Abstract
Granulosa cells (GCs) are essential components of follicles and are involved in regulating the process of follicles development. However, comparative studies on GCs isolated from different staged follicles have not been conducted in goose. The aim of the present study was to identify the growth characteristics of goose GCs from pre-hierarchical (6–10 mm) and hierarchical (F4–F2, F1) follicles. Our results showed that the three cohorts of cells had different tolerance to collagenase and had noticeable morphological differences. The F1 granulosa layers were fully digested by 0.1% collagenase, while higher concentration (0.3%) was used for both F4–F2 and pre-hierarchical granulosa layers. In the state of suspension, the diameter of F1 individual cell was larger than the other two cohorts. However, after adhering to the culture plate, cells of F1 just had changes in the diameter accompanied by small bright spots, while both pre-hierarchical and F4–F2 GCs proliferated rapidly with spreading and irregularly shaped voids. Furthermore, all attached cells could be stained by the follicle-stimulating hormone receptor antibody. Analyses of both growth curve and the mRNA expression profiles of genes related to cellular proliferation, apoptosis, and steroidogenesis suggested that three cohorts of in vitro cultured GCs had different physiological viability and functions. Taken together, the present study not only revealed differences of the growth characteristics among three cohorts of goose GCs from pre-hierarchical, F4–F2 and F1 follicles, but also optimized the in vitro culture system of geese different staged GCs.
Keywords: Cell culture, Geese, Granulosa cells, Growing characteristics, Gene expression
Introduction
Granulosa cells (GCs) are the key follicular somatic compartments surrounding the oocytes and play an essential role in maintaining normal ovarian functions [1,2]. GCs are involved in a broad range of cellular physiological processes during the development of follicles, including mechanical support, nutrient intake [3,4], theca cell differentiation [5], and steroidogenesis [6,7]. In particular, its own proliferation, differentiation or apoptosis has a determinative effect on the fate of developing follicles [8].
In laying chickens, ovarian follicles are usually arranged in a strict follicular hierarchy where follicles are mainly divided into pre-hierarchical (<8 mm in diameter) and hierarchical (>9 mm in diameter; designated as F5–F1, F5 < F4 < F3 < F2 < F1) follicles [9]. During the development of follicles, the granulosa layer increases in its cell number and surface area, and changes simultaneously occur in the thickness and number of granulosa layers [10,11]. Meanwhile, GCs go through the morphological change from flattened to cuboidal cells with follicular maturation [11]. In addition, the process of follicular development is closely associated with the degree of GCs differentiation. In general, GCs are arrested in an undifferentiated state during the pre-hierarchical phase but become differentiated when entering into the hierarchy [12,13]. Notably, the steroidogenic capacity of GCs also varies with follicular growth and development, with the maximum in secreting progesterone (P4) in the largest hierarchical follicle (F1) [14]. Pawlowska et al. [15] have shown that levels of steroidogenic acute regulatory protein (StAR) and cholesterol side chain cleavage (CYP11A1) mRNAs remained constantly low in pre-hierarchical GCs but increased gradually in the hierarchy with the maturation of follicles. Similarly, very low levels of 3β-hydroxysteroid dehydrogenase (3β-HSD) mRNA were observed in GCs of pre-hierarchical follicles in contrast with its highest levels in the F1 GCs [14,15]. Notably, GCs prior to follicle selection are more susceptible to undergoing apoptosis compared with those of hierarchical follicles [8,16,17]. Collectively, these data reveal morphological and functional differences among GCs of follicles at different developmental stages.
In order to better understand the molecular and cellular mechanisms controlling avian follicular development, it is of importance to establish in vitro culture models of GCs from follicles at different stages of development. Although some studies have successfully isolated and cultured GCs from either pre- or hierarchical follicles [9,18–25], a comparative study on the growing characteristics of in vitro cultured GCs isolated from different sized follicles as well as the optimization of each culture condition is still required. The objectives of the present study were to first optimize the in vitro culture condition of each cohort of goose GCs (namely pre-hierarchical (6–10 mm), F4–F2, and F1 GCs respectively) including the concentrations of both collagenase and fetal bovine serum (FBS), and then to compare their growing characteristics during in vitro culture through morphological observation, growing curve and investigation of expression of genes related to cellular proliferation, apoptosis, and steroidogenesis.
Materials and methods
Animals and isolation of GCs
The healthy maternal line of Tianfu meat geese (Anser cygnoides, 35–45 weeks) during the period of egg laying were used in the present study. The geese were kept under natural light and temperature condition at the Waterfowl Breeding Experimental Farm of Sichuan Agricultural University (Sichuan, China), and were provided with unlimited access to feed and water. Individual laying cycles were monitored for each goose throughout the laying sequence. Geese were killed by cervical dislocation 7–9 h before oviposition.
Follicles from each ovary were dissected and subsequently washed with ice-cold sterile phosphate buffered saline (PBS, pH 7.4. Solarbio), and morphologically normal follicles were characterized based on a previously described report [26]. The granulosa layers from both F4–F2 and F1 follicles were isolated according to the method introduced by Gilbert et al. [18]. Briefly, a cut (about 1 cm long) was made with a scalpel blade along the stigma of follicles, and then the follicular compartments were inverted into a dish containing PBS. Next, the residual yolk was washed out and granulosa layers were collected. As to the pre-hierarchical follicles, follicles were first made a cut with a scalpel blade. Next, two crooked tweezers were utilized to clamp the incision and then to split the follicular walls gently. Then, a tweezer was used to clamp one incision and follicular walls were washed with PBS until granulosa layers were peeled off from the follicular walls. After collecting the granulosa layers from different sized follicles, they were cut into pieces and transferred into 15 ml of centrifuge tubes, respectively, and 0.1% or 0.3% type II collagenase (Sigma) was used to disperse those granulosa layers. During the digestion, the tubes were shaken at 37°C in a water bath for no more than 3 min, until the granulosa layers were fully dispersed. Dulbecco modified Eagle medium/nutrient and F-12/1:1 (DMEM/F12, HyClone) was utilized to dilute the concentration of collagenase. The dispersed cells were finally filtered through a 200-mesh sieve and centrifuged for 10 min at 1000 rpm. Both freshly dispersed and suspended cells were captured under a microscope (Olympus, Tokyo, Japan) and the latter’s diameter was measured using the OPTPro software. All procedures using birds in the present study were carried out according to the Guide of the Faculty Animal Care and Use Committee, Sichuan Agricultural University, Sichuan, China.
