Abstract
Laminarin, a linear β-1,3 glucan (mean degree of polymerization of 33) was extracted and purified from the brown alga Laminaria digitata. Its elicitor activity on tobacco (Nicotiana tabacum) was compared to that of oligogalacturonides with a mean degree of polymerization of 10. The two oligosaccharides were perceived by suspension-cultured cells as distinct chemical stimuli but triggered a similar and broad spectrum of defense responses. A dose of 200 μg mL−1 laminarin or oligogalacturonides induced within a few minutes a 1.9-pH-units alkalinization of the extracellular medium and a transient release of H2O2. After a few hours, a strong stimulation of Phe ammonia-lyase, caffeic acid O-methyltransferase, and lipoxygenase activities occurred, as well as accumulation of salicylic acid. Neither of the two oligosaccharides induced tissue damage or cell death nor did they induce accumulation of the typical tobacco phytoalexin capsidiol, in contrast with the effects of the proteinaceous elicitor β-megaspermin. Structure activity studies with laminarin, laminarin oligomers, high molecular weight β-1,3–1,6 glucans from fungal cell walls, and the β-1,6–1,3 heptaglucan showed that the elicitor effects observed in tobacco with β-glucans are specific to linear β-1,3 linkages, with laminaripentaose being the smallest elicitor-active structure. In accordance with its strong stimulating effect on defense responses in tobacco cells, infiltration of 200 μg mL−1 laminarin in tobacco leaves triggered accumulation within 48 h of the four families of antimicrobial pathogenesis-related proteins investigated. Challenge of the laminarin-infiltrated leaves 5 d after treatment with the soft rot pathogen Erwinia carotovora subsp. carotovora resulted in a strong reduction of the infection when compared with water-treated leaves.
The outcome of any plant/pathogen interaction depends on complex cascades of recognition, attack, and defense reactions at the plant/microbe interface. Within minutes of pathogen recognition, a variety of early events occurs in the host, such as ion fluxes across the plasma membrane, cascades of phosphorylations and dephosphorylations, and production of reactive oxygen species (Dixon et al., 1994). Within hours these events are followed by a broad spectrum of metabolic modifications that include: (a) stimulation of the phenylpropanoid and fatty acid pathways, (b) production of defense-specific chemical messengers such as salicylic acid (SA) or jasmonates, and (c) accumulation of components with antimicrobial activities such as phytoalexins and pathogenesis-related (PR) proteins (Kombrink and Somssich, 1995).
Signal molecules from the pathogen or from the host that are able to trigger defense responses are known as elicitors. Many of the elicitors of defense reactions in plants are oligosaccharides, and are among the first biologically active oligosaccharides (oligosaccharins) to be characterized (Albersheim et al., 1983). Only a few such oligosaccharins have been fully characterized in terms of structure and spectrum of biological activities. These oligosaccharins include β-1,3–1,6 glucans, xyloglucans, oligogalacturonides, and chitin-derived oligomers (Côté and Hahn, 1994). The relevance of some of these oligosaccharides as biological signals acting in vivo in the defense system is supported by their possible natural occurrence during plant-microbe interactions (Fritig et al., 1998). There is some evidence that oligosaccharides are able to enhance non-host plant resistance against pathogens, and it is thought that mimicking pathogen attack with such nonspecific elicitors could become an alternative strategy in crop plant protection (Lyon et al., 1996).
β-1,3–1,6 Glucans were recognized to be actively involved in plant-pathogen interactions during the mid-1970s (Ayers et al., 1976a). Evidence was then obtained from crude or only partially purified fungal cell wall fractions, such as the Phytophthora sojae cell wall hydrolysate (referred to as Psg) glucan, a heterogeneous β-1,3–1,6 glucan extracted from the mycelial walls of P. sojae f. sp. glycinea (Sharp et al., 1984a). The elicitor activity of the Psg glucan was mainly studied in leguminous plants but it was also reported to induce a Gly-rich protein in tobacco (Nicotiana tabacum) (Brady et al., 1993) and antiviral protection (Kopp et al., 1989). A pure glucan heptasaccharide (Sharp et al., 1984b) prepared from Psg glucan induced the synthesis of phytoalexins in soybean (Glycine max) (Sharp et al., 1984a). The minimal structural requirements for the elicitation of phytoalexin synthesis in soybean by this glucan were established as a succession of five β-1,6-linked glucosyl residues with two side branches of β-1,3 Glc (Cheong et al., 1991). Specific binding sites for the β-1,6–1,3 heptaglucan from P. sojae have since been described in soybean (Cheong et al., 1991), alfalfa (Medicago sativa), bean (Phaesoleus vulgarus), lupine (Lupinus albus), and pea (Pisum sativum) (Cosio et al., 1996; Côté et al., 2000).
