Abstract
Understanding the genetic architecture is a prerequisite for crop improvement. The current research aimed to characterize the extent of genetic variation of drought tolerance harbored in a global collection of 159 chrysanthemum cultivars over 2 years. An average subordinate function value (ASFV), integrating the wilting index, the fresh weight retention rate, and the survival rate after re-watering under two drought-stressed trials, was used to quantify the level of drought tolerance. The performance of ASFV was generally correlated between the 2 years; and a high magnitude (0.95) of broad-sense heritability, coupled with the moderate genetic advance, was estimated for the ASFV. By applying MLM model with both population structure and kinship matrix as covariates association mapping identified 16 markers linked to drought tolerance, with the proportion of the phenotypic variation explained by an individual marker ranging from 4.4 to 7.6%. Of the eight markers predictive across the 2 years, four (E11M24-9, E3M2-8, E1M5-5, and EST-SSR34-3) were identified as favorable alleles for drought tolerance. Several cultivars that carry at least three of the four favorable alleles were identified as potential donor parents for future improvement of the drought tolerance. The findings provide an insight into the genetic basis of the drought tolerance in chrysanthemum and will, therefore, aid in developing new cultivars with enhanced tolerance against drought stress.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1258-3) contains supplementary material, which is available to authorized users.
Keywords: Association mapping, Chrysanthemum, Drought tolerance, Favorable allele, Genetic architecture
Introduction
Drought is a prominent abiotic stress that drastically retards plant growth and production (Juenger 2013; Lu et al. 2010; Tavakol et al. 2016); and the adverse effect of reduced water availability is likely to be further exacerbated by the impending climate changes (Ahuja et al. 2010; Cattivelli et al. 2008), particularly for herbaceous plants. Hence, it is a pressing demand, in this context, to develop varieties expressing a superior level of tolerance to drought stress, for crop breeders. Chrysanthemum (Chrysanthemum morifolium Ramat.), one of the most commercially important ornamental crops across the world, is no exception, especially in a greenhouse or some areas subjected to frequent drought. Water scarcity not only limits the growth of chrysanthemum plants, but also drastically affects the quality of flowers. Considering water management, the chrysanthemum production is highly labor-intensive. It is, therefore, essential to breed for new chrysanthemums with acceptable drought tolerance, for the sake of sustainable production in chrysanthemum.
Remarkable progress has been made in understanding the mechanisms of drought tolerance in chrysanthemum. The morphological, physiological and transcriptional responses of chrysanthemum to water-reduced soil have been depicted in some detail (Sun et al. 2013; Xu et al. 2013; Zhang et al. 2005). The related species, C. indicum and Ajania przewalskii, are relatively tolerant to drought, and the variation for series of relevant physiological and biochemical indices has been noted among their hybrid progenies derived from the wide crosses, chrysanthemum × C. indicum (Sun et al. 2010), and chrysanthemum × A. przewalskii (Deng et al. 2012). Significant efforts, in addition, in discovery of functional genes responsible for drought tolerance, have been accomplished in chrysanthemum thus far (Chen et al. 2011, 2012; Li et al. 2015; Song et al. 2014; Yang et al. 2014). Despite recent progress, the genetic architecture of drought tolerance has not as yet been adequately investigated in chrysanthemum.
Past attempts to reveal the genetic determination of traits of commercial interest in chrysanthemum have markedly relied on biparental linkage mapping, which has successfully uncovered massive quantitative trait loci (QTL) underlying inflorescence-related traits (Zhang et al. 2011), plant architecture (Peng et al. 2015; Zhang et al. 2012), flowering time (Zhang et al. 2013), and aphid resistance (Wang et al. 2014). This mapping strategy requires the construction of appropriate mapping population(s), a time-consuming process, and the subsequent genotyping with enormous markers, which is limited to the allelic variations harbored between the two parents. As a result of the complex genetic backgrounds, including high heterozygosity, self-incompatibility, and genome complexity, whereas, linkage mapping is challenging and not particularly efficient for chrysanthemum as yet. In contrast, the genome-wide association study (GWAS) approach surmount the restrictions in biparental mapping and provides an effective alternative to discover marker/trait association or genomic regions governing expression of complex quantitative traits, from a pre-established panel of genetic resources. Potentially, GWAS enables excellent resolution due to an increased number of recombination events, occurred during the evolution of plant species (Zanke et al. 2015). The application of GWAS in chrysanthemum has hitherto been confined to the genetic dissection of a few horticultural traits (Klie et al. 2016; Li et al. 2012, 2016) and waterlogging tolerance (Su et al. 2016).
In the current study, we are positioned to investigate the variability of drought tolerance in a global collection of 159 cut chrysanthemums over two years, and to perform GWAS based on the acquisition of 707 informative molecular markers recently reported by Li et al. (2016). The purposes of this study were to decipher the genetic architecture of drought tolerance, and in the meantime to identify elite alleles or markers contributing to drought tolerance, and finally to figure out the superior entries showing desirable tolerance to drought, with an intention for their use as breeding parents. Knowledge derived from this study will provide an insight into the genetics of drought tolerance and, therefore, be beneficial to the improvement of chrysanthemum’s tolerance against drought stress.
