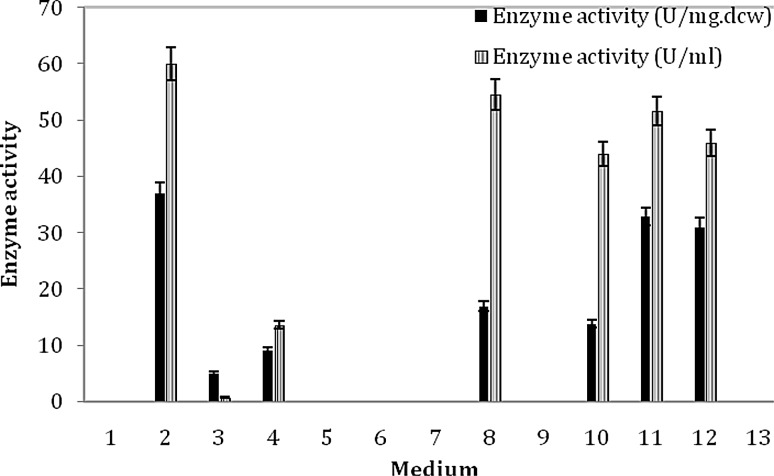
The Bacillus sp. APB-6 was grown in 13 media (pH 7.5) at 30 °C and the results are illustrated in Fig. 1. Among the media used, Medium No. 2 containing glucose, yeast extract, peptone, K2HPO4, MgSO4, urea, and CoCl2 (Nagasawa et al. 1991), proved to be the best medium for the production of nitrile hydratase (37.04 ± 2.85 U mg−1 dcw and 60 ± 2.5 U ml−1) with maximum microbial growth.
Fig. 1.
Selection of growth medium
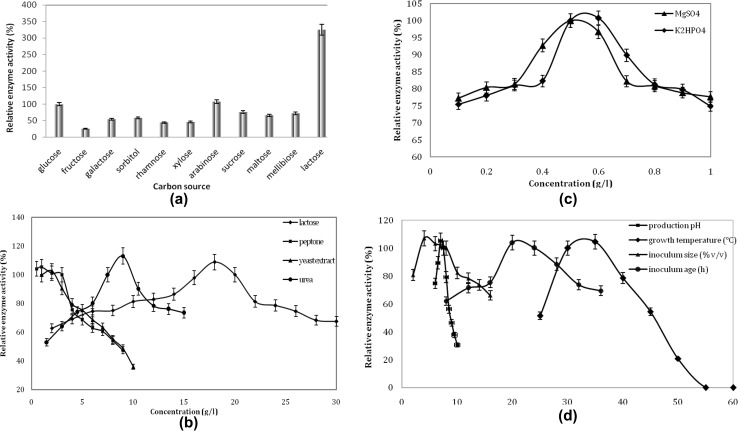
It is evident from the inference that the Nhase of the Bacillus sp. APB-6 is Co++ containing enzyme as it was only expressed in the presence of CoCl2. This nature of enzyme has been reported by many researchers in earlier investigations (Bhalla et al. 1992; Mauger et al. 1988; Prasad and Bhalla 2010; Watanabe et al. 1987). Carbon is a main constituent of cellular material of prokaryotes and eukaryotic cells; therefore, selection of carbon source has a profound effect on the growth of microorganisms. Although the bacterium was able to utilize all the carbon sources for its growth, addition of lactose exhibited maximum enzyme production (Fig. 2a). To the best of our knowledge, first time lactose has been observed as optimal carbon source for nitrile hydratase production. Most of the earlier studies have reported glucose as an optimal carbon source for Nhase production from Bacillus subtilis ZJB-063 (Zheng et al. 2008), Brevibacterium sp. R312 (Nagasawa et al. 1986), Rhodococcus sp. N-774 (Watanabe et al. 1987), and Serratia marcescens ZJB-09104 (Jin et al. 2010). Besides, glycerol has also been used as a carbon source previously for production of Nhase (Raj et al. 2006).
Fig. 2.
