Abstract
Patients with interstitial cystitis/bladder pain syndrome (IC/BPS) can potentially develop symptom flares after exposure to minor bladder irritants such as subclinical bacterial infection. To reproduce this symptom onset, we intravesically instilled a sub-noxious dose of uropathogenic E. coli component lipopolysaccharide (LPS) in young URO-OVA/OT-I mice, a transgenic autoimmune cystitis model that spontaneously develops bladder inflammation at ≥10 weeks of age. Female URO-OVA/OT-I mice (6-weeks old) were treated intravesically with phosphate-buffered saline (PBS) or PBS containing a sub-noxious dose (1 μg) of LPS. Mice were evaluated for bladder inflammation, pelvic pain, and voiding dysfunction at days 1, 7, and 14 post-treatment. Mice treated with LPS but not PBS developed early bladder inflammation with increased macrophage infiltration. Accordingly, the inflamed bladders expressed increased levels of mRNA for proinflammatory cytokines (IL-1β and IL-6) and pain mediator (substance P precursor). In addition, LPS-treated mice exhibited pelvic pain and voiding dysfunction such as increased urinary frequency and reduced bladder capacity. These functional changes sustained up to day 14 tested. Our results indicate that a single sub-noxious dose of intravesical LPS triggers early bladder inflammation and symptom onset in URO-OVA/OT-I mice, providing a useful model for IC/BPS symptom flare study.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic inflammatory condition of the urinary bladder characterized by the hallmark symptoms of pelvic pain, urinary frequency and urgency1. During their lifespan, IC/BPS patients often experience symptom exacerbations (flares). These IC/BPS flares can vary in frequency, severity, and duration and severely affect patients’ quality of life2. Multiple factors can be a flare trigger including stress, sexual activity, diet, alcohol, and exercise, among others2. Urinary tract infection (UTI) has also been noted to be a potential flare trigger in IC/BPS patients2–4. While the actual incidence of significant bacteriuria was low based on standard urine culture3, sequencing analysis of DNA extracted from patients’ urine revealed the presence of numerous bacterial species5. These observations suggest that subclinical bacterial infection can be a potential risk factor for bladder inflammation and symptom flares in IC/BPS patients.
The etiology of IC/BPS remains elusive but has been associated with various conditions including autoimmunity. Patients with IC/BPS have been observed to develop antinuclear and anti-urothelium autoantibodies6, overexpress HLA-DR molecules on the urothelium7, and coexist with some autoimmune disorders such as systemic lupus erythematosus, Sjogren’s syndrome, and rheumatoid arthritis1,6,8,9. Immunosuppressive drugs have also been used to treat IC/BPS and demonstrated to be beneficial for some patients10,11. Moreover, bladder histology data have revealed a role of cell-mediated inflammatory mechanisms in IC/BPS12,13. Hence, autoimmune inflammation is likely a component of the pathophysiology of IC/BPS in subgroups of patients.
Animal models with experimental autoimmune cystitis (EAC) have been actively used in IC/BPS research since the 1990s14–20. These EAC models can be developed through immunization with bladder tissue components in rodents and used to reproduce many clinical correlates of human IC/BPS such as cystitis pain and voiding dysfunction18–20. We previously used genetic engineering technology to develop a transgenic EAC model (URO-OVA) that expresses the membrane form of the model antigen ovalbumin (OVA) as a self-antigen on the urothelium21. URO-OVA mice develop bladder inflammation at day 7 after adoptive transfer of OVA-specific CD8+ T cells from OT-I mice21–25 and resemble many clinical features of human IC/BPS including pelvic pain, voiding dysfunction, and increased mast cell counts21,24,26. Subsequently, we crossed URO-OVA mice with OT-I mice to generate URO-OVA/OT-I mice that spontaneously develop bladder inflammation at ≥10 weeks of age21,23. In this study, we used URO-OVA/OT-I mice to investigate whether intravesical administration of a sub-noxious dose of uropathogenic Escherichia coli (E. coli) component lipopolysaccharide (LPS) triggers early bladder inflammation and symptom onset in the animal model.
LPS is a cell wall component of Gram-negative bacteria including uropathogenic E. coli and acts as a virulence factor (endotoxin) to elicit strong inflammatory responses during bacterial infection27. Intravesical administration of LPS has been demonstrated to induce bladder inflammation characterized by edema and leukocytic infiltration in mice28,29, which was associated with increased expression of neuro-inflammatory factors in the bladder such as tumor necrosis factor (TNF)-α, substance P (SP), and nerve growth factor (NGF)30,31. These neuro-inflammatory factors are thought to play important roles in mediating cystitis pain30,31. Prior studies also demonstrated that intravesical LPS-induced bladder inflammation had the similar features of edema and leukocytic infiltration in rats, which was associated with increased expression of macrophage migration inhibitory factor (MIF) in the bladder32. Other inflammatory factors such as the bradykinin-1 receptor (a bradykinin receptor), neurokinin-1 receptor (an SP receptor), and nuclear factor-kappa B (a nuclear transcription factor) were also observed to involve in LPS-induced bladder inflammation and functional changes33–35. LPS is commonly used at a dose of 15 µg for intravesical induction of bladder inflammation in C57BL/6 mice29,31,35. We previously observed no induction of bladder inflammation in C57BL/6 mice after intravesical instillation of LPS at a dose of 1 µg36. Here we report that this low dose of intravesical LPS triggers early bladder inflammation and symptom onset in URO-OVA/OT-I mice (the same C57BL/6 genetic background), mimicking symptom flares seen in IC/BPS patients.