Materials and methods
Plant materials
A global collection of 159 cut chrysanthemum cultivars, developed multifariously in China, Japan, South Korea, Europe, plus some accessions of unknown provenance, were deployed in this study (Table S1). The full set showed a wealth of genetic diversity, without any known direct kinship, as was described in more detail in a latest research with emphasis on horticultural traits (Li et al. 2016). All entries are being maintained at Nanjing Agricultural University’s Chrysanthemum Germplasm Resource Preserving Centre (Nanjing, China).
Drought stress trials
Drought stress was imposed on the 10-leaf rooted cuttings of the association mapping panel in the spring of 2012 and 2013. The rooted cuttings were laid out in a randomized complete block design with three replications under greenhouse conditions. There are two drought stress trials, namely by exposure to dehydration in the air and by planting in the medium without watering.
The approach of exposure to dehydration in the air was to get the rooted cutting out of the medium, remove residual medium from its root part and place it in the air to dehydrate gradually. Phenotypic performance of the plantlet was photographed and recorded during the dehydration in the air for 1, 2 and 3 h, respectively, which was used to grade the wilting index (WI). The main morphological characteristics of the WI scaled from 1 (slight wilting) to 5 (severe wilting), as described in Fig. S1. In the meantime, the fresh weight retention rate (FWRR) was measured according to the following equation: FWRR = DW/FW, where FW represented the fresh weight of the plantlet before exposure treatment, and DW the dehydration weight after the exposure for 1.5 h. The measurements were run on four plantlets per entry.
Another drought treatment was to withhold water. All 10-leaf rooted cuttings were planted in a greenhouse in a 1:1 mixture of perlite and vermiculite and irrigated until soil water content reached full soil moisture capacity before the treatment. Phenotypic data of each accession were observed during the stress treatment on a daily basis. The WI of each accession was graded after water withheld for 4 days. The drought stress was maintained until most plantlets showed visible symptoms of wilt for 5 days and then finished by replenishing water. The survival rate (SR) of each entry was counted after re-watered for 1 week.
Evaluation for drought tolerance
The indicators, FWRR and WI under in-air dehydration, and SR and WI under planting with water withholding, were integrated to measure the drought tolerance for each entry, by the subordinate function values (SFV) of each indicator via the following equation: if an indicator was positively correlated with drought tolerance, Uij = (Xij − Xjmin)/(Xjmax − Xjmin); otherwise Uij = 1 − (Xij − Xjmin)/(Xjmax − Xjmin), where Uij represents the SFV of the jth indicator for the ith variety, Xij the average value of the jth indicator for the ith variety, Xjmin and Xjmax, respectively, the minimum and maximum value of the jth indicator across the whole panel. The average of SFV (ASFV) across all the indicators derived from two separate experiments was deployed to determine the drought tolerance of the ith variety. A greater ASFV was considered to indicate a stronger tolerant variety.
The descriptive statistics, correlation analysis and relevant analysis of variance from the phenotypic data over the 2 years were performed independently using SPSS software (http://www-01.ibm.com/software/cn/analysis/spss). Broad-sense heritability (hB2), the phenotypic (PCV) and genotypic (GCV) variation coefficients and genetic advance (△G) were estimated according to Su et al. (2016).
Association mapping and elite allele identification
The ASFV of the 2 years was, independently, used in the genome-wide association mapping. The association mapping was performed based on the 707 informative SRAP, SCoT and EST-SSR markers, latterly acquired by Li et al. (2016). Mixed linear model (MLM) module implemented in TASSEL software (Bradbury et al. 2007), with both effects of population structure (Q) and kinship (K) taken into account (Yu and Buckler 2006), was adopted for detection of the alleles significant associated with drought tolerance, quantified by the value of the aforementioned ASFV. The parametric Q and K information were according to Li et al. (2016). As commonly adopted in previous studies (Zheng et al. 2015; Li et al. 2016; Su et al. 2016), the significant threshold for calling a marker/trait association was set at P < 0.01. The proportion of the phenotypic variation explained (PVE) by a given marker was derived from the relevant R2 value. As described by Breseghello and Sorrells (2016), the phenotypic effect of an individual marker was estimated by comparing the mean phenotypic value (ASFV) of the accessions carrying the marker with that of members lacking it. The markers associated with a positive phenotypic effect on the ASFV will be considered candidate elite alleles for drought tolerance.
Results
Variability of drought tolerance
Descriptive statistics for drought tolerance represented by ASFV, of the 159 cut chrysanthemum cultivars over the 2 years (2012 and 2013), are depicted in Tables 1 and S1, respectively. The ASFV of drought tolerance varied in a range of 0.23–0.87 and 0.20–0.92, respectively, in 2012 and 2013. The ASFV averaged 0.57 in 2012 and 0.54 in 2013, with a significant magnitude (P < 0.01) of the correlation between the 2 years. The broad-sense heritability, hB2, was estimated at 0.95 and the phenotypic (PCV) and genotypic coefficient of variation (GCV) were 26.73 and 26.11%, respectively. The absolute and relative genetic advances were individually calculated at 37.80 and 68.11% at P = 0.01 (Table 2).
Table 1.
Descriptive statistics for ASFVs of drought tolerance among the 159 varieties, the P value in ANOVA and the number of varieties in each group
| Year | Number of entries | Maximum | Minimum | Mean | SD | |||
|---|---|---|---|---|---|---|---|---|
| HT | MT | ST | S | |||||
| 2012 | 15 | 54 | 71 | 19 | 0.87 | 0.23 | 0.57 | 0.15 |
| 2013 | 9 | 54 | 71 | 25 | 0.92 | 0.20 | 0.54 | 0.15 |
SD standard deviation, CV coefficients of variation, hB2 broad-sense heritability, HT highly tolerant, MT moderately tolerant, ST slightly tolerant, S susceptible
Table 2.