Culture of GCs
The GCs were harvested from pre-hierarchical (6–10 mm), F4–F2, and F1 follicles. The culture medium used in the study consisted of DMEM/F12, 10% FBS (Gibco), 1% streptomycin, and penicillin mixture (Gibco). The cells resuspended with above medium were seeded on different culture plates and then incubated at 37°C under 5% CO2 in humidified air to allow the cells to reach a confluence. After incubation of around 6 h, the medium was replaced by fresh medium. The six-well plates (∼15 × 105 cells/well) were used for cell immunofluorescence assay, and the 12-well plates (∼10 × 105 cells/well) were used for qPCR. In addition, the 96-well plates (∼5 × 105 cells/well) were used for the collection of images and the growth curve of GCs. For three groups of GCs cultured in vitro, each experiment was conducted in triplicate.
Morphological observation and cell viability assay
Before analysis of cell viability, the morphology of GCs was observed per 24 h for a total period of 168 h, and the field was randomly selected to capture using a microscope (Olympus, Tokyo, Japan). Cell viability was analyzed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Amresco) method. Briefly, the cells were treated with 0.5–1 mg/ml MTT for additional 4 h at different time points (24, 48, 72, 96, 120, 144, and 168 h), with ten replicates and two negative controls for each cohort at each point. Then, MTT was removed and DMSO (Solarbio) was inserted with 150 µl/well, and the plate was shaken for 10 min [27]. The absorbance of cells at 490 nm was measured using an automatic enzyme immunoassay analyzer.
Immunofluorescence assay
Follicle-stimulating hormone receptor (FSHR) immunostaining was used to identify GCs from different sized follicles after 72 h of incubation, since FSHR is exclusively present in avian GCs [28]. In brief, each well was washed three times with PBS to remove the culture medium. Then, the GCs were fixed with 4% paraformaldehyde (Solarbio) for 30 min, and penetrated with 0.1% Triton-X (Amresco) for 15 min. After that, the GCs were blocked with 4% BSA (Solarbio) for 1 h and washed with PBS. The rabbit-anti-goose-FSHR primary antibody (diluted 1:200 with 1% BSA; Bioss) was then added to the wells and incubated overnight at 4°C. After that, the cells were washed three times with PBS and incubated with a FITC-goat anti-rabbit IgG antibody (diluted 1:500 with 1% BSA; Boster) at 37°C for 2 h. Finally, the nuclei was labeled with DAPI (4,6-diamidino-2-phenylindole; 10 µg/ml in PBS; BiYunTian Biotechnology) (Solarbio) for 10 min and images were taken using a florescence microscope (Nikon). For three groups of GCs cultured in vitro, each experiment was conducted in triplicate.
RNA extraction and real-time PCR
According to the manufacturer’s protocol, total RNA was extracted using TRIzol reagent (Invitrogen), and the quality was assessed by spectrophotometric absorbance at 260/280 nm. First-strand cDNA was synthesized from 1 µg of total RNA using a cDNA synthesis kit (TaKaRa) following the manufacturer’s instructions. Reactions of quantitative real-time PCR (qPCR) were performed in a CFX96TM Real-Time system (Bio-Rad, CA, U.S.A.) using the SYBR PrimerScriptTM real-time PCR kit (TaKaRa). The procedure included 1 cycle of 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and primer-specific annealing temperature for 30 s. An 80-cycle melting curve was performed, starting at a temperature of 85°C and increasing by 0.5 every 10 s to determine primer specificity. Only one product of the desired size was identified and one single peak was observed in a melting curve for each primer. Each sample was repeated three times and the relative mRNA expression of genes was normalized to β-actin and GAPDH using the 2− ΔΔCt method [29]. The primers designed for qPCR are shown in Table 1.
Table 1. Primer pairs for real-time quantitative PCR.
| Gene | Sequence (5′-3′) | Tm (°C) | Size (bp) |
| 3β-HSD | F: GACCTGGGGTTTGGAATTGAG | 60 | 170 |
| R: TAGGAGAAGGTGAATGGGGTGT | |||
| StAR | F: AGAATCTTGACCTCTTTGACGCTG | 60 | 87 |
| R: GAGACGGTGGTGGATAACGGA | |||
| CYP11A1 | F: AGGGAGAAGTTGGGTGTCTACGA | 60 | 89 |
| R:CGTAGGGCTTGTTGCGGTAGT | |||
| Caspase-3 | F: CTGGTATTGAGGCAGACAGTGG | 62 | 158 |
| R: CAGCACCCTACACAGAGACTGAA | |||
| Bcl-2 | F:GATGCCTTCGTGGAGTTGTATG | 60 | 100 |
| R: GCTCCCACCAGAACCAAAC | |||
| CCND2 | F: TTCATCGCCCTTTGTGCC | 60 | 80 |
| R: ATTGCTCCCACGCTTCCA | |||
| p21 | F: TGAGGCAACACCTGGAAGAAG | 60 | 207 |
| R: CCTTAGATGGGACCTTGTGGG | |||
| 1GAPDH | F: TTTCCCCACAGCCTTAGCA | 60 | 90 |
| R: GCCATCACAGCCACACAGA | |||
| 1β-Actin | F: CAACGAGCGGTTCAGGTGT | 59.6 | 92 |
| R: TGGAGTTGAAGGTGGTCTCG |
F: sense primers; R: antisense primers.