Effect of medium components and culture conditions: a carbon source, b lactose (2–30 g l−1), peptone (0.5–9.0 g l−1), yeast extract (1–10 g l−1), and urea (1.5–15 g l−1); c MgSO4 (0.1–1.0 g l−1) and K2HPO4 (0.1–1.0 g l−1); and d production pH (pH 6–10), production temperature (25–60 °C), inoculum age (8–36 h), and inoculum size (2–16% v/v)
Maximum Nhase production was observed with 18.0 g l−1 lactose (Fig. 2b), while further increase in concentration of lactose leads to decrease in the enzyme production, possibly due to either catabolic repression as increase in concentration of sugar in medium exhibits repression effect on bacterial operon due to decrease in the concentration of c-AMP or due to increase in osmotic concentration of the medium as compared to cell. Maximum Nhase production was observed in the presence of lactose (18.0 g l−1), peptone (1.0 g l−1), yeast extract (2.0 g l−1), urea (9.0 g l−1), K2HPO4 (0.6 g l−1), and MgSO4.7H2O (0.5 g l−1) (Fig. 2b, c). CoCl2 is the vital component in production medium and 0.01 g l−1 CoCl2 was observed optimum for Nhase production in Bacillus sp. APB-6 (data not shown). Some previous studies have also used similar concentration of CoCl2 in the growth medium for Nhase production (Gilligan et al. 1993; Mauger et al. 1988; Nagasawa et al. 1991; Yamaki et al. 1997). Nhase of R. rhodochrous J1 (Nagasawa et al. 1988), Rhodococcus rhodochrous M8 (Pogorelova et al. 1996), and R. rhodochrous NHB 2 (Sankhian et al. 2003) also contained Co as prosthetic group. Optimum Nhase production was achieved when Bacillus sp. APB-6 was grown in selected production medium at pH 7.0 and 35 °C for 24 h using 8% (v/v) 20 h grown preculture (Fig. 2d). Similarly, several studies have been reported for optimal Nhase production in the pH range of 7.0–8.0 and majority of the organism were mesophiles (Gilligan et al. 1993; Kobayashi et al. 1996; Lee et al. 1993; Nagasawa et al. 1986, 1988; Raj et al. 2006; Watanabe et al. 1987).
Substrate specificity of the nitrile hydratase of Bacillus sp. APB-6
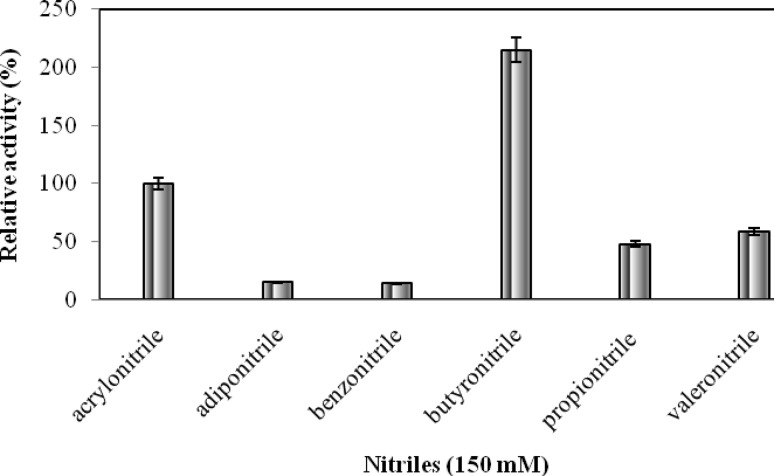
Nhase activity of Bacillus sp. APB-6 was used for the hydrolysis of various nitriles (acrylonitrile, adiponitrile, benzonitrile, butyronitrile, propionitrile, and valeronitrile) to evaluate its substrate specificity. All selected nitriles were used at a similar concentration (150 mM) in different reactions keeping other ingredients of the reaction mixture constant. The reactions were performed as described in preceding section and each product was analyzed through HPLC using their respective standards. Nhase of Bacillus sp. APB-6 was observed to be more active for aliphatic substrates in comparison to aromatic nitriles (Fig. 3). The previous studies have reported Nhase with diverse substrate specificities. Nhase with broad substrate specificity have been reported from R. rhodochrous NHB-2 (Sankhian et al. 2003), Bacillus subtilis ZJB-063 (Zheng et al. 2008) whereas Brevibacterium sp. R312 (Nagasawa et al. 1986), and Pseudonocardia thermophila JCM 3095 (Yamaki et al. 1997) produced Nhase with specificity for aliphatic nitriles.
