Abstract
Rationale
Attentional processing deficits are a core feature of schizophrenia, likely contributing to the persistent functional and occupational disability observed in patients with schizophrenia. The pathophysiology of schizophrenia is hypothesized to involve dysregulation of NMDA receptor-mediated glutamate transmission, contributing to disruptions in normal dopamine transmission. Preclinical investigations often use NMDA receptor antagonists, such as phencyclidine (PCP), to induce cognitive disruptions relevant to schizophrenia. We sought to test the ability of partial dopamine D2/D3 agonists, cariprazine and aripiprazole, to attenuate PCP-induced deficits in attentional performance.
Objectives
The objective of this study is to determine whether systemic administration of cariprazine or aripiprazole attenuated 5-choice serial reaction time task (5-CSRTT) deficits induced by repeated exposure to PCP.
Methods
We utilized a repeated PCP-treatment regimen (2 mg/kg, subcutaneous [s.c.], once daily for 5 days) in rats to induce deficits in the 5-CSRTT. Rats were pre-treated with cariprazine (0.03, 0.1, or 0.3 mg/kg, oral [p.o.]) or aripiprazole (1, 3, or 10 mg/kg, p.o.) to determine whether they prevented PCP-induced deficits in the 5-CSRTT performance.
Results
PCP treatment increased inappropriate responding in the 5-CSRTT, elevating incorrect, premature, and timeout responses. Cariprazine treatment reduced PCP-induced increases in inappropriate responding. However, at higher doses, cariprazine produced non-specific response suppression, confounding interpretation of the attenuated PCP-induced deficits. Aripiprazole treatment also attenuated PCP-induced deficits; however, unlike cariprazine treatment, aripiprazole reduced correct responding and increased omissions.
Conclusions
Cariprazine and aripiprazole both demonstrated potential in attenuating PCP-induced deficits in the 5-CSRTT performance. While both compounds produced non-specific response suppression, these effects were absent when 0.03 mg/kg cariprazine was administered.
Keywords: Cariprazine, 5-CSRTT, Aripiprazole, Schizophrenia, Cognition, PCP
Introduction
Schizophrenia is a chronic disorder associated with psychotic disturbances, negative symptoms, and cognitive dysfunction. Cognitive deficits are a prominent feature of schizophrenia. Indeed, cognitive impairments are evident before the onset of psychotic outbreaks, persist despite therapeutic intervention, and appear to be a stable feature of the illness. As cognitive impairments are largely unresponsive to currently available medications and are thought to significantly contribute to the functional disability associated with the disorder, the identification of novel and efficacious therapeutic strategies is essential.
The National Institute of Mental Health’s Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) initiative identified several cognitive domains that are disrupted in patients with schizophrenia, including attention and vigilance (Kern et al. 2004; Young and Geyer 2015). Attention is a cognitive process that allows an individual to detect, select, and process sensory stimuli (Maunsell and Treue 2006). Impairments in attention have been associated with schizophrenia since the earliest descriptions of the disorder (Kraepelin 1921). Furthermore, attentional processing is suggested to form an underlying basis of several higher-order cognitive processes (Riedel et al. 2006), many of which were also implicated by the MATRICS initiative as being disrupted in schizophrenia (Kern et al. 2004). Thus, attentional deficits in schizophrenia may contribute to the spectrum of cognitive dysfunction observed in patients and the associated functional disabilities. Identifying the mechanism(s) that contribute to attentional deficits, and subsequent therapeutic strategies to improve attentional functioning, may be a key to improving the patients’ quality of life.
N-methyl-d-aspartate (NMDA) glutamate receptor hypofunction is hypothesized to contribute to the pathophysiology of schizophrenia, as NMDA receptor antagonists can induce schizophrenia-like symptoms in otherwise healthy individuals that recapitulate aspects of positive, negative, and cognitive symptomatology (Corlett et al. 2007; Krystal et al. 1994; Pomarol-Clotet et al. 2006). Indeed, it has recently been demonstrated that patients with schizophrenia may exhibit alterations in the expression of key functional subunits, potentially leading to endogenous NMDA receptor dysfunction in schizophrenia (Weickert et al. 2013). In addition to glutamate transmission, cortical hypofunction and sub-cortical hyperfunction of dopamine transmission are reported in patients with schizophrenia (Howes et al. 2015). Importantly, dopamine transmission is implicated in attentional performance (Barnes et al. 2012; Boulougouris and Tsaltas 2008; Carli and Invernizzi 2014; Granon et al. 2000; Nieoullon 2002), and augmenting dopamine transmission has been suggested as a potential target in the development of procognitive therapeutics for the treatment of patients with schizophrenia (Gray and Roth 2007; Ibrahim and Tamminga 2011). Furthermore, alterations in glutamate transmission can impact normal dopaminergic transmission, suggesting that hypotheses that involve these neurotransmitter systems and schizophrenia pathogenesis may not be mutually exclusive. Indeed, NMDA receptor antagonists have been used in experimental animals to induce deficits in cognitive processing relevant to schizophrenia (Grayson et al. 2015; Jentsch and Roth 1999; Neill et al. 2010; Neill et al. 2014; Pratt et al. 2008). Moreover, NMDA receptor antagonism produces alterations in dopamine transmission (Adams et al. 2002; Kapur and Seeman 2002). Hence, the use of experimental animal models that utilize NMDA receptor antagonists to induce deficits in attentional processing, coupled with dopaminergic manipulations to improve attention, may provide insights into the mechanism(s) that contribute to attention deficits in patients with schizophrenia.
The 5-choice serial reaction time task (5-CSRTT) is a well-validated and widely used behavioral procedure that tests attentional deficits in experimental rodents (Lustig et al. 2012), and the neural substrates involved in task performance have been well described (Chudasama and Robbins 2004; Robbins 2002). Previous investigations revealed that the administration of NMDA receptor antagonists (i.e., phencyclidine [PCP] or ketamine) to experimental animals impairs attentional processing (Amitai and Markou 2009, 2010; Amitai et al. 2007; Barnes et al. 2014; Nikiforuk and Popik 2014; Thomson et al. 2011). However, acute administration of PCP disrupts the 5-CSRTT performance in a generalized manner that is difficult to interpret as a specific cognitive impairment (Amitai and Markou 2010; Carli and Invernizzi 2014). Therefore, researchers find it advantageous to use a NMDA antagonist-treatment regimen that allows testing after a washout period or after repeated exposure to allow tolerance to the non-specific disruptions to develop (Barnes et al. 2014; Barnes et al. 2016; Nikiforuk and Popik 2014; Thomson et al. 2011). For instance, we have previously shown that a repeated PCP-treatment regimen induces cognitive-specific deficits in 5-CSRTT performance (Amitai and Markou 2009, 2010; Amitai et al. 2007). Deficits in 5-CSRTT performance were largely attributed to an increase in incorrect responses, omissions, and premature responses. These findings suggest that aberrant glutamatergic transmission may contribute to the attentional deficits evident in patients with schizophrenia. Furthermore, PCP-induced deficits in 5-CSRTT performance were attenuated by clozapine (Amitai et al. 2007) but not quetiapine treatment (Amitai and Markou 2009). Interestingly, clozapine displays a greater receptor occupancy than quetiapine for striatal dopamine receptors (Tauscher et al. 2004). This occupancy difference may account for the differing efficacy in ameliorating PCP-induced deficits. Using a pharmacological strategy that attenuates PCP-induced deficits in attentional performance by augmenting dopamine transmission may represent an effective therapeutic strategy for patients with schizophrenia.