Housekeeping gene for data normalization.
Data analysis
Analyses of data from cell diameter, cell viability, and the relative genes mRNA expression levels were subjected to ANOVA, and the means were assessed for significant differences using the Tukey’s test. All results were expressed as mean ± S.D. and a P-values below 0.05 was considered statistically significant. All statistical analyses were carried out using SAS 8.1 (SAS Institute, Cary NC, U.S.A.).
Results
Morphological differences of GCs
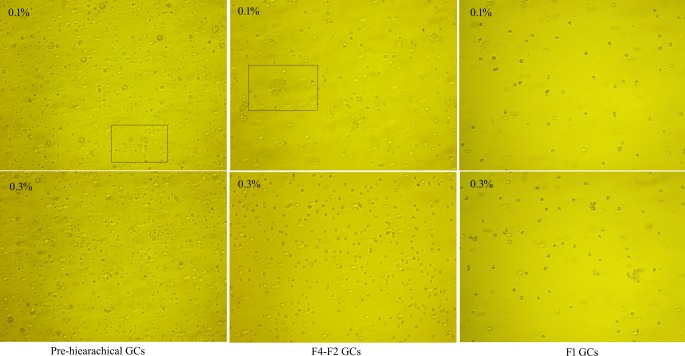
Three cohorts of granulosa layers were isolated from different sized follicles according to the methods mentioned in the “Materials and methods” section. Although 0.1% and 0.3% type II collagenase were able to fully disperse the F1 granulosa layers, 0.3% collagenase led to cell loss and decreased cell viability during digestion (Figure 1). In contrast, the pre-hierarchical and F4–F2 granulosa layers could only be fully dispersed by 0.3% collagenase which did not impair cell viability (Figure 1). Thus, we finally used 0.1% collagenase to digest the F1 granulosa layers while 0.3% for the pre-hierarchical and F4–F2 granulosa layers.
Figure 1. Digestion of GCs from follicles at different developmental stages.
The concentrations of collagenase are 0.1% and 0.3%, and the morphological differences among three cohorts of GCs cultured in vitro were observed under a microscope (×100). The rectangular box indicates the granulosa layer without being fully digested.
“F1 GCs” represents the GCs from the largest follicle (F1) and “F4–F2 GCs” represents the GCs from the fourth to the second largest follicles (F4–F2).
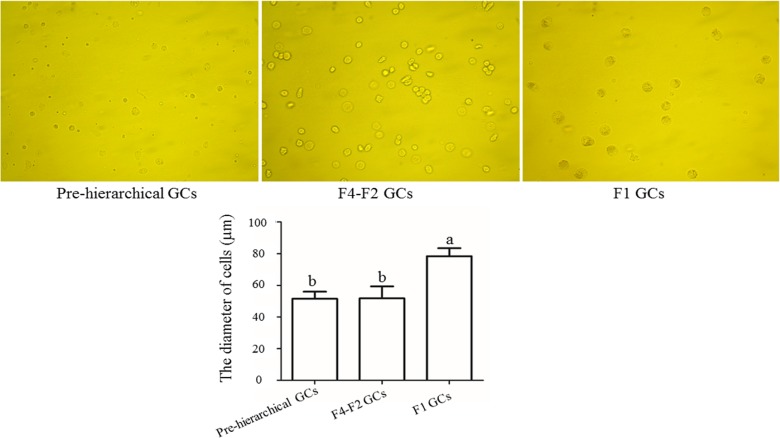
Microscopic examination showed that the freshly suspended cells presented either round or oval shapes. Noticeably, the diameter of F1 GCs was larger than that of the other two cohorts of GCs (Figure 2, P<0.05).
Figure 2. Differences in the diameter of freshly suspended GCs from follicles at different developmental stages.
Morphological differences among three cohorts of GCs cultured in vitro were observed under a microscope (×400). The diameter of three cohorts of GCs is displayed below and different lowercase letters indicate significant differences at P<0.05.
“F1 GCs” represents the GCs from the largest follicle (F1) and “F4–F2 GCs” represents the GCs from the fourth to the second largest follicles (F4–F2).