Results
URO-OVA/OT-I mice develop early bladder inflammation upon intravesical instillation of a single sub-noxious dose of LPS
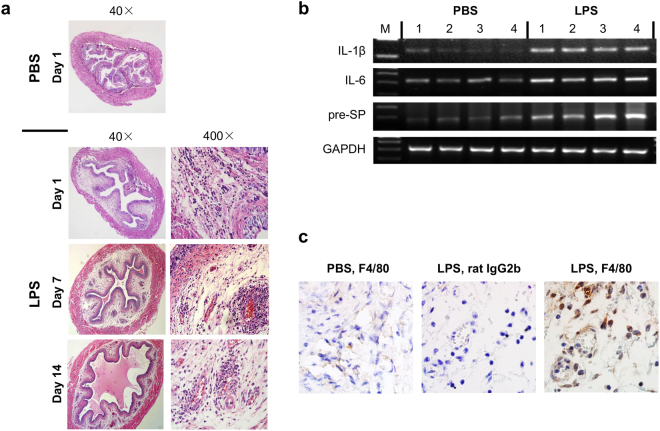
During the normal life history, URO-OVA/OT-I mice do not develop bladder inflammation until ≥10 weeks of age21,23. However, compared to the control 6-week-old URO-OVA/OT-I mice treated with intravesical phosphate buffered saline (PBS) (n = 11; score: - for all mice), 10 of 12 of sex- and age-matched URO-OVA/OT-I mice developed clear bladder inflammation (n = 12; score: - for 2, +for 2, ++for 2, and +++ for 6) 24 hours after intravesical instillation of a single sub-noxious dose of LPS (1 μg of LPS) (Fig. 1a, Table 1). The bladder inflammation sustained up to day 14 tested, although the intensity of bladder inflammation slightly reduced at the late timepoint (day 7: n = 8, score: - for 0, + for 1, ++ for 2, and +++ for 5; day 14: n = 8, score: - for 0, + for 2, ++ for 3, and +++ for 3) (Fig. 1a, Table 1). The LPS-treated bladders exhibited interstitial edema, mucosal hyperemia, and cellular infiltration in the lamina propria. In parallel with bladder histopathology, the inflamed bladders (day 1) expressed elevated levels of mRNAs for IL-1β, IL-6, and substance P precursor (pre-SP) as detected by RT-PCR (Fig. 1b) and increased F4/80 positive cell (macrophage) infiltration as detected by immunohistochemistry (Fig. 1c). These observations indicate that the bladders of URO-OVA/OT-I mice are highly sensitive to minor irritants and readily develop early inflammation upon stimulation with a single sub-noxious dose of LPS.
Figure 1.
URO-OVA/OT-I mice develop early bladder inflammation upon intravesical instillation of a single sub-noxious dose of LPS. (a) URO-OVA/OT-I mice develop early bladder inflammation. The bladders of 6-week-old URO-OVA/OT-I mice were instilled with 100 µl PBS or 1 µg of LPS in 100 µl PBS. At days 1, 7 and 14 post-treatment with LPS the bladders were collected and processed for histological H&E staining. PBS-treated bladders were collected and processed for histological analysis at day 1 post-treatment. Magnification: x40 and x400. The images are representative of 11 PBS-treated bladders and 12, 8 and 8 LPS-treated bladders for day 1, 7 and 14, respectively. (b) The inflamed bladders of URO-OVA/OT-I mice express increased inflammatory factor mRNAs after LPS treatment. The bladder total RNAs were extracted 24 hours after intravesical PBS or LPS treatment and analyzed by RT-PCR for IL-1ß, IL-6, and substance P precursor (pre-SP) mRNAs. GAPDH was used as an internal control. Four bladders for each group are presented. M: a 100 bp DNA ladder. This figure panel is cropped from 4 different gels run and exposed in the same experimental conditions. Their corresponding full-length gels are presented in Supplementary Figures 1–4. (c) The inflamed bladders of URO-OVA/OT-I mice show increased macrophage infiltration after LPS treatment. The bladder immunohistochemistry was performed 24 hours after intravesical PBS or LPS treatment. Left panel, the bladder of a mouse treated with intravesical PBS and stained with a rat anti-mouse F4/80 antibody (IgG2b); Middle panel, the bladder of a mouse treated with intravesical LPS and stained with a control rat IgG2b; Right panel, the bladder of a mouse treated with intravesical LPS and stained with a rat anti-mouse F4/80 antibody (IgG2b). The images are representative of 4–5 mice for each group. Magnification: x1,000.
