Abstract
Erythroid Krüppel-like Factor (EKLF/KLF1) is an erythroid-enriched transcription factor that plays a global role in all aspects of erythropoiesis, including cell cycle control and differentiation. We queried whether its mutation might play a role in red cell malignancies by genomic sequencing of the KLF1 transcription unit in cell lines, erythroid neoplasms, dysplastic disorders, and leukemia. In addition, we queried published databases from a number of varied sources. In all cases we only found changes in commonly notated SNPs. Our results suggest that if there are mutations in KLF1 associated with erythroid malignancies, they are exceedingly rare.
Introduction
Erythroid Krüppel-like Factor (EKLF/KLF1) is a red cell-enriched, zinc finger DNA binding protein that interacts with its cognate 5′CCMCRCCCN3′ element at target promoters and enhancers1. Its roles in ß-like globin gene regulation during terminal erythroid differentiation have been well-established using genetic, biochemical, and molecular approaches2,3. Specific functional properties and expression characteristics of EKLF, along with recognition of its surprisingly broad role prior to and during red cell differentiation (reviewed in4–8), provide the conceptual basis for the present study.
First, single amino acids and their modifications are critically important for EKLF protein-protein interactions and its function as an activator or repressor9–12, raising the possibility that mutations at these sites or within their consensus sequences could have dramatic functional effects on gene expression control.
Second, subtle altering of EKLF cellular levels can change the erythroid cell cycle status from proliferation to differentiation, an effect mediated, at least in part, by its direct activation of the p21, p18, p27, and E2f2 genes13–17. Induction of p21 is reminiscent of a similar up-regulation that has been observed with KLF418 and with KLF619, known tumor suppressors that function in a p53-independent manner and that are frequently inactivated or downregulated in human cancer20.
Third, EKLF mRNA is highly restricted in its expression pattern during development to erythropoietic organs such as the yolk sac, fetal liver, adult bone marrow, and red pulp of the spleen1,21. Although most abundant in the erythroid cell, EKLF is also highly expressed in the megakaryocyte/erythroid progenitor (MEP)22. Its level is downregulated as MEPs differentiate towards the megakaryocytic lineage yet remains high in the erythroid lineage22. By using both gain- and loss-of-function approaches, we12,22 and others23–25 have found that the expression levels of EKLF impacts the bipotential lineage decisions that are made by the MEP; specifically, EKLF inhibits the formation of megakaryocyte colony and cell numbers while at the same time stimulating erythroid differentiation.
Fourth, there are now links between KLF1 mutation and altered mammalian hematology26–28. For example, the semi-dominant mouse mutation Nan (neonatal anemia), which presents with hereditary spherocytosis, was mapped to a single amino acid change (E339D) within the second zinc finger of EKLF29–31. The mutation alters the DNA binding specificity of EKLF such that it no longer binds promoters of a subset of its DNA targets29. In addition, recognition of a novel target DNA sequence by Nan-EKLF leads to ectopic expression of genes not normally expressed in the red cell, yielding a neomorphic phenotype with cellular and systemic consequences32,33.
Excitingly, this murine mutation has converged with human disease, particularly a subtype of congenital dyserythropoietic anemia (CDA)34–39, where the same amino acid is altered (albeit to another charged residue, lysine) in human EKLF/KLF1. These patients are severely anemic with highly elevated HbF and reticulocyte levels, membrane abnormalities, severe hemolytic anemia, erythroid hyperplasia with dyserythropoiesis, splenomegaly, and growth delay36. KLF1/E325K is recognized as a characteristic feature of CDA type IV40.
Additionally, the regulation of some genes are uniquely sensitive to haploinsufficient levels of KLF1 (Lu, Bcl11a, HbA2), leading to altered genetic expression patterns and hematologic parameters in humans, of which hereditary persistence of fetal hemoglobin (HPFH) is particularly relevant to clinical outcome4,5,26,41–43.
Collectively these functional properties suggest that genetic mutation or altered levels of KLF1 may also be a causative factor for a specific subset of hematopoietic disease. We focused our attention on two types of disorders. Myeloproliferative neoplasms (MPN) are chronic hematological malignancies that yield an excessive proliferation of blood cells with normal differentiation44,45. On the other hand, unrestricted proliferation and impaired differentiation are characteristic of acute myeloid leukemia (AML)46,47. Our test hypothesis is that dysregulation of KLF1 function may contribute or lead to either of these human malignancies.
Results
Chromosomal associations
19p13.13, the chromosomal locus of KLF1, has been associated with variation in blood cell traits in meta-analysis studies48. Recent studies provide an extensive catalogue of SNP variants of consequence for red cell parameters49. Perusal of the data enable us to extract the ones most relevant to KLF1 as summarized in Table 1, suggesting these KLF1 gene variants are significantly associated with altered MCH, MCV, and MCHC red cell indices, and RBC and RET numbers. In combination with studies summarized in the Introduction, we felt justified to address whether mutagenic changes in KLF1 might also be associated with aberrant or malignant red cell parameters.
Table 1.