Fig. 3.
Substrate specificity
Reaction conditions
The reaction parameters such as time course of enzyme reaction, substrate concentration, and enzyme–substrate concentration were carried out in 40 mM Tris–HCl buffer (pH 8.0). Selection of buffer system has not been included in this communication as the results of this parameter included in another study (unpublished data).
Time course of enzyme reaction at different temperatures
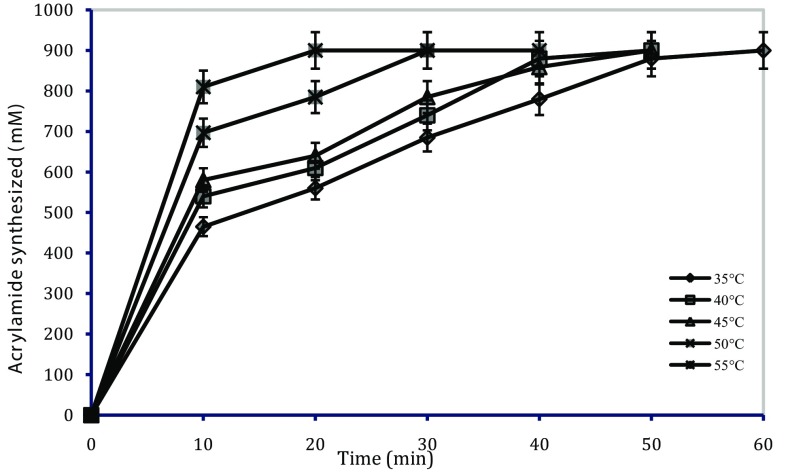
To find out the best time and temperature combination for maximum conversion of substrates into product, time course of enzyme reaction at different temperatures (35, 40, 45, 50, and 55 °C) was investigated by terminating the reaction at different intervals of time, viz., 10, 20, 30, 40, 60, 80, 100, and 120 min. Samples were withdrawn on regular interval of time and the amount of product formed (mM) was analyzed by HPLC. It was observed that the complete conversion of acrylonitrile using free cells occurred in 1 h and 20 min at 35 and 55 °C, respectively. Free cells were not stable for long at higher temperature; therefore, temperature 35 °C was selected for batch experiments for their longer operational stability and reusability. The results obtained are summarized in Fig. 4.
Fig. 4.
Time course of enzyme reaction at different temperatures for conversion of acrylonitrile to acrylamide using free cells (0.15 mg dcw ml−1)
Enzyme–substrate concentration for higher conversion
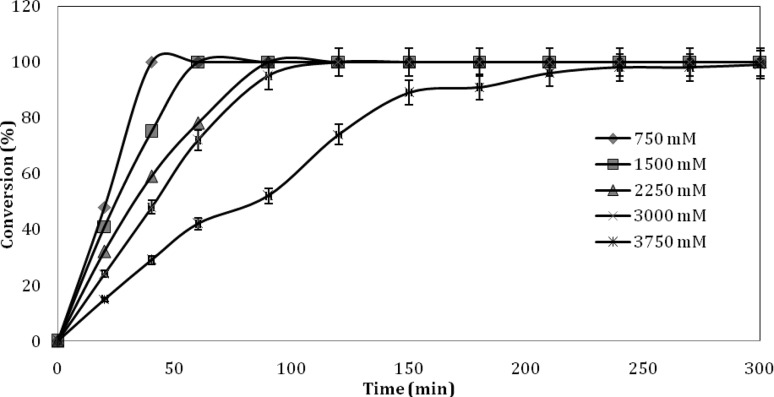
To achieve higher bench scale productivity of acrylamide production from acrylonitrile, the conversion was carried out in separate experiments using increased substrate and enzyme concentrations. The experiments were carried out in optimized reaction conditions as described in the previous section. The results obtained using immobilized cells are illustrated in Fig. 5. A complete conversion of 3 × 103 mM acrylonitrile was achieved in 90 min at 35 °C using immobilized cells equivalent to 500 mg dcw l−1. Likewise, complete conversion of the substrate was also achieved in 60 min with same quantity of unimmobilized dry cell weight. However, nearly complete conversion of 3.75 × 103 mM substrate was also achieved when incubation time was increased further. The conversion of higher concentration of acrylonitrile hardly increased with reaction time (data not shown).