Table 1.
Bladder response to a single sub-noxious dose of intravesical LPS in URO-OVA/OT-I mice.
| Bladder Inflammatory Score | ||||
|---|---|---|---|---|
| − | + | ++ | +++ | |
| PBS Day 1 (n = 11) | 11 | 0 | 0 | 0 |
| LPS Day 1 (n = 12) | 2 | 2 | 2 | 6 |
| LPS Day 7 (n = 8) | 0 | 1 | 2 | 5 |
| LPS Day 14 (n = 8) | 0 | 2 | 3 | 3 |
LPS-induced early bladder inflammation is associated with pelvic pain in URO-OVA/OT-I mice
Six-week-old URO-OVA/OT-I mice were intravesically instilled with a single sub-noxious dose of LPS (1 μg of LPS) and evaluated for pelvic pain using von Frey filament stimulation at days 1, 7, and 14 post-treatment (Fig. 2a, Supplementary Table S1). Sex- and age-matched URO-OVA/OT-I mice treated with intravesical PBS at day 1 post-treatment were included for comparison. Compared to PBS-treated mice (n = 8), LPS-treated mice exhibited significantly increased pelvic response at day 1 (n = 8; 0.4 g: 32.5 ± 3.66 vs 18.75 ± 3.504, p < 0.05; 1 g: 47.5 ± 4.91 vs 26.25 ± 2.631, p < 0.01; 4 g: 58.75 ± 5.154 vs 32.5 ± 3.66, p < 0.01). This increased pelvic response sustained at day 7 (n = 19; 0.4 g: 33.684 ± 2.779, p < 0.01; 1 g: 45.79 ± 3.361, p < 0.01; 4 g: 62.105 ± 3.296, p < 0.01) and up to day 14 (n = 10; 4 g: 49.0 ± 6.227, p < 0.01). There were no significant differences in tactile sensitivity (50% threshold) of the plantar region of the hind paw between PBS- and LPS-treated groups (Fig. 2b; p = 0.871 by ANOVA). These observations indicate that URO-OVA/OT-I mice readily develop pelvic-restricted pain upon intravesical stimulation with a single sub-noxious dose of LPS in this experimental setting.
Figure 2.
LPS-induced early bladder inflammation is associated with pelvic pain in URO-OVA/OT-I mice. (a) Six-week-old URO-OVA/OT-I mice were treated intravesically with 1 µg of LPS in 100 µl PBS and evaluated for pelvic response to von Frey filament stimulation at days 1 (n = 8), 7 (n = 19) and 14 (n = 10) post-treatment. Sex- and age-matched URO-OVA/OT-I mice treated with intravesical PBS at day 1 post-treatment (n = 8) were included for comparison. Data are shown as mean ± SEM percent of response frequency. *p < 0.05 and **p < 0.01 as compared to the PBS-treated group. (b) The same LPS-treated URO-OVA/OT-I mice exhibited no significant changes in tactile sensitivity (50% threshold) of the plantar region of the hind paw at 1, 7, and 14 days as compared to the PBS-treated URO-OVA/OT-I mice.
LPS-induced early bladder inflammation is associated with voiding dysfunction in URO-OVA/OT-I mice
Six-week-old URO-OVA/OT-I mice were intravesically instilled with a single sub-noxious dose of LPS (1 μg of LPS) and evaluated for voiding habits using micturition cages at days 1, 7, and 14 post-treatment. Sex- and age-matched URO-OVA/OT-I mice treated with intravesical PBS at day 1 post-treatment were included for comparison. There were no significant changes in voiding habits after a single intravesical PBS treatment compared to baseline voiding habits in the animal model (Supplementary Table S2). Compared to PBS-treated mice (n = 5), LPS-treated mice exhibited significant changes in voiding habits at day 1 post-treatment (n = 7) (Fig. 3, Table 2). These changes included decreased average volume voided per micturition (0.154 ± 0.012 vs 0.28 ± 0.033, p < 0.0001), decreased maximum volume voided per micturition (0.337 ± 0.054 vs 0.56 ± 0.048, p = 0.004), and increased total number of voids (9.429 ± 0.571 vs 5.2 ± 0.583, p < 0.0001). The number of voids in both light and dark periods was also significantly increased (in light: 3.571 ± 0.571 vs 2.0 ± 0.316, p = 0.019; in dark: 5.857 ± 0.404 vs 3.2 ± 0.49, p = 0.01). This voiding dysfunction sustained at days 7 (n = 10) and 14 (n = 5) (Table 2), although the numbers of voids in the light and dark periods were not statistically significant between the groups at these timepoints. The total voided volumes in 24 hours were similar between PBS- and LPS-treated groups (PBS day 1: 1.467 ± 0.254 g; LPS day 1: 1.471 ± 0.203 g; LPS day 7: 1.203 ± 0.152 g; LPS day 14: 1.224 ± 0.214 g; p = 0.6383 by ANOVA). These observations indicate that URO-OVA/OT-I mice readily develop voiding dysfunction upon intravesical stimulation with a single sub-noxious dose of LPS.