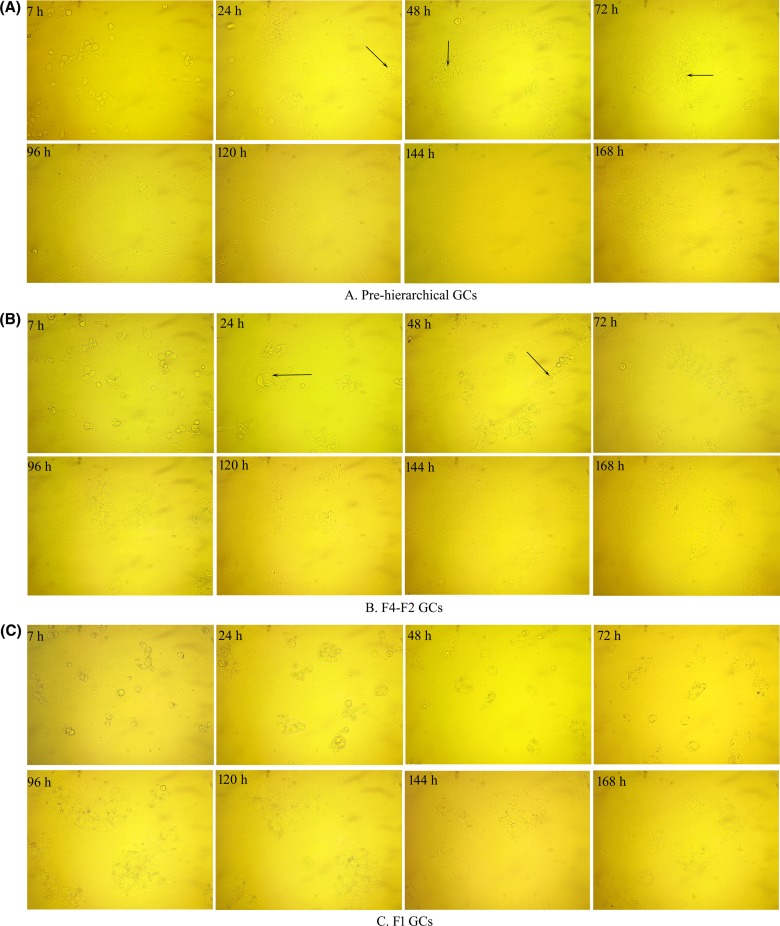
As shown in Figure 3, all cultured cells adhered to the bottom of plates and were shown as roundness or oval after 7 h of incubation (Figure 3A–C). Pre-hierarchical GCs spread more rapidly than the other GCs and displayed filamentous extensions at 24 h of culture (Figure 3A). Irregularly shaped voids were observed in both F4–F2 and pre-hierarchical GCs (Figure 3A,B; 24 h), but F1 GCs only increased in cell size (Figure 3C; 24 h). During the incubation from 48 to 72 h, those irregular voids seen in pre-hierarchical and F4–F2 GCs became smaller but their number increased gradually, and the cells from the two stages proliferated significantly (Figure 3A,B; 48–72 h) while F1 GCs still changes in cell size (Figure 3C; 48–72 h). At 96 h of culture, F1 GCs began to fuse by spreading all over the bottom of plates (Figure 3C), while the pre-hierarchical and F4–F2 GCs increased in cellular density although their layers became thinner with increasing time of culture (Figure 3A,B; 96 h). When cultured to 120 h, the F1 GCs started to be senescent (Figure 3C), while the adhered cell layers of F4–F2 and pre-hierarchical GCs were extremely thin and grew over each other (Figure 3A,B). In addition, numerous tiny bright spots were observed in F1 GCs (Figure 3C; 24–120 h) but not in F4–F2 and pre-hierarchical GCs (Figure 3A,B).
Figure 3. Morphological differences among three cohorts of adhered GCs.
Changes in the morphology of three cohorts of GCs during the in vitro culture period from 7 to 168 h were observed under a microscope (×400). Arrows indicate the irregularly shaped voids. “A/B/C” represents the GCs of pre-hierarchical, F4–F2, and F1 follicles respectively.
“F1 GCs” represents the GCs from the largest follicle (F1) and “F4–F2 GCs” represents the GCs from the fourth to the second largest follicles (F4–F2).
Growth activity of GCs cultured in vitro
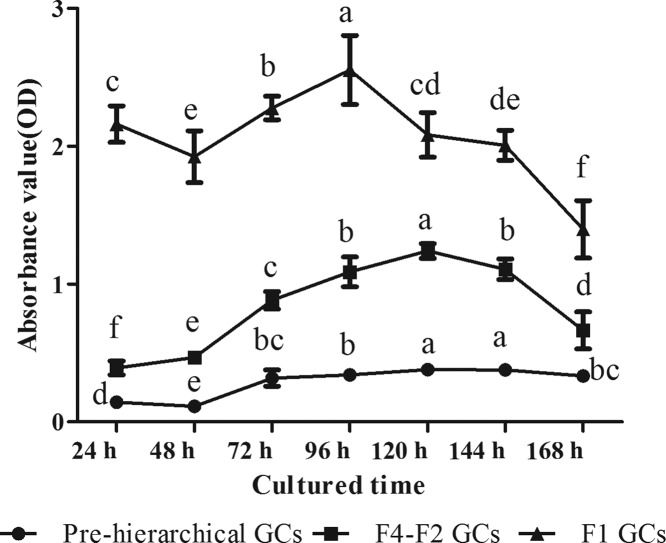
Growth curve showed that all GCs entered the logarithmic phase at 48 h of culture, and it went on for ~48 h for F1 GCs and 72 h for pre-hierarchical and F4–F2 GCs (Figure 4). During this period, cell activity increased fast and significantly (Figure 3A,B; 48–120 h; Figure 3C; 48–96 h) (P<0.05). However, activity of F1 GCs decreased gradually at 120 h while that of either F4–F2 or pre-hierarchical GCs decreased at 144 h of culture (Figure 4).
Figure 4. Growth curve of three cohorts of in vitro cultured GCs.
Different lowercase letters indicate significant differences at P<0.05 among different culture time within the same cohort of cells.
“F1 GCs” represents the GCs from the largest follicle (F1) and “F4–F2 GCs” represents the GCs from the fourth to the second largest follicles (F4–F2).