KLF1 loci associated with RBC traits.
| Associated Blood Index | rsID | BP (GRCh37) | REF/ALT | MAF (%) | Univariable Analysis | |||
|---|---|---|---|---|---|---|---|---|
| Estimate of Additive Allelic Effect | Standard Error of Estimator | −log10 P | Unadjusted R2 | |||||
| RET# | rs3817621 | 12998205 | G/C | 23.9 | −0.038 | 0.0042 | 18.6 | 0.000527 |
| RET% | rs3817621 | 12998205 | G/C | 23.9 | −0.046 | 0.0042 | 27 | 0.000776 |
| RBC# | rs56397034 | 13000550 | G/C | 38.9 | −0.049 | 0.0036 | 41.3 | 0.001166 |
| MCV | rs56397034 | 13000550 | G/C | 38.9 | 0.068 | 0.0036 | 78.5 | 0.002203 |
| MCH | rs56397034 | 13000550 | G/C | 38.9 | 0.071 | 0.0036 | 85.3 | 0.002411 |
| MCHC | rs11085824 | 13001547 | A/G | 37.6 | 0.029 | 0.0035 | 15.8 | 0.000407 |
SNPs (rsID) at or near the KLF1 gene associated with red blood cell indices are tabulated with respect to chromosome 19 location (Ch37), percent minor allele frequency (MAF), and nucleotide change compared to the reference genome (REF/ALT). Univariable analysis indicates the direction and significance of the allelic effect on the index parameter. Data from49.
Genomic analyses of KLF1 in selected populations
The complete human KLF1 transcription unit is only 3.5 kB50, enabling us to interrogate its proximal promoter, 5′ UTR, introns, exons, and 3′ UTR by eight overlapping amplimers. The proximal promoter contains highly conserved transcription factor binding sequences that are critical for its expression in erythroid cells51–55. To begin our evaluation of human KLF1 genomic status, we focused first on sequencing human leukemia cell lines that retain erythroid and/or megakaryocytic features56,57. These lines are derived from CML, AML-M6 or -M7 patients (F36P, HEL, JK1, K562, KMOE2, KU812, LAMA84, OCIM1, TF1), and include those with mixed erythroid/megakaryocytic features (CMK, KG1, Meg01). We conclude from genomic sequence comparison of these lines to the 1000 Genomes project58,59 that the KLF1 genomic changes observed represent known single nucleotide polymorphisms (SNPs), but no novel mutations (Table 2).
Table 2.
SUMMARY of genomic sequence analyses.
| Sample Type | rsID | BP (GRCh38) | number | REF/ALT | MAF (%) | Location | Predicted effect*** |
|---|---|---|---|---|---|---|---|
| cell lines | rs3817621 | 12887391 | 8 | G/C | 32.5 | **promoter (−188) | |
| rs112631212 | 12886115 | 1 | T/G | 1.44 | **M39L-class 1 | likely benign | |
| rs2072597 | 12885926 | 10 | A/G | 44.4 | **S102P-class 1 | likely benign | |
| rs16978757 | 12884608 | 1 | G/A | 5.61 | 3′UTR | ||
| rs16978754 | 12884589 | 1 | T/C | 5.59 | 3′UTR | ||
| MPN | rs115672848 | 12888141 | 1 | C/T | *0.14 | promoter (−938) | |
| rs3817621 | 12887391 | 5 | G/C | 32.5 | **promoter (−188) | ||
| rs79334031 | 12887288 | 2 | C/T | 1.58 | **promoter (−85) | ||
| rs112631212 | 12886115 | 7 | T/G | 1.44 | **M39L-class 1 | likely benign | |
| rs2072597 | 12885926 | 12 | A/G | 44.4 | **S102P-class 1 | likely benign | |
| rs2072596 | 12885686 | 3 | A/G | 4.95 | **F182L-class 1 | likely benign**** | |
| rs16978757 | 12884608 | 1 | G/A | 5.61 | 3′UTR | ||
| rs16978754 | 12884589 | 1 | T/C | 5.59 | 3′UTR | ||
| MDS | rs201870270 | 12887780 | 1 | delA | *0.8 | promoter (−577) | |
| rs3817621 | 12887391 | 15 | G/C | 32.5 | **promoter (−188) | ||
| rs79334031 | 12887288 | 4 | C/T | 1.58 | **promoter (−85) | ||
| rs112631212 | 12886115 | 1 | T/G | 1.44 | **M39L-class 1 | likely benign | |
| rs2072597 | 12885926 | 21 | A/G | 44.4 | **S102P-class 1 | likely benign | |
| rs182276666 | 12885919 | 1 | G/A | *0.08 | **A104V-class 1 | likely benign | |
| rs2072596 | 12885686 | 2 | A/G | 4.95 | **F182L-class 1 | likely benign**** | |
| rs16978754 | 12884589 | 2 | T/C | 5.59 | 3′UTR | ||
| AMKL | rs3817621 | 12887391 | 1 | G/C | 32.5 | **promoter | |
| rs2072597 | 12885926 | 5 | A/G | 44.4 | **S102P-class 1 | likely benign |
Tabulation of all KLF1 SNPs (rsID) found in the present study, grouped together based on cell types as described in the Results. Included are the number of examples of each change, along with chromosome 19 location (Ch38), nucleotide change compared to the reference genome (REF/ALT), and percent minor allele frequency (MAF). Location with respect to the KLF1 transcription unit (promoter, coding region, 3′UTR) are as indicated, along with the amino acid change. “Class 1” refers to the tabulation in26, indicating that any amino acid change is likely benign, a conclusion supported by the “Predicted effect” based on other criteria66,67. KLF1 transcription initiation is at BP = 12887203 in Ch38 (based on50,54).