Figure 3.
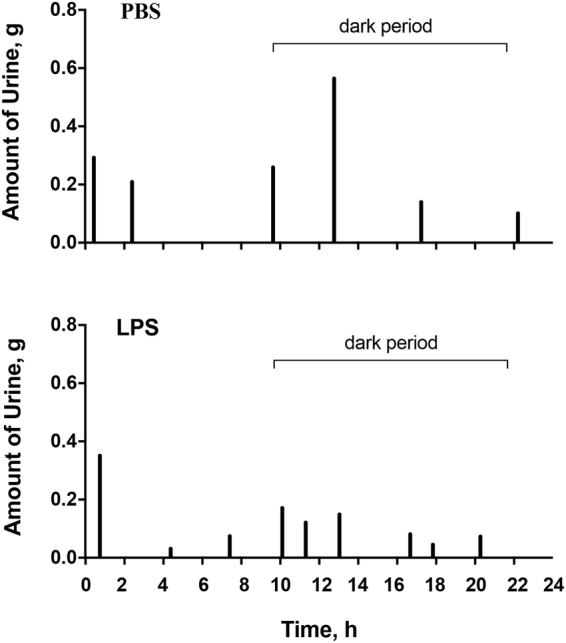
LPS-induced early bladder inflammation is associated with voiding dysfunction in URO-OVA/OT-I mice. Six-week-old URO-OVA/OT-I mice were treated intravesically with 100 μl PBS (top panel) or 1 µg of LPS in 100 µl PBS (bottom panel). After 24 hours, mice were evaluated for voiding habits using micturition cages. The results are representative of 5 PBS- and 7 LPS-treated mice.
Table 2.
Changes in voiding habits after a single sub-noxious dose of intravesical LPS in URO-OVA/OT-I mice (p-value: One-way ANOVA with LSD post test).
| PBS Day 1 (n = 5) | LPS Day 1 (n = 7) | LPS Day 7 (n = 10) | LPS Day 14 (n = 5) | p-value | |
|---|---|---|---|---|---|
| Average volume voided per micturition, g | 0.28 ± 0.033 | 0.15 ± 0.012 | 0.17 ± 0.015 | 0.17 ± 0.019 | 0.0008 |
| Maximum volume voided per micturition, g | 0.56 ± 0.048 | 0.34 ± 0.054 | 0.32 ± 0.037 | 0.29 ± 0.043 | 0.0044 |
| Total number of voids | 5.2 ± 0.583 | 9.43 ± 0.571 | 7.3 ± 0.578 | 7.4 ± 0.748 | 0.0023 |
| in light | 2.0 ± 0.316 | 3.57 ± 0.571 | 2.7 ± 0.260 | 3.0 ± 0.447 | 0.1087 |
| in dark | 3.2 ± 0.490 | 5.86 ± 0.404 | 4.4 ± 0.541 | 4.4 ± 1.030 | 0.0645 |
Discussion
IC/BPS is one of the most refractory diseases in urology today, and the effort to develop animal models that can reproduce the clinical correlates of the human disease is greatly needed for therapeutic development. Since the etiology of IC/BPS remains elusive and many factors can be causative for the disease, animal models with diverse pathological pathways have been developed26,37,38. Due to their presence of OVA “self” antigen on the urothelium and endogenous OVA-specific CD8+ T cells in the immune system, URO-OVA/OT-I mice spontaneously develop bladder inflammation at ≥10 weeks of age21,23. Although these mice show no bladder inflammation prior to 10 weeks of age, the autoimmune condition renders the mice hypersensitive to otherwise sub-noxious bladder irritative stimuli such as a low dose of LPS. We have observed that URO-OVA/OT-I mice exhibit a low threshold trigger for producing exaggerated responses to a single sub-noxious dose of LPS. Intravesical instillation of LPS at 1 µg efficiently induced early bladder inflammation, pelvic pain, and voiding dysfunction in the animal model. This low dose of LPS used for cystitis induction in URO-OVA/OT-I mice was only 1/15 dose commonly used for cystitis induction in C57BL/6 mice29,31,35. This feature of the URO-OVA/OT-I model is clinically relevant, as a subclinical bacterial infection is suggested to be a potential risk factor for symptom flares in some IC/BPS patients2–4.
