Abstract
AIM
To investigate patient adherence to surveillance endoscopy after index esophageal variceal hemorrhage and the extent to which adherence influences outcomes.
METHODS
We reviewed the records of patients with cirrhosis admitted to the medical intensive care unit between 2000 and 2014 for first time esophageal variceal hemorrhage treated with endoscopic variceal ligation who were subsequently discharged and scheduled for surveillance endoscopy at our medical center. Demographic and clinical data were obtained through the medical records, including etiology of cirrhosis, completion of variceal obliteration, attendance at surveillance endoscopy, zip code of primary residence, distance from home to hospital, insurance status, rehospitalization for variceal hemorrhage, beta-blocker at discharge, pharmacologically treated psychiatric disorder, and transplant free survival.
RESULTS
Of 99 consecutive survivors of esophageal variceal bleeding, the minority (33) completed variceal obliteration and fewer (12) adhered to annual surveillance. Completion of variceal obliteration was associated with fewer rehospitalizations for variceal rebleeding (27% vs 56%, P = 0.0099) and when rehospitalizations occurred, they occurred later in those who had completed obliteration (median 259 d vs 207 d, P = 0.0083). Incomplete adherence to endoscopic surveillance was associated with more rehospitalizations for variceal rebleeding compared to those fully adherent to annual endoscopic surveillance (51% vs 17%, P = 0.0328). Those adherent to annual surveillance were more likely to be insured privately or through Medicare compared to those who did not attend post-hospital discharge endoscopy (100% vs 63%, P = 0.0119).
CONCLUSION
Most patients do not complete variceal obliteration after index esophageal variceal hemorrhage and fewer adhere to endoscopic surveillance, particularly the uninsured and those insured with Medicaid.
Keywords: Liver cirrhosis, Endoscopy, Esophageal varices, Secondary prevention, Patient adherence
Core tip: We investigated adherence to surveillance endoscopy in 99 consecutive patients with cirrhosis who survived esophageal variceal bleeding, and the extent to which adherence influenced outcomes. We found that the minority (33%) completed variceal obliteration and fewer (12%) underwent annual surveillance. Completion of obliteration was associated with fewer and later rehospitalizations for variceal rebleeding. Those non-adherent to annual surveillance were more likely to be uninsured or to have coverage through Medicaid assistance. Our findings identify potential markers for socioeconomic factors that limit endoscopic adherence following variceal hemorrhage and lead to adverse outcomes. New approaches are needed to overcome barriers to adherence.
INTRODUCTION
Gastrointestinal variceal hemorrhage is a major and dramatic complication of cirrhosis, with a hospitalization-associated mortality rate of 15%-20%[1,2]. Survivors of initial variceal hemorrhage have a 60% probability of rebleeding within 1-2 years and a 33% mortality rate if no further treatment is sought after hemostasis[2,3]. Controlled clinical trials have demonstrated that the most effective strategy to reduce rebleeding from varices is serial endoscopic variceal ligation (EVL), with a goal of variceal obliteration, in combination with non-selective beta adrenergic receptor blockers[2-4]. Combined endoscopic and pharmacologic therapy, in this way, reduces rebleeding rates to as low as 14%[5]. In a real-world setting, the success of this strategy is likely to be influenced by patient adherence to endoscopic surveillance, but this has not been formally studied.
There is growing evidence that cirrhotic patients often do not receive evidence-based treatments for disease related complications[6]. Despite recommendations to perform an esophagogastroduodenoscopy (EGD) to screen for gastroesophageal varices after a diagnosis of cirrhosis is made[2], a study of United States military veterans with newly diagnosed cirrhosis due to hepatitis C found only a third of patients had an EGD within one year after diagnosis, and 46% had still not undergone an EGD at 6 years[7]. Moreover, in those with endoscopic evidence of esophageal varices but no history of hemorrhage, only 60% were either placed on beta-blockers or underwent EVL for primary prophylaxis of variceal bleeding[6].
Similar gaps in care have been observed in cirrhotics with a history of gastrointestinal variceal bleeding. In one study, referral for surveillance endoscopy was placed in just 65% of patients at the time of discharge following hospitalization for esophageal variceal hemorrhage[8]. In another study, between 74%-93% of patients with an acute variceal hemorrhage had either post-hospital discharge esophageal variceal obliteration or were placed on a beta-blocker, while 44%-59% had post-discharge esophageal variceal obliteration alone[9]. Even if orders for beta-blockers and surveillance endoscopy are placed, patient-related factors may influence adherence to measures to prevent recurrent variceal hemorrhage. This study was undertaken in survivors of hospitalization for esophageal variceal hemorrhage to identify, in a real-world setting, potential factors that prevent patients from returning for surveillance endoscopy, and to examine if such patients have different outcomes than those who adhere to endoscopy following index variceal hemorrhage.