Cariprazine is FDA approved to treat adults with schizophrenia and manic or mixed episodes associated with bipolar disorder. Cariprazine is a dopamine D3/D2 receptor partial agonist that preferentially binds to the dopamine D3 receptor (Kiss et al. 2010). It has shown procognitive and prosocial effects in rodents, improving PCP-induced deficits in executive functioning, working memory, recognition memory, and social interaction (Neill et al. 2016; Zimnisky et al. 2013). Interestingly, dopamine D3 receptor expression is most abundant in mesolimbic regions (Kiss et al. 2010) that may be involved in attentional processing (Feja et al. 2014; St. Peters et al. 2011). Cariprazine may therefore modulate 5-CSRTT performance and attenuate PCP-induced attentional deficits by altering mesolimbic dopamine D3 receptor activity. Aripiprazole is an FDA approved compound for the treatment of schizophrenia that has D2 receptor partial agonist activity and a similar pharmacological profile to cariprazine (Kiss et al. 2010). However, unlike cariprazine, it shows very low occupancy of D3 receptors within its antipsychotic-like effective doses that occupy D2 receptors to a high extent (≥ 80%) (Gyertyán et al. 2011). Interestingly, a disruption of the 5-CSRTT performance induced by the infusion of the NMDA receptor antagonist 3-(R)-2-carboxypiperazin-4-propyl-1-phosphonic acid (CPP) into the medial prefrontal cortex was attenuated by aripiprazole (Carli et al. 2011). Aripiprazole has also shown efficacy in the treatment of cognitive and negative symptoms in experimental animal models (Nagai et al. 2009; Wilson and Koenig 2014). Therefore, the aim of the present study was to identify whether systemic administration of cariprazine or aripiprazole attenuated 5-CSRTT deficits induced by repeated exposure to PCP and to explore eventual differences between these compounds in this model.
Experimental procedures
Animals
Male Wistar rats (weighing approximately 225 g; n = 96) were purchased from Charles River Laboratories (Raleigh, NC) and housed two per cage in a climate-controlled room on a 12 h/12 h reversed light-dark cycle (lights on at 6:00 p.m.); all behavioral testing was conducted in the animals’ dark cycle. No environmental enrichment was provided. Food and water were available ad libitum until behavioral testing began, during which time access to food was restricted. All experiments were conducted in accordance with the guidelines from the National Institutes of Health and the Association for the Assessment and Accreditation of Laboratory Animal Care and were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Drugs
Phencyclidine hydrochloride (PCP) (Sigma Aldrich, MO) was dissolved in 0.9% saline and administered by a subcutaneous (s.c.) injection of a volume of 2 ml/kg and a concentration of 2 mg/kg. Aripiprazole (Forest Laboratories, NY) was suspended in 2% Tween 80 in distilled H2O and administered by oral (p.o.) gavage (1, 3, or 10 mg/kg). Cariprazine HCl (Forest Laboratories, NY) was also dissolved in 2% Tween 80 and administered p.o. (0.03, 0.1, or 0.3 mg/kg). Drugs were administered according to the treatment regimen described as follows by an experimenter blind to all treatment groups throughout testing.
Behavioral apparatus
Training and testing were conducted in operant conditioning boxes (Med Associates, St Albans, VT). Each box was enclosed in a wooden sound-attenuating chamber and contained a curved rear wall with nine response apertures, each containing a photobeam at the entrance of each aperture to detect nose pokes and a 3-W yellow light to provide a visual stimulus. Metal inserts blocked four response apertures, leaving holes 1, 3, 5, 7, and 9 free for exploration. On the opposite wall, a food magazine connected to a food hopper enabled the delivery of food pellets (45 mg rodent pellet, Test Diet 5TUM, Richmond, IN), which was signaled by the illumination of a 3-W bulb in the food magazine. A photobeam detected head entries into the food magazine. Each box was controlled by a PC running the MedPC software (Med Associates).
Training
Animals were food restricted to 85% of their free-feeding body weight, although water access was available ad libitum. Food restriction continued throughout training and testing. Training was conducted as previously described (Amitai et al. 2007). Briefly, training began by habituating animals to the 5-CSRTT chambers for 20 min for 2 days. This initial habituation was followed by two 20-min sessions in which a food pellet was non-contingently delivered every 20 s. During these initial training sessions, each response aperture was baited with food pellets to encourage exploration. Response training began with all response apertures illuminated in a session lasting 30 min. Response in any aperture delivered a reward pellet; criteria to move onto the next stage was > 70 completed trials.
Animals were then trained in the 5-CSRTT procedure; one response aperture was illuminated for each trial. A response in the illuminated aperture resulted in the delivery of a reward pellet. A response in any aperture that was not illuminated was deemed an incorrect response and resulted in a 5-s timeout period (house light extinguished and no food reward delivered). Failure to respond (omission), responding during the inter-trial interval (ITI) before the visual stimulus was presented (premature response), or repeated responses after a correct response (perseverative response) also resulted in a timeout. Responses made during the timeout period restarted the 5-s timeout period and were recorded as a timeout response. These measures are summarized in Table 1. The stimulus duration was initially 30 s and progressively decreased (20, 10, 5, 2.5, 1.5 s) as individual animals reached the predetermined criteria (> 70 trials completed, > 70% accuracy, and < 20% omissions) until animals reached the target stimulus duration of 1 s. Each session lasted 30 min or when 100 trials had been completed, whichever occurred first.
Table 1.