We recently reported that the same single sub-noxious dose of intravesical LPS induced acute bladder inflammation in a monocyte chemoattractant protein (MCP)-1 expressing transgenic cystitis model (URO-MCP-1)36. The bladder inflammation developed in URO-MCP-1 mice was also associated with pelvic pain and voiding dysfunction seen in IC/BPS patients36. Despite its constitutive expression of MCP-1 by the urothelium, the URO-MCP-1 model does not spontaneously develop bladder inflammation in the unmanipulated state. However, like the URO-OVA/OT-I model, the URO-MCP-1 model develops bladder phenotypic and functional changes upon intravesical instillation of 1 µg LPS36. Our observations indicate that a single sub-noxious dose of intravesical LPS is capable of triggering bladder inflammation and symptom onset in both IC/BPS-like animal models, supporting the hypothesis that a subclinical bacterial infection can be causative for bladder inflammation and symptom flares in human IC/BPS.
The URO-OVA/OT-I model develops early bladder inflammation, pelvic pain, and voiding dysfunction such as increased urinary frequency and reduced bladder capacity following intravesical instillation of a single sub-noxious dose of LPS. Although it is evident that a single sub-noxious dose of intravesical LPS triggers early bladder inflammation and symptom onset in the URO-OVA/OT-I model, the mechanisms by which LPS triggers bladder phenotypic and functional changes have not been identified. We have observed that the inflamed bladders of URO-OVA/OT-I mice expressed increased levels of CD8 and interferon (IFN)-γ mRNAs, suggesting that activation of endogenous OVA-specific CD8+ T cells may play an important role in LPS-induced early bladder inflammation and symptom onset in the animal model. In addition, the increased expressions of IL-1ß, IL-6 and pre-SP mRNAs in the inflamed bladders suggested that multiple inflammatory pathways, such as the Toll-like receptor (TLR), inflammasome and SP pathways, gained activation and contributed to the phenotypic and functional changes in the animal model. We plan to explore the mechanisms underlying the bladder phenotypic and functional changes in the animal model.
In this study we used intravesical E. coli LPS to mimic bladder infection by uropathogenic E. coli. LPS is the major component of the outer membrane of Gram-negative bacteria including E. coli. and acts as an endotoxin to elicit strong inflammatory responses during bacterial infection27. A study revealed that E. coli LPS inoculation was equally potent to, if not stronger than, E. coli inoculation for the induction of IL-6, nitric oxide (NO), and inducible NO synthase (iNOS) expressions in mouse bladders39. In addition, protein kinase C (PKC) activation and detrusor contraction were similarly increased in the bladders of mice treated with intravesical E. coli LPS or intravesical E. coli39. Other studies revealed that, similar to E. coli bladder infection40,41, intravesical E. coli LPS induced bladder pain and voiding dysfunction in mice40,42. These observations indicate that intravesical E. coli LPS can reproduce many features of E. coli bladder infection. However, it should be noted that E. coli LPS inoculation is not equivalent to E. coli inoculation, as E. coli LPS inoculation cannot reproduce bacterial colonization and associated pathological changes seen in E. coli inoculation.
The limitations of this study include the gender restriction of the URO-OVA/OT-I model, as only female mice were used due to a higher incidence of IC/BPS in females than males in humans1,43. The other limitations of this study include the lack of direct evaluation of bladder nociception as well as the lack of analysis of bladder inflammatory factors at the protein levels. In addition to exploring the mechanisms of the URO-OVA/OT-I model, our future studies will include the use of this animal model for therapeutic development, as URO-OVA/OT-I mice are responsive to anti-inflammatory agents such as dimethyl sulfoxide (DMSO)23, the only agent approved for intravesical treatment of IC/BPS by the Food and Drug Administration (FDA).
In summary, our results indicate that a single sub-noxious dose of intravesical LPS triggers early bladder inflammation and symptom onset in URO-OVA/OT-I mice, providing a useful model for IC/BPS symptom flare study.
Materials and Methods
Ethics statement
All animal procedures were approved by University of Iowa Animal Care and Use Committee (Permit Number: 1308153) and performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Mice
URO-OVA/OT-I mice were generated through crossbreeding of URO-OVA mice, a transgenic EAC model previously developed in our laboratory21, with OT-I mice (Jackson Laboratories, Bar Harbor, ME), a transgenic line that expresses CD8+ T cell receptor specific for the H2-Kb/OVA257–264 epitope25. Both URO-OVA and OT-I mice are of C57BL/6 genetic background. URO-OVA/OT-I mice (F1 generation) retain the urothelial OVA expression and endogenous OVA-specific CD8+ T cells and develop spontaneous bladder inflammation at ≥10 weeks of age21,23. All mice were housed in a pathogen-free facility at the University of Iowa Animal Care Facility. Female mice were used due to a higher incidence of IC/BPS in females than males in humans1,43.