MATERIALS AND METHODS
Study population
We reviewed the medical records of patients (age ≥ 18 years) who were admitted consecutively to the medical intensive care unit of the University of Vermont Medical Center (UVMMC), a tertiary care center, with an admission diagnosis of gastrointestinal (GI) hemorrhage, melena, hematemesis, or bleeding esophageal varices, for which they underwent EGD from July 1, 2000 to December 31, 2014 with follow up through June 30, 2015. The medical records and EGD reports were then reviewed to identify those with esophageal varices on initial EGD[1]. Bleeding was attributed to esophageal varices if at least one of the following criteria was met: (1) Identification of actively bleeding esophageal varices; (2) esophageal varices identified with stigmata of recent hemorrhage; or (3) clinical presentation consistent with upper GI hemorrhage (e.g., melena and/or hematemesis), large esophageal varices present, and no alternative etiology for GI bleeding identified on EGD. Patients treated for bleeding esophageal varices were provided information at discharge regarding the time of follow up endoscopy through patient instructions. Similar instructions were provided following each subsequent endoscopy.
Inclusion criteria were: (1) Cirrhosis (defined by any of the following International Classification of Diseases 9th Revision diagnostic codes: 571.2, 571.5, or 571.6); (2) index esophageal variceal hemorrhage (i.e., no prior history of variceal bleeding); and (3) EVL. Exclusion criteria were: (1) Transjugular intrahepatic portosystemic shunt (TIPS) for control of bleeding at index bleed; (2) primary residence in county outside of local endoscopy region; (3) death at index bleed; (4) age < 18 years; (5) presence of comorbid illness with limited survival (e.g., metastatic cancer, end stage heart or lung disease); (6) non-esophageal variceal bleed (e.g., gastric), and (7) sclerotherapy.
Measurements and outcomes
The study design was approved by the University of Vermont Committee on Human Research in the Medical Sciences (CHRMS 15-134). Demographic and clinical data were obtained through the medical records, including age, gender, date of index variceal hemorrhage, etiology of cirrhosis, recent significant alcohol consumption (greater than 7 drinks per week for women, greater than 14 drinks per week for men), completion of variceal obliteration, attendance at surveillance EGD, zip code of primary residence, distance from home to hospital, insurance status, rehospitalization for variceal hemorrhage, beta-blocker at discharge, pharmacologically treated psychiatric disorder, and transplant free survival.
Completion of variceal obliteration was defined as endoscopic eradication of varices with the first EGD of the series occurring within 6 mo following hospital discharge. Adherence to surveillance endoscopy was defined as undergoing EGD at intervals no greater than every 1 year after completion of variceal obliteration. The severity of liver disease at the time of admission was assessed by the Model for End-stage Liver Disease (MELD) score[10] and Child-Turcotte-Pugh class[11].
The following outcomes were assessed: (1) Beta adrenergic blockade at hospital discharge; (2) appearance at initially scheduled outpatient EGD; (3) completion of variceal obliteration; (4) adherence to surveillance EGD after variceal obliteration; (5) rehospitalization for gastrointestinal variceal bleeding; and (6) transplant free survival.
Statistical analysis
Analyses were performed with GraphPad Prism (Version 6.0). Differences between groups were determined by Fisher’s exact test for categorical variables, by Mann-Whitney U test for continuous non-parametric variables, and by Student’s t test for continuous parametric variables. Kaplan-Meier curves for both survival and time to rehospitalization were compared using the log-rank test. A value of P < 0.05 was deemed statistically significant.
RESULTS
Characteristics of the Study population
Between July 1, 2000 and December 31, 2014, there were 347 consecutive individuals with cirrhosis admitted to the medical intensive care unit for gastrointestinal hemorrhage, melena, hematemesis, or bleeding esophageal varices. Of these, 205 had an esophageal variceal bleed. Ultimately, 99 met the study entry criteria. Reasons for exclusion included primary residence outside of local endoscopy region (63 admissions), death or TIPS at index bleed (17 admissions), incomplete records (14 admissions), and comorbid illness with limited survival (12 admissions).
As shown in Table 1, the median age of the study population was 55 years; 60% of the study population was male, the median admission MELD score was 13, 54% of the study population was Child-Turcotte-Pugh class B, and 39% had a pharmacologically treated psychiatric disorder. The most common etiology of liver disease was alcoholic cirrhosis (63%), of which 16% (10 of 62) had concomitant hepatitis C. The median distance from the hospital was 24.9 kilometers. Nearly a third of patients had no health insurance or had financial health coverage through Medicaid (a government-sponsored health plan that assists with medical costs in selected low income individuals). At hospital discharge, 87% of patients were on a beta-blocker.
Table 1.