Description of the behavioral measures assessed in the 5-CSRTT
| Measure | Description |
|---|---|
| Accuracy | Correct responses / (correct + incorrect) × 100 |
| Percent correct | Correct response / (correct + incorrect + omissions) × 100 |
| Percent incorrect | Incorrect response / (correct + incorrect + omissions) × 100 |
| Percent omission | Omitted responses / (correct + incorrect + omissions) × 100 |
| Premature | Response made during ITI prior to stimulus onset |
| Perseverative | Initial inappropriate repeat response following a correct response |
| Timeout | Subsequent inappropriate repeat responses made during the timeout period |
| Correct latency | Time taken to make a correct response |
| Reward latency | Time take to collect the food reward |
Further information describing these measures can be found in Amitai and Markou (2010)
Experimental design
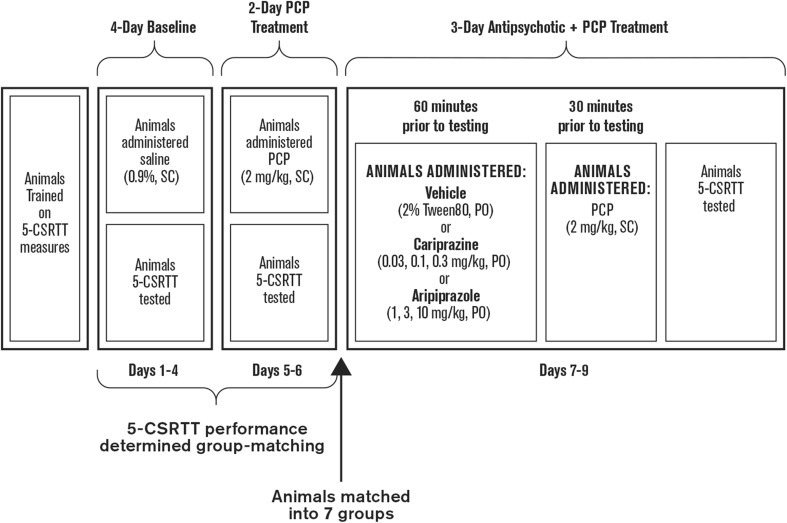
Once trained, animals were split into seven groups (Fig. 1). All animals were administered saline (0.9%, s.c.) 30 min before 5-CSRTT testing for 4 days. Then, all animals were administered PCP (2 mg/kg, s.c.) for 5 consecutive days. Using the 4 days of saline treatment and the initial 2 days of PCP treatment, the performance of the seven treatment groups was balanced to minimize any difference in behavioral performance (in the absence and presence of PCP) between groups before aripiprazole or cariprazine were administered. Group-matching was based on response accuracy, premature responding, percent correct, percent omissions, correct latency, and reward latency, as previously described (Amitai et al. 2007). After group-matching, the animals were administered vehicle (2% Tween 80, p.o.), aripiprazole (1, 3, or 10 mg/kg, p.o.), or cariprazine (0.03, 0.1, or 0.3 mg/kg, p.o.) 60 min before 5-CSRTT testing. After 30 min, all animals were administered PCP (2 mg/kg, s.c.), and 5-CSRTT testing was initiated after a 30-min pre-treatment time.
Fig. 1.
Experimental study design. 5-CSRTT 5-choice serial reaction time test, PCP phencyclidine, PO oral gavage, SC subcutaneous
Data analysis
The baseline performance, consisting of 4 days of saline treatment, and the final 3 days of the PCP + cariprazine or aripiprazole treatment were averaged. Data were analyzed by two-way repeated measures ANOVA (day as repeated measures and treatment as the between-subject variable), followed by a Fisher’s LSD post hoc test where appropriate. The a priori hypothesis was that repeated PCP treatment would disrupt incorrect responding, according to previous observations (Amitai and Markou 2009; Amitai et al. 2007). Sample size was selected based on prior experience from our lab with this treatment regimen and behavioral procedure. As a result, no formal power analysis was performed. Data were expressed as means ± SEM, analyzed in Statsoft Statistica v8, and displayed in GraphPad Prism v5.
Results
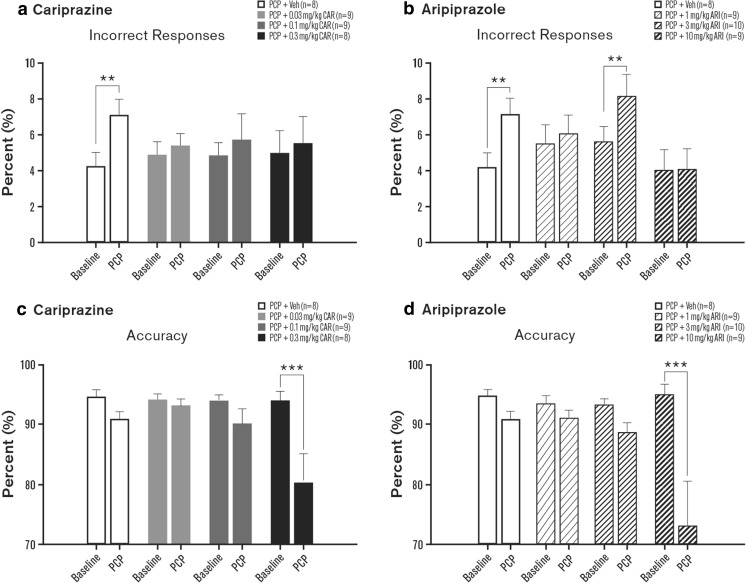
Percent incorrect responses, percent correct responses, accuracy, and percent omissions were evaluated in rats. Although the day × treatment interaction for percent incorrect responding failed to reach significance [F(3, 30) = 1.75, p = 0.18], preplanned comparisons demonstrated that PCP alone significantly increased incorrect responding compared to baseline (p < 0.01; Fig. 2a, Table 2). This increase in incorrect responding was not observed in cariprazine-treated (0.01–0.3 mg/kg) animals (Fig. 2a, Table 2). Significant interactions were evident for percent incorrect responses in the aripiprazole treatment experiments [F(3, 32) = 3.32, p < 0.05]. PCP alone increased the percentage of incorrect responses (p < 0.01), but this effect was attenuated when aripiprazole (1 or 10 mg/kg) was administered (Fig. 2b, Table 2). At 3 mg/kg, aripiprazole was not effective in attenuating the PCP-induced increase in incorrect responses (p < 0.01; Fig. 2b, Table 2).
Fig. 2.
Effects of cariprazine and aripiprazole on PCP-induced alterations in attentional processing: incorrect responses (a, b) and response accuracy (c, d). Measures are shown as means ± SEM; p values are based on ANOVA planned comparisons (cariprazine; incorrect responses) or post hoc Fisher least significant difference tests (cariprazine; response accuracy, and aripiprazole). **p < 0.01 compared to baseline. ARI aripiprazole, CAR cariprazine, PCP phencyclidine, Veh vehicle
Table 2.