Cystitis induction
Female URO-OVA/OT-1 mice (6 weeks old) were anesthetized through intraperitoneal (IP) injection of a mixture solution of ketamine (87.5 mg/kg) and xylazine (12.5 mg/kg). The bladder was then catheterized via the urethra with a 24 gauge, 3/4′′long plastic intravenous catheter (Smiths Medical, Southington, CT), instilled with 1 µg of LPS (E. coli 055:B5, Sigma-Aldrich, St. Louis, MO) in 100 µl PBS, and retained for 1 hour. Control mice were instilled with 100 µl PBS alone in the bladders.
Bladder histology
Bladders were collected and processed for standard histological hematoxylin and eosin (H&E) staining as described previously21. Also, bladder inflammation was scored in a blinded manner based on infiltration of inflammatory cells in the lamina propria and the presence of interstitial edema as described previously21.
Bladder immunohistochemistry
Bladder immunohistochemistry was performed as described previously36. Biotinylated rat anti-mouse F4/80 antibody (BioLegend, San Diego, CA; clone: CI:A3-1; rat IgG2b) was used to detect macrophages in the bladder tissue sections, while biotinylated rat IgG2b (BioLegend, San Diego, CA; clone: RTK4530;) was used as a control. Slides were developed using streptavidin-horseradish peroxidase complex (SAv-HRP) and diaminobenzidine (DAB) substrate solution (BD PharMingen), counterstained with hematoxylin solution, and photographed using an Olympus BX-51 microscope.
Pelvic pain analysis
Pelvic pain was evaluated using 5 selected von Frey filaments (Stoelting Co., Wood Dale, IL) and presented as the percentage of positive response to each filament as described previously36. Tactile sensitivity of the plantar region of the hind paw was also evaluated using the same von Frey filaments and presented as the 50% withdrawal threshold to the filament stimulation as described previously36.
Voiding habit analysis
Voiding habits were evaluated using micturition cages (Columbus Instruments, Columbus, OH) as described previously24,36. Urinary frequency, voided volume per micturition, and total urine volume were recorded and analyzed using Oxymax software (Columbus Instruments, Columbus, OH) as described previously24,36.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
As described previously24,36, total RNAs were extracted from the bladder using Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA), followed by cDNA synthesis using Invitrogen SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) and Oligo dT. PCR amplification was then performed on cDNA products using Taq DNA polymerase (New England Biolabs, Ipswich, MA) and sequence-specific primer pairs: 5′-GCCCATCCTCTGTGACTCAT-3′ and 5′-AGGCCACAGGTATTTTGTCG-3′ (230 bp) for IL-1ß, 5′-GTTCTCTGGGAAATCGTGGA-3′ and 5′-GGAAATTGGGGTAGGAAGGA-3′ (339 bp) for IL-6, 5′-GCCAATGCAGAACTACGAAA-3′ and 5′-GCTTGGACAGCTCCTTCATC-3′ (280 bp) for tachykinin-1 (substance P precursor), and 5′-GTTCCAGTATGACTCCACT-3′ and 5′-GTGCAGGATGCATTGCTG-3′ (321 bp) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The housekeeping gene GAPDH was amplified for 25 cycles, while other molecules were amplified for 40 cycles. The PCR products were run on 1% agarose gels, stained with ethidium bromide, and imaged by Gel Doc EZ Imager (Bio-Rad Laboratories, Hercules, CA).
Statistical analysis
Data were analyzed using Statistics Package for Social Sciences (SPSS 13.0, Chicago, IL), presented as mean ± SEM for both pelvic pain and voiding habit changes, and compared by Student’s t-test (two groups) or ANOVA followed by LSD post hoc tests (multiple groups). A value of p < 0.05 was considered statistically significant.
Data Availability Statement
All data are fully available without restriction.
Electronic supplementary material
Acknowledgements
We thank Dr. Timothy Ratliff for providing the micturition cages and Ms. Bridget Fahey for editorial review of the manuscript. This work was supported in part by grants U01DK082344 and R01DK100891 from the National Institutes of Diabetes and Digestive and Kidney Diseases.