Summary of baseline patient characteristics n (%)
| Demographics/baseline measures | Number (n = 99) |
| Male | 59 (60) |
| Age, median (range), yr | 55 (31-82) |
| Etiology of cirrhosis | |
| Alcohol | 62 (63) |
| Hepatitis C | 12 (12) |
| Other | 25 (25) |
| Recent alcohol use | 46 (46) |
| Distance from hospital, median (range), kilometers | 24.9 (0.8-95.1) |
| MELD score, median (range) | 13 (6-27) |
| Child-turcotte-pugh classification | |
| A | 24 (24) |
| B | 53 (54) |
| C | 22 (22) |
| Index bleed in 2007 or earlier | 44 (44) |
| Insurance | |
| Medicaid1 or uninsured | 31 (31) |
| Medicare or private insurance | 68 (69) |
| Comorbid psychiatric disorder | 39 (39) |
| Length of stay, median (range), d | 5 (2-75) |
| Beta-blocker at discharge | 86 (87) |
Government-sponsored health plan that assists with medical costs in selected low income individuals. MELD: Model for end-stage liver disease.
Completion of variceal obliteration and clinical characteristics
Overall, 53 of 99 patients (53%) came for an initial surveillance EGD after hospital discharge, as scheduled, and 33 patients (33%) completed variceal obliteration (Table 2). Achievement of obliteration required a median of 2 EGD sessions after hospital discharge (range 1-6). The median time to first surveillance EGD was 44 d (range 10 to 171 d). There were no significant differences in the proportion of patients who completed esophageal variceal obliteration with respect to gender, age, etiology of cirrhosis, length of index hospital stay, recent alcohol use, distance from residence to hospital, MELD score, Child-Turcotte-Pugh class, health insurance status, comorbid psychiatric disorders, or beta blocker at time of discharge (Table 3). Similarly, no significant differences in demographic features were observed between those completing variceal obliteration and those without endoscopic follow-up following hospital discharge (Supplemental Table 1). In addition, despite codification of guidelines for management of gastroesophageal varices by the American Association for the Study of Liver Diseases in 2007[2], the proportion of patients who completed esophageal variceal obliteration before and after 2007 did not significantly differ.
Table 2.
Outcomes of the study population n (%)
| Demographics/baseline measures | Number (n = 99) |
| Incomplete obliteration | 66 (66) |
| No post-discharge endoscopy | 46 (46) |
| Completed obliteration | 33 (33) |
| Adhered to 1-yr surveillance | 12 (12) |
| Rehospitalized for variceal bleed | 46 (46) |
Table 3.
Characteristics of those completing variceal obliteration n (%)
| Measures | Obliteration (n = 33) | No obliteration (n = 66) | P value |
| Male | 18 (55) | 41 (62) | 0.5186 |
| Age, median (range), yr | 53 (34-78) | 54 (31-82) | 0.3934 |
| Etiology of cirrhosis | |||
| Alcohol | 19 (58) | 43 (65) | 0.5125 |
| Hepatitis C | 4 (12) | 8 (12) | 1.0000 |
| Other | 10 (30) | 15 (23) | 0.4657 |
| Recent alcohol use | 15 (45) | 31 (47) | 1.0000 |
| Distance from hospital, median (range), kilometers | 24.9 (0.8-80.3) | 20.9 (1.4-95.1) | 0.6892 |
| MELD score, median (range) | 12 (6-18) | 13.5 (7-27) | 0.1615 |
| Child-turcotte-pugh classification | |||
| A | 11 (33) | 13 (20) | 0.1451 |
| B | 17 (52) | 36 (54) | 0.8325 |
| C | 5 (15) | 17 (26) | 0.3080 |
| Index bleed in 2007 or earlier | 15 (45) | 29 (44) | 1.0000 |
| Medicaid1 or uninsured | 7 (21) | 24 (36) | 0.1687 |
| Comorbid psychiatric disorder | 9 (27) | 30 (45) | 0.1260 |
| Length of stay, median (range), d | 6 (2-33) | 5 (3-75) | 0.5619 |
| Beta-blocker at discharge | 30 (91) | 56 (85) | 0.5348 |
Government-sponsored health plan that assists with medical costs in selected low income individuals. MELD: Model for end-stage liver disease.
Adherence to surveillance endoscopy and clinical characteristics
Complete adherence to annual endoscopic surveillance was achieved in 12 of 99 patients (12%). Those adherent to annual endoscopic surveillance were more likely to be insured privately or through Medicare (a national health plan with universal coverage for age 65 or greater) when compared with those with incomplete adherence to annual surveillance (100% vs 64%, P = 0.016, Table 4) and to those without endoscopic follow up after hospital discharge (100% vs 63%, P = 0.0119, Table 5). Non-significant differences were observed in the proportion of patients who adhered to annual endoscopic surveillance (vs the proportion with incomplete adherence) when the index variceal bleed occurred in 2007 or earlier (17% vs 48%, P = 0.0607). There were otherwise no significant differences in the proportion of patients who adhered to surveillance endoscopy with respect to gender, age, etiology of cirrhosis, length of hospital stay, distance from residence to hospital, MELD score, Child-Turcotte-Pugh Class, recent alcohol use at time of admission, comorbid psychiatric disorders, or beta-blocker at time of discharge.