Yet it should not be overlooked that β-glucans are found in numerous, distinct structural features. It was shown that, depending on the defense response under investigation, the oligosaccharide structures recognized by the plant cells were not the same, even among preparations of fungal origin (Rouhier et al., 1995). Moreover, Kobayashi et al. (1993) reported the induction of phytoalexin biosynthesis in alfalfa by a pyridylaminated hepta-β-1,3–1,6 linear glucan derived from brown algal laminarin, the non-modified hepta-β-glucoside being far less active. This glucan also proved to be active in the bean cotyledon assay (Tai et al., 1996a) and the essential minimal structure for biological activity was shown to be a β-1,3–1,6 triglucoside (Tai et al., 1996b). Cyclic β-1,3–1,6 glucans, from the symbiotic bacterium Bradyrhizobium japonicum, were also shown to elicit isoflavonoid production in soybean (Miller et al., 1994), whereas Mithöfer et al. (1996) found a suppressor activity of phytoalexin production by this β-glucan. In rice (Oryza sativa) suspension cells, β-1,3 glucan oligomers with a degree of polymerization (DP) over 4 were shown to stimulate chitinase activity, and DP 6 also acted as an elicitor of Phe ammonia-lyase (PAL) activity (Inui et al., 1997). Glucans were reported to enhance resistance against viruses (Kopp et al., 1989) or fungi (Reglinski et al., 1994).
In the above-mentioned studies, however, only a few defense markers were investigated, so that no causal link can be drawn between the elicitation of defense responses by glucans and the establishment of resistance. In this context, we have undertaken an investigation of the elicitation of defenses as well as the stimulation of resistance in tobacco after application of laminarin, an essentially linear glucan composed of ca. 33 β-1,3-linked Glc residues. We report here a comprehensive analysis of the induced defense reactions, including extracellular medium alkalinization, release of H2O2, stimulation of PAL activity and lipoxygenase (LOX) activity, and accumulation of SA. The spectrum of elicitor activity of laminarin was compared with that of oligogalacturonides, well-known oligosaccharins that have been shown to induce defense-related responses in several plants (Côté et al., 1998). The results indicate that both laminarin and β-1,3 glucan oligomers are elicitors of defense reactions in tobacco cells as potent as oligogalacturonides. We also show that tobacco leaves infiltrated with laminarin synthesize PR proteins and develop a resistance against infection by the bacterial pathogen Erwinia carotovora subsp. carotovora.
RESULTS
Laminarin Induces a Strong, Rapid, and Saturable Extracellular Alkalinization Response in Tobacco cv Bright Yellow (BY) Cells
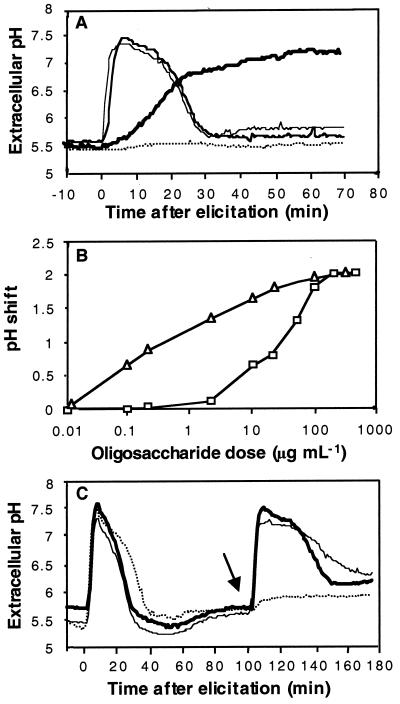
Tobacco cell suspension cultures responded to incubation with purified laminarin with a rapid and transient alkalinization of the incubation medium (Fig. 1A). Medium alkalinization was detected as early as 30 s after addition of laminarin, with the maximum pH shift reached after 5 min. It was followed by a gradual re-acidification of the culture medium, back to its original pH value, 30 min after addition of the elicitor. The kinetics of extracellular medium alkalinization were parallel to those recorded for oligogalacturonides. In particular, the maximal pH shift observed for laminarin, 1.9 pH units, was as high as that induced by a saturating concentration of oligogalacturonides. In contrast, necrosis-inducing concentrations of the basic elicitin, β-megaspermin, induced a late but sustained alkalinization of the suspension medium (Fig. 1A).
Figure 1.
Alkalinization of the culture medium of tobacco cv BY suspension cells in response to elicitation with laminarin or with oligogalacturonides. Representative data from 1 of 5 replicates are presented. A, Time courses of pH variation after treatment of suspension cells at time 0 with 200 μg mL−1 laminarin (light line), 200 μg mL−1 oligogalacturonides (medium line), or 50 nm β-megaspermin (bold line). Controls consisted of cells treated with the same volume of water (dotted line). B, Alkalinization dose responses in tobacco cells treated with laminarin (▫) or with oligogalacturonides (▵). The basal pH value of the culture medium was 5.5. C, Refractory state analysis. After a first treatment with a given oligosaccharide at time 0, the same or a different oligosaccharide fraction was added at the time indicated by the arrow. The different combinations were: 200 μg mL−1 laminarin followed by 200 μg mL−1 laminarin (dotted line), 200 μg mL−1 laminarin followed by 200 μg mL−1 oligogalacturonides (light line), and 200 μg mL−1 oligogalacturonides followed by 200 μg mL−1 laminarin (bold line).
The alkalinization dose response curves obtained with laminarin and oligogalacturonides are compared in Figure 1B. The maximal pH shift was reached with 200 μg mL−1 of laminarin and with 20 μg mL−1 of oligogalacturonides. No pH shift was observed with concentrations of laminarin of 0.1 μg mL−1, whereas the same dose of oligogalacturonides still induced a 0.6-pH-unit alkalinization . The molecular concentrations required to induce a half-maximal alkalinization response were 6.6 μm (35 μg mL−1) and 224 nm (0.4 μg mL−1) for laminarin and oligogalacturonides, respectively. In all experiments, the alkalinization kinetics remained similar for the two oligosaccharides over the complete concentration range.