Fig. 5.
Effect of substrate concentration on enzyme activity by immobilized cells
Bioconversion of acrylonitrile to acrylamide
Bacillus sp. APB-6 Nhase-mediated bench scale conversion of acrylonitrile was performed at 1 l scale in BIOFLO C-32 Fermenter (New Brunswick Scientific, USA). The reaction mixture consisted of acrylonitrile (3.0 × 103 mM), Tris buffer (40 mM, pH 8.0), and cells equivalent to 500 mg dcw. The amount of water required for the hydration reaction was also added in the reaction vessel. The reaction was carried out at 35 °C and constant agitation of 120 rpm. Samples were withdrawn from the reaction mixture with lapse of time and analyzed by HPLC as mentioned in the preceding section. The cells were separated from the reaction mixture on completion of the reaction; the product formed was isolated and lyophilized (Flexi-Dry MP™, FTS USA) to recover crystallized product. HPLC analysis of reaction mixture on completion of reaction or acrylamide recovered (lyophilized) showed that the product acrylamide was not contaminated with substrate (acrylonitrile) or acrylic acid (a product of amidase activity). A complete conversion of acrylonitrile with approximately 85% yield of white and crystalline acrylamide was achieved in 60 and 90 min using free and immobilized cells, respectively. The productivity achieved using free and immobilized cells (1 batch) was 426 and 284 g h−1g−1 dcw, respectively. In a previous study, productivity of acrylamide production was 31.25 g h−1 g−1 dcw using free cells of R. rhodochrous PA-34 (Raj et al. 2006). Similarly, acrylamide production was also achieved using Nhase of Brevibacterium sp. CH2 with time and space productivity of 20 g h−1 g−1 cells (Lee et al. 1993). Consequently, the productivity achieved using Nhase of Bacillus sp. APB-6 for production of acrylamide was higher; and hence, the Bacillus sp. APB-6 Nhase proved to be a potential source for bioconversion of other nitriles to valuable amides.
The immobilized cells of Bacillus sp. APB-6 were tested for the reusability so as to make the biocatalysts applicable for repeated cycles of production of acrylamide from acrylonitrile. The decrease in enzyme activity for each cycle was determined assuming activity of beads in the first cycle as 100%. The immobilized cells retained similar activity up to three batches and could be reused up to eight batches making the biocatalyst useful for commercial purposes.
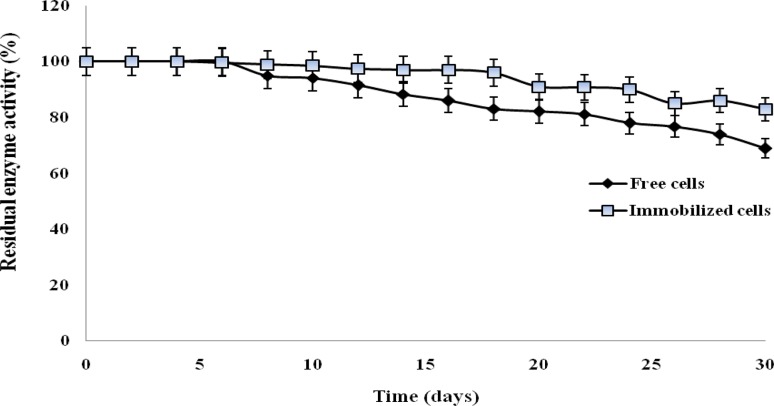
Shelf life of enzyme
Shelf life of enzyme is an essential factor for long-term operational stability. To study the storage stability, the free and immobilized cells were stored for 30 days at 4 °C and tested for enzyme activity at an interval of 2 day. The results obtained have been shown in Fig. 6. Only 17% loss of activity was observed with immobilized cells on storage at 4 °C, whereas 69% residual activity was retained by unimmobilized nitrile hydratase of Bacillus sp. APB-6. The results indicated that the immobilized cells have stronger storage stability than free cells.
Fig. 6.