Table 4.
Characteristics of those completing variceal obliteration and surveillance at 1-yr intervals n (%)
| Measures | 1-yr surveillance (n = 12) | Incomplete adherence (n = 87) | P value |
| Male | 7 (58) | 52 (60) | 1.0000 |
| Age, median (range), yr | 55 (45-78) | 54 (31-82) | 0.1936 |
| Etiology of cirrhosis | |||
| Alcohol | 6 (50) | 56 (64) | 0.3560 |
| Hepatitis C | 2 (17) | 10 (12) | 0.6364 |
| Other | 4 (33) | 21 (24) | 0.4918 |
| Recent alcohol use | 15 (45) | 42 (48) | 0.3726 |
| Distance from hospital, median (range), kilometers | 30.4 (3.7-80.3) | 13.7 (0.8-95.1) | 0.3898 |
| MELD score, median (range) | 11.5 (6-17) | 13 (7-27) | 0.2543 |
| Child-turcotte-pugh classification | |||
| A | 3 (25) | 21 (24) | 1.0000 |
| B | 6 (50) | 47 (54) | 1.0000 |
| C | 3 (25) | 19 (22) | 0.7259 |
| Index bleed in 2007 or earlier | 2 (17) | 42 (48) | 0.0607 |
| Medicaid1 or uninsured | 0 (0) | 31 (36) | 0.0160 |
| Comorbid psychiatric disorder | 5 (42) | 34 (39) | 1.0000 |
| Length of stay, median (range), d | 6 (3-7) | 5 (2-75) | 0.8808 |
| Beta-blocker at discharge | 12 (100) | 74 (85) | 0.3572 |
Government-sponsored health plan that assists with medical costs in selected low income individuals. MELD: Model for end-stage liver disease.
Table 5.
Characteristics of those adherent to surveillance at 1-yr intervals vs no endoscopic follow up n (%)
| Measures | 1-yr surveillance (n = 12) | No post-discharge EGD (n = 46) | P value |
| Male | 7 (58) | 29 (63) | 0.7518 |
| Age, median (range), yr | 55 (45-78) | 54.5 (31-82) | 0.4237 |
| Etiology of cirrhosis, | |||
| Alcohol | 6 (50) | 28 (61) | 0.5273 |
| Hepatitis C | 2 (17) | 6 (13) | 0.6649 |
| Other | 4 (33) | 12 (26) | 0.7200 |
| Recent alcohol use | 4 (33) | 21 (46) | 0.5255 |
| Distance from hospital, median (range), kilometers | 30.4 (3.7-80.3) | 20.9 (1.5-95.1) | 0.4965 |
| MELD score, median (range) | 11.5 (6-17) | 13 (7-26) | 0.1707 |
| Child-turcotte-pugh classification | |||
| A | 3 (25) | 9 (20) | 0.6983 |
| B | 6 (50) | 27 (59) | 0.7455 |
| C | 3 (25) | 10 (21) | 1.0000 |
| Index bleed in 2007 or earlier | 2 (17) | 19 (41) | 0.1790 |
| Medicaid1 or uninsured | 0 (0) | 17 (37) | 0.0119 |
| Comorbid psychiatric disorder | 5 (42) | 19 (41) | 1.0000 |
| Length of stay, median (range), d | 6 (3-7) | 5 (3-75) | 0.6455 |
| Beta-blocker at discharge | 12 (100) | 38 (80) | 0.1851 |
Government-sponsored health plan that assists with medical costs in selected low income individuals. MELD: Model for end-stage liver disease; EGD: Esophagogastroduodenoscopy.
Rehospitalization for variceal bleeding
Rehospitalization for gastrointestinal variceal bleeding occurred in 46% of patients (median time to rehospitalization 250 d). More hospitalizations for variceal rebleeding occurred in those who did not complete obliteration (56% vs 27%, P = 0.0099), and these rehospitalizations occurred earlier (median 207 d vs 259 d, P = 0.0083, Figure 1). Incomplete adherence to endoscopic surveillance was associated with more rehospitalizations for variceal rebleeding compared to those fully adherent to annual endoscopic surveillance (51% vs 17%, P = 0.0328).
Figure 1.
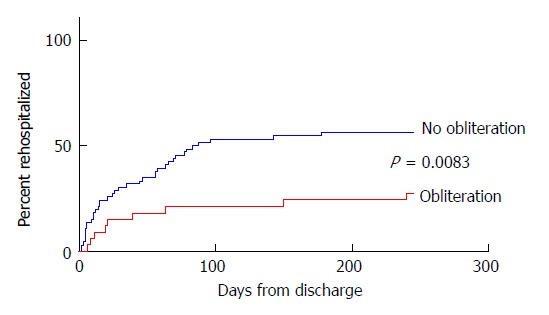
Time until rehospitalization for variceal rebleeding. The results shown represent the time from admission for index variceal esophageal variceal hemorrhage to rehospitalization for variceal rebleeding in those completing variceal obliteration (median 259 d to rehospitalization) and those not completing obliteration (median 207 d to rehospitalization, P = 0.0083).