5-CSRTT performance
| Measure | PCP + Veh | PCP + cariprazine | PCP + aripiprazole | |||||
|---|---|---|---|---|---|---|---|---|
| 0.03 mg/kg | 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | 3 mg/kg | 10 mg/kg | |||
| Incorrect responses (%) | Baseline | 4.3 (0.8) | 4.9 (0.8) | 4.9 (0.7) | 5.0 (1.2) | 5.8 (1.1) | 5.6 (0.8) | 4.0 (1.2) |
| Treatment | 7.1 (0.9)* | 5.4 (0.7) | 5.7 (1.4) | 5.5 (1.5) | 6.0 (1.1) | 8.2 (1.2)* | 4.1 (1.2) | |
| Correct reponses (%) | Baseline | 78.4 (3.0) | 79.2 (3.3) | 75.6 (2.1) | 79.2 (3.3) | 79.4 (2.5) | 79.2 (2.3) | 80.3 (3.2) |
| Treatment | 71.4 (3.0) | 76.6 (3.4) | 51.6 (2.6)* | 36.4 (6.0)* | 65.2 (4.9)* | 66.9 (3.0)* | 38.7 (5.2)* | |
| Accuracy (%) | Baseline | 94.7 (1.1) | 94.1 (1.0) | 94.0 (0.9) | 93.9 (1.6) | 93.4 (1.3) | 93.2 (1.1) | 95.0 (1.7) |
| Treatment | 90.8 (1.3) | 93.1 (1.0) | 90.1 (2.4) | 80.3 (4.9)* | 91.0 (1.3) | 88.6 (1.7) | 73.0 (7.5)* | |
| Omissions (%) | Baseline | 17.3(2.6) | 16.0 (3.1) | 19.5 (2.0) | 15.7 (2.9) | 15.0 (2.3) | 15.2 (1.7) | 15.8 (2.4) |
| Treatment | 21.5 (2.1) | 18.0 (3.1) | 42.6 (2.8)* | 58.2 (6.8)* | 28.8 (5.1)* | 24.9 (2.5)* | 57.2 (5.7)* | |
| Trials completed, n | Baseline | 99.1 (0.6) | 99.1 (0.6) | 98.9 (0.7) | 96.6 (2.5) | 99.0 (0.5) | 99.4 (0.4) | 99.1 (0.9) |
| Treatment | 89.1 (5.6) | 97.7 (1.0) | 79.2 (5.9)* | 49.1 (9.8)* | 86.6 (6.3) | 94.6 (2.5) | 56.1 (9.6)* | |
| Premature responses, n | Baseline | 6.7 (1.4) | 10.3 (1.1) | 8.3 (1.7) | 9.7 (2.6) | 11.0 (3.1) | 9.3 (1.7) | 6.8 (1.2) |
| Treatment | 12.8 (3.3)* | 10.1 (1.8) | 3.9 (1.4) | 2.5 (1.2)* | 9.4 (2.4) | 8.2 (2.4) | 2.7 (0.9) | |
| Timeout responses, n | Baseline | 6.8 (1.1) | 7.5 (1.4) | 5.3 (1.0) | 7.8 (1.6) | 7.0 (2.3) | 8.8 (1.6) | 6.2 (1.3) |
| Treatment | 12.9 (3.2)* | 9.4 (1.3) | 4.6 (1.6) | 4.3 (1.5) | 6.6 (1.3) | 14.0 (2.7)* | 5.0 (1.6) | |
| Correct latency (s) | Baseline | 0.8 (0.0) | 0.8 (0.0) | 0.8 (0.0) | 0.8 (0.0) | 0.8 (0.0) | 0.9 (0.0) | 0.8 (0.0) |
| Treatment | 0.8 (0.0) | 0.8 (0.0) | 1.0 (0.0) | 1.0 (0.1) | 0.9 (0.1) | 0.9 (0.0) | 0.9 (0.1) | |
| Reward latency (s) | Baseline | 1.8 (0.1) | 1.7 (0.2) | 2.1 (0.2) | 2.1 (0.3) | 1.8 (0.1) | 2.0 (0.2) | 1.7 (0.2) |
| Treatment | 1.6 (0.2) | 1.5 (0.1) | 2.5 (0.3) | 1.8 (0.3) | 1.7 (0.1) | 2.0 (0.2) | 1.5 (0.2) | |
| Perseverative responses, n | Baseline | 6.5 (1.0) | 5.8 (1.2) | 6.8 (1.4) | 9.8 (1.8) | 7.2 (0.7) | 8.7 (2.1) | 7.0 (0.8) |
| Treatment | 7.3 (1.9) | 7.2 (0.8) | 5.6 (1.2) | 2.8 (1.2)* | 8.1 (2.3) | 8.0 (1.2) | 4.7 (1.6) | |
Measures are shown as mean ± SEM
5-CSRTT 5-choice serial reaction time task, PCP phencyclidine, Veh vehicle
*p < 0.05
For response accuracy, significant interactions were evident with cariprazine [F(3, 30) = 3.81, p < 0.05] and aripiprazole [F(3, 32) = 5.51, p < 0.01]. Accuracy was significantly reduced by PCP and 0.3 mg/kg cariprazine (p < 0.001; Table 2; Fig. 2c) and by PCP and 10 mg/kg aripiprazole (p < 0.001; Fig. 2d), but not by PCP alone. Similarly, the analysis of the percentage of correct responses also resulted in significant interactions for cariprazine [F(3, 30) = 13.68, p < 0.01] and aripiprazole [F(3, 32) = 9.99, p < 0.001]. As this measure was not reduced with PCP alone, this effect was the result of fewer correct responses made in animals that received PCP and cariprazine (0.1 and 0.3 mg/kg, p < 0.001 each) or PCP and aripiprazole (all three doses, p < 0.05–p < 0.001) (Table 2).
For percent omissions, a significant interaction was evident with both cariprazine [F(3, 30) = 14.67, p < 0.001] and aripiprazole [F(3, 32) = 11.49, p < 0.001]. No increase was observed in animals treated with PCP alone. Omissions were increased in the groups that received PCP and cariprazine (0.1 or 0.3 mg/kg; p < 0.001 each) or PCP and aripiprazole (all three doses, p < 0.05 to p < 0.001) (Table 2).
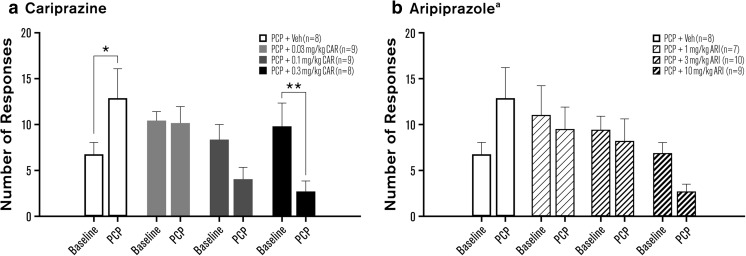
In the 5-CSRTT, motoric impulsivity is reflected by the number of premature responses before stimulus presentation, although this measure could also reflect timing capabilities (Cope et al. 2016). Premature responding was significantly disrupted with cariprazine [F(3, 30) = 6.04, p < 0.01] and aripiprazole [F(3, 32) = 3.06, p < 0.05]. Compared to baseline, premature responding was significantly increased in PCP-treated animals (p < 0.05; Fig. 3a, Table 2). No significant difference from baseline was evident in two cariprazine treatment groups (0.03, 0.1 mg/kg). The highest dose of cariprazine (0.3 mg/kg) significantly reduced premature responding compared to baseline performance (p < 0.01; Fig. 3a, Table 2). In the aripiprazole experiments, premature responses approached a significant increase in PCP-treated animals (p = 0.06) and were decreased in animals treated with PCP and 1 mg/kg aripiprazole (p < 0.05; Fig. 3b, Table 2). However, this effect likely reflected the unusually high baseline level of premature responses rather than a reduction per se. Balancing the high level of baseline premature responding in the PCP and 1 mg/kg aripiprazole group (by removing two animals from this group) with that of all other groups at baseline diminished the interaction, and the effect just failed to reach statistical significance [F(3, 30) = 2.78, p = 0.058]. Aripiprazole attenuated the PCP-induced increase in premature responding at all doses tested (Fig. 3b, Table 2).