Shelf life of enzyme containing free cells and immobilized cells
Conclusion
Bacillus sp. APB-6 Nhase was proved as an important catalyst in the development of an efficient bench scale bioprocess for conversion of acrylonitrile to acrylamide using free and immobilized cells with high yield and productivity. Optimum cultivation of Bacillus sp. APB-6 for production of Co++ containing nitrile hydratase was achieved when organism was cultured in a medium constituting lactose, peptone, yeast extract, MgSO4, K2HPO4, urea, and CoCl2, and incubated at 35 °C for 24 h using 8% (v/v) 20 h grown preculture. The bioconversion of acrylonitrile to acrylamide was performed in BIOFLO C-32 fermenter at 1 l scale in reaction medium containing Tris buffer (40 mM, pH 8.0), dry cell weight concentration 500 mg dcw, and substrate concentration 3 × 103 mM. Immobilized cells showed stability and a good level of reusability up to eight batches for bioconversion. The complete bioconversion was achieved successfully using free cells with a recovery of 362 g h−1 g−1 dcw; and therefore, the biocatalytic process developed may be suitable for higher scale applications.
Acknowledgements
The authors acknowledge the Department of Biotechnology, Ministry of Science and Technology, New Delhi, India, and Indian Council of Medical Research, New Delhi, India for the financial support in the form of research fellowships to Mr. Rajendra Singh and Mr. Deepak Pandey, respectively.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Contributor Information
Rajendra Singh, Email: 911ras@gmail.com.
Duni Chand, Email: dunichand_2000@yahoo.com.
References
- Acrylamide-Chemical Economics handbook. https://www.ihs.com/products/acrylamide-chemical-economics-handbook.html. Accessed 3 Aug 2015
- Bhalla TC, Miura A, Wakamoto A, Ohba Y, Furuhashi K. Asymmetric hydrolysis of α-aminonitriles to optically active amino acids by a nitrilase of Rhodococcus rhodochrous PA-34. Appl Microbiol Biotechnol. 1992;37:184–190. doi: 10.1007/BF00178168. [DOI] [Google Scholar]
- Egelkamp R, Schneider D, Hertel R, Daniel R. Nitrile-degrading bacteria isolated from compost. Front Environ Sci. 2017;5:56. doi: 10.3389/fenvs.2017.00056. [DOI] [Google Scholar]
- Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem. 2003;51(16):4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- Gilligan T, Yamada H, Nagasawa T. Production of S-(+)-2-phenylpropionic acid from (R,S)-2 phenylpropionitrile by the combination of nitrile hydratase and stereoselective amidase in Rhodococcus equi TG 328. Appl Microbiol Biotechnol. 1993;39:720–725. doi: 10.1007/BF00164456. [DOI] [PubMed] [Google Scholar]
- Hjort CM, Godtfredsen SE, Emborg C. Isolation and characterization of a nitrile hydratase from a Rhodococcus sp. J Chem Technol Biotechnol. 1990;48:217–226. doi: 10.1002/jctb.280480211. [DOI] [Google Scholar]
- Jin LQ, Liu ZQ, Zheng YG, Shen YC. Identification and characterisation of Serratia marcescens ZJB-09104, a nitrile-converting bacteria. World J Microbiol Biotechnol. 2010;26:817–823. doi: 10.1007/s11274-009-0238-5. [DOI] [Google Scholar]
- Kierstan MPJ, Coughlan MP. Immobilization of cells and enzymes by gel entrapment. Immobilized cells and enzymes-a practical approach. Oxford: IRL Oxford; 1985. pp. 43–45. [Google Scholar]
- Kobayashi M, Shimizu S. Metalloenzyme nitrile hydratase: structure, regulation and application to biotechnology. Nat Biotechnol. 1998;16:733–736. doi: 10.1038/nbt0898-733. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Komeda H, Nagasawa T, Yamada H, Shimizu S. Occurrence of amidases in the industrial microbe Rhodococcus rhodochrous J1. Biosci Biotech Biochem. 1993;57:1949–1950. doi: 10.1271/bbb.57.1949. [DOI] [Google Scholar]