Identification of GCs using FSHR immunostaining
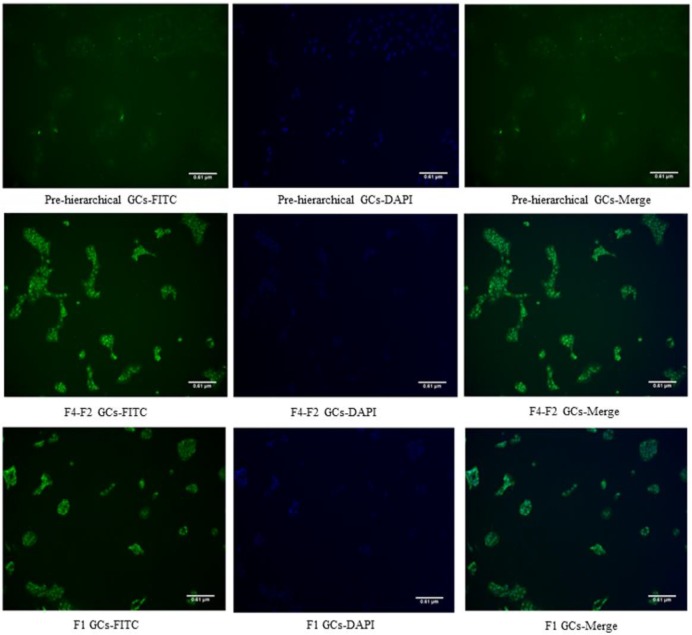
The cultured GCs at 72 h were used for immunofluorescence assay, and a FSHR primary antibody (rabbit-anti-goose) was utilized to identify GCs. Our results showed that all GCs were marked with a green outline (Figure 5), indicating that there was no other cell contamination.
Figure 5. Immunofluorescence identification of three cohorts of GCs using the FSHR antibody.
“F1 GCs” represents the GCs from the largest follicle (F1) and “F4–F2 GCs” represents the GCs from the fourth to the second largest follicles (F4–F2).
Expression patterns of several genes related to basic functions of GCs during in vitro culture
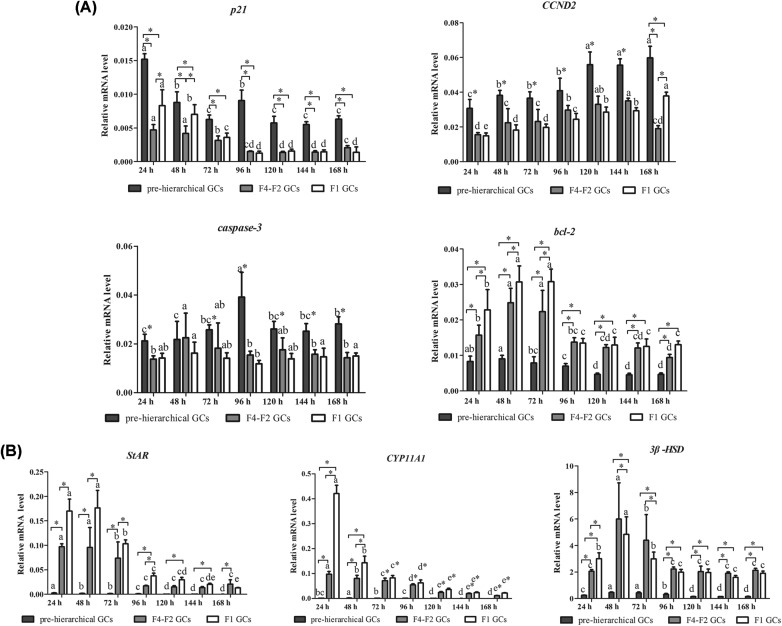
Our results showed that the expression levels of genes related to proliferation and apoptosis including Cyclin D2 (CCND2), p21, and caspase-3 were significantly higher in pre-hierarchical than in hierarchical GCs (P<0.05), and there was no significant difference between F1 and F4–F2 GCs (P>0.05). However, expression of p21 in F1 GCs was higher than in F4–F2 GCs (P<0.05). In addition, levels of bcl-2 (anti-apoptotic) were markedly higher in hierarchical than in pre-hierarchical GCs (P<0.05). For either F1 or F4–F2 GCs, bcl-2 mRNA levels increased significantly during the incubation period from 24 to 72 h (P<0.05), followed by an abrupt decline at 96 h of culture, and then remained statistically unchanged (P>0.05). The mRNA levels of p21 gradually decreased with increasing time of culture while CCND2 presented an opposite trend. As for bcl-2 and caspase-3, their expression showed a tendency of increasing early and decreasing later during incubation (Figure 6A).
Figure 6. Expression patterns of several genes related to basic functions of GCs from different sized follicles during in vitro culture.
(A) and (B) represent expression of proliferation, apoptosis genes, and expression of steroidogenesis genes mRNA respectively. Bars with different lowercase latter are significantly different for the same type of cell during the cultured time in vitro (P<0.05). * indicates significant differences among GCs of three different stages in the same cultured time (P<0.05 and P<0.01 respectively). GAPDH and β-actin were used as internal controls.
“F1 GCs” represents the GCs from the largest follicle (F1) and “F4–F2 GCs” represents the GCs from the fourth to the second largest follicles (F4–F2).
In addition, the expression levels of several steroidogenic genes including StAR, CYP11A1, and 3β-HSD were higher in hierarchical than in pre-hierarchical GCs during in vitro culture (P<0.05, Figure 6B). The pre-hierarchical GCs had lower expression levels of StAR and CYP11A1 but relatively higher levels of 3β-HSD. Besides, expression of steroidogenic genes was significantly higher in F1 than in F4–F2 GCs at 24 h of culture (P<0.05, Figure 6B). As the cells were cultured from 48 to 72 h, expression of 3β-HSD was significantly higher in F4–F2 than in F1 GCs (P<0.05, Figure 6B), while expression of StAR and CYP11A1 reached the maximum in F1 GCs (P<0.05, Figure 6B). After that, there was no significant difference in the levels of those genes between F1 and F4–F2 GCs (P>0.05). With increasing time of culture, expression of StAR and CYP11A1 gradually decreased, while that of 3β-HSD increased first and then decreased in each cohort of GCs (Figure 6B).
Discussion
In the avian ovary, GCs play essential roles during the processes of follicular recruitment, selection, maturation, and ovulation. Previous researchers have used 0.1% collagenase to disperse the F3–F1 granulosa layers or 0.3% collagenase for the F4–F2 granulosa layers in chicken [9,23]. Based on previous reports [9,19–25], in the present study we further improved the methods used for isolation and culture of GCs from geese follicles at different developmental stages through optimizing the concentrations of collagenase and FBS. The results showed that the F1 granulosa layers were fully dispersed by 0.1% collagenase, but a much higher concentration (0.3%) of collagenase was required to digest the granulosa layers of both pre-hierarchical and F4–F2 follicles (Figure 1).