Author Contributions
Y.L. designed and supervised the study. P.K., S.X., and Y.W. performed the experiments. S.X. and Y.W. performed all statistical analyses. P.K., S.X., Y.W. and Y.L. drafted the article. P.K., S.X., Y.W., M.O., S.L., C.B., A.S., K.K., and Y.L. reviewed and approved the final version of this manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Paul Kogan, Suming Xu and Yaoqin Wang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24833-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hanno PM, Erickson D, Moldwin R, Faraday MM. American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J. Urol. 2015;193:1545–53. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 2.Sutcliffe S, et al. Urological chronic pelvic pain syndrome flares and their impact: qualitative analysis in the MAPP network. Int. Urogynecol J. 2015;26:1047–60. doi: 10.1007/s00192-015-2652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanford E, McMurphy C. There is a low incidence of recurrent bacteriuria in painful bladder syndrome/interstitial cystitis patients followed longitudinally. Int. Urogynecol J. Pelvic Floor Dysfunct. 2007;18:551–4. doi: 10.1007/s00192-006-0184-9. [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Shoskes D, Irvine-Bird K. Clinical phenotyping of women with interstitial cystitis/painful bladder syndrome: a key to classification and potentially improved management. J. Urol. 2009;182:155–60. doi: 10.1016/j.juro.2009.02.122. [DOI] [PubMed] [Google Scholar]
- 5.Nickel JC, et al. Assessment of the Lower Urinary Tract Microbiota during Symptom Flare in Women with Urologic Chronic Pelvic Pain Syndrome: A MAPP Network Study. J. Urol. 2016;195:356–62. doi: 10.1016/j.juro.2015.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nat. Clin. Pract. Urol. 2007;4:484–91. doi: 10.1038/ncpuro0874. [DOI] [PubMed] [Google Scholar]
- 7.Liebert M, et al. Evidence for urothelial cell activation in interstitial cystitis. J. Urol. 1993;149:470–5. doi: 10.1016/S0022-5347(17)36121-9. [DOI] [PubMed] [Google Scholar]
- 8.van de Merwe JP, Yamada T, Sakamoto Y. Systemic aspects of interstitial cystitis, immunology and linkage with autoimmune disorders. Int. J. Urol. 2003;10(Suppl):S35–8. doi: 10.1046/j.1442-2042.10.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 9.Peeker R, Atanasiu L, Logadottir Y. Intercurrent autoimmune conditions in classic and non-ulcer interstitial cystitis. Scand. J. Urol. Nephrol. 2003;37:60–3. doi: 10.1080/00365590310008721. [DOI] [PubMed] [Google Scholar]
- 10.Bouchelouche K, Nordling J. Recent developments in the management of interstitial cystitis. Curr. Opin. Urol. 2003;13:309–13. doi: 10.1097/00042307-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Taneja R, Jawade KK. A rational combination of intravesical and systemic agents for the treatment of interstitial cystitis. Scand. J. Urol. Nephrol. 2007;41:511–5. doi: 10.1080/00365590701435918. [DOI] [PubMed] [Google Scholar]
- 12.Erickson DR, Belchis DA, Dabbs DJ. Inflammatory cell types and clinical features of interstitial cystitis. J. Urol. 1997;158:790–3. doi: 10.1016/S0022-5347(01)64317-9. [DOI] [PubMed] [Google Scholar]
- 13.MacDermott JP, Miller CH, Levy N, Stone AR. Cellular immunity in interstitial cystitis. J. Urol. 1991;145:274–8. doi: 10.1016/S0022-5347(17)38313-1. [DOI] [PubMed] [Google Scholar]
- 14.Bullock AD, Becich MJ, Klutke CG, Ratliff TL. Experimental autoimmune cystitis: a potential murine model for ulcerative interstitial cystitis. J. Urol. 1992;148:1951–6. doi: 10.1016/S0022-5347(17)37091-X. [DOI] [PubMed] [Google Scholar]
- 15.Luber-Narod J, et al. Experimental autoimmune cystitis in the Lewis rat: a potential animal model for interstitial cystitis. Urol. Res. 1996;24:367–73. doi: 10.1007/BF00389795. [DOI] [PubMed] [Google Scholar]
- 16.Mitra S, Dagher A, Kage R, Dagher RK, Luber-Narod J. Experimental autoimmune cystitis: further characterization and serum autoantibodies. Urol. Res. 1999;27:351–6. doi: 10.1007/s002400050162. [DOI] [PubMed] [Google Scholar]
- 17.Phull H, et al. Angiotensin II plays a role in acute murine experimental autoimmune cystitis. Br. J. Urol. Int. 2007;100:664–7. doi: 10.1111/j.1464-410X.2007.07035.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin YH, et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. Br. J. Urol. Int. 2008;102:1724–30. doi: 10.1111/j.1464-410X.2008.07891.x. [DOI] [PubMed] [Google Scholar]