*rare (<1%); **noted previously in reference26 as implicated in hypomorphic KLF1 expression; ***based on references66,67; ****PolyPhen suggests ‘possibly damaging’ due to cross-KLF family conservation of F (phenylalanine) at this position, possibly by decreasing its stability98.
We then directly assessed primary human DNA samples from a selected cohort of patients. Given that KLF1 levels may be playing a directive role in erythroid/megakaryocyte bipotential decisions, we focused on myeloproliferative neoplasms (MPNs) as cells whose aberrant properties might result from expression of mutated KLF1. MPNs are a heterogeneous group of clonal hematological malignancies that are characterized by hypercellular bone marrow, panmyelosis, and a gradual evolution to myelofibrosis (MF) and acute leukemia60,61. These disorders are clonal and yield an excessive proliferation of blood cells that exhibit normal differentiation44. We were particularly interested in two subtypes of MPNs: polycythemia vera (PV) and essential thrombocythemia (ET), which represent abnormalities in the proliferation of erythroid and megakaryocytic lineage respectively45. We therefore sequenced genomic DNA from a collection of individuals with PV (eighteen), ET (eleven), and MF (five) samples. No novel mutations were identified, only SNPs (Table 2). Although rs115672848 is a rare variant, we found no evidence for its selective enrichment.
Many KLF1 target genes overlap with the expression signature identified in the differential analysis of myelodysplastic syndrome (MDS) patients that vary in their response to lenalidomide treatment62. We hypothesized that the mutational status of KLF1 may provide a mechanistic basis to explain the differential expression signature in these patients, and potentially predict whether they will respond to lenalidomide. We analyzed 26 samples each of responders and non-responders. All variants identified in these samples were known SNPs (Table 2), and none partitioned significantly to either of the two differentially responding groups. There were no significant difference in clinical parameters between the most common rs3817621 (p = 0.44) and rs2072597 (p = 0.53) SNPs. In addition, while rs201870270 and rs182276666 are rare variants, they are not selectively enriched.
We next examined ten acute megakaryoblastic leukemia (AMKL) samples63, including four from patients with Down syndrome (DS) and six from those without DS. AMKL is an aggressive form of leukemia where a majority of the expanding cells are abnormal megakaryoblasts64, and whose DNA methylation patterns are distinct between DS and non-DS cells65. Again, all samples contain known KLF1 SNPs (Table 2).
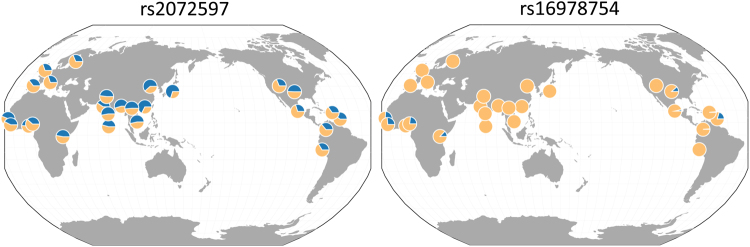
As indicated in Table 2, many of these variants have been noted before26. The ones that result in non-synonymous coding changes are predicted not to affect KLF1 function66,67 (with the possible exception of rs2072596 as highlighted in Table 2). However, it is noteworthy that a geographical analysis68,69 shows that, unlike ones whose variation is common and universal (e.g., rs2072597), some with an overall low frequency (~5%) are nonetheless highly enriched (~20%) prevalently and exclusively in selected genetic or geographic sub-populations while completely absent in all others (e.g., rs16978754) (Fig. 1).
Figure 1.
Geographical distribution68,69 of two KLF1 SNPs from Table 2 as examples of widely dispersed (rs2072597; >40%) or limited (rs16978754; ~5%) MAFs. Blue pie conveys a given MAF percentage out of 100% across the indicated global populations. rs16978754 is common (~20%) only in the Gambian, Sierra Leone, and Nigerian populations on the African continent, and beyond is commonly detected only in the African Caribbean and African American populations; otherwise it is not detectable.
Alternative analyses
Given our hypothesis, we were surprised by the absence of mutations associated with our target samples/sources. To expand our analysis, we also considered an in silico approach and queried published data from whole genome and exome analyses. These global analyses enable tabulation of altered genes to be accumulated in an unbiased manner. Consistent with our own directed sequencing studies, perusal of recent MPN70,71, acute erythroleukemia (250 samples total in two studies72,73), and AML tabulations74, along with those derived from The Cancer Genome Atlas (TCGA) data sets75,76 do not reveal a role for KLF1 in any case in these blood cancers. KLF1 is not one of 142 driver genes identified from an analysis of 1699 pediatric leukemias and tumors77. An shRNA screen of AML cells lines also did not implicate a role for KLF178, and KLF1 does not appear in differential analyses related to predicting therapy resistance in AML79. Examination of COSMIC data (v83)80 indicates that, out of 3478 curated hematopoietic and lymphoid samples, only a single coding sequence variant was observed (F27V) in a CLL patient81, one predicted to be benign66,67.