Fig. 3.
Effects of cariprazine (a) and aripiprazole (b) on PCP-induced impulsivity: premature responses. Measures are shown as mean ± SEM. p values are based on post hoc Fisher least significant difference test (cariprazine) or ANOVA planned comparison tests (aripiprazole). *p < 0.05; **p < 0.01 compared to baseline. ARI aripiprazole, CAR cariprazine, PCP phencyclidine, Veh vehicle. aTwo outliers were removed from the PCP + 1 mg/kg aripiprazole group
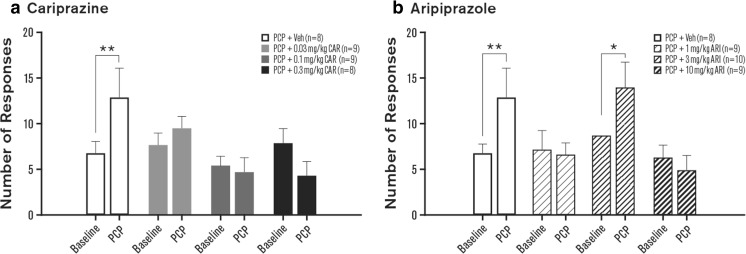
A surrogate measure for cognitive flexibility in the 5-CSRTT is the number of perseverative responses and timeout responses. Cariprazine significantly influenced perseverative responding [F(3, 30) = 6.02, p < 0.001]. However, this effect was not driven by a PCP-induced increase. Rather, perseverative responding was significantly reduced after cariprazine treatment (0.3 mg/kg, p < 0.001; Table 2). No effect on perseverative responding was observed with aripiprazole [F(3, 32) = 0.55, ns]. The number of timeout responses was significantly influenced by both cariprazine [F(3, 30) = 4.30, p < 0.05] and aripiprazole [F(3, 32) = 3.37, p < 0.05]. Timeout responding was significantly increased in the PCP-treated group compared to baseline (p < 0.01); no significant changes from baseline were observed with any cariprazine dose (Fig. 4a, Table 2). In contrast, the PCP-induced increase in timeout responding (p < 0.01) was attenuated in animals receiving 1 and 10 mg/kg aripiprazole (Fig. 4b, Table 2). A significant increase in timeout responding was evident in animals receiving PCP and 3 mg/kg aripiprazole (p < 0.05; Fig. 4b, Table 2).
Fig. 4.
Effects of cariprazine (a) and aripiprazole (b) on PCP-induced cognitive flexibility: timeout responses. Measures are shown as means ± SEM. p values are based on post hoc Fisher least significant difference test. *p < 0.05; **p < 0.01 compared to baseline. ARI aripiprazole, CAR cariprazine, PCP phencyclidine, Veh vehicle
When the number of completed trials was analyzed, significant interactions were evident with cariprazine [F(3, 30) = 11.59, p < 0.001] and aripiprazole [F(3, 32) = 7.37, p < 0.001]. PCP alone had no effect on trials completed; therefore, the significant interactions were attributable to the significant reduction of the number of trials completed within a session with PCP and cariprazine (0.1 mg/kg, p < 0.01; 0.3 mg/kg, p < 0.001) or with PCP and aripiprazole (10 mg/kg, p < 0.001) (Table 2).
In the 5-CSRTT, a surrogate measure for processing speed can be evaluated by comparing correct latency (latency to correct response) and reward latency (time to reward retrieval). In the cariprazine treatment experiments, no interaction was observed for correct latency [F(3, 30) = 2.19, p = 0.1], but a main effect of Day was present [F(1, 30) = 24.69, p < 0.001]. ANOVA did not reveal any effect on reward latency [F(3, 30) = 1.01, NS]. Hence, cariprazine treatment slowed processing speed without affecting overall motoric capability. For aripiprazole, a main effect of Day was also evident when correct latency was analyzed [F(1, 32) = 12.27, p < 0.01], indicating that the latency to make a correct response was elevated in all groups. No significant effect was observed for reward latency, although a main effect of Day approached significance [F(1, 32) = 3.66, p < 0.1] (Table 2).
Discussion
The 5-CSRTT assesses a range of cognitive domains, including aspects of sustained attention, motoric impulsivity, speed of processing, and cognitive flexibility. In this study, we have shown that a repeated PCP treatment regimen disrupted several of these domains in rats in ways that are similar to those observed in patients with schizophrenia. Namely, PCP treatment significantly increased the number of incorrect, premature, and timeout responses, which can be considered proxy measurements of attention deficits, motoric impulsivity, and cognitive inflexibility, respectively, symptoms that are frequently observed in patients with schizophrenia (Amitai and Markou 2010). Moreover, these effects occurred without affecting measures such as completed trials or reward latency, suggesting PCP-induced deficits were not the result of non-specific performance alterations. In addition, all three cariprazine doses significantly diminished the PCP-induced increases in incorrect, premature, and timeout responses. However, the two higher cariprazine doses also significantly reduced the number of trials completed, percent accuracy, and number of correct responses, suggesting that these doses resulted in a non-specific suppression of responses. This non-specific response suppression appears to be a characteristic of antipsychotic drugs, as similar non-specific effects due to high doses of antipsychotic compounds were also reported in an earlier study (Amitai et al. 2007). Importantly, the lowest cariprazine dose (0.03 mg/kg) improved the 5-CSRTT performance without inducing these non-specific disruptions, suggesting that this dose is effective in attenuating PCP-induced impairments in cognition. Of note, this cariprazine dose is lower than the antipsychotic-like effective dose (ED50 = 0.09 mg/kg) that attenuated PCP-induced increases in locomotor activity in a previous study (Gyertyán et al. 2011). It should be noted, however, that some effects presented here were marginal and only statistically significant when a priori pre-planned comparisons were applied. Nonetheless, the findings of the present study provide support for the hypothesis that low doses of cariprazine may be effective against cognitive symptoms of schizophrenia.