- 19.Altuntas CZ, et al. Autoimmunity to uroplakin II causes cystitis in mice: a novel model of interstitial cystitis. Eur. Urol. 2012;61:193–200. doi: 10.1016/j.eururo.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bicer F, et al. Chronic pelvic allodynia is mediated by CCL2 through mast cells in an experimental autoimmune cystitis model. Am. J. Physiol. Renal. Physiol. 2015;308:F103–13. doi: 10.1152/ajprenal.00202.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Evanoff DP, Chen X, Luo Y. Urinary bladder epithelium antigen induces CD8+ T cell tolerance, activation, and autoimmune response. J. Immunol. 2007;178:539–46. doi: 10.4049/jimmunol.178.1.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Deyoung BR, Chen X, Evanoff DP, Luo Y. RDP58 inhibits T cell-mediated bladder inflammation in an autoimmune cystitis model. J. Autoimmun. 2008;30:257–65. doi: 10.1016/j.jaut.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim R, Liu W, Chen X, Kreder K, Luo Y. Intravesical dimethyl sulfoxide inhibits acute and chronic bladder inflammation in transgenic experimental autoimmune cystitis models. J. Biomed. Biotechnol. 2011;2011:937061. doi: 10.1155/2011/937061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, et al. Evidence for the Role of Mast Cells in Cystitis-Associated Lower Urinary Tract Dysfunction: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Animal Model Study. PLoS One. 2016;11(12):e0168772. doi: 10.1371/journal.pone.0168772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke SR, et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell. Biol. 2000;78:110–7. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 26.Lai H, et al. Animal Models of Urologic Chronic Pelvic Pain Syndromes: Findings From the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Urology. 2015;85:1454–65. doi: 10.1016/j.urology.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerde TJ, Bjorling DE, Steinberg H, Warner T, Saban R. Determination of mouse bladder inflammatory response to E. coli lipopolysaccharide. Urol. Res. 2000;28:269–73. doi: 10.1007/s002400000114. [DOI] [PubMed] [Google Scholar]
- 29.Saban MR, et al. LPS-sensory peptide communication in experimental cystitis. Am. J. Physiol. Renal. Physiol. 2002;282:F202–10. doi: 10.1152/ajprenal.0163.2001. [DOI] [PubMed] [Google Scholar]
- 30.Bjorling DE, et al. Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. Br. J. Urol. Int. 2001;87:697–702. doi: 10.1046/j.1464-410x.2001.02138.x. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez RR, et al. Modulating bladder neuro-inflammation: RDP58, a novel anti-inflammatory peptide, decreases inflammation and nerve growth factor production in experimental cystitis. J. Urol. 2005;173:630–4. doi: 10.1097/01.ju.0000143192.68223.f7. [DOI] [PubMed] [Google Scholar]
- 32.Meyer-Siegler KL, Ordorica RC, Vera PL. Macrophage migration inhibitory factor is upregulated in an endotoxin-induced model of bladder inflammation in rats. J. Interferon Cytokine Res. 2004;24:55–63. doi: 10.1089/107999004772719918. [DOI] [PubMed] [Google Scholar]
- 33.Busser BW, Hammond TG, Bjorling DE, Saban R. Lipopolysaccharide upregulates bradykinin 1 receptors in the isolated mouse bladder. J. Urol. 1998;160:2267–73. doi: 10.1016/S0022-5347(01)62308-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang XC, et al. Nuclear factor kappa B mediates lipopolysaccharide-induced inflammation in the urinary bladder. J. Urol. 2000;163:993–8. doi: 10.1016/S0022-5347(05)67870-6. [DOI] [PubMed] [Google Scholar]
- 35.Saban MR, Nguyen NB, Hammond TG, Saban R. Gene expression profiling of mouse bladder inflammatory responses to LPS, substance P, and antigen-stimulation. Am. J. Pathol. 2002;160:2095–110. doi: 10.1016/S0002-9440(10)61159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu S, et al. Transgenic Mice Expressing MCP-1 by the Urothelium Demonstrate Bladder Hypersensitivity, Pelvic Pain and Voiding Dysfunction: A Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network Animal Model Study. PLoS One. 2016;11:e0163829. doi: 10.1371/journal.pone.0163829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bjorling DE, Wang ZY, Bushman W. Models of inflammation of the lower urinary tract. Neurourol. Urodyn. 2011;30:673–82. doi: 10.1002/nau.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westropp JL, Buffington CA. In vivo models of interstitial cystitis. J. Urol. 2002;167:694–702. doi: 10.1016/S0022-5347(01)69129-8. [DOI] [PubMed] [Google Scholar]
- 39.Weng TI, Wu HY, Lin PY, Liu SH. Uropathogenic Escherichia coli-induced inflammation alters mouse urinary bladder contraction via an interleukin-6-activated inducible nitric oxide synthase-related pathway. Infect. Immun. 2009;77:3312–9. doi: 10.1128/IAI.00013-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudick CN, et al. Host-pathogen interactions mediating pain of urinary tract infection. J. Infect. Dis. 2010;201:1240–9. doi: 10.1086/651275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen JM, Klumpp DJ. Mechanisms of pain from urinary tract infection. Int. J. Urol. 2014;21(Suppl 1):26–32. doi: 10.1111/iju.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takezawa K, et al. Combination of bladder ultrasonography and novel cystometry method in mice reveals rapid decrease in bladder capacity and compliance in LPS-induced cystitis. Am. J. Physiol. Renal. Physiol. 2014;307:F234–41. doi: 10.1152/ajprenal.00043.2014. [DOI] [PubMed] [Google Scholar]
- 43.Clemens JQ, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J. Urol. 2005;174:576–80. doi: 10.1097/01.ju.0000165170.43617.be. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are fully available without restriction.