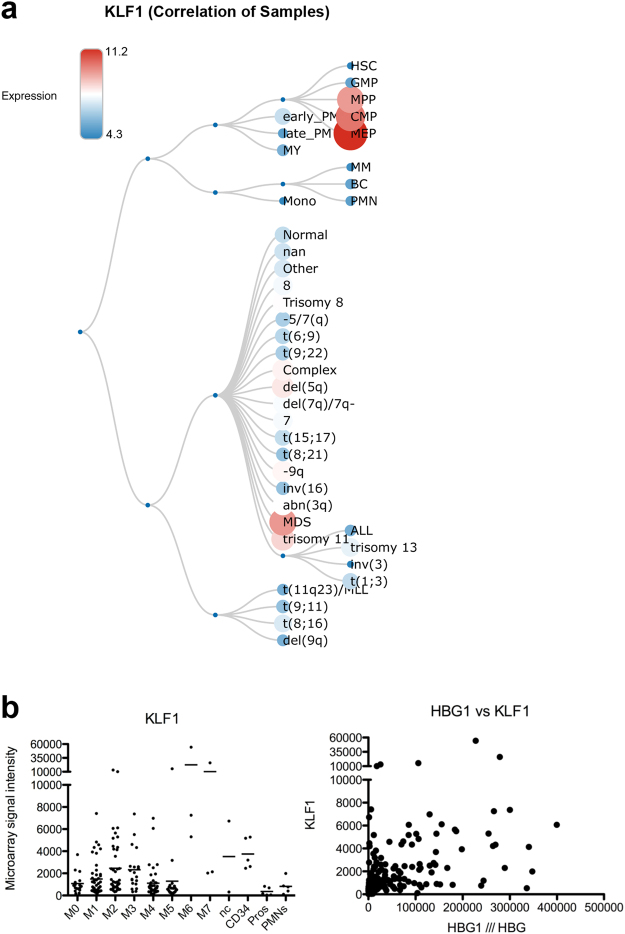
We finally considered whether variation in levels of KLF1, rather than a mutated form, might be correlated with a particular blood cancer. This hypothesis arises from two bits of data. One, it is known that some genes are uniquely sensitive to haploinsufficient levels KLF1 (reviewed in4,26). Two, KLF1 levels vary considerably across various malignancies as judged by tabulation of RNA sequence datasets82 (Fig. 2a). To further address this idea we queried a series of 200 AML samples to see whether KLF1 levels correlate with a particular AML subtype83. We were surprised by two observations (Fig. 2b). First, there is a wide range in KLF1 expression within the AML M6 and M7 categories that generally would be anticipated to express elevated levels of KLF1. Second, other AML subtypes, including M0-M5, contain samples that express similar high levels of KLF1. This was not expected as KLF1 is most highly enriched in erythroid, not myeloid, cells. Clearly, normal cellular and genetic control mechanisms are altered in many of these patient samples, as KLF1 levels do not even correlate with HBG expression (Fig. 2b), a target that is normally repressed by KLF1 and whose levels are indirectly proportional to that of KLF142. We conclude there is no apparent correlation between KLF1 levels and malignancy status across a range of AML malignancy subtypes.
Figure 2.
Variation of KLF1 levels across aberrant erythroid sources. (a) BloodSpot (82; http/servers.binf.ku.dk/bloodspot/?gene=KLF1&dataset=MERGED_AML) analysis of relative KLF1 levels in samples across dysplastic and leukemic sources, as well as that seen during normal hematopoiesis (concordant with murine studies22). (b) Left - Analysis of KLF1 expression in AML samples derived from a range of subtypes (M0-M7), also compared to CD34, promyelocytes (pros) and polymorphonuclear leukocytes (PMNs). nc = not categorized. Right – Graph showing lack of correlation between KLF1 and HBG levels in the same set of AML samples. Note that HBG expression within the five highest-expressing KLF1 samples vary tremendously.
Discussion
Given the molecular and biochemical properties of KLF1, along with its regulation of selected downstream target gene expression, it remains surprising that our study did not uncover any mutations associated with erythroid dyplasia or malignancy. A simple explanation is that an insufficient number of samples have been analyzed. Although this possibility cannot be excluded, we directly analyzed nearly 150 samples, and in addition perused numerous databases without success. In any case, other explanations come to mind.
For instance, we have not considered whether the epigenetic status of the KLF1 gene may be playing a role in its regulation that may be of consequence to malignancy. For example, 5mC modification at the KLF1 locus inversely correlates with its expression when compared across a number of cell types84,85. In addition, the level and extent of 5hmC modification at the KLF1 locus is also inversely correlated with its expression as the CD34+ cell differentiates to the mature erythroid cell86. Intriguingly, the KLF1 gene exhibits a synergistic “type III/cluster 3” pattern of expression control such that KLF1 transcript levels are dramatically increased in Tet2/Dnmt3a double knockout cells87. Of relevance to the present discussion, increased KLF1 levels in a subset of AML is only seen in samples from patients with mutations in both genes87. The DNA modification locales overlap regions demonstrated to be important for KLF1 expression control7,54,55. These studies demonstrate that the DNA modification status of controlling regions in the KLF1 gene is important for establishing its optimal level of expression. However, the causative versus correlative nature of KLF1 gene epigenetic modification and aberrant erythropoiesis will remain challenging to tease out.
One final explanation for our findings is that KLF1 functions during late stages of erythropoiesis, which may circumscribe any search for an effect at early stages8. In other words, it is known that terminal maturation of erythroid cells, particularly at the transition from orthochromatic to reticulocyte stage, is completely dependent on KLF117. Part of the explanation for this requirement is KLF1 regulation of cell cycle inhibitors such as p18 and p27 specifically at this late stage. It is notable that these genes are not dependent on KLF1 at an earlier stage, for example in proliferating erythroblasts; indeed, there are no cell cycle differences when comparing such cells from WT vs KLF1-null17. Given that the blood cell disorders that we tested exhibit unrestricted proliferation, it remains possible that mutated KLF1 would not have a causative effect on cell cycle in this context in any case.