Genetic parameters for ASFV, based on a combined data set obtained from the two experiments over 2 years
| h B 2 | Coefficient of variation (%) | △G (%) | △Gʹ (%) | |||
|---|---|---|---|---|---|---|
| PCV | GCV | P = 5% | P = 1% | P = 5% | P = 1% | |
| 0.95 | 26.73 | 26.11 | 29.17 | 37.80 | 52.56 | 68.11 |
hB2 broad sense of heritability, PCV phenotypic coefficient of variation, GCV genetic coefficient of variation, △G absolute genetic advance, △Gʹ relative genetic advance
Based on the ASFV, drought tolerance of the panel was classified into four scales: ASFV ≥ 0.80, highly tolerant (HT); 0.80 > ASFV ≥ 0.60, moderately tolerant (MT); 0.60 > ASFV ≥ 0.40, slightly tolerant (ST); and ASFV < 0.40, susceptible (S). There were 15 and 9 HT accessions, both 54 MT varieties, both 71 ST varieties, and 19 and 25 S varieties in 2012 and 2013, respectively (Table S1, Table 1). Approximately 33% of the panel consistently showed high or moderate tolerance to drought over the 2 years; of which, four cultivars, namely ‘Qx102’, ‘Nannong Xiangbin’, ‘Nannong Xuefeng’ and ‘Nannong Ziguan’, expressed notably a constant higher level of drought tolerance based on the performance of ASFV.
Association mapping
The markers linked to the drought tolerance are summarized in Table 3. By calling a marker/trait association at the significant threshold P < 0.01, 13 markers for drought tolerance were detected in 2012 and 11 markers in 2013 by MLM-modeled association mapping, with an average explanation of 5.6% of the total variation (individually 4.4–7.6%). Corrected by the Bonferroni adjustment (1.4E−05), whereas, none of these remained significant. The 13 drought tolerance-associated markers identified in 2012 explained individually 4.40–6.83%, with an average of 5.43% of the total phenotypic variation, while the phenotypic variation, accounted for by each of the 11 associated markers detected in 2013, ranged from 4.52 to 7.57% and averaged at 5.82%, suggestive of the quantitative nature of polygenic inheritance for drought tolerance. Of these associated markers, eight were consistently expressed across the 2 years. EST-SSR27-8 showed the highest explanation (7.57%) of the total phenotypic variation, at the lowest P values (P 7.00E−04), nonetheless expressed only in the year of 2013.
Table 3.
Markers significantly associated with drought tolerance at P < 0.01, identified using a MLM model with population structure and kinship as covariates
| Marker | Year 2012 | Year 2013 | Reported for other traitsc | ||||
|---|---|---|---|---|---|---|---|
| P a | R2 (%)b | Phenotypic effects | P | R2 (%) | Phenotypic effects | ||