To investigate the presence of two distinct perception systems for oligogalacturonides and laminarin, tobacco cv BY cells were monitored for the establishment of a refractory state, as described by Felix et al. (1998). After an initial treatment with 200 μg mL−1 of laminarin, a subsequent addition of the same oligosaccharide at the end of the pH shift did not trigger a second alkalinization response, whereas these cells remained fully reactive to oligogalacturonides (Fig. 1C). An initial stimulation with oligogalacturonides similarly did not prevent a subsequent response to laminarin.
Laminarin Induces a Broad Range of Defense-Related Responses in Tobacco Cells
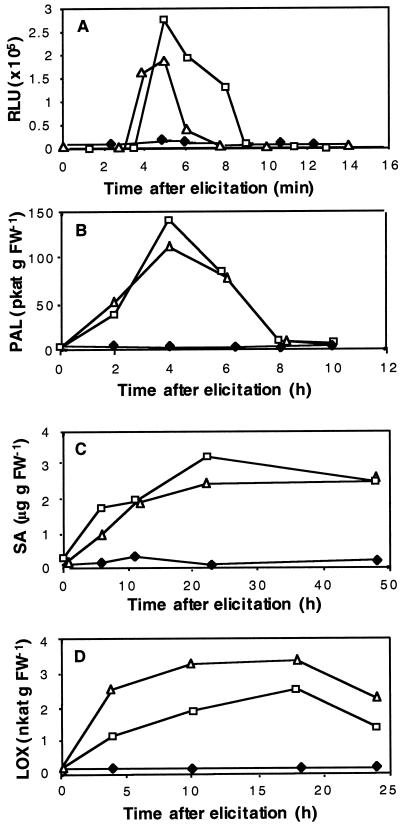
Extracellular medium alkalinization is an early event that may or may not be associated with the elicitation of other responses (Felix et al., 1993). The question of whether the perception of laminarin, as shown by extracellular alkalinization, was followed by induction of defense responses was therefore addressed. To this end, tobacco cells were challenged by either laminarin or oligogalacturonides at the saturating concentration of 200 μg mL−1, then assayed for a variety of typical defense markers. Cells responded to the addition of these elicitors with a rapid release of H2O2. This burst lasted for several minutes (Fig. 2A) and no further H2O2 release was observed during the next 3-h period (data not shown). Treatment of the cells with diphenyleneiodonium, an inhibitor of NADPH oxidase (O'Donnell et al., 1993), completely abolished the elicitor-induced release of H2O2 (data not shown). A strong and transient induction of PAL activity was observed upon treatment with laminarin or oligogalacturonides, with a maximum activity at 4 h after the addition of elicitors (Fig. 2B). Caffeic acid O-methyltransferase (COMT), one of the enzymes involved in lignin synthesis (Maury et al., 1999), was up-regulated 2-fold as early as 2 h after treatment and for at least 25 h (data not shown). The level of SA, a defense secondary signal derived from the phenylpropanoid pathway (Mauch-Mani and Slusarenko, 1996), was low in control tobacco cv BY cells, but increased sharply in response to the addition of either laminarin or pectin oligosaccharides within 6 h and remained high for at least 48 h (Fig. 2C).
Figure 2.
Defense reactions of tobacco cv BY- suspended cells in response to elicitation with laminarin or with oligogalacturonides. Time course curves of H2O2 release into the culture medium (A), PAL activity (B), SA accumulation (C), and LOX activity (D) in tobacco cv BY cells treated with 200 μg mL−1 laminarin (▫), 200 μg mL−1 oligogalacturonides (▵), or water (♦). Representative data from 1 of 4 replicates are presented.
LOX activity, a putative control point for fatty acid-derived signaling pathways (Blée, 1998), was also stimulated within 4 h of incubation with laminarin or oligogalacturonides, with the maximal induction reached between 10 and 20 h after treatment (Fig. 2D). Capsidiol, one of the major sesquiterpenoid phytoalexins in tobacco (Kuc, 1995), was reported to be produced in high amounts in tobacco cells treated with the elicitin cryptogein (Milat et al., 1991). We observed capsidiol in tobacco cells treated with the elicitin β-megaspermin, whereas none was detectable after treatments with laminarin (up to 200 μg mL−1) even after several days (data not shown).
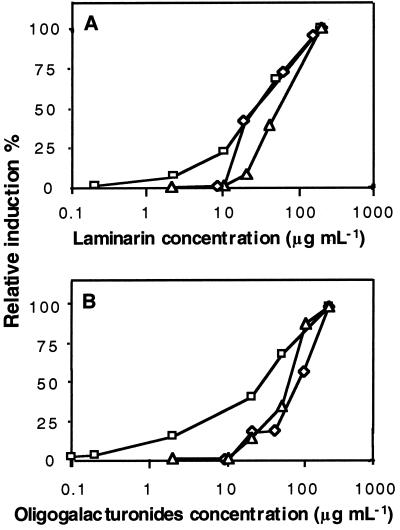
The response of defense markers PAL, SA, and LOX to laminarin or oligogalacturonides was determined by dose response experiments. In contrast with the extracellular medium alkalinization response, the dose response curves of both oligosaccharides were very similar (Fig. 3). It is interesting that PAL induction was not tightly correlated with the increases in SA levels. Although PAL was clearly stimulated at oligosaccharide doses as low as 2 to 10 μg mL−1, doses higher than 10 μg mL−1 were required to induce a detectable increase in SA. Induction of LOX activity also required an elicitor threshold concentration exceeding 10 μg mL−1.