In spite of these considerations, we are still left with the example of the monoallelic mutation in KLF1 that leads to CDA type IV, with its dominant effect on erythroid cell properties, including proliferation34,36,39. Nonetheless, our results suggest that if there are any KLF1 mutants implicated in erythroid malignancy, they are quite rare.
Methods
K562 cells were from our original lab stock88; all other cell lines were purchased from either the ATCC or DSMZ. K562 cells merit additional discussion. There are inconsistencies in the literature as to whether KLF1 is expressed in this cell line, with some studies indicating low to nil50,88,89, and others showing detectable levels90. There is a large body of work on its use as a cotransfection reporter line whose utility is dependent on lack of KLF1 expression (studies that began with88). This line was established decades ago91, and early on was noted to exhibit variability92,93. We have noted major differences in transfection efficiency upon comparing our lab K562 stock with the ATCC K562 line (unpublished observations) although we did not find any KLF1 genomic sequence differences. We suggest labs are working with dissimilar isolates, differing in levels of GATA1 and/or KLF194.
Patient samples were procured after informed consent and IRB approval within the individual institutions (Mount Sinai School of Medicine, Columbia University Medical Center, New York Blood Center, Northwestern University School of Medicine). All methods were performed in accordance with the relevant institutional guidelines and regulations. Mononuclear cells from MPN patients were provided by the Myeloproliferative Disorders Research Consortium Tissue Bank Core C.
Genomic DNA was isolated from all samples using a Qiagen DNeasy Blood & Tissue Kit. PCR primers spanning the complete KLF1 transcription unit were used to amplify eight overlapping regions across the locus (Supplemental Table 1). These were individually sequenced in both directions (with their corresponding PCR forward and reverse primer) using a 96-well format (Macrogen USA). With regards to comparison of clinical parameters in MDS samples, for continuous variables satisfying the normality assumption, a two-tailed unpaired t test was used.
Discovery of any nucleotide change(s) followed alignment to the GRCh38 reference sequence using Vector NTI ContigExpress software (ThermoFisher Scientific). SNPs and any associated parameters were identified from the 1000 Genomes databases58,59,68. Other datasets queried included BloodSpot82, cBioPortal95, GGV69, UK10K96,97, COSMIC80, and NCBI public resources.
Electronic supplementary material
Acknowledgements
This work was supported by PHS grants R21 CA133608 and R01 DK46865 to JJB, and by a Cooley’s Anemia Fellowship to MNG. The Myeloproliferative Disorders Research Consortium Tissue Bank Core C was supported by grant P01 CA108671. We thank Dr John Martignetti for discussion and comments on the manuscript, and Dr Tim Ley for discussion.
Author Contributions
M.N.G. performed experiments, M.N.G. and J.J.B. designed the study, analyzed data, and wrote the manuscript, R.W., A.M.A., J.D.C., R.H., and A.R. provided patient samples and discussion. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24962-3.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller IJ, Bieker JJ. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol. Cell Biol. 1993;13:2776–2786. doi: 10.1128/MCB.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins A. Erythroid Kruppel like factor: from fishing expedition to gourmet meal [In Process Citation] Int J Biochem Cell Biol. 1999;31:1175–92. doi: 10.1016/S1357-2725(99)00083-7. [DOI] [PubMed] [Google Scholar]
- 3.Bieker, J. J. EKLF and the development of the erythroid lineage. in Transcription Factors: Normal and Malignant Development of Blood Cells (eds. Ravid, K. & Licht, J.D.) 71–84 (Wiley-Liss, New York, 2000).
- 4.Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118:2044–54. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg J, Patrinos GP, Felice AE, Philipsen S. Erythroid phenotypes associated with KLF1 mutations. Haematologica. 2011;96:635–8. doi: 10.3324/haematol.2011.043265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tallack MR, Perkins AC. KLF1 directly coordinates almost all aspects of terminal erythroid differentiation. IUBMB Life. 2010;62:886–90. doi: 10.1002/iub.404. [DOI] [PubMed] [Google Scholar]