Gilbert et al. [30] have shown that changes in the diameter of granulosa cells were dependent on follicular size, which was also related to corresponding changes in the cell shape. With regard to the freshly dispersed GCs, we found that the diameter of F1 cells was significantly larger than that of either pre-hierarchical or F4–F2 ones (P<0.05, Figure 2). During the incubation from 7 to 72 h, the F1 GCs only increased in their diameter, however, GCs of pre-hierarchical and F4–F2 follicles spread more rapidly until fusion, coupled with the formation of filamentous extensions and irregularly shaped voids. After that, the F1 cells began to attach and fuse together, while the pre-hierarchical and F4–F2 GCs continued proliferating with their cell layers becoming much thinner (Figure 3A–C). The growth curve displayed that all groups of GCs reached the logarithmic phase at 48 h of culture, and it lasted 48 and 72 h for the F1 and the other GCs respectively (Figure 4). Thereafter, the cells came into the plateau stage within the incubation of 120–144 h, followed by the senescence phase (Figure 4). These results altogether suggested that the three populations of geese GCs showed both similar and different growth characteristics during in vitro culture, which was to a larger extent consistent with the phenomenon seen in other cell types [31]. Additionally, the identification of isolated geese GCs were verified by IF assay with the specific FSHR primary antibody, showing that no other cell contaminations were present in all three cohorts of GCs (Figure 5). Thus, it was concluded that the three populations of geese GCs were successfully isolated and cultured under our optimal condition and exhibited different growth characteristics during in vitro culture.
It has been well recognized that changes in the morphology of GCs were closely related to some biological processes such as cell proliferation, apoptosis, and steroidogenesis [32–34]. To further explore differences in the basic functions of the three cohorts of GCs, expression of several key genes associated with cell proliferation and apoptosis was first determined. Among these genes, CCND2 and p21 were recognized as their roles in controlling cell cycle by stimulating and inhibiting cell proliferation respectively [35,36], while bcl-2 and caspase-3 function as an anti- and pro-apoptotic gene respectively [16,17]. In the present study, results from the expression of caspase-3 and bcl-2 further demonstrated that the pre-hierarchical GCs were much more susceptible to apoptosis than the hierarchical ones, which were in accordance with others’ reports [16,17,37,38]. Besides, the pre-hierarchical GCs also exhibited a high proliferation rate, as demonstrated by both the expression of CCND2 and p21 (Figure 6A) and the observed morphological changes during in vitro culture (Figure 3A). Since the mRNA expression of bcl-2 reached its maximum in the F1 GCs, indicating that these GCs were much more resistant to apoptosis. In contrast with CCND2, the mRNA expression levels of p21 were higher in F1 than F4–F2 GCs, which indicated that F4–F2 GCs proliferated more quickly than F1 ones, which were also demonstrated by their morphological changes (Figure 3C). Additionally, the mRNA expression of key steroidogenic genes (e.g. CYP11A1, StAR, and 3β-HSD) [14,39–42] were also investigated to further define the steroidogenic characteristics of GCs from different sized follicles. The results showed that the expression of CYP11A1 and StAR in the F1 GCs was highest at 72 h of culture, but was extremely low in pre-hierarchical ones during in vitro culture. However, expression of 3β-HSD, a key gene controlling P4 production [14,39], reached the maximum in the F4–F2 GCs but was relatively lower in pre-hierarchical ones when cultured to 72 h. These results were consistent with previous studies [14,40,43], suggesting that large amounts of P4 were mainly produced in hierarchical follicles with the maximum in the F1 GCs, followed by the F4–F2 GCs, whereas the pre-hierarchical GCs were not able to produce P4. Taken together, differences observed in the expression patterns of key genes related to proliferation, apoptosis, and steroidogenesis during in vitro culture further supported the notion that there were significant differences in both morphological and functional characteristics among geese GCs isolated from different sized follicles.
In conclusion, the present study not only established an optimal culture condition for each cohort of GCs isolated from geese follicles at different developmental stages, but also revealed the differences in their morphological and functional characteristics during in vitro culture. These data may contribute to a better understanding of the mechanisms controlling avian follicle growth and development.
Supporting information
Abbreviations
- 3β-HSD
3β-hydroxysteroid dehydrogenase
- FSHR
follicle-stimulating hormone receptor
- GC
granulosa cell
Funding
This work was supported by the National Natural Science Funds of China [31672424]; the Ministry of Agriculture of the People’s Republic of China [CARS-42-4]; and the Ministry of Science and Technology of the People’s Republic of China (MOST) [2015BAD03B06].
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
Conceptualization: Shenqiang Hu and Xiang Gan; Data analysis: Yan Deng; Funding acquisition: Jiwen Wang; Investigation: Yan Deng, Xiang Gan, Da Chen, Huilan Huang, Junsong Yuan, and Jiamin Qiu. Methodology: Yan Deng; Project administration: Jiwen Wang and Jiwei Hu; Supervision: Jiwen Wang; Visualization: Yan Deng; Writing (original draft): Yan Deng; Writing (review and editing): Yan Deng and Shenqiang Hu.