Figure 3.
Dose dependence of defense responses in tobacco cv BY cells treated with laminarin (A) or with oligogalacturonides (B). PAL activity (▫), LOX activity (⋄), SA accumulation (▵). An arbitrary value of 100 was given to the maximal induction level (see Fig. 2) of these defense markers in response to a saturating oligosaccharide concentration of 200 μg mL−1. For each applied concentration of a given oligosaccharide, the relative inductions of the defense markers are given as a percentage of these maximal induction levels. Representative data from 1 of 3 replicates are presented.
Laminaripentaose Is the Shortest Linear β-1,3 Glucan with Elicitor Activity in Tobacco Cells
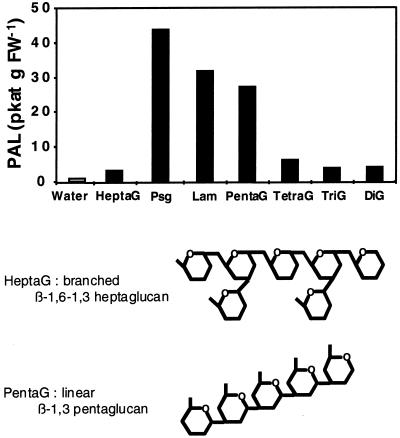
To better understand the structure-activity relationships of β-1,3 glucan oligosaccharides in eliciting defense reactions in tobacco, laminarin and linear β-1,3 glucans of various polymerization degrees, as well as glucan fractions obtained by the partial hydrolysis of mycelial walls of P. sojae f.sp glycinea, were compared for their PAL-inducing activity. The Psg, an efficient inducer of the accumulation of phytoalexins in the soybean cotyledon assay, contains linear β-1,3 glucans as well as β-1,3–1,6-branched glucan fragments, ranging in size from short oligomers to Mr as high as 100,000 (Sharp et al., 1984a). At the concentration of 50 μg mL−1, the half-maximum PAL-stimulating dose for laminarin, the Psg crude glucan fraction displayed an elicitor activity comparable to that of laminarin (Fig. 4). It is surprising, however, that the β-1,6–1,3 heptaglucan purified from the Psg glucan, shown to be the smallest structural motif required for elicitor activity in soybean (Sharp et al., 1984b), was not active in tobacco. In contrast, laminaripentaose, i.e. the laminarin oligomer made of five β-1,3-linked glucosyl residues, was a potent elicitor of PAL activity in tobacco cells. The laminarin tetramer and trimer, however, displayed weak and no activity, respectively.
Figure 4.
Structure-activity relationships of glucans in eliciting PAL activity in tobacco cells. The glucan fractions included: laminarin (Lam), laminarin oligomers, namely laminaripentaose through laminaribiose (referred to as pentaG through diG); a mycelial, cell wall extract from P. sojae (Psg) obtained as described by Ayers et al. (1976b); and the β-1,6–1,3 glucan heptasaccharide (HeptaG) purified from the latter glucan fraction (Sharp et al., 1984a, 1984b). The structures of the β-1,6–1,3 heptaglucan and the laminarin DP5 are represented. The induction of PAL activity was measured 4 h after the addition of glucans, applied at 50 μg mL−1. Representative data from 1 of 2 replicates are presented.
Laminarin Triggers the Accumulation of PR Proteins in Tobacco Leaves But Does Not Induce Cell Death
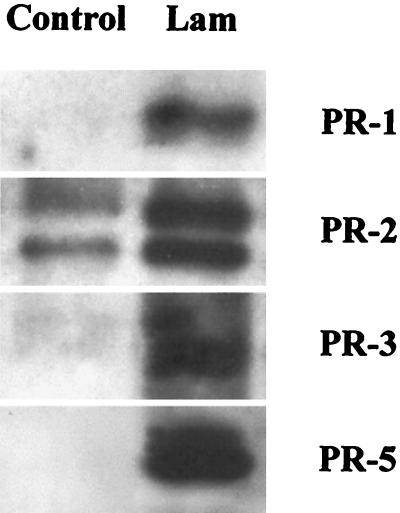
The relevance of laminarin as an elicitor of defense responses in planta was examined using tobacco cv Samsun NN plants. The accumulation of four families of PR proteins that are typical markers of defense responses in tobacco (Stintzi et al., 1993; Fritig et al., 1998) was monitored by immunodetection in extracts from leaves 48 h after infiltration with 200 μg mL−1 of laminarin. The oligosaccharide elicitor induced the production of the four examined PR families (Fig. 5): PR1, PR2 (glucanases), PR3 (chitinases), and PR5 (thaumatin-like proteins).
Figure 5.