- Kobayashi M, Fujita T, Shimizu S. Hyper induction of nitrile hydratase acting on indole-3-acetonitrile in Agrobacterium tumefaciens. Appl Microbiol Biotechnol. 1996;45:176–181. doi: 10.1007/s002530050667. [DOI] [Google Scholar]
- Lan Y, Zhang X, Liu Z, Zhou L, Shen R, Zhong X, Cui W, Zhou Z. Overexpression and characterization of two types of nitrile hydratases from Rhodococcus rhodochrous J1. PloS ONE. 2017 doi: 10.1371/journal.pone.0179833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Choi SK, Chang HN. Bench scale production of acrylamide using resting cells of Brevibacterium sp. CH2 in a fed batch reactor. Enzyme Microbiol Technol. 1993;15:979–984. doi: 10.1016/0141-0229(93)90175-2. [DOI] [PubMed] [Google Scholar]
- Martinez S, Kuhna ML, Russell JT, Holza RC, Elgren TE. Acrylamide production using encapsulated nitrile hydratase from Pseudonocardia thermophila in a sol–gel matrix. J Mol Catal B Enzym. 2014;100:19–24. doi: 10.1016/j.molcatb.2013.11.014. [DOI] [Google Scholar]
- Martinez S, Yang X, Bennett B, Holz RC. A cobalt-containing eukaryotic nitrile hydratase. Biochim Biophys Acta. 2017;1865(1):107–112. doi: 10.1016/j.bbapap.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Mauger J, Nagasawa T, Yamada H. Nitrile hydratase catalyzed production of isonicotinamide, picolinamide and pyrazinamide from 4-cyanopyridine, 2-cyanopyridine and cyanopyrazine in Rhodococcus rhodochrous J1. J Biotechnol. 1988;8:87–95. doi: 10.1016/0168-1656(88)90071-5. [DOI] [Google Scholar]
- Mersinger LJ, Hann EC, Cooling FB, Gavagan JE, Bassat AB, Wu S, Petrillo KL, Payne MS, Di-Cosimo R. Production of acrylamide using alginate immobilized E. coli expressing Comamonas testosteroni 5-MGAM-4D nitrile hydratase. Adv Synth Catal. 2005;347:1125–1131. doi: 10.1002/adsc.200505039. [DOI] [Google Scholar]
- Nagasawa T, Ryuno K, Yamada H. Nitrile hydratase of Brevibacterium R312-purification and characterization. Biochem Biophys Res Commun. 1986;139:1305–1312. doi: 10.1016/S0006-291X(86)80320-5. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Mathew CD, Mauger J, Yamada H. Nitrile hydratase-catalyzed production of nicotinamide from 3-cyanopyridine in Rhodococcus rhodochrous J1. Appl Environ Microbiol. 1988;54:1766–1769. doi: 10.1128/aem.54.7.1766-1769.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Takeuchi K, Nardidei V, Mihara Y, Yamada H. Optimum culture conditions for the production of cobalt-containing nitrile hydratase by Rhodococcus rhodochrous J1. Appl Microbiol Biotechnol. 1991;34:783–788. doi: 10.1007/BF00169350. [DOI] [Google Scholar]
- Pandey D, Singh R, Chand D. An improved bioprocess for synthesis of acetohydroxamic acids using DTT (dithiothreitol) treated resting cells of Bacillus sp. APB-6. Bioresour Technol. 2011;102(11):6579–6586. doi: 10.1016/j.biortech.2011.03.071. [DOI] [PubMed] [Google Scholar]
- Piotraschke E, Nurk A, Golunsky B, Kasche V. Genetic construction of catalytically active cross-species heterodimer penicillin G amidase. Biotechnol Lett. 1994;16:119–124. doi: 10.1007/BF01021656. [DOI] [Google Scholar]
- Pogorelova TE, Ryabchenko LE, Sunzov NI, Yanenko AS. Cobalt-dependent transcription of the nitrile hydratase gene in Rhodococcus rhodochrous M8. FEMS Microbiol Lett. 1996;144:191–195. doi: 10.1111/j.1574-6968.1996.tb08529.x. [DOI] [Google Scholar]