- 7.Yien YY, Bieker JJ. EKLF/KLF1, a tissue-restricted integrator of transcriptional control, chromatin remodeling, and lineage determination. Mol Cell Biol. 2013;33:4–13. doi: 10.1128/MCB.01058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gnanapragasam MN, Bieker JJ. Orchestration of late events in erythropoiesis by KLF1/EKLF. Curr Opin Hematol. 2017;24:183–190. doi: 10.1097/MOH.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Kadam S, Emerson BM, Bieker JJ. Site-specific acetylation by p300 or CREB binding protein regulates erythroid Kruppel-like factor transcriptional activity via its interaction with the SWI-SNF complex. Mol Cell Biol. 2001;21:2413–22. doi: 10.1128/MCB.21.7.2413-2422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sengupta T, Chen K, Milot E, Bieker JJ. Acetylation of EKLF is essential for epigenetic modification and transcriptional activation of the beta-globin locus. Mol Cell Biol. 2008;28:6160–70. doi: 10.1128/MCB.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Bieker JJ. Stage-specific repression by the EKLF transcriptional activator. Mol Cell Biol. 2004;24:10416–24. doi: 10.1128/MCB.24.23.10416-10424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siatecka M, Xue L, Bieker JJ. Sumoylation of EKLF Promotes Transcriptional Repression and Is Involved in Inhibition of Megakaryopoiesis. Mol Cell Biol. 2007;27:8547–8560. doi: 10.1128/MCB.00589-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tallack MR, Keys JR, Perkins AC. Erythroid Kruppel-like factor regulates the G1 cyclin dependent kinase inhibitor p18INK4c. J Mol Biol. 2007;369:313–21. doi: 10.1016/j.jmb.2007.02.109. [DOI] [PubMed] [Google Scholar]
- 14.Pilon AM, et al. Failure of terminal erythroid differentiation in EKLF-deficient mice is associated with cell cycle perturbation and reduced expression of E2F2. Mol Cell Biol. 2008;28:7394–401. doi: 10.1128/MCB.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tallack MR, Keys JR, Humbert PO, Perkins AC. EKLF/KLF1 controls cell cycle entry via direct regulation of E2f2. J Biol Chem. 2009;284:20966–74. doi: 10.1074/jbc.M109.006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siatecka M, Lohmann F, Bao S, Bieker JJ. EKLF directly activates the p21WAF1/CIP1 gene by proximal promoter and novel intronic regulatory regions during erythroid differentiation. Mol Cell Biol. 2010;30:2811–2822. doi: 10.1128/MCB.01016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnanapragasam MN, et al. EKLF/KLF1-regulated cell cycle exit is essential for erythroblast enucleation. Blood. 2016;128:1631–41. doi: 10.1182/blood-2016-03-706671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowland BD. & Peeper, D.S. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]
- 19.Narla G, et al. A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Cancer Res. 2005;65:1213–22. doi: 10.1158/0008-5472.CAN-04-4249. [DOI] [PubMed] [Google Scholar]
- 20.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southwood CM, Downs KM, Bieker JJ. Erythroid Kruppel-like Factor (EKLF) exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Devel. Dyn. 1996;206:248–259. doi: 10.1002/(SICI)1097-0177(199607)206:3<248::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Frontelo P, et al. Novel role for EKLF in megakaryocyte lineage commitment. Blood. 2007;110:3871–3880. doi: 10.1182/blood-2007-03-082065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouilloux F, et al. EKLF restricts megakaryocytic differentiation at the benefit of erythrocytic differentiation. Blood. 2008;112:576–84. doi: 10.1182/blood-2007-07-098996. [DOI] [PubMed] [Google Scholar]
- 24.Tallack MR, Perkins AC. Megakaryocyte-erythroid lineage promiscuity in EKLF null mouse blood. Haematologica. 2010;95:144–7. doi: 10.3324/haematol.2009.010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isern J, Fraser ST, He Z, Zhang H, Baron MH. Dose-dependent regulation of primitive erythroid maturation and identity by the transcription factor Eklf. Blood. 2010;116:3972–80. doi: 10.1182/blood-2010-04-281196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins A, et al. Kruppeling erythropoiesis: an unexpected broad spectrum of human red blood cell disorders due to KLF1 variants. Blood. 2016;127:1856–62. doi: 10.1182/blood-2016-01-694331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waye JS, Eng B. Kruppel-like factor 1: hematologic phenotypes associated with KLF1 gene mutations. Int J Lab Hematol. 2015;37(Suppl 1):78–84. doi: 10.1111/ijlh.12356. [DOI] [PubMed] [Google Scholar]
- 28.Keller J, et al. Novel mutations in KLF1 encoding the In(Lu) phenotype reflect a diversity of clinical presentations. Transfusion. 2018;58:196–199. doi: 10.1111/trf.14378. [DOI] [PubMed] [Google Scholar]
- 29.Siatecka M, et al. Severe anemia in the Nan mutant mouse caused by sequence-selective disruption of erythroid Kruppel-like factor. Proc Natl Acad Sci USA. 2010;107:15151–6. doi: 10.1073/pnas.1004996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White RA, et al. Hematologic characterization and chromosomal localization of the novel dominantly inherited mouse hemolytic anemia, neonatal anemia (Nan) Blood Cells Mol Dis. 2009;43:141–8. doi: 10.1016/j.bcmd.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Heruth DP, et al. Mutation in erythroid specific transcription factor KLF1 causes Hereditary Spherocytosis in the Nan hemolytic anemia mouse model. Genomics. 2010;96:303–7. doi: 10.1016/j.ygeno.