Accumulation in tobacco leaves of PR proteins determined by immunodetection 48 h after infiltration with laminarin. Leaves were infiltrated at discrete spots with water (Control) or with 200 μg mL−1 laminarin (Lam), allowed to recover for 2 d, and protein was extracted from the infiltrated areas. Proteins were separated by SDS-PAGE, electrotransfered to a nylon membrane, and immunodetected with antibodies raised against the PR proteins families known as PR-1, PR-2, PR-3, and PR-5, respectively (Dorey et al., 1998). Data are representative of two independent experiments.
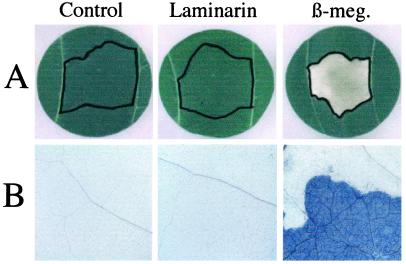
In contrast with the situation resulting from a treatment with the hypersensitive response (HR)-inducing elicitin, β-megaspermin, visual inspection of water- or laminarin-infiltrated leaf tissue revealed no damage or cell death (Fig. 6A). Because some situations of systemic acquired resistance have been related to the presence of micro-HR lesions (Alvarez et al., 1998), microscopic examination was also performed after staining with trypan blue. As shown for the positive control, β-megaspermin, dead cells were revealed by their inability to exclude the trypan blue dye and thus appeared as more intensely stained blue (Fig. 6B). No micro-HR lesions in the mesophyll tissue were detected after infiltration with water or laminarin.
Figure 6.
Treatment of tobacco leaves with laminarin causes no tissue damage or cell death. Representative data of three independent experiments are shown. A, Tobacco leaves were infiltrated (zones delineated with black pencil) with 200 μL of 200 μg mL−1 laminarin, water (Control), or with 50 nm of β-megaspermin (β-meg.), a necrosis-inducing proteinaceous elicitor used as a positive control. The photographs were taken 5 d after infiltration. B, Microscopic examination (15× magnification) after trypan blue staining of the corresponding infiltrated zones in A. The microphotographs are cropped to show a portion of an infiltrated necrotic zone (bright blue) and a portion of the surrounding zone.
Laminarin Protects Tobacco Leaves against the Pathogenic Bacterium E. carotovora
The question of whether the induction of defense reactions in tobacco results in a protection against pathogens was then investigated using the bacterium E. carotovora. Tobacco cv Samsun NN plants were injected with water or with a laminarin solution (one leaf per plant), allowed to recover for 5 d, and were then reinoculated with the pathogenic bacterium. Results obtained in two independent experiments are shown in Table I. For 18 of the 21 control plants (water infiltrated), marked maceration characteristic of soft rot symptoms (Fig. 7, left) occurred and spread from the inoculated area, almost covering the whole leaf by 24 h following bacterial inoculation. In contrast, upon injection with laminarin, only six of the treated plants showed maceration symptoms, whereas the other 15 plants exhibited minor symptoms of infection only, i.e. a slightly translucent area restricted to the zone infiltrated with the bacteria (Fig. 7, right). The maceration symptoms characteristic of the soft rot disease observed in most of the control leaves and in one-third of the laminarin-treated leaves were accompanied by a 10-fold increase in the bacterial populations in planta, whereas the bacterial populations in the leaves protected by laminarin (restricted symptoms) were not significantly different from those of uninoculated plants (Table I). Thus in those leaves treated with laminarin that showed no maceration symptoms, there was also no multiplication of E. carotovora in the infiltrated zone.
Table I.
Infiltration with laminarin protects tobacco against soft-rot disease
| No. of Plants
|
Symptoms | Bacterial Population (× 106)
|
|||
|---|---|---|---|---|---|
| Experiment A | Experiment B | Experiment A | Experiment B | ||
| Water-treated plants | 12/12 | 9 /9 | None | 1.6 ± 0.3 | 2.5 ± 0.5 |
| Water-treated plants plus E. carotovora | 11/12 | 7 /9 | Maceration | 21 ± 6 | 20 ± 4.8 |
| 1/12 | 2 /9 | None | 1.5 ± 0.5 | 1.1 ± 0.7 | |
| Laminarin-treated plants plus E. carotovora | 4/12 | 2 /9 | Maceration | 19 ± 4.5 | 20 ± 4.5 |
| 8/12 | 7 /9 | Restricted symptoms | 1.1 ± 0.1 | 1.4 ± 0.4 | |
Leaves from 30-d-old tobaccos were infiltrated with water or laminarin before inoculation with a suspension of E. carotovora 5 d later or were infiltrated with water only. Symptoms (see Fig. 7) and bacterial multiplication were determined 24 h after bacterial inoculation. Results of two independent experiments are presented.
Figure 7.

Laminarin-induced restriction of soft rot disease symptoms in tobacco. Left, Characteristic maceration symptoms observed on leaves of 30-d-old tobacco plants 24 h after infiltration with a suspension of E. carotovora. Right, Restricted symptoms observed on a portion (see Table I) of plants pretreated with 50 μL of 200 μg mL−1 laminarin and infiltrated 5 d later into the same zones with E. carotovora. Pictures were taken 24h after bacterial inoculation.