Survival
Survival data were available for 88 of 99 (89%) patients. Of this population, 30 had transplant free survival at the close of the study (34%), 56 died (64%) and 2 patients underwent liver transplantation. Of the 66% who died or received liver transplantation, the median time to the event was 995 d (mean 1380 d). There were no significant differences in median time to death/transplant in those completing obliteration (median 1072 d, range 123 d-4917 d) compared with those not completing obliteration (median 1118 d, range 24-4549 d, P = 0.2585) and those without post-discharge endoscopy (median 679 d, range 24-4401 d, P = 0.9601).
DISCUSSION
Despite abundant evidence summarized in practice guidelines[2], concerning the efficacy of endoscopic management in survivors of esophageal variceal bleeding, a striking finding of our study is that 66% of patients did not complete variceal obliteration and even fewer adhered to endoscopic surveillance for this major cirrhosis-related problem. Moreover, patients who did not complete variceal obliteration were more likely to develop recurrent variceal bleeding and were rehospitalized significantly sooner for this complication. Similarly, those non-adherent to annual endoscopic variceal surveillance had significantly more rehospitalizations for variceal rebleeding. Collectively, these observations reinforce the importance of variceal obliteration and annual endoscopic surveillance for prevention of variceal rebleeding[2,5,12]. The suboptimal adherence to preventative therapies in the outpatient setting, as observed in our study, mirrors those of studies in hospitalized cirrhotic patients, which have demonstrated that a minority of such individuals receive evidence-based treatments to prevent disease related complications[13,14]. Two broad reasons may account for gaps in cirrhosis-related quality care: decisions made by health professionals, and decisions made by patients. In this study, the gaps were attributable to patient attendance at already scheduled endoscopic sessions. Our findings, in a real world setting, are consistent with the results of controlled clinical trials[2-4,12,15], which have demonstrated that variceal obliteration and surveillance variceal ligation in patients who have recovered from esophageal variceal hemorrhage reduces hospitalizations for recurrent bleeding from esophageal varices. In addition, our observations, which suggest that adherence to endoscopic surveillance did not influence transplant-free survival over the duration of the study, are consistent with the results of a large meta-analysis, which suggested that beta-blocker use (and not endoscopic intervention) is the dominant factor that improves survival rates following hospitalization for esophageal variceal bleeding[16].
The principal factor we found to be associated with decreased adherence to surveillance EGD and variceal obliteration was health plan coverage. In particular, we found that those uninsured or insured by Medicaid were significantly less likely to maintain full adherence to annual surveillance endoscopy. This observation mirrors an association between Medicaid and/or lack of health plan coverage and reduced adherence to health care interventions in other contexts. Specifically, among patients with cirrhosis, those insured by Medicaid have been shown to have a greater all-cause 30 d rehospitalization rate[17] and reduced adherence to hepatocellular carcinoma (HCC) surveillance imaging, with just 17% undergoing follow-up ultrasound in a 15-mo period[18]. Suboptimal utilization of preventative care is not unique to those with cirrhosis and Medicaid, as screening modalities for cervical cancer, breast cancer, and colon cancer[19-21] have all been shown to be under-utilized by those insured by Medicaid when compared to those privately insured. In 2012, the United States passed the Patient Protection and Affordable Care Act which allowed states to choose whether to expand Medicaid coverage. States which chose not to expand Medicaid had lower cancer screening rates, particularly amongst federally qualified health centers, while those expanding Medicaid had an increase in early stage cancer diagnosis in the working-age population[22], suggesting that reducing financial barriers to care by expanding public health insurance coverage increases utilization of services that have been shown to improve healthcare outcomes[23]. Additional socioeconomic factors that may interfere with utilization of preventative measures in this population include lack of transportation[24,25], work hours conflicting with medical office hours[26], and reduced health literacy[27]. Additionally, patient knowledge of their medical problems has been shown to correlate with improved adherence to HCC surveillance ultrasound[24], creating another barrier to adherence for a population at risk for reduced health literacy.
By contrast, factors shown in other contexts to influence adherence to medical management regimens, such as distance to the hospital[28,29], psychiatric comorbidities[30], and alcohol use[31], were not found to be associated with adherence to surveillance or completion of variceal obliteration in our study. This latter conclusion must be taken with caution, given that our study took place at a single center with a large rural referral population and the population’s median distance from residence to the hospital was small for a rural region. Additionally, the sample size may not have been sufficiently powered to detect a significant association between psychiatric disorders and endoscopic non-adherence.