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillinder KR, et al. Promiscuous DNA-binding of a mutant zinc finger protein corrupts the transcriptome and diminishes cell viability. Nucleic Acids Res. 2017;45:1130–1143. doi: 10.1093/nar/gkw1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planutis A, et al. Neomorphic effects of the neonatal anemia (Nan-Eklf) mutation contribute to deficits throughout development. Development. 2017;144:430–440. doi: 10.1242/dev.145656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnaud L, et al. A dominant mutation in the gene encoding the erythroid transcription factor KLF1 causes a congenital dyserythropoietic anemia. Am J Hum Genet. 2010;87:721–7. doi: 10.1016/j.ajhg.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singleton BK, et al. Mutations in the second zinc finger of human EKLF reduce promoter affinity but give rise to benign and disease phenotypes. Blood. 2011;118:3137–45. doi: 10.1182/blood-2011-04-349985. [DOI] [PubMed] [Google Scholar]
- 36.Jaffray JA, et al. Erythroid transcription factor EKLF/KLF1 mutation causing congenital dyserythropoietic anemia type IV in a patient of Taiwanese origin: Review of all reported cases and development of a clinical diagnostic paradigm. Blood Cells Mol Dis. 2013;51:71–5. doi: 10.1016/j.bcmd.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de-la-Iglesia-Inigo S, et al. A case of congenital dyserythropoietic anemia type IV. Clin Case Rep. 2017;5:248–252. doi: 10.1002/ccr3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortolano, R., Forouhar, M., Warwick, A. & Harper, D. A Case of Congenital Dyserythropoeitic Anemia Type IV Caused by E325K Mutation in Erythroid Transcription Factor KLF1. J Pediatr Hematol Oncol (2017). [DOI] [PubMed]
- 39.Ravindranath, Y. et al. KLF1 E325K-associated Congenital Dyserythropoietic Anemia Type IV: Insights Into the Variable Clinical Severity. J Pediatr Hematol Oncol (2017). [DOI] [PMC free article] [PubMed]
- 40.Iolascon A, Esposito MR, Russo R. Clinical aspects and pathogenesis of congenital dyserythropoietic anemias: from morphology to molecular approach. Haematologica. 2012;97:1786–94. doi: 10.3324/haematol.2012.072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singleton BK, Burton NM, Green C, Brady RL, Anstee DJ. Mutations in EKLF/KLF1 form the molecular basis of the rare blood group In(Lu) phenotype. Blood. 2008;112:2081–8. doi: 10.1182/blood-2008-03-145672. [DOI] [PubMed] [Google Scholar]
- 42.Bieker JJ. Putting a finger on the switch. Nat Genet. 2010;42:733–4. doi: 10.1038/ng0910-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu, D. et al. Erythroid Kruppel-like factor mutations are relatively more common in a thalassemia endemic region and ameliorate the clinical and hematological severity of beta-thalassemia. Blood (2014). [DOI] [PMC free article] [PubMed]
- 44.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–8. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;118:1723–35. doi: 10.1182/blood-2011-02-292102. [DOI] [PubMed] [Google Scholar]
- 46.Rosenbauer F, Koschmieder S, Steidl U, Tenen DG. Effect of transcription-factor concentrations on leukemic stem cells. Blood. 2005;106:1519–24. doi: 10.1182/blood-2005-02-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003;3:89–101. doi: 10.1038/nrc989. [DOI] [PubMed] [Google Scholar]
- 48.Ganesh SK, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet. 2009;41:1191–8. doi: 10.1038/ng.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Astle WJ, et al. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell. 2016;167:1415–1429 e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bieker JJ. Isolation, genomic structure, and expression of human Erythroid Kruppel-like Factor (EKLF) DNA and Cell Biol. 1996;15:347–352. doi: 10.1089/dna.1996.15.347. [DOI] [PubMed] [Google Scholar]
- 51.Crossley M, Tsang AP, Bieker JJ, Orkin SH. Regulation of the erythroid Kruppel-like factor (EKLF) gene promoter by the erythroid transcription factor GATA-1. J. Biol. Chem. 1994;269:15440–15444. [PubMed] [Google Scholar]
- 52.Chen X, Reitman M, Bieker JJ. Chromatin structure and transcriptional control elements of the erythroid Kruppel-like factor (EKLF) gene. J Biol Chem. 1998;273:25031–40. doi: 10.1074/jbc.273.39.25031. [DOI] [PubMed] [Google Scholar]
- 53.Xue L, Chen X, Chang Y, Bieker JJ. Regulatory elements of the EKLF gene that direct erythroid cell-specific expression during mammalian development. Blood. 2004;103:4078–83. doi: 10.1182/blood-2003-09-3231. [DOI] [PubMed] [Google Scholar]
- 54.Lohmann F, Bieker JJ. Activation of Eklf expression during hematopoiesis by Gata2 and Smad5 prior to erythroid commitment. Development. 2008;135:2071–82. doi: 10.1242/dev.018200. [DOI] [PubMed] [Google Scholar]
- 55.Lohmann F, et al. TheDEK Oncoprotein Is a Critical Component of the EKLF/KLF1 Enhancer in Erythroid Cells. Mol Cell Biol. 2015;35:3726–38. doi: 10.1128/MCB.00382-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stamatoyannopoulos, G. & Grosveld, F. Hemoglobin Switching. in The Molecular Bases of Blood Diseases (eds Stamatoyannopoulos, G., Majerus, P. W., Perlmutter, R. M. & Varmus, H.) 135–182 (W. B. Saunders Co., Philadelphia, 2001).