DISCUSSION
Laminarin Induces a Variety of Early- and Late-Defense Responses in Tobacco-Suspended Cell Cultures
A large array of defense responses was induced in tobacco cv BY cells following treatment with laminarin. The immediate responses were the extracellular alkalinization of the culture medium (Fig. 1), indicating the activation of ionic fluxes, followed by a NADPH oxidase-dependent release of H2O2 in the culture medium (Fig. 2A). Later responses, i.e. within hours of the addition of the elicitor, included the activation of the phenylpropanoid pathway, shown by a marked enhancement of PAL activity (Fig. 2B) and by the stimulation of COMT, as well as by the sustained accumulation of the signal molecule SA (Fig. 2C). Laminarin similarly activated the fatty acid pathway, as shown by the marked increase in LOX activity (Fig. 2D). These observations show that elicitation with laminarin does mobilize the metabolic machinery of tobacco cells, triggering a variety of events characteristic of signal perception and the initiation and development of the multiple-step cascade of defense responses in plants.
Although they were perceived as distinct chemical stimuli (Fig. 1C), β-1,3 glucans and oligogalacturonides had globally similar elicitor effects, both in terms of kinetics and amplitude of stimulation of defense responses (Fig. 2). At the saturating dose, however, laminarin was slightly more efficient in inducing the oxidative burst, stimulating PAL activity and increasing SA levels, whereas oligogalacturonides were better inducers of LOX activity. In dose response studies the two oligosaccharide elicitors showed strikingly similar effects (Fig. 3), except for the induction of extracellular medium alkalinization (Fig. 1B). Culture medium alkalinization in response to various doses of β-1,3 glucans correlated well with PAL induction. In contrast, oligogalacturonides at concentrations lower than 0.2 μg mL−1 triggered a 0.6-pH-unit shift with no further induction of later defense responses. Such an elicitor perception not followed by the triggering of defense responses has been observed in chitin-treated tomato (Lycopersicon esculentum) cells (Felix et al., 1993) and in oligogalacturonide-treated tobacco cells (Lapous et al., 1998). Therefore, observation of extracellular alkalinization is not systematically correlated to the activation of downstream defense events. It is interesting that for both oligosaccharides, PAL activity was induced at elicitor doses markedly lower than those required for the stimulation of LOX activity and SA accumulation (Fig. 3). This result indicates that a threshold of PAL activity has to be reached before SA accumulates.
Laminarin Induces the Accumulation of PR Proteins and Stimulates the Resistance of Tobacco Leaves against Microbial Diseases
Consistent with its strong elicitor activity in suspended cell cultures, laminarin also induced defense reactions in tobacco leaf tissue. A marked overproduction was observed within 2 d of treatment for all of the four families of PR proteins that were tested (Fig. 5). These PR proteins have well-described antimicrobial activities against different pathogens (Fritig et al., 1998). Based on their hydrolytic activities (Van Loon and Van Strien, 1999), they might also play an important role in the amplification of defense reactions through release of β-1,3 glucans (Keen and Yoshikawa, 1983) and chitin oligosaccharides (Kurosaki et al., 1988) de novo from the pathogen and host cell walls.
Using soft rot disease as a model pathosystem, we show here that infiltration of laminarin into leaf tissue protects tobacco against local infections by the pathogenic bacterium E. carotovora. Bacteria did not spread beyond the inoculated zone and their multiplication was inhibited in planta (Fig. 7, Table I). This result is consistent with the observations of Palva et al. (1992), who showed that the E. carotovora cell number in tobacco is proportional to the severity of symptoms, with the bacteria spreading in the plant tissue along with the maceration front. Such a protective effect of laminarin is reminiscent of those observed after treatment of plants with systemic acquired resistance inducers SA (Palva et al., 1994) or benzothiadiazole and 2,6-dichloroisonicotinic acid (Siegrist et al., 1997). These treatments enhanced the defense markers' PR proteins, and they were correlated with a strong reduction of infection symptoms and bacterial growth in planta. As demonstrated recently, oligosaccharides may exert a direct control of pathogenicity of invading microorganisms. Bouarab et al. (1999) have shown that specific oligocarrageenan structures triggered the synthesis of virulence proteins and enhanced pathogenecity of the green alga Acrochaete operculata. Yet because coinoculation of E. carotovora with laminarin did not affect the bacterium virulence (data not shown) and as shown by the accumulation in tobacco leaves of various antimicrobial PR proteins, it is likely that the laminarin-induced resistance to E. carotovora results from the stimulation of the plant natural defense metabolism.
Despite its marked effects on the defense system of tobacco cells and leaves, laminarin induced no HR, even at the microscopic level (Fig. 6). This finding is consistent with findings by others that resistance to pathogen invasion is not necessarily associated with cell death (Yu et al., 1998; Gilchrist, 1999). Treatments with either SA (Palva et al., 1994) or benzothiadiazole and 2,6-dichloroisonicotinic acid (Siegrist et al., 1997) were capable of conferring protection against bacteria without inducing HR.
Linear β-1,3 Glucan Oligosaccharides Are Potent Defense Signals in Plants
Altogether it appears that laminarin, like oligogalacturonides, is a potent elicitor in tobacco of a multi-component panel of defense reactions that mimics the responses observed during natural resistance (Kombrink and Somssich, 1995) and enhances resistance to bacterial invasion. Laminarin consists of linear, mannitol- or Glc-terminated chains of β-1,3-linked Glc residues, with occasional β-1,6-linked branches (Maeda and Nisizawa, 1968; Elyakova and Zvyagintseva, 1974; Usui et al., 1979; Read et al., 1996). The DPs of laminarins range from 7 to 19 and 16 to 31, respectively, indicating a structural polydispersity, from essentially linear β-1,3 glucans to branched β-1,3-β-1,6 glucans with an average of three branches per molecule. In particular, the laminarin from Laminaria digitata was reported to contain less than 2% of β-1,6-linked glucosyl residues (Nelson and Lewis, 1974), a feature confirmed by the high-performance anion-exchange chromatography with pulsed-amperometric detection (HPAEC-PAD) and NMR analyses of the laminarin samples investigated here (Lépagnol-Descamps et al., 1998).