- Polyacrylamides Market (Type-Anionic, Cationic, and Non-ionic; Form-Powder, Emulsion, and Gel; Application-Waste Water Treatment, Oil & Gas Extraction, Mining, Agriculture, Paper & Pulp, Paints & Coatings, Textiles, and Food)—Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2017–2025. https://www.transparencymarketresearch.com/polyacrylamide-market.html. Accessed 4 Mar 2018
- Prasad S, Bhalla TC. Nitrile hydratases (NHases): at the interface of academia and industry. Biotechnol Adv. 2010;28:725–741. doi: 10.1016/j.biotechadv.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Pratush A, Seth A, Bhalla TC. Expression of nitrile hydratase gene of mutant 4D strain of Rhodococcus rhodochrous PA 34 in Pichia pastoris. Biocatal Biotransform. 2017 doi: 10.1007/s12010-012-9790-9. [DOI] [PubMed] [Google Scholar]
- Raj J, Prasad S, Bhalla TC. Rhodococcus rhodochrous PA-34: a potential biocatalyst for acrylamide synthesis. Process Biochem. 2006;41:1359–1363. doi: 10.1016/j.procbio.2006.01.022. [DOI] [Google Scholar]
- Raj J, Sharma NN, Prasad S, Bhalla TC. Acrylamide synthesis using agar entrapped cells of Rhodococcus rhodochrous PA-34 in a partitioned fed batch reactor. J Industr Microbiol Technol. 2008;35:35–40. doi: 10.1007/s10295-007-0263-z. [DOI] [PubMed] [Google Scholar]
- Raj J, Prasad S, Sharma NN, Bhalla TC. Bioconversion of acrylonitrile to acrylamide using polyacrylamide entrapped cells of Rhodococcus rhodochrous PA-34. Folia Microbiol. 2010;55:442–446. doi: 10.1007/s12223-010-0074-x. [DOI] [PubMed] [Google Scholar]
- Robas N, Zouheiry H, Branlant G, Branlant C. Improved penicillin amidase production using a genetically engineered mutant of Escherichia coli ATCC 11105. Biotechnol Bioeng. 1993;41:14–24. doi: 10.1002/bit.260410104. [DOI] [PubMed] [Google Scholar]
- Sankhian UD, Kumar H, Chand D, Kumar D, Bhalla TC. Nitrile hydratase of Rhodococcus rhodochrous NHB-2: optimization of conditions for production of enzyme and conversion of acrylonitrile to acrylamide. Asian J Microbiol Biotech Environ Sci. 2003;5(2):217–223. [Google Scholar]
- Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3Biotech 6:174. 10.1007/s13205-016-0485-8 [DOI] [PMC free article] [PubMed]
- Velankar H, Clarke KG, Preez RD, Cowan DA, Stephanie G, Burton SG. Developments in nitrile and amide biotransformation processes. Trends Biotechnol. 2010;28(11):561–569. doi: 10.1016/j.tibtech.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu Z, Cui W, Zhou Z. Establishment of bioprocess for synthesis of nicotinamide by recombinant Escherichia coli expressing high-molecular-mass nitrile hydratase. Appl Biochem Biotechnol. 2017;182(4):1458–1466. doi: 10.1007/s12010-017-2410-y. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Satoh Y, Enomoto K, Seki S, Sakashita K. Optimal conditions for cultivation of Rhodococcus sp. N-774 and for conversion of acrylonitrile to acrylamide by whole cells. Agric Biol Chem. 1987;51:3201–3206. doi: 10.1080/00021369.1987.10868478. [DOI] [Google Scholar]
- Yamada H, Kobayashi M. Nitrile hydratase and its application to industrial production of acrylamide. Biosci Biotech Biochem. 1996;60:1391–1400. doi: 10.1271/bbb.60.1391. [DOI] [PubMed] [Google Scholar]
- Yamaki T, Oikawa T, Ito K, Nakamura T. Cloning and sequencing of nitrile hydratase gene from Pseudonocardia thermophila JCM 3095. J Ferment Bioeng. 1997;83:474–477. doi: 10.1016/S0922-338X(97)83004-8. [DOI] [Google Scholar]
- Zheng YG, Chen J, Liu ZQ, Wu MH, Xing LY, Shen YC. Isolation, identification and characterization of Bacillus subtilis ZJB-063, a versatile nitrile-converting bacterium. Appl Microbiol Biotechnol. 2008;77:985–993. doi: 10.1007/s00253-007-1236-x. [DOI] [PubMed] [Google Scholar]