- 57.Drexler HG, Matsuo Y, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for the study of erythroleukemia. Leuk Res. 2004;28:1243–51. doi: 10.1016/j.leukres.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Abecasis GR, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sudmant PH, et al. An integrated map of structural variation in 2,504 human genomes. Nature. 2015;526:75–81. doi: 10.1038/nature15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111:4841–51. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 61.Nangalia J, Green AR. Myeloproliferative neoplasms: from origins to outcomes. Blood. 2017;130:2475–2483. doi: 10.1182/blood-2017-06-782037. [DOI] [PubMed] [Google Scholar]
- 62.Ebert BL, et al. An erythroid differentiation signature predicts response to lenalidomide in myelodysplastic syndrome. PLoS Med. 2008;5:e35. doi: 10.1371/journal.pmed.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wechsler J, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32:148–52. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 64.Wickrema A, Crispino JD. Erythroid and megakaryocytic transformation. Oncogene. 2007;26:6803–15. doi: 10.1038/sj.onc.1210763. [DOI] [PubMed] [Google Scholar]
- 65.Malinge S, et al. Development of acute megakaryoblastic leukemia in Down syndrome is associated with sequential epigenetic changes. Blood. 2013;122:e33–43. doi: 10.1182/blood-2013-05-503011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–81. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 67.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Auton A, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcus JH, Novembre J. Visualizing the geography of genetic variants. Bioinformatics. 2017;33:594–595. doi: 10.1093/bioinformatics/btw643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schischlik F, Kralovics R. Mutations in myeloproliferative neoplasms - their significance and clinical use. Expert Rev Hematol. 2017;10:961–973. doi: 10.1080/17474086.2017.1380515. [DOI] [PubMed] [Google Scholar]
- 71.Vainchenker W, Kralovics R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood. 2017;129:667–679. doi: 10.1182/blood-2016-10-695940. [DOI] [PubMed] [Google Scholar]
- 72.Ping N, et al. Exome sequencing identifies highly recurrent somatic GATA2 and CEBPA mutations in acute erythroid leukemia. Leukemia. 2017;31:195–202. doi: 10.1038/leu.2016.162. [DOI] [PubMed] [Google Scholar]
- 73.Grossmann V, et al. Acute erythroid leukemia (AEL) can be separated into distinct prognostic subsets based on cytogenetic and molecular genetic characteristics. Leukemia. 2013;27:1940–3. doi: 10.1038/leu.2013.144. [DOI] [PubMed] [Google Scholar]
- 74.Bodini M, et al. The hidden genomic landscape of acute myeloid leukemia: subclonal structure revealed by undetected mutations. Blood. 2015;125:600–5. doi: 10.1182/blood-2014-05-576157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kandoth C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma X, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature. 2018;555:371–376. doi: 10.1038/nature25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H, et al. The EMT regulator ZEB2 is a novel dependency of human and murine acute myeloid leukemia. Blood. 2017;129:497–508. doi: 10.1182/blood-2016-05-714493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ng SW, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–437. doi: 10.1038/nature20598. [DOI] [PubMed] [Google Scholar]
- 80.Forbes SA, et al. COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res. 2017;45:D777–D783. doi: 10.1093/nar/gkw1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Landau DA, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–26. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bagger FO, et al. BloodSpot: a database of gene expression profiles and transcriptional programs for healthy and malignant haematopoiesis. Nucleic Acids Res. 2016;44:D917–24. doi: 10.1093/nar/gkv1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ley TJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taskesen E, et al. Two splice-factor mutant leukemia subgroups uncovered at the boundaries of MDS and AML using combined gene expression and DNA-methylation profiling. Blood. 2014;123:3327–35. doi: 10.1182/blood-2013-07-512855. [DOI] [PubMed] [Google Scholar]
- 85.Fluhr, S. et al. Epigenetic dysregulation of the erythropoietic transcription factor KLF1 and the beta-like globin locus in juvenile myelomonocytic leukemia. Epigenetics, 1–9 (2017). [DOI] [PMC free article] [PubMed]
- 86.Madzo J, et al. Hydroxymethylation at gene regulatory regions directs stem/early progenitor cell commitment during erythropoiesis. Cell Rep. 2014;6:231–244. doi: 10.1016/j.celrep.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, et al. DNMT3A and TET2 compete and cooperate to repress lineage-specific transcription factors in hematopoietic stem cells. Nat Genet. 2016;48:1014–23. doi: 10.1038/ng.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donze D, Townes TM, Bieker JJ. Role of Erythroid Krüppel-like Factor (EKLF) in human g- to ß-globin switching. J. Biol. Chem. 1995;270:1955–1959. doi: 10.1074/jbc.270.4.1955. [DOI] [PubMed] [Google Scholar]
- 89.Trakarnsanga K, et al. Induction of adult levels of beta-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL. Haematologica. 2014;99:1677–85. doi: 10.3324/haematol.2014.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang Y, Kim YW, Yun J, Shin J, Kim A. KLF1 stabilizes GATA-1 and TAL1 occupancy in the human beta-globin locus. Biochim Biophys Acta. 2015;1849:282–9. doi: 10.1016/j.bbagrm.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 91.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–34. [PubMed] [Google Scholar]
- 92.Lozzio BB, Lozzio CB. Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leuk Res. 1979;3:363–70. doi: 10.1016/0145-2126(79)90033-X. [DOI] [PubMed] [Google Scholar]
- 93.Dimery IW, et al. Variation amongst K562 cell cultures. Exp Hematol. 1983;11:601–10. [PubMed] [Google Scholar]
- 94.Ulirsch JC, et al. Systematic Functional Dissection of Common Genetic Variation Affecting Red Blood Cell Traits. Cell. 2016;165:1530–1545. doi: 10.1016/j.cell.2016.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walter K, et al. The UK10K project identifies rare variants in health and disease. Nature. 2015;526:82–90. doi: 10.1038/nature14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geihs M, et al. An interactive genome browser of association results from the UK10K cohorts project. Bioinformatics. 2015;31:4029–31. doi: 10.1093/bioinformatics/btv491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Khamphikham, P. et al. Genetic variation of Kruppel-like factor 1 (KLF1) and fetal hemoglobin (HbF) levels in beta(0)-thalassemia/HbE disease. Int J Hematol (2017). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.