Therefore, the structure-activity relationships in Figure 4 suggest that the glucan oligosaccharide motifs recognized by tobacco as defense signals are linear with β-1-3 linkages. This conclusion is supported by the fact that a linear chain of five β-1,3-linked Glc units triggered a significant PAL activity, whereas a β-1,6–1,3 heptaglucan consisting of five β-1,6-linked glucosides with two β-1,3 side branches was not active. The rather small minimal length for recognition of β-glucan elicitors in tobacco, five Glc residues, is consistent with other observations indicating that oligoglucans shorter than seven glycosidic residues are elicitor active in various unrelated plants, including soybean (Cheong et al., 1991; Miller et al., 1994), bean (Tai et al., 1996b), alfalfa (Kobayashi et al., 1993), and rice (Inui et al., 1997). Based on the induction of PAL activity, linear β-1,3 glucans were also elicitor active in wheat (Triticum aestivum) and tomato but not in parsley (Petroselinum crispum) cultured cells (data not shown).
The biological relevance for the involvement of linear β-1,3 glucan oligomers in pathogen recognition by plants stems from the fact that, besides cellulose and chitin, fungal cell walls are made of glucans that mainly consist of a backbone of β-1,3 Glc residues with only a few β-1,6 branches (Ruiz-Herrera, 1992). In addition, callose is deposited at the infection site within minutes of pathogen invasion of the plant (Kauss, 1985), and it might be a relevant source of β-1,3-linked glucan oligomers of plant origin. Glucanases secreted by the host and the pathogen, possibly regulated by glucanase inhibitor proteins (Ham et al., 1997), are thought to hydrolyze fungal cell wall components, thus generating β-1,3-linked glucan oligosaccharides (Keen and Yoshikawa, 1983). Glucanases probably affect the host callose as well, as shown by the finding that β-1,3 glucanase-deficient plants accumulate more callose at virus infection sites (Beffa et al., 1996). β-1,3 Glucan oligomers appear as common inducers of defense events, i.e. they are recognized in a variety of plants such as tobacco, wheat (data not shown), alfalfa (Kobayashi et al., 1993), bean (Tai et al., 1996a, 1996b), and rice (Inui et al., 1997), whereas the activity of the β-1,6–1,3 heptaglucan motif seems to be in large part restricted to leguminous plants (Cosio et al., 1996; Côté et al., 2000).
In conclusion, we show here that laminarin, the oligomeric β-1,3 glucan naturally abundant in marine brown alga, elicits a variety of defense reactions in tobacco, conferring resistance to the soft rot disease agent, E. carotovora. That storage polysaccharides of marine brown alga are recognized as defense signals in land green plants of course is a fortuitous coincidence. It happens that laminarin is a structural analog of the linear β-1,3 glucan oligosaccharides naturally involved in the cell-cell recognition mechanisms in land plant-pathogen interactions, either exogenous (resulting from the degradation of fungal cell walls) or endogenous (callose fragments) to the host. Yet, as such, laminarin and laminarin oligomers are potent defense elicitors, both in other dicots (tomato and bean) and in monocots (wheat and rice), and these β-1,3 glucans thus might become interesting, alternative tools for disease control in agronomic crops.
MATERIALS AND METHODS
Plant Material
Tobacco (Nicotiana tabacum cv Samsun NN) plants were grown from seed in a greenhouse under controlled conditions and used after 2 months of culture (eight leaves). Suspended cell cultures of tobacco cv BY (Narváez-Vásquez, 1991) were maintained in Murashige and Skoog medium at pH 5.8 (Duchefa, Haarlem, The Netherlands), supplemented with 0.2 g L−1 of 2,4-D, 1 mg L−1 of thiamin, 100 mg L−1 of myo-inositol, 200 mg L−1 of KH2PO4, and 30 g L−1 of Suc. Cells were grown in the dark on a rotary shaker (120 rpm, 25°C) and subcultured weekly. Elicitation experiments were performed with 5-mL aliquots of cells after 6 d of subculture.
Preparation of Elicitors
Laminarin was extracted and purified from the marine brown alga Laminaria digitata by Laboratoires Goëmar as follows. L. digitata sporophytes were harvested in Brittany in late summer, extracted with hot water (100 kg of fresh algae in 200 L, 70°C) for 2 h, and the aqueous extracts were fractionated by ultrafiltration, using 1-m2 membranes with a cutoff of 300 kD (TAMI Industries, Nyons, France) and a flow rate of approximately 9.8 L h−1. The ultrafiltrate was then filtrated with a cutoff of 1 kD and the resulting retentate was desalted and lyophilized. Yields typically amounted to 10% of the kelp dry weight. The average molecular mass of laminarin samples was 5,300 g mol−1, as measured by molecular size chromatography coupled with a refractometric detector, corresponding to an average DP of 33 glycosidic units. Purity, size, and structure were further analyzed by natural abundance 13C NMR spectroscopy and HPAEC-PAD (Lépagnol-Descamps et al., 1998), confirming that L. digitata laminarin is an essentially linear β-1,3 glucan. Laminarin oligosaccharides varying in size from DP 2 through 5, i.e. laminaribiose through laminaripentaose, were purchased from Sigma (St. Louis). The β-1,6–1,3 heptaglucan (Sharp et al., 1984a) and the Psg void glucan (Ayers et al., 1976b) were gifts from M.G. Hahn (Complex Carbohydrate Research Center, Athens, GA).