References
- 1.Monniaux D., Michel P., Postel M. and Clément F. (2016) Multiscale modeling of ovarian follicular development: From follicular morphogenesis to selection for ovulation. Biol. Cell 108, 149–160 10.1111/boc.201500087 [DOI] [PubMed] [Google Scholar]
- 2.Gougeon A. (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev. 17, 121 10.1210/edrv-17-2-121 [DOI] [PubMed] [Google Scholar]
- 3.Wojtysiak D., Okólski A. and Sechman A. (2011) Structure and steroidogenic activity of the granulosa layer of F1 preovulatory ovarian follicles of the hen (Gallus domesticus). Folia Biol. (Krakow) 59, 59–64 10.3409/fb59_1-2.59-64 [DOI] [PubMed] [Google Scholar]
- 4.Johnson A.L. (2014) The avian ovary and follicle development: some comparative and practical insights. Turk. J. Vet. Anim. Sci. 38, 660–669 10.3906/vet-1405-6 [DOI] [Google Scholar]
- 5.Tajima K., Orisaka M., Yata H., Goto K., Hosokawa K. and Kotsuji F. (2006) Role of granulosa and theca cell interactions in ovarian follicular maturation. Microsc. Res. Tech. 69, 450–458 10.1002/jemt.20304 [DOI] [PubMed] [Google Scholar]
- 6.Krysko D.V., Mussche S., Leybaert L. and D’Herde K. (2004) Gap junctional communication and connexin43 expression in relation to apoptotic cell death and survival of granulosa cells. J. Histochem. Cytochem. 52, 1199–1207 10.1369/jhc.3A6227.2004 [DOI] [PubMed] [Google Scholar]
- 7.Johnson A.L. and Woods D.C. (2009) Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 163 (1-2), 12–17 10.1016/j.ygcen.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 8.Manabe N., Goto Y., Matsuda-Minehata F., Inoue N., Maeda A., Sakamaki K. et al. (2004) Regulation mechanism of selective atresia in porcine follicles: regulation of granulosa cell apoptosis during atresia. J. Reprod. Develop. 50, 493–514 10.1262/jrd.50.493 [DOI] [PubMed] [Google Scholar]
- 9.Wei Q., Zhu G., Cui X., Kang L., Cao D. and Jiang Y. (2013) Expression of CCT6A mRNA in chicken granulosa cells is regulated by progesterone. Gen. Comp. Endocrinol. 189, 15–23 10.1016/j.ygcen.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 10.Dong X., Liu H.H., Wang J.W., Xiao Q.H., Yuan X., Li L. et al. (2014) Histological and developmental study of prehierarchical follicles in geese. Folia Biol. (Krakow) 62, 171–177 10.3409/fb62_3.171 [DOI] [PubMed] [Google Scholar]
- 11.Brawtal R. (2002) The initiation of follicle growth: the oocyte or the somatic cells? Mol. Cell. Endocrinol. 187, 11–18 10.1016/S0303-7207(01)00699-2 [DOI] [PubMed] [Google Scholar]
- 12.Johnson A.L. and Woods D.C. (2009) Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation. Gen. Comp. Endocrinol. 163, 12–17 10.1016/j.ygcen.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 13.Johnson A.L. (2015) Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 94, 781–785 10.3382/ps/peu008 [DOI] [PubMed] [Google Scholar]
- 14.Nitta H., Mason J.I. and Bahr J.M. (1993) Localization of 3 beta-hydroxysteroid dehydrogenase in the chicken ovarian follicle shifts from the theca layer to granulosa layer with follicular maturation. Biol. Reprod. 48, 110–116 10.1095/biolreprod48.1.110 [DOI] [PubMed] [Google Scholar]
- 15.Pawlowska K. and Sechman A. (2010) Expression of StAR protein and steroidogenic enzyme mRNAs in ovarian follicles of the domestic hen (Gallus gallus domesticus). Endocrine 22, P479 [Google Scholar]
- 16.Johnson A.L., Bridgham J.T., Witty J.P. and Tilly J.L. (1996) Susceptibility of avian ovarian granulosa cells to apoptosis is dependent upon stage of follicle development and is related to endogenous levels of bcl-xlong gene expression. Endocrinology 137, 2059–2066 10.1210/endo.137.5.8612548 [DOI] [PubMed] [Google Scholar]
- 17.Johnson A.L. and Bridgham J.T. (2000) Caspase-3 and -6 expression and enzyme activity in hen granulosa cells1. Biol. Reprod. 62, 589–598 10.1095/biolreprod62.3.589 [DOI] [PubMed] [Google Scholar]
- 18.Gilbert A.B., Evans A.J., Perry M.M. and Davidson M.H. (1977) A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J. Reprod. Fertil. 50, 179–181 10.1530/jrf.0.0500179 [DOI] [PubMed] [Google Scholar]
- 19.Jin Y., Zhang C., Lin X. and Zeng W. (2006) Prostaglandin involvement in follicle-stimulating hormone-induced proliferation of granulosa cells from chicken prehierarchical follicles. Prostaglandins Other Lipid Mediat. 81, 45–54 10.1016/j.prostaglandins.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Chen F., Jiang X., Chen X., Liu G. and Ding J. (2007) Effects of downregulation of inhibin alpha gene expression on apoptosis and proliferation of goose granulosa cells. J. Genet. Genomics 34, 1106–1113 10.1016/S1673-8527(07)60126-X [DOI] [PubMed] [Google Scholar]
- 21.Xiao Y., Ni Y., Huang Y., Wu J., Grossmann R. and Zhao R. (2011) Effects of kisspeptin-10 on progesterone secretion in cultured chicken ovarian granulosa cells from preovulatory (F1-F3) follicles. Peptides 32, 2091–2097 10.1016/j.peptides.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 22.Johnson A.L., Solovieva E.V. and Bridgham J.T. (2012) Relationship between steroidogenic acute regulatory protein expression and progesterone production in hen granulosa cells during follicle development. Biol. Reprod. 67, 1313–1320 10.1095/biolreprod67.4.1313 [DOI] [PubMed] [Google Scholar]