Aripiprazole treatment resulted in a slightly different attention response profile. Two aripiprazole doses (1 and 10 mg/kg) significantly reversed the PCP-induced increases in incorrect and timeout responses. However, all three aripiprazole doses also decreased the percentage of correct responses and increased the number of omissions, suggesting that aripiprazole may directly impair select forms of attention, resulting in a reduction in stimulus detection. While there was an apparent dose-dependent reduction in premature response in aripiprazole-treated animals, the interaction for this measure did not reach statistical significance. While it may be possible that aripiprazole improves PCP-induced increases in motoric impulsivity, further experiments and replication are required before firm conclusions can be made. The effects of drug treatment on 5-CSRTT performance were not evaluated in the absence of PCP; therefore, it is unclear to what extent any or all of these effects of aripiprazole are due to its action on PCP-induced responses vs its direct effects on 5-CSRTT performance, or an interaction between the two. These findings are similar to those of an earlier study, which used the competitive NMDA receptor antagonist CPP to induce cognitive deficits in rats that underwent the 5-CSRTT (Carli et al. 2011). Aripiprazole (1 and 3 mg/kg) ameliorated CPP-induced decreases in accuracy and increases in perseverative overresponding, suggesting that aripiprazole can affect both the attention and the cognitive flexibility domains of cognition at these doses. Furthermore, these data support the doses chosen for the present study, although lower doses could be tested in the future.
Our findings are consistent with those reported in previous studies in which second-generation antipsychotics attenuated PCP- or CPP-induced cognitive deficits in the 5-CSRTT (Amitai et al. 2007; Carli et al. 2011). Clozapine reversed chronic PCP-induced decreases in accuracy and increases in premature responses in the 5-CSRTT, but had no effect on measures of cognitive flexibility (Amitai et al. 2007). In a later study, quetiapine failed to modulate any PCP-induced cognitive deficits modeled by the 5-CSRTT (Amitai and Markou 2009). Olanzapine (0.3 and 1.0 mg/kg) reduced CPP-induced attention deficits and premature responding, but did not affect perseverative responses (Carli et al. 2011). Taken together, these studies indicate that different antipsychotics can specifically modulate discrete cognitive domains. Clinical studies have also shown that different antipsychotics are effective against different aspects of cognition in patients with schizophrenia (Meltzer and McGurk 1999; Sharma and Mockler 1998; Strous et al. 2006; Velligan et al. 2002). At the dose that specifically attenuated PCP-induced cognitive deficits, cariprazine exerted broad effects on multiple cognitive domains, suggesting that cariprazine may be of use in patients with schizophrenia who exhibit a range of cognitive symptoms.
The diverse effects of different antipsychotic compounds could be related to their varied receptor pharmacology profiles. Clozapine, quetiapine, and olanzapine are dopamine D2 receptor and serotonin 5-HT2A receptor antagonists (Bymaster et al. 1996; Ellenbroek and Cesura 2015; Moore et al. 1992; Schmidt et al. 2001). In contrast, cariprazine and aripiprazole are partial agonists at dopamine D2 and D3 receptors and serotonin 5-HT1A receptors and antagonists at 5-HT2A receptors (Kiss et al. 2010). Importantly, alterations in glutamate transmission can impact dopamine levels, as NMDA receptor antagonism results in aberrant dopamine transmission (Adams et al. 2002; Kapur and Seeman 2002; Pouvreau et al. 2016). Moreover, NMDA antagonism induces upregulation of striatal dopamine D2 receptors (Nair et al. 1998). Striatal dopamine D2 receptor overexpression produces motivational and cognitive impairment associated with schizophrenia (Kellendonk et al. 2006; Simpson et al. 2011) and alterations in the PFC inhibitory transmission (Li et al. 2011) reminiscent to those observed after the PCP treatment. Increased expression of striatal dopamine D2 receptors may, therefore, contribute to the disruptive effects of NMDA antagonists on attentional processes and provide a potential mechanism for the behavioral attenuation when compounds that modulate the activity of dopamine D2-like receptors are administered. While dopamine D2 receptors are expressed throughout the brain, high dopamine D3 receptor expression occurs in several particular regions of the brain, including the prefrontal cortex, nucleus accumbens, and ventral tegmental area, that are associated with negative, cognitive, and mood symptoms of schizophrenia (Gross and Drescher 2012). Because the compounds in this study were administered systemically, it is not possible to pinpoint the exact region of the brain in which they function to modulate cognitive impairment. However, an impaired cognition associated with schizophrenia is thought to be associated with hypofunction of the prefrontal cortex, including reduced dopaminergic activity (Goldman-Rakic et al. 2004; Slifstein et al. 2015).
The fact that cariprazine acts as a partial agonist at D3 and D2 receptors may have important implications for its therapeutic profile that makes its use preferable to that of pure D3/D2 receptor antagonists. It has been hypothesized that a general dysregulation of dopaminergic systems in patients with schizophrenia can result in both hyperactivity in circuits associated with positive symptoms and hypoactivity in those associated with cognitive and negative symptoms (Maia and Frank 2017). Partial agonists, by definition, have the ability to constrain activity in neurotransmitter-receptor circuits within a certain range, potentially allowing for the normalization of both hyperactive and hypoactive circuits. Therefore, the partial agonist properties of cariprazine may allow for the simultaneous treatment of the multiple symptom domains of schizophrenia, acting as an antagonist to reduce dopaminergic hyperactivity associated with positive symptoms and as an agonist to increase dopaminergic activity in brain regions associated with negative and cognitive symptoms.
Based on the preferential distribution of D3 receptors in corticolimbic circuits (Sokoloff et al. 1990), it has been suggested that dopamine antagonists displaying high affinity for both D3 and D2 receptors may yield a favorable antipsychotic therapeutic profile in terms of maximizing efficacy (Gyertyán et al. 2008; Kiss et al. 2008). Cariprazine exhibits such a binding profile, having a higher affinity and selectivity for the D3 vs the D2 receptor. In contrast, aripiprazole has a higher affinity and selectivity for the D2 vs the D3 receptor, as measured in various in vitro assays (de Bartolomeis et al. 2015; Kiss et al. 2010). Moreover, in the in vivo rodent studies, cariprazine but not aripiprazole showed high occupancy of both D2 and D3 receptors at antipsychotic-like effective doses (Gyertyán et al. 2011), indicating that potentially, cariprazine would have a greater impact on D3 receptor activity than aripiprazole. Interestingly, while the low and high doses of aripiprazole attenuated PCP-induced impairments in incorrect responding, the medium dose was not effective. Dopamine transmission-mediated modulation of cognitive processing is extremely sensitive to an optimal range of activity (Floresco 2013). While further investigations are necessary to elucidate the precise mechanism underlying this unusual effect, it may reflect aripiprazole displaying optimal or sub-optimal effects on dopamine transmission depending on the dose administered. The absence of this usual effect in cariprazine treated rats may also reflect differences in D2 and D3 receptor selectivity, but the precise mechanism for this dissociation is unknown and requires further investigation. Cariprazine was previously shown to reverse behavioral, cognitive, and social deficits in PCP-induced animal models of schizophrenia (Gyertyán et al. 2011; Neill et al. 2016; Watson et al. 2016; Zimnisky et al. 2013). These effects are likely due to cariprazine’s partial agonist activity on the D3 receptor, as cariprazine significantly ameliorated cognitive deficits induced by acute administration of PCP in wild-type mice, but not in D3 receptor knockout mice (Zimnisky et al. 2013). The more specific effect of cariprazine compared with aripiprazole on impaired cognition induced by PCP in this study may therefore be due to its preferential activity at the D3 receptor. Future studies using dopamine D3 receptor knockout mice in the 5-CSRTT are necessary to determine whether the effects of cariprazine are specific to the D3 receptor.