Lessons can be learned from studies that have addressed suboptimal adherence to guideline-based measures in other health conditions. For example, cardiac rehabilitation after an index myocardial infarction, a class I A recommendation from the American College of Cardiology and American Heart Association, has been shown to reduce both mortality and rehospitalization at 1 year[32]. Cardiac rehabilitation utilization after a qualifying hospitalization has been shown to be low, with just 18.7% attending at least 1 session after hospital discharge[33]. In response, tools and programs have been developed to address this gap in care. One such tool has been an automatic electronic medical record based “opt out” referral system, which was shown to double cardiac rehabilitation attendance[34]. A similar increase in utilization of ultrasound screening for HCC was seen by mailing invitations to patients[35]. Financial incentives have been shown to increase cardiac rehabilitation adherence[32] and to increase smoking abstinence during pregnancy with high rates of sustained abstinence at 24 wk post-partum[36]. Another intervention that may improve adherence is minimizing lead-time between visits with providers and subsequently scheduled studies. Adherence to HCC surveillance ultrasound has been shown to correlate with shortened lead-times, prompting the authors to recommend scheduling ultrasounds on the same day as appointments with health professionals as a means to improve adherence by both reducing lead-time and transportation barriers[28]. These and other approaches should be considered to improve endoscopic adherence among patients with cirrhosis after an index esophageal variceal hemorrhage. This area is ripe for future study and is likely to translate into long term improvement of disease-related outcomes.
ARTICLE HIGHLIGHTS
Research background
Esophageal variceal hemorrhage is a significant complication of cirrhosis and is associated with a high mortality rate. Current guidelines recommend a combination of non-selective beta adrenergic receptor blockers with endoscopic variceal ligation as the most effective way of reducing variceal rebleeding. It is increasingly recognized that cirrhotic patients often do not receive evidence-based treatments for disease related complications.
Research motivation
Whereas prior studies have focused on gaps in cirrhosis-related quality care attributable to decisions made by healthcare professionals, this study focused specifically on patient factors which may impact adherence to endoscopic variceal surveillance. By identifying a patient population at risk of poor adherence, we hope to spur future studies which will assess interventions to promote adherence to improve disease-related outcomes.
Research objectives
We sought to identify potential factors, in a real world setting, which may prevent patients from completing variceal obliteration and adhering to surveillance endoscopy following their first esophageal variceal hemorrhage.
Research methods
We performed a retrospective review of the records of patients with cirrhosis admitted to the medical intensive care unit between 2000 and 2014 for first time esophageal variceal hemorrhage treated with endoscopic variceal ligation who were subsequently discharged and scheduled for surveillance endoscopy at our medical center. Demographic and clinical data were obtained through the medical records. Differences between groups were determined by Fisher’s exact test for categorical variables, by Mann-Whitney U test for continuous non-parametric variables, and by Student’s t test for continuous parametric variables. Kaplan-Meier curves for both survival and time to rehospitalization were compared using the log-rank test.
Research results
Of 99 patients included in the study, 33% completed variceal obliteration and 12% adhered to annual surveillance. Completion of variceal obliteration was associated with fewer rehospitalizations for variceal rebleeding (27% vs 56%, P = 0.0099) and when rehospitalizations occurred, they occurred later in those who had completed obliteration (median 259 d vs 207 d, P = 0.0083). Incomplete adherence to endoscopic surveillance was associated with more rehospitalizations for variceal rebleeding compared to those fully adherent to annual endoscopic surveillance (51% vs 17%, P = 0.0328). Those adherent to annual surveillance were more likely to be insured privately or through Medicare (a national government-sponsored health plan that provides universal coverage for age 65 or greater) compared to those who did not attend post-hospital discharge endoscopy (100% vs 63%, P = 0.0119).
Research conclusions
We found that the minority of survivors of esophageal variceal bleeding completed variceal obliteration and fewer adhered to annual surveillance. Those completing variceal obliteration had fewer and later rehospitalizations for variceal rebleeding. Incomplete adherence to endoscopic surveillance was associated with more frequent rehospitalizations for variceal rebleeding. Collectively, these observations reinforce the importance of variceal obliteration and annual endoscopic surveillance for prevention of variceal rebleeding. Incomplete adherence to endoscopic surveillance was associated with lack of health care insurance or health care insurance through Medicaid (a government-sponsored health plan that defrays medical expenses in selected low income individuals). This population has been shown in prior studies to have greater financial barriers to healthcare, poor health literacy, and limited transportation, all of which may be contributing to the decreased endoscopic adherence observed in our study.
Research perspectives
This study provides a link between health care coverage vulnerability, a marker of lower socioeconomic status, and reduced adherence to endoscopic surveillance following esophageal variceal bleeding. Future research should attempt to improve adherence in this population using interventions which have been shown to be successful in other fields, such as scheduling procedures on the same day as a preexisting appointment, using text message appointment reminders, or even using financial incentives.