| EST-SSR131-4 | 0.0034 | 5.59 | − 0.01 | Capitulum diameter, ray floret number | |||
| EST-SSR27-8 | 0.0043 | 5.30 | − 0.02 | 7.00E−04 | 7.57 | − 0.03 | |
| EST-SSR34-2 | 0.0013 | 6.83 | 0.00 | 0.0015 | 6.63 | − 0.03 | |
| EST-SSR34-3 | 0.0035 | 5.55 | 0.10 | 0.0025 | 6.00 | 0.10 | Flooding tolerance |
| E1M5-5 | 0.0031 | 5.87 | 0.00 | 0.0043 | 5.42 | 0.01 | |
| E2M2-9 | 0.0076 | 4.83 | 0.02 | ||||
| E3M2-8 | 0.0030 | 5.86 | 0.02 | 0.0040 | 5.47 | 0.01 | |
| E3M2-1 | 0.0049 | 5.25 | − 0.05 | Flooding tolerance | |||
| E7M22-2 | 0.0095 | 4.51 | 0.03 | ||||
| E8M21-5 | 0.0084 | 4.60 | 0.03 | ||||
| E9M24-2 | 0.0093 | 4.40 | − 0.02 | 0.0016 | 6.59 | − 0.03 | |
| E10M21-1 | 0.0090 | 4.46 | 0.02 | Flooding tolerance | |||
| E11M23-11 | 0.0066 | 4.82 | − 0.05 | Flooding tolerance | |||
| E11M24-9 | 0.0020 | 6.36 | 0.02 | 0.0023 | 6.19 | 0.02 | Flower neck length |
| E15M21-4 | 0.0028 | 6.00 | − 0.05 | 0.0028 | 5.99 | − 0.05 | |
| E15M21-9 | 0.0091 | 4.52 | − 0.03 | ||||
Mining favorable alleles
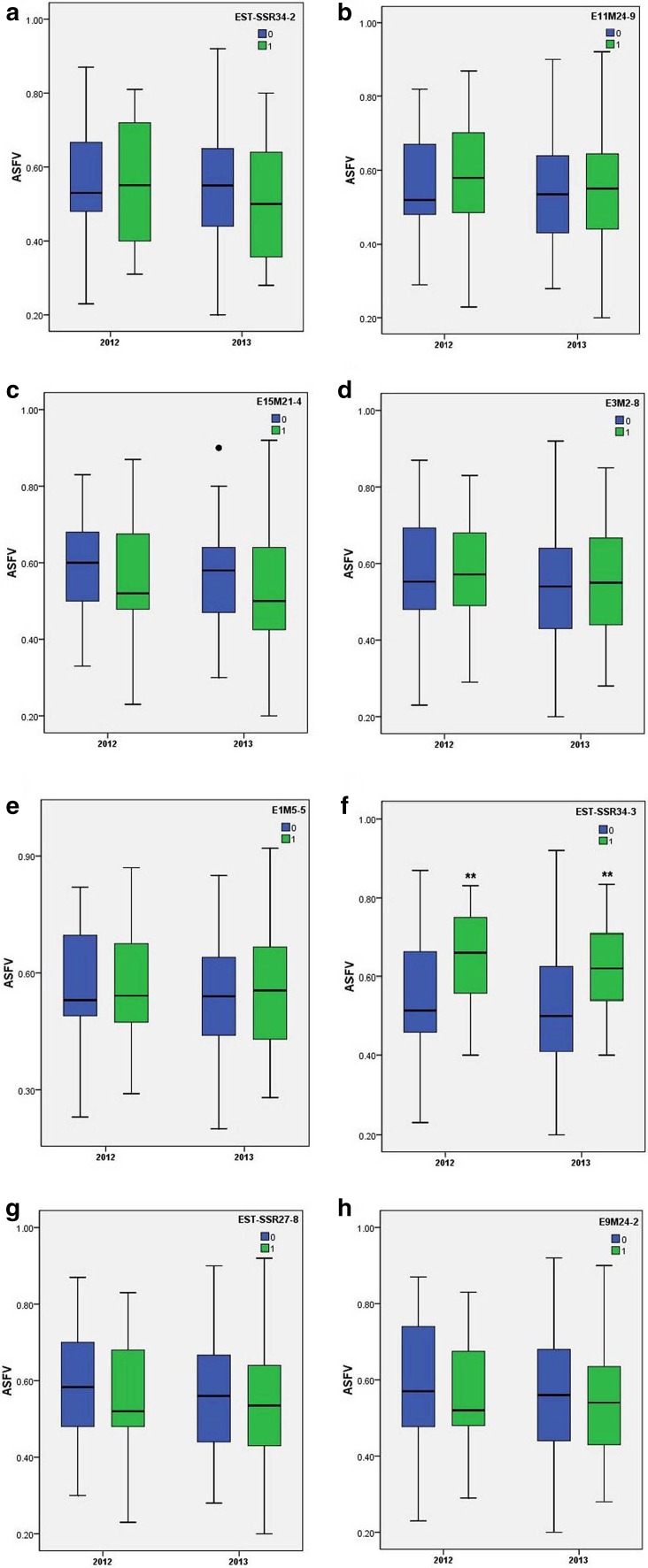
The mean ASFV of all accessions either carrying or not carry a given associated marker is summarized in Table 3. Focusing on the eight markers associated with drought tolerance in both 2 years, the mean ASFV of entries either carrying or not carrying an associated marker is shown in Fig. 1. As a higher ASFV implied a superior drought tolerance, the presence of markers with a positive ASFV would be of worth to marker-assisted selection breeding. Of the eight markers, three (namely E15M21-4, EST-SSR27-8, and E9M24-2) were uniformly negative in phenotypic effect over the 2 years (Fig. 1c, g, h), thus should be discarded. EST-SSR34-2 carried by 36 accessions should be validated further, due to its contrasting phenotypic effects across the 2 years (Fig. 1a). The remaining four markers, E11M24-9, E3M2-8, E1M5-5 and EST-SSR34-3, all had positive effects on ASFV over the 2 years (Fig. 1b, d, e, f), with EST-SSR34-3 expressing the most positive phenotypic effect at P < 0.001 by independent samples t test. Six accessions carrying all the four favorable alleles and 15 accessions harboring three of the four were identified; of which the four accessions, ‘Qx007’, ‘Qx079’, ‘Nannong Xuefeng’, and ‘Mundial Improved’, showed robust ASFV > 0.7 across the 2 years (Table 4).
Fig. 1.
Box plots showing variation for ASFV represented by entries grouped into marker allele type. Boxes colored blue and green refer, respectively, to entries lacking and carrying a marker linked to drought tolerance. **Significant difference at P < 0.01
Table 4.