In summary, cariprazine exhibited a better overall cognitive profile than aripiprazole in the attenuation of PCP-induced deficits in the 5-CSRTT performance. Although higher tested doses of both compounds appeared to induce non-specific effects, the lowest dose of cariprazine significantly reversed the inappropriate responses induced by PCP and may therefore have potential for improving attentional impairment and other cognitive defects associated with schizophrenia.
Acknowledgements
Writing and editorial support for the manuscript was provided by Jennifer Fetting, PhD, of Prescott Medical Communications Group, Chicago, IL, a contractor of Allergan. All experiments in the study comply with the current laws of the country in which they were performed.
Compliance with ethical standards
All experiments were conducted in accordance with the guidelines from the National Institutes of Health and the Association for the Assessment and Accreditation of Laboratory Animal Care and were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Conflict of interest
This study was funded by Forest Laboratories, LLC, an Allergan affiliate, and Gedeon Richter Plc. Both companies participated in the study design, analysis, and interpretation of the data, and the decision to submit the paper for publication. The authors have full control of all data and agree to allow the journal to review the data if requested. N. Adham is an employee of Allergan. B. Kiss is an employee of Gedeon Richter. I. Gyertyán was an employee of Gedeon Richter Plc at the time of the study. During the last 3 years, A. Markou received research contract support from Astra-Zeneca, Bristol-Myers-Squibb, and Forest Laboratories, and honoraria from AbbVie, Germany. S. Barnes and J. Young report no conflict of interest.
Footnotes
The original version of this article was revised due to a retrospective open access order.
Athina Markou is deceased.
Change history
3/21/2018
The article The Effects of Cariprazine and Aripiprazole on PCP-Induced Deficits on Attention Assessed in the 5-Choice Serial Reaction Time Task, written by Samuel A. Barnes, Jared W. Young, Athina Markou, Nika Adham, István Gyertyán, Béla Kiss, was originally published electronically.
References
- Adams BW, Bradberry CW, Moghaddam B. NMDA antagonist effects on striatal dopamine release: microdialysis studies in awake monkeys. Synapse. 2002;43:12–18. doi: 10.1002/syn.1114. [DOI] [PubMed] [Google Scholar]
- Amitai N, Markou A. Increased impulsivity and disrupted attention induced by repeated phencyclidine are not attenuated by chronic quetiapine treatment. Pharmacol Biochem Behav. 2009;93:248–257. doi: 10.1016/j.pbb.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 2010;68:5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology. 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Neill JC. D1 receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology. 2012;220:129–141. doi: 10.1007/s00213-011-2460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Sawiak SJ, Caprioli D, Jupp B, Buonincontri G, Mar AC, Harte MK, Fletcher PC, Robbins TW, Neill JC, Dalley JW. Impaired limbic cortico-striatal structure and sustained visual attention in a rodent model of schizophrenia. Int J Neuropsychopharmacol. 2014;18:1–12. doi: 10.1093/ijnp/pyu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Young JW, Bate ST, Neill JC. Dopamine D1 receptor activation improves PCP-induced performance disruption in the 5C-CPT by reducing inappropriate responding. Behav Brain Res. 2016;300:45–55. doi: 10.1016/j.bbr.2015.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Tomasetti C, Iasevoli F. Update on the mechanism of action of aripiprazole: translational insights into antipsychotic strategies beyond dopamine receptor antagonism. CNS Drugs. 2015;29:773–799. doi: 10.1007/s40263-015-0278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Carli M, Invernizzi RW. Serotoninergic and dopaminergic modulation of cortico-striatal circuit in executive and attention deficits induced by NMDA receptor hypofunction in the 5-choice serial reaction time task. Front Neural Circuits. 2014;8:58. doi: 10.3389/fncir.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Calcagno E, Mainolfi P, Mainini E, Invernizzi RW. Effects of aripiprazole, olanzapine, and haloperidol in a model of cognitive deficit of schizophrenia in rats: relationship with glutamate release in the medial prefrontal cortex. Psychopharmacology. 2011;214:639–652. doi: 10.1007/s00213-010-2065-7. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Psychopharmacological approaches to modulating attention in the five-choice serial reaction time task: implications for schizophrenia. Psychopharmacology. 2004;174:86–98. doi: 10.1007/s00213-004-1805-y. [DOI] [PubMed] [Google Scholar]
- Cope ZA, Halberstadt AL, van Enkhuizen J, Flynn AD, Breier M, Swerdlow NR, Geyer MA, Young JW. Premature responses in the five-choice serial reaction time task reflect rodents' temporal strategies: evidence from no-light and pharmacological challenges. Psychopharmacology. 2016;233:3513–3525. doi: 10.1007/s00213-016-4389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Fletcher PC. From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol. 2007;21:238–252. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cesura AM. Antipsychotics and the dopamine–serotonin connection. In: Celanire S, Poli S, editors. Small molecule therapeutics for schizophrenia. Cham: Springer International Publishing; 2015. pp. 1–49. [Google Scholar]
- Feja M, Hayn L, Koch M. Nucleus accumbens core and shell inactivation differentially affects impulsive behaviours in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;54:31–42. doi: 10.1016/j.pnpbp.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology. 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–1215. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33:1100–1119. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GraysonB, BarnesSA, MarkouA, PiercyC, PoddaG, NeillJC (2015) Postnatal phencyclidine (PCP) as a neurodevelopmental animal model of schizophrenia pathophysiology and aymptomatology: a review. Curr Top Behav Neurosci [DOI] [PubMed]
- Gross G, Drescher K (2012) The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Handb Exp Pharmacol:167–210 [DOI] [PubMed]
- Gyertyán I, Sághy K, Laszy J, Elekes O, Kedves R, Gémesi LI, Pásztor G, Zajer-Balázs M, Kapás M, Ágai Csongor E, Domány G, Kiss B, Szombathelyi Z. Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: II. Behavioural characterisation of RG-15. Naunyn Schmiedeberg's Arch Pharmacol. 2008;378:529–539. doi: 10.1007/s00210-008-0311-x. [DOI] [PubMed] [Google Scholar]