ACKNOWLEDGMENTS
We would like to thank Philip Ades, Eric Ganguly, James Vecchio, and Richard Zubarik of UVMMC and Nicholas Lim of the University of Minnesota for their helpful discussions. We also thank the Jeffords Institute for Quality and Operational Effectiveness at UVMMC for assistance in assembling the subject database.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: The study design was reviewed and approved by the University of Vermont Institutional Review Board (CHRMS 15-134) with a waiver of informed consent.
Informed consent statement: Patients were not required to give informed consent to the study as the research involved no more than minimal risk to the individual. Waiver of consent was approved by the University of Vermont Institutional Review Board.
Conflict-of-interest statement: The authors declare no conflicts-of-interest related to this article.
Data sharing statement: No additional data are available.
Peer-review started: February 7, 2018
First decision: February 28, 2018
Article in press: April 11, 2018
P- Reviewer: Goral V, Ruiz-Margain A, Sterpetti AV S- Editor: Cui LJ L- Editor: A E- Editor: Huang Y
Contributor Information
Brendan T Everett, Gastroenterology and Hepatology Unit, University of Vermont Medical Center, Burlington, VT 05401, United States.
Steven D Lidofsky, Gastroenterology and Hepatology Unit, University of Vermont Medical Center, Burlington, VT 05401, United States. steven.lidofsky@uvm.edu.
References
- 1.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823–832. doi: 10.1056/NEJMra0901512. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 3.Bosch J, García-Pagán JC. Prevention of variceal rebleeding. Lancet. 2003;361:952–954. doi: 10.1016/S0140-6736(03)12778-X. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez R, Zamora J, Gomez-Camarero J, Molinero LM, Bañares R, Albillos A. Meta-analysis: Combination endoscopic and drug therapy to prevent variceal rebleeding in cirrhosis. Ann Intern Med. 2008;149:109–122. doi: 10.7326/0003-4819-149-2-200807150-00007. [DOI] [PubMed] [Google Scholar]
- 5.de la Peña J, Brullet E, Sanchez-Hernández E, Rivero M, Vergara M, Martin-Lorente JL, Garcia Suárez C. Variceal ligation plus nadolol compared with ligation for prophylaxis of variceal rebleeding: a multicenter trial. Hepatology. 2005;41:572–578. doi: 10.1002/hep.20584. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan PM, Kramer JR, El-Serag HB, Asch SM, Assioun Y, Bacon BR, Kanwal F. The quality of care provided to patients with varices in the department of Veterans Affairs. Am J Gastroenterol. 2014;109:934–940. doi: 10.1038/ajg.2013.487. [DOI] [PubMed] [Google Scholar]
- 7.Flemming JA, Saxena V, Shen H, Terrault NA, Rongey C. Facility- and Patient-Level Factors Associated with Esophageal Variceal Screening in the USA. Dig Dis Sci. 2016;61:62–69. doi: 10.1007/s10620-015-3865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh H, Targownik LE, Ward G, Minuk GY, Bernstein CN. An assessment of endoscopic and concomitant management of acute variceal bleeding at a tertiary care centre. Can J Gastroenterol. 2007;21:85–90. doi: 10.1155/2007/296435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlansky B, Lee B, Hartwell L, Urquhart J, Willis B, Zaman A. Guideline adherence and outcomes in esophageal variceal hemorrhage: comparison of tertiary care and non-tertiary care settings. J Clin Gastroenterol. 2012;46:235–242. doi: 10.1097/MCG.0b013e318227422d. [DOI] [PubMed] [Google Scholar]
- 10.Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 11.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 12.Lo GH, Lai KH, Cheng JS, Chen MH, Huang HC, Hsu PI, Lin CK. Endoscopic variceal ligation plus nadolol and sucralfate compared with ligation alone for the prevention of variceal rebleeding: a prospective, randomized trial. Hepatology. 2000;32:461–465. doi: 10.1053/jhep.2000.16236. [DOI] [PubMed] [Google Scholar]
- 13.Ghaoui R, Friderici J, Visintainer P, Lindenauer PK, Lagu T, Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014;34:204–210. doi: 10.1111/liv.12225. [DOI] [PubMed] [Google Scholar]
- 14.Lim N, Lidofsky SD. Impact of physician specialty on quality care for patients hospitalized with decompensated cirrhosis. PLoS One. 2015;10:e0123490. doi: 10.1371/journal.pone.0123490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnan A, Srinivasan V, Venkataraman J. Variceal recurrence, rebleeding rates and alterations in clinical and laboratory parameters following post-variceal obliteration using endoscopic sclerotherapy. J Dig Dis. 2012;13:596–600. doi: 10.1111/j.1751-2980.2012.00633.x. [DOI] [PubMed] [Google Scholar]