Entries carried associated at least three of the four favorable alleles and their performance of drought tolerance
| Entry | E11M24-9 | E3M2-8 | E1M5-5 | EST-SSR34-3 | ASFV and grade of drought tolerance | ||
|---|---|---|---|---|---|---|---|
| 2012 | 2013 | ||||||
| Grand White | ✓ | ✓ | ✓ | 0.63 MT | 0.67 MT | ||
| Grand Rose | ✓ | ✓ | ✓ | ✓ | 0.67 MT | 0.60 MT | |
| Qx007 | ✓ | ✓ | ✓ | 0.81 HT | 0.72 MT | ||
| Tigerrag | ✓ | ✓ | ✓ | 0.48 ST | 0.55 ST | ||
| Grand Orange.deep | ✓ | ✓ | ✓ | ✓ | 0.47 ST | 0.42 ST | |
| Grand Splendid | ✓ | ✓ | ✓ | ✓ | 0.68 MT | 0.58 ST | |
| Qx042 | ✓ | ✓ | ✓ | 0.50 ST | 0.46 ST | ||
| Puma White | ✓ | ✓ | ✓ | 0.66 MT | 0.67 MT | ||
| Qx044 | ✓ | ✓ | ✓ | 0.52 ST | 0.50 ST | ||
| Mundial improved | ✓ | ✓ | ✓ | ✓ | 0.76 MT | 0.72 MT | |
| Finch | ✓ | ✓ | ✓ | 0.55 ST | 0.50 ST | ||
| Ariggs | ✓ | ✓ | ✓ | ✓ | 0.48 ST | 0.44 ST | |
| Puma Sunny | ✓ | ✓ | ✓ | 0.43 ST | 0.42 ST | ||
| Vyking Orange | ✓ | ✓ | ✓ | 0.48 ST | 0.50 ST | ||
| Vyking Dark | ✓ | ✓ | ✓ | 0.64 MT | 0.56 ST | ||
| Qx075 | ✓ | ✓ | ✓ | 0.60 MT | 0.53 ST | ||
| Qx079 | ✓ | ✓ | ✓ | ✓ | 0.83 HT | 0.76 MT | |
| Winter White | ✓ | ✓ | ✓ | 0.66 MT | 0.67 MT | ||
| Qx083 | ✓ | ✓ | ✓ | 0.50 ST | 0.43 ST | ||
| Qx106 | ✓ | ✓ | ✓ | 0.75 MT | 0.62 MT | ||
| Noa | ✓ | ✓ | ✓ | 0.40 ST | 0.40 ST | ||
| Monalisa Yellow | ✓ | ✓ | ✓ | 0.63 MT | 0.67 MT | ||
| Qx068 | ✓ | ✓ | ✓ | 0.50 ST | 0.40 ST | ||
| Jingyun | ✓ | ✓ | ✓ | 0.76 MT | 0.68 MT | ||
| Nannong Gongfen | ✓ | ✓ | ✓ | 0.66 MT | 0.60 MT | ||
| Nannong Xuefeng | ✓ | ✓ | ✓ | 0.80 HT | 0.80 HT | ||
Discussion
The evaluation of drought tolerance
The drought tolerance is a complex trait that is controlled by multiple genes (Hao et al. 2011; Ravi et al. 2011; Yu et al. 2013), and in addition, the environmental factors, including the developmental stage of the plants, the severity and duration of tolerance, and the weather conditions, can all influence the plant drought tolerance (Witcombe et al. 2008). To date, various evaluation methods and phenotypic or physiological indexes, the effectiveness of which varies with the stress severity, have been proposed for the quantifying of drought tolerance at the different growth stages (Hao et al. 2011; Mardeh et al. 2006; Yu et al. 2013). However, no agreement has been reached regarding the criteria for measuring drought tolerance; probably this is because drought tolerance was not an independent trait by plant breeders and also the tolerance mechanism was fairly polygenic in nature (Bahrami et al. 2014). Compared with other developmental stages, in addition, evaluation of drought tolerance at seedling stage possesses a variety of advantages, e.g. time-saving, easy operation, high efficiency, and high repeatability due to more manageability of environmental conditions (Kato et al. 2008).
Wilting, defined as the loss of rigidity and often leading to a flaccid state due to the turgor pressure falling to zero (Taiz and Zeiger 2010), is the most common visualized sign of drought stress in plant, and therefore, wilting index (WI) provides an easy and rapid measurement for whole-plant responses to drought stress (Hu et al. 2011; Pungulani et al. 2013). In the current study, an average subordinate function value (ASFV) of drought tolerance, which integrated WI, FWRR, and SR in drought-stressed plantlets, was adopted to characterize variation in drought tolerance among the chrysanthemum accessions. Phenotypic data from the two drought-stressed experiments, in-air dehydration and planting without watering, were combined to calculate the ASFV of drought tolerance. The former method was to test the rate of water loss and the latter to investigate the response to drought stress and survival rate after re-watering. Here, considerable variability for drought tolerance, with ASFV ranging from 0.2 to 0.9, was found in the investigated chrysanthemums, and the classification of drought tolerance was significantly correlated (P < 0.01) (data not shown) between 2 years. The divergent phenotypic responses of the entries to drought stress are crucial for marker–trait association mapping. Noteworthily, the broad-sense heritability (hB2) was estimated at 0.95. A high hB2 of such size, together with the moderate genetic advance demonstrates the possibility of improving drought tolerance on the basis of AFSVs in chrysanthemum at early generation.
Association mapping for drought tolerance and comparison with other traits in chrysanthemum
Association mapping has been effectively used to find associations between potential targeted loci and complex drought tolerance (Bac-Molennar et al. 2016; Lu et al. 2010; Yu et al. 2013; Zhang et al. 2016). In this study, based on a global collection of cut chrysanthemums an MLM model in which population structure and kinship are considered as covariates was applied to identify associated alleles for drought tolerance. Compared with GLM model, the MLM model can reduce spurious associations, and thus more robust despite often resulting in less associated markers (Su et al. 2016). Here, the MLM-based association mapping identified 13 markers for drought tolerance represented by ASFV in 2012 and 11 in 2013 (P < 0.01). The proportion of the phenotypic variance explained (PVE) by an individual marker ranged from 4.40 to 7.57% (Table 3), suggestive of the polygenic basis of drought tolerance at molecular level. Of the detected associated markers eight were replicated across both years, reinforcing to some extent the significant correlation between the phenotypic data of the 2 years.