- Gyertyán I, Kiss B, Sághy K, Laszy J, Szabó G, Szabados T, Gémesi LI, Pásztor G, Zájer-Balázs M, Kapás M, Csongor EA, Domány G, Tihanyi K, Szombathelyi Z. Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents. Neurochem Int. 2011;59:925–935. doi: 10.1016/j.neuint.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29:97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HM, Tamminga CA. Schizophrenia: treatment targets beyond monoamine systems. Annu Rev Pharmacol Toxicol. 2011;51:189–209. doi: 10.1146/annurev.pharmtox.010909.105851. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kern RS, Green MF, Nuechterlein KH, Deng B-H. NIMH-MATRICS survey on assessment of neurocognition in schizophrenia. Schizophr Res. 2004;72:11–19. doi: 10.1016/j.schres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kiss B, Laszlovszky I, Horváth A, Némethy Z, Schmidt E, Bugovics G, Fazekas K, Gyertyán I, Ágai-Csongor E, Domány G, Szombathelyi Z. Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: I. Neurochemical characterisation of RG-15. Naunyn Schmiedeberg's Arch Pharmacol. 2008;378:515–528. doi: 10.1007/s00210-008-0308-5. [DOI] [PubMed] [Google Scholar]
- Kiss B, Horváth A, Némethy Z, Schmidt É, Laszlovszky I, Bugovics G, Fazekas K, Hornok K, Orosz S, Gyertyán I, Ágai-Csongor É, Domány G, Tihanyi K, Adham N, Szombathelyi Z. Cariprazine (RGH-188), a dopamine D3 receptor-preferring, D3/D2 dopamine receptor antagonist–partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther. 2010;333:328–340. doi: 10.1124/jpet.109.160432. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. J Nerv Ment Dis. 1921;54:384. doi: 10.1097/00005053-192110000-00104. [DOI] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:12107–12112. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Kozak R, Sarter M, Young JW, Robbins TW. CNTRICS final animal model task selection: control of attention. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Frank MJ. An integrative perspective on the role of dopamine in schizophrenia. Biol Psychiatry. 2017;81:52–66. doi: 10.1016/j.biopsych.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell JH, Treue S. Feature-based attention in visual cortex. Trends Neurosci. 2006;29:317–322. doi: 10.1016/j.tins.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Moore NA, Tye NC, Axton MS, Risius FC. The behavioral pharmacology of olanzapine, a novel “atypical” antipsychotic agent. J Pharmacol Exp Ther. 1992;262:545–551. [PubMed] [Google Scholar]
- Nagai T, Murai R, Matsui K, Kamei H, Noda Y, Furukawa H, Nabeshima T. Aripiprazole ameliorates phencyclidine-induced impairment of recognition memory through dopamine D1 and serotonin 5-HT1A receptors. Psychopharmacology. 2009;202:315–328. doi: 10.1007/s00213-008-1240-6. [DOI] [PubMed] [Google Scholar]
- Nair VD, Savelli JE, Mishra RK. Modulation of dopamine D2 receptor expression by an NMDA receptor antagonist in rat brain. J Mol Neurosci. 1998;11:121–126. doi: 10.1385/JMN:11:2:121. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. 2014. [DOI] [PubMed] [Google Scholar]
- Neill JC, Grayson B, Kiss B, Gyertyán I, Ferguson P, Adham N. Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol. 2016;26:3–14. doi: 10.1016/j.euroneuro.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/S0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Nikiforuk A, Popik P. The effects of acute and repeated administration of ketamine on attentional performance in the five-choice serial reaction time task in rats. Eur Neuropsychopharmacol. 2014;24:1381–1393. doi: 10.1016/j.euroneuro.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, McKenna PJ, Bullmore ET, Fletcher PC. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvreau T, Tagliabue E, Usun Y, Eybrard S, Meyer F, Louilot A. Neonatal prefrontal inactivation results in reversed dopaminergic responses in the shell subregion of the nucleus accumbens to NMDA antagonists. ACS Chem Neurosci. 2016;7:964–971. doi: 10.1021/acschemneuro.6b00087. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Winchester C, Egerton A, Cochran SM, Morris BJ. Modelling prefrontal cortex deficits in schizophrenia: implications for treatment. Br J Pharmacol. 2008;153:S465–S470. doi: 10.1038/bjp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel WJ, Mehta MA, Unema PJ. Human cognition assessment in drug research. Curr Pharm Des. 2006;12:2525–2539. doi: 10.2174/138161206777698882. [DOI] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Schmidt AW, Lebel LA, Howard HR, Jr, Zorn SH. Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol. 2001;425:197–201. doi: 10.1016/S0014-2999(01)01188-8. [DOI] [PubMed] [Google Scholar]
- Sharma T, Mockler D. The cognitive efficacy of atypical antipsychotics in schizophrenia. J Clin Psychopharmacol. 1998;18:12S–19S. doi: 10.1097/00004714-199804001-00004. [DOI] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, Kandel ER, Balsam PD. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry. 2011;69:928–935. doi: 10.1016/j.biopsych.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifstein M, van de Giessen E, Van Snellenberg J, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- St. Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011;31:9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Kupchik M, Roitman S, Schwartz S, Gonen N, Mester R, Weizman A, Spivak B. Comparison between risperidone, olanzapine, and clozapine in the management of chronic schizophrenia: a naturalistic prospective 12-week observational study. Hum Psychopharmacol. 2006;21:235–243. doi: 10.1002/hup.764. [DOI] [PubMed] [Google Scholar]
- Tauscher J, Hussain T, Agid O, Verhoeff NPL, Wilson AA, Houle S, Remington G, Zipursky RB, Kapur S. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161:1620–1625. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- Thomson DM, McVie A, Morris BJ, Pratt JA. Dissociation of acute and chronic intermittent phencyclidine-induced performance deficits in the 5-choice serial reaction time task: influence of clozapine. Psychopharmacology. 2011;213:681–695. doi: 10.1007/s00213-010-2020-7. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin R, Miller AL. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res. 2002;53:239–248. doi: 10.1016/S0920-9964(01)00268-7. [DOI] [PubMed] [Google Scholar]
- Watson DJ, King MV, Gyertyán I, Kiss B, Adham N, Fone KC. The dopamine D(3)-preferring D(2)/D(3) dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur Neuropsychopharmacol. 2016;26:208–224. doi: 10.1016/j.euroneuro.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Fung SJ, Catts VS, Schofield PR, Allen KM, Moore LT, Newell KA, Pellen D, Huang XF, Catts SV, Weickert TW. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol Psychiatry. 2013;18:1185–1192. doi: 10.1038/mp.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Koenig JI. Social interaction and social withdrawal in rodents as readouts for investigating the negative symptoms of schizophrenia. Eur Neuropsychopharmacol. 2014;24:759–773. doi: 10.1016/j.euroneuro.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Developing treatments for cognitive deficits in schizophrenia: the challenge of translation. J Psychopharmacol. 2015;29:178–196. doi: 10.1177/0269881114555252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimnisky R, Chang G, Gyertyán I, Kiss B, Adham N, Schmauss C. Cariprazine, a dopamine D3-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology. 2013;226:91–100. doi: 10.1007/s00213-012-2896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]