- 16.Albillos A, Zamora J, Martínez J, Arroyo D, Ahmad I, De-la-Peña J, Garcia-Pagán JC, Lo GH, Sarin S, Sharma B, et al. Stratifying risk in the prevention of recurrent variceal hemorrhage: Results of an individual patient meta-analysis. Hepatology. 2017;66:1219–1231. doi: 10.1002/hep.29267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singal AG, Rahimi RS, Clark C, Ma Y, Cuthbert JA, Rockey DC, Amarasingham R. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013;11:1335–1341.e1. doi: 10.1016/j.cgh.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer LB, Kappelman MD, Sandler RS, Hayashi PH. Surveillance for hepatocellular carcinoma in a Medicaid cirrhotic population. J Clin Gastroenterol. 2013;47:713–718. doi: 10.1097/MCG.0b013e318286fd97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuBard CA, Schmid D, Yow A, Rogers AB, Lawrence WW. Recommendation for and receipt of cancer screenings among medicaid recipients 50 years and older. Arch Intern Med. 2008;168:2014–2021. doi: 10.1001/archinte.168.18.2014. [DOI] [PubMed] [Google Scholar]
- 20.Guessous I, Dash C, Lapin P, Doroshenk M, Smith RA, Klabunde CN; National Colorectal Cancer Roundtable Screening Among the 65 Plus Task Group. Colorectal cancer screening barriers and facilitators in older persons. Prev Med. 2010;50:3–10. doi: 10.1016/j.ypmed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Peterson NB, Han J, Freund KM. Inadequate follow-up for abnormal Pap smears in an urban population. J Natl Med Assoc. 2003;95:825–832. [PMC free article] [PubMed] [Google Scholar]
- 22.Soni A, Hendryx M, Simon K. Medicaid Expansion Under the Affordable Care Act and Insurance Coverage in Rural and Urban Areas. J Rural Health. 2017;33:217–226. doi: 10.1111/jrh.12234. [DOI] [PubMed] [Google Scholar]
- 23.Choi SK, Adams SA, Eberth JM, Brandt HM, Friedman DB, Tucker-Seeley RD, Yip MP, Hébert JR. Medicaid Coverage Expansion and Implications for Cancer Disparities. Am J Public Health. 2015;105 Suppl 5:S706–S712. doi: 10.2105/AJPH.2015.302876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, Yopp AC, Parikh ND, Marrero JA, Singal AG. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875–884. doi: 10.1002/hep.28770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Norton EC, Stearns SC. Transportation brokerage services and Medicaid beneficiaries’ access to care. Health Serv Res. 2009;44:145–161. doi: 10.1111/j.1475-6773.2008.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastroberti M, Stein JE. Barriers to timely mammography. HMO Pract. 1996;10:104–107. [PubMed] [Google Scholar]
- 27.Patel MR, Kruger DJ, Cupal S, Zimmerman MA. Effect of Financial Stress and Positive Financial Behaviors on Cost-Related Nonadherence to Health Regimens Among Adults in a Community-Based Setting. Prev Chronic Dis. 2016;13:E46. doi: 10.5888/pcd13.160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, Baytarian M, Fox R, Hunt K, Pedrosa M, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65:864–874. doi: 10.1002/hep.28765. [DOI] [PubMed] [Google Scholar]
- 29.Endo N, Goto A, Suzuki T, Matsuda S, Yasumura S. Factors associated with enrollment and adherence in outpatient cardiac rehabilitation in Japan. J Cardiopulm Rehabil Prev. 2015;35:186–192. doi: 10.1097/HCR.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 30.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 31.Mancebo A, González-Diéguez ML, Navascués CA, Cadahía V, Varela M, Pérez R, Rodrigo L, Rodríguez M. Adherence to a Semiannual Surveillance Program for Hepatocellular Carcinoma in Patients With Liver Cirrhosis. J Clin Gastroenterol. 2017;51:557–563. doi: 10.1097/MCG.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 32.Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, Shepard DS, Thomas RJ. Increasing Cardiac Rehabilitation Participation From 20% to 70%: A Road Map From the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466. [DOI] [PubMed] [Google Scholar]
- 34.Grace SL, Russell KL, Reid RD, Oh P, Anand S, Rush J, Williamson K, Gupta M, Alter DA, Stewart DE; Cardiac Rehabilitation Care Continuity Through Automatic Referral Evaluation (CRCARE) Investigators. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med. 2011;171:235–241. doi: 10.1001/archinternmed.2010.501. [DOI] [PubMed] [Google Scholar]
- 35.Singal AG, Tiro JA, Marrero JA, McCallister K, Mejias C, Adamson B, Bishop WP, Santini NO, Halm EA. Mailed Outreach Program Increases Ultrasound Screening of Patients With Cirrhosis for Hepatocellular Carcinoma. Gastroenterology. 2017;152:608–615.e4. doi: 10.1053/j.gastro.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cahill K, Hartmann-Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database Syst Rev. 2015:CD004307. doi: 10.1002/14651858.CD004307.pub5. [DOI] [PubMed] [Google Scholar]