Compared with the markers latterly reported by Su et al. (2016) and Li et al. (2016), four drought tolerance-related markers, namely E10M21-1, E11M23-11, E3M2-1, and EST-SSR34-3, were found to be simultaneously associated with waterlogging tolerance, and two markers (E11M24-9 and EST-SSR131-4) associated with some horticultural traits (Table 3), a situation which can occur as a consequent of either linkage or pleiotropy. Previous researches indicate that linkage becomes more likely between alleles underlying unrelated traits (Li et al. 2016; Ravi et al. 2011; Su et al. 2016; Yang et al. 2010; Wang et al. 2012). Since the drought tolerance in the present study and the waterlogging tolerance lately reported by Su et al. (2016) are both water-related abiotic stresses that can be correlated in regulatory mechanisms, we would suggest the common markers are much more likely the results of pleiotropy. In contrast, drought tolerance is different from the horticultural traits reported by Li et al. (2016); it is, therefore, more likely that linkage rather than pleiotropy would be responsible for the markers shared by these unrelated traits. After further validation, the common markers can be useful in selecting for the aforementioned traits simultaneously. Say, it is encouraging that EST-SSR34-3 acts a positive effect on both drought and waterlogging tolerance. Taking this one step further, if effect of the marker is further consistently validated in different genetic backgrounds, it would allow for achievement of chrysanthemums with both improved drought and waterlogging tolerance at one stroke.
Favorable alleles and the possibility of application in marker-assisted selection for drought tolerance
To a plant breeder markers associated with a gene that controls variation for a target trait are potentially invaluable (Su et al. 2016). In the current study, a dozen associated markers were identified for drought tolerance represented by ASFV. Of particular note are the four markers, E11M24-9, E3M2-8, E1M5-5 and EST-SSR34-3, which could make accurate prediction of performance of drought tolerance over different years. Unlike biparental linkage population, natural germplasm harbor multiple alleles at any given locus; as a consequence, association mapping enable the identification of favorable alleles in a large panel of crops resources rather than the favorable entries which are identified by a phenotypic screen (Wang et al. 2012; Su et al. 2016). Based on the identified favorable alleles here, the four entries ‘Qx007’, ‘Qx079’, ‘Nannong Xuefeng’, and ‘Mundial Improved’ that showed stronger drought tolerance with ASFV > 0.7 should be underscored as potential donor parents for future improving the drought tolerance of chrysanthemum. Prior to application in molecular marker-assisted selection, further validation in multiple genetic backgrounds and environments will be required to confirm the robustness of these alleles’ predicative advantage.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 Five wilting index (WI) scales of phenotypic variation in the response of chrysanthemum rooted cuttings exposed to drought stress (JPG 578 KB)
Table S1 The panel of 159 chrysanthemum accessions, showing provenance, ASFV and grade of drought tolerance (DOCX 39 KB)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant nos. 31572152, 31370699 and 31272196).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1258-3) contains supplementary material, which is available to authorized users.
References
- Ahuja I, de Vos RC, Bones AM, Hall RD. Plant molecular stress responses face climate change. Trend Plant Sci. 2010;15(12):664–674. doi: 10.1016/j.tplants.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Bac-Molennar JA, Granier C, Keurentjes JJB, Vreugdenhil D. Genome-wide association mapping of time-dependent growth response to moderate drought stress in Arabidopsis. Plant Cell Environ. 2016;39:88–102. doi: 10.1111/pce.12595. [DOI] [PubMed] [Google Scholar]
- Bahrami F, Arzani A, Karimi V. Evaluation of yield-based drought tolerance indices for screening safflower genotypes. Agron J. 2014;106(4):1219–1224. doi: 10.2134/agronj13.0387. [DOI] [Google Scholar]
- Bradbury PJ, Zhang ZW, Kroon DE, Casstevens RM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23:2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- Breseghello F, Sorrells ME. Association analysis as a strategy for improvement of quantitative traits in plants. Crop Sci. 2016;46(3):1323–1330. doi: 10.2135/cropsci2005.09-0305. [DOI] [Google Scholar]
- Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, Marè C, Tondelli A, Stanca AM. Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crop Res. 2008;105:1–14. doi: 10.1016/j.fcr.2007.07.004. [DOI] [Google Scholar]
- Chen S, Cui X, Chen Y, Gu C, Miao H, Gao H, Chen F, Liu Z, Guan Z, Fang W. CgDREBa transgenic chrysanthemum confers drought and salinity tolerance. Environ Exp Bot. 2011;74:255–260. doi: 10.1016/j.envexpbot.2011.06.007. [DOI] [Google Scholar]