- 23.Zhu G., Li K., Yang C., Zhang X., Wang M. and Jiang Y. (2012) Differential expression of CTGF in pre- and post-ovulatory granulosa cells in the hen ovary is regulated by TGFβ1 and gonadotrophins. Gen. Comp. Endocrinol. 178, 314–322 10.1016/j.ygcen.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 24.Hu S., Gan C., Wen R., Xiao Q., Gou H., Liu H. et al. (2014) Role of leptin in the regulation of sterol/steroid biosynthesis in goose granulosa cells. Theriogenology 82, 677–685 10.1016/j.theriogenology.2014.05.025 [DOI] [PubMed] [Google Scholar]
- 25.Wen R., Hu S., Xiao Q., Han C., Gan C., Gou H. et al. (2015) Leptin exerts proliferative and anti-apoptotic effects on goose granulosa cells through the PI3K/Akt/mTOR signaling pathway. J. Steroid Biochem. Mol. Biol. 149, 70–79 10.1016/j.jsbmb.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 26.Kovacs J., Forgo V. and Peczely P. (1992) The fine structure of the follicular cells in growing and atretic ovarian follicles of the domestic goose. Cell Tissue Res. 267, 561–569 10.1007/BF00319379 [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Wang F., Tao X. and Cheng H. (2012) Ammonia-containing dimethyl sulfoxide: an improved solvent for the dissolution of formazan crystals in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Anal. Biochem. 421, 324–326 10.1016/j.ab.2011.10.043 [DOI] [PubMed] [Google Scholar]
- 28.Woods D.C. and Johnson A.L. (2005) Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection. Biol. Reprod. 72, 643–650 10.1095/biolreprod.104.033902 [DOI] [PubMed] [Google Scholar]
- 29.Livak K.J. and Schmittgen T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 30.Gilbert A.B., Hardie M.A., Perry M.M., Dick H.R. and Wells J.W. (1980) Cellular changes in the granulosa layer of the maturing ovarian follicle of the domestic fowl. Br. Poult. Sci. 21, 257–263 10.1080/00071668008416667 [DOI] [PubMed] [Google Scholar]
- 31.Gan X., Chen D., Deng Y., Yuan J., Kang B., Qiu J. et al. (2017) Establishment of an in vitro culture model of theca cells from hierarchical follicles in ducks. Biosci. Rep. 37, BSR20160491 10.1042/BSR20160491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C.S., Mrksich M., Huang S., Whitesides G.M. and Ingber D.E. (1997) Geometric control of cell life and death. Science 276, 1425–1428 10.1126/science.276.5317.1425 [DOI] [PubMed] [Google Scholar]
- 33.Carnegie J.A., Byard R., Dardick I. and Tsang B.K. (1988) Culture of granulosa cells in collagen gels: the influence of cell shape on steroidogenesis. Biol. Reprod. 38, 881–890 10.1095/biolreprod38.4.881 [DOI] [PubMed] [Google Scholar]
- 34.Da S.B.P., Jayasooriya G.S., Mora J.M., Mobberley M., Ryder T.A., Baithun M. et al. (2008) Effect of cell shape and packing density on granulosa cell proliferation and formation of multiple layers during early follicle development in the ovary. J. Cell Sci. 121, 3890–3900 10.1242/jcs.036400 [DOI] [PubMed] [Google Scholar]
- 35.Cannon J.D., Cherianshaw M., Lovekampswan T. and Chaffin C.L. (2007) Granulosa cell expression of G1/S phase cyclins and cyclin-dependent kinases in PMSG-induced follicle growth. Mol. Cell. Endocrinol. 264, 6–15 10.1016/j.mce.2006.09.015 [DOI] [PubMed] [Google Scholar]
- 36.Sherr C.J. and Roberts J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 10.1101/gad.13.12.1501 [DOI] [PubMed] [Google Scholar]
- 37.Tilly J.L., Kowalski K.I., Johnson A.L. and Hsueh A.J.W. (1991) Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrine Soc. 129, 2799–2801 10.1210/endo-129-5-2799 [DOI] [PubMed] [Google Scholar]
- 38.Johnson J.L. (2000) Granulosa cell apoptosis: conservation of cell signaling in an avian ovarian model system. Biol. Signals Recept. 9, 96–101 10.1159/000014628 [DOI] [PubMed] [Google Scholar]
- 39.Marrone B.L. and Sebring R.J. (1989) Quantitative cytochemistry of 3 beta-hydroxysteroid dehydrogenase activity in avian granulosa cells during follicular maturation. Biol. Reprod. 40, 1007–1011 10.1095/biolreprod40.5.1007 [DOI] [PubMed] [Google Scholar]
- 40.Tilly J.L., Kowalski K.I. and Johnson A.L. (1991) Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol. Reprod. 44, 305–314 10.1095/biolreprod44.2.305 [DOI] [PubMed] [Google Scholar]
- 41.Z L. and AL J. (1993) Regulation of P450 cholesterol side-chain cleavage messenger ribonucleic acid expression and progesterone production in hen granulosa cells. Biol. Reprod. 49, 463–469 10.1095/biolreprod49.3.463 [DOI] [PubMed] [Google Scholar]
- 42.Johnson A.L. and Bridgham J.T. (2001) Regulation of steroidogenic acute regulatory protein and luteinizing hormone receptor messenger ribonucleic acid in hen granulosa cells. Endocrinology 142, 3116–3124 10.1210/endo.142.7.8240 [DOI] [PubMed] [Google Scholar]
- 43.Lovell T.M., Gladwell R.T., Groome N.P. and Knight P.G. (2002) Differential effects of activin A on basal and gonadotrophin-induced secretion of inhibin A and progesterone by granulosa cells from preovulatory (F1-F3) chicken follicles. Reproduction 124, 649–657 10.1530/rep.0.1240649 [DOI] [PubMed] [Google Scholar]