- Chen L, Chen Y, Jiang J, Chen S, Chen F, Guan Z, Fang W. The constitutive expression of Chrysanthemum dichrum ICE1 in Chrysanthemum grandiflorum improves the level of low temperature, salinity and drought tolerance. Plant Cell Rep. 2012;31:1747–1758. doi: 10.1007/s00299-012-1288-y. [DOI] [PubMed] [Google Scholar]
- Deng Y, Jiang J, Chen S, Huang C, Fang W, Chen F. Drought tolerance of intergeneric hybrids between Chrysanthemum morifolium and Ajania przewalskii. Sci Hortic. 2012;148:17–22. doi: 10.1016/j.scienta.2012.09.021. [DOI] [Google Scholar]
- Hao Z, Li X, Xie C, Weng J, Li M, Zhang D, Liang X, Liu L, Liu S, Zhang S. Identification of functional genetic variations underlying drought tolerance in maize using SNP markers. J Integr Plant Biol. 2011;53(8):641–652. doi: 10.1111/j.1744-7909.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- Hu B, Fu X, Zhang T, Wan Y, Li X, Huang Y, Dai L, Luo X, Xie J. Genetic analysis on characteristics to measure drought resistance using Dongxiang wild rice (Oryza rufupogon Griff.) and its derived backcross inbred lines population at seedling stage. Agric Sci China. 2011;10(11):1653–1664. doi: 10.1016/S1671-2927(11)60164-8. [DOI] [Google Scholar]
- Juenger TE. Natural variation and genetic constraints on drought tolerance. Curr Opin Plant Biol. 2013;16:274–281. doi: 10.1016/j.pbi.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Kato Y, Hirotsu S, Nemoto K, Yamagishi J. Identification of QTLs controlling rice drought tolerance at seedling stage in hydroponic culture. Euphytica. 2008;260:423–430. doi: 10.1007/s10681-007-9605-1. [DOI] [Google Scholar]
- Klie M, Menz I, Linde M, Thomas D. Strigolactone pathway genes and plant architecture: association analysis and QTL detection for horticultural traits in chrysanthemum. Mol Genet Genomics. 2016;291:957–969. doi: 10.1007/s00438-015-1155-y. [DOI] [PubMed] [Google Scholar]
- Li R, Wang C, Dai S, Luo X, Li B, Zhu J, Lu J, Liu Q. The association analysis of phenotypic traits with SRAP markers in chrysanthemum. Sci Agric Sin. 2012;45(7):1355–1364. [Google Scholar]
- Li P, Song A, Gao C, Wang L, Wang Y, Sun J, Jiang J, Chen F, Chen S. Chrysanthemum WRKY gene CmWRKY17 negatively regulates salt stress tolerance in transgenic chrysanthemum and Arabidopsis plants. Plant Cell Rep. 2015;34:1365–1378. doi: 10.1007/s00299-015-1793-x. [DOI] [PubMed] [Google Scholar]
- Li P, Zhang F, Chen S, Jiang J, Wang H, Su J, Fang W, Guan Z, Chen F. Genetic diversity, population structure and association analysis in cut chrysanthemum (Chrysanthemum morifolium Ramat.) Mol Genet Genom. 2016;291(3):1117–1125. doi: 10.1007/s00438-016-1166-3. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang S, Shah T, Xie C, Hao Z, Li X, Farkhari M, Ribaut JM, Rong T, Xu Y. Joint linkage-linkage disequilibrium mapping is a powerful approach to detecting quantitative trait loci underlying drought tolerance in maize. Proc Nat Acad Sci USA. 2010;107(45):19585–19590. doi: 10.1073/pnas.1006105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardeh SS, Ahmadi A, Poustini K, Mohammadi V. Evaluation of drought resistance indices under various environmental conditions. Field Crop Res. 2006;98(2–3):222–229. doi: 10.1016/j.fcr.2006.02.001. [DOI] [Google Scholar]
- Peng H, Zhang F, Jiang J, Chen S, Fang W, Guan Z, Chen F. Identification of quantitative trait loci for branching traits of spray cut chrysanthemum. Euphytica. 2015;202:385–392. doi: 10.1007/s10681-014-1259-1. [DOI] [Google Scholar]
- Pungulani LLM, Millner JP, Williams WM, Banda M. Improvement of leaf wilting scoring system in cowpea (Vigna unguiculata (L) Walp.): from qualitative scale to quantitative index. Aust J Crop Sci. 2013;7(9):1262–1269. [Google Scholar]
- Ravi K, Vadez V, Isobe S, Mir RR, Guo Y, Nigam SN, Gowda MV, Radhakrishnan T, Bertioli DJ, Knapp SJ, Varshney RK. Identification of several small main-effect QTLs and a large number of epistatic QTLs for drought tolerance related traits in groundnut (Arachis hypogaea L.) Theor Appl Genet. 2011;122:1119–1132. doi: 10.1007/s00122-010-1517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song A, An J, Guan Z, Jiang J, Chen F, Lou W, Fang W, Liu Z, Chen S. The constitutive expression of a two transgene construct enhances the abiotic stress tolerance of chrysanthemum. Plant Physiol Biochem. 2014;80:114–120. doi: 10.1016/j.plaphy.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Su J, Zhang F, Li P, Guan Z, Fang W, Chen F. Genetic variation and association mapping of waterlogging tolerance in chrysanthemum. Planta. 2016;244(6):1241–1252. doi: 10.1007/s00425-016-2583-6. [DOI] [PubMed] [Google Scholar]
- Sun C, Chen F, Teng N, Liu Z, Fang W, Hou X. Interspecific hybrids between Chrysanthemum grandiflorum (Ramat.) Kitamura and C. indicum (L.) Des Moul. and their drought tolerance evaluation. Euphytica. 2010;174:51–60. doi: 10.1007/s10681-009-0117-z. [DOI] [Google Scholar]
- Sun J, Gu J, Zeng J, Hang S, Song A, Chen F, Fang W, Jiang J, Chen F. Changes in leaf morphology, antioxidant activity and photosysnthesis capacity in two different drought-tolerant cultivars of chrysanthemum during and after water stress. Sci Hortic. 2013;161:429–458. doi: 10.1016/j.scienta.2013.07.015. [DOI] [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. 5. Sunderland: Sinauer Assoc; 2010. [Google Scholar]
- Tavakol E, Elbadry N, Tondelli A, Cattivelli L, Rossini L. Genetic dissection of heading date and yield under Mediterranean dry climate in barley (Hordeum vulgare L.) Euphytica. 2016;212:343–353. doi: 10.1007/s10681-016-1785-0. [DOI] [Google Scholar]
- Wang P, Zhou G, Cui K, Li Z, Yu S. Clustered QTL for source leaf size and yield traits in rice (Oryza sativa L.) Mol Breed. 2012;29:99–113. doi: 10.1007/s11032-010-9529-7. [DOI] [Google Scholar]
- Wang C, Zhang F, Guan Z, Chen S, Jiang J, Fang W, Chen F. Inheritance and molecular markers for aphid (Macrosiphoniella sanbourni) resistance in chrysanthemum (Chrysanthemum morifolium Ramat.) Sci Hortic. 2014;180:220–226. doi: 10.1016/j.scienta.2014.10.038. [DOI] [Google Scholar]
- Witcombe JR, Hollington PA, Howarth CJ, Reader S, Steele KA. Breeding for abiotic stresses for sustainable agriculture. Philos Trans R Soc B. 2008;363:703–716. doi: 10.1098/rstb.2007.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Gao S, Yang Y, Huang M, Cheng L, Wei Q, Fei Z, Gao J, Hong B. Transcriptome sequencing and whole genome expression profiling of chrysanthemum under dehydration stress. BMC Genom. 2013;14:662. doi: 10.1186/1471-2164-14-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Guo Y, Yan J, Zhang J, Song T, Rocheford T, Li J. Major and minor QTL and epistasis contribute to fatty acid compositions and oil concentration in high-oil maize. Theor Appl Genet. 2010;120:665–678. doi: 10.1007/s00122-009-1184-1. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma C, Xu Y, Wei Q, Imtiaz M, Lan H, Gao S, Cheng L, Wang M, Fei Z, Hong B, Gao J. A zinc finger protein regulates flowering time and abiotic stress tolerance in chrysanthemum by modulating gibberellin biosynthesis. Plant Cell. 2014;26:2038–2054. doi: 10.1105/tpc.114.124867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Buckler ES. Genetic association mapping and genome organization of maize. Curr Opin Biotechnol. 2006;17:155–160. doi: 10.1016/j.copbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Yu X, Bai G, Liu S, Luo N, Wang Y, Richmond DS, Pijut PM, Jackson SA, Yu J, Jiang Y. Association of candidate genes with drought tolerance traits in diverse perennial ryegrass accessions. J Exp Bot. 2013;64(6):1537–1551. doi: 10.1093/jxb/ert018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanke CD, Ling J, Plieske J, Kollers S, Ebmeyer E, Korzun V, Argillier O, Stiewe G, Hinze M, Neumann F, Eichhorn A, Polley A, Jaenecke C, Ganal MW, Röder MS. Analysis of main effect QTL for thousand grain weight in European winter wheat (Triticum aestivum L.) by genome-wide association mapping. Front Plant Sci. 2015;6:644. doi: 10.3389/fpls.2015.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hong B, Li J, Gao J. A simple method to evaluate the drought tolerance of ground-cover chrysanthemum (Dendranthema × grandiflorum) rooted cuttings. Sci Agric Sin. 2005;37(4):789–796. [Google Scholar]
- Zhang F, Chen S, Chen F, Fang W, Chen Y, Li F. SRAP-based mapping and QTL detection for inflorescence-related traits in chrysanthemum (Dendranthema morifolium) Mol Breeding. 2011;27:11–23. doi: 10.1007/s11032-010-9409-1. [DOI] [Google Scholar]
- Zhang F, Jiang J, Chen S, Chen F, Fang W. Mapping single-locus and epistatic quantitative trait loci for plant architectural traits in chrysanthemum. Mol Breed. 2012;30:1027–1036. doi: 10.1007/s11032-011-9686-3. [DOI] [Google Scholar]
- Zhang F, Chen S, Jiang J, Guan Z, Fang W, Chen F. Genetic mapping of quantitative trait loci underlying flowering time in chrysanthemum (Chrysanthemum morifolium) PLoS One. 2013;8(12):e83023. doi: 10.1371/journal.pone.0083023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Zhong K, Tong H, Shahid MQ, Li J. Associaiton mapping for aluminum tolerance in a core collection of rice landraces. Front Plant Sci. 2016;7:1415. doi: 10.3389/fpls.2016.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Wang J, Zhao H, Sun J, Guo L, Zou D. Genetic structure, linkage disequilibrium and association mapping of salt tolerance in japonica rice germplasm at the seedling stages. Mol Breed. 2015;35(7):1–16. doi: 10.1007/s11032-015-0342-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Five wilting index (WI) scales of phenotypic variation in the response of chrysanthemum rooted cuttings exposed to drought stress (JPG 578 KB)
Table S1 The panel of 159 chrysanthemum accessions, showing provenance, ASFV and grade of drought tolerance (DOCX 39 KB)