Oligogalacturonides were obtained enzymatically as follows. Pectin from apple (25 g, P2157 Sigma) was suspended in 5 L of phosphate-citrate buffer (20 mm, pH 5). Pectin lyase (20 U, P7052 Sigma) was added to 2 L of the pectin solution and incubated for 30 min at 40°C with gentle stirring. The reaction mixture was then subjected to ultrafiltration with a Pellicon system (Millipore, Bedford, MA), using a 0.46-m2 membrane with a cutoff of 30 kD. The flow rate was approximately 500 mL h−1 and the pectin solution was added until exhaustion of the initial solution. After 2 h, 20 U of enzyme was supplemented and the ultrafiltrate was submitted to another ultrafiltration step using a membrane with a cutoff of 500 D. The resulting ultrafiltrate was finally lyophilized and stored. The molecular size distribution of oligo-GalUAs was determined by HPAEC-PAD (Spiro et al., 1993) using a chromatograph in a D×500 configuration (Dionex, Sunyvale, CA), with a 50-μL injection loop, a Carbopac PA100 column (4 × 250 mm), and an electrochemical detector fitted with a gold electrode (E1 = 0.05 V, 0.4 s; E2 = 0.75 V, 0.2 s; and E3 = −0.15 V, 0.4 s). Elution was performed at 1 mL min−1, first for 20 min with a linear gradient from 30% to 100% (w/v) of acetate buffer (1 m; pH 7.2), and subsequently for 10 min with 100% (w/v) acetate buffer. Oligogalacturonides ranged from DP 4 through 15 with a mean DP of 10.
The proteinaceous elicitor β-megaspermin was purified from the culture medium of the fungus Phytophthora megasperma H20 as described previously (Baillieul et al., 1995).
Measurements of Defense Reactions in Tobacco-Suspended Cell Cultures
All measurements were made on tobacco cells treated with elicitor or water after 6 d of subculture. The pH variations of the culture medium were recorded by introducing a computer-combined glass electrode (Physcope software; Inforlab, Chelles, France) into 5 mL of the cell suspension culture. The pH measurements, performed every 30 s, started 10 min before treatment with elicitors or water. The amounts of H2O2 released in the culture medium were assayed by chemiluminescence, using ferricyanide-catalyzed luminol oxidation as described by Jabs et al. (1997), and modified as follows. Following treatment with water of each elicitor, 20 μL of culture medium was added every minute to 100 μL of 250 μm of luminol and 100 μL of 5.6 mm K3(Fe(CN6)) in a 50-mm potassium phosphate buffer (pH 7.9). Chemiluminescence was then recorded for 10 s with a luminometer (TR717; PE Applied Biosystem, Courtaboeuf, France).
For measurements of PAL and LOX activities, cells were harvested by filtration, frozen in liquid nitrogen, and kept at −80°C until analysis. PAL and COMT activities were measured as described by Legrand et al. (1976). LOX activity was determined using the method described by Bohland et al. (1997), with some modifications. Cells (ca. 400 mg) were ground in a mortar in the presence of 0.8 mL of ice-cold Tris [tris(hydroxymethyl)aminomethane] buffer (0.1% [w/v] Triton X-100, 3 mm EDTA, 0.04% [w/v] Na2S2O5, and 0.1% [w/v] polyvinylpyrrolidone; pH 6.8). The homogenate was centrifuged at 13,000g at 5°C for 15 min. Supernatants were assayed for LOX activity spectrophotometrically at 234 nm, using linolenic acid as the substrate. The reaction mixture contained 100 μm of linolenic acid, 0.1 m Tris buffer (pH 6.8), and 100 μL of supernatant. After an initial incubation period of 1 min to allow the reaction rate to stabilize, increase in absorbance was followed for 8 min and the rate of increase was calculated from the initial linear portion of the curve. SA analysis was performed with the method described in Baillieul et al. (1995).
Variations in the absolute values of PAL and LOX activity as well as in SA contents were noticed between individual experiments. Percent stimulation values were constant in each experiment; therefore, variation in absolute values was probably due to unavoidable, slight variations in the growth conditions of suspended cells. Three to five replicates were performed for each experiment.
Measurements of Defense Reactions in Tobacco Plants
Accumulation of PR proteins was measured in leaves from three different tobacco plants. In every plant, one-half of a fully developed leaf was infiltrated with 200 μL of laminarin solution, whereas the other half was infiltrated with water as a control, using a syringe without needle. Protein extraction, SDS-PAGE, and immunoblotting were performed as described previously (Baillieul et al., 1995). Protein extract aliquots corresponding to 2 mg of fresh leaf tissue were electrophoresed, blotted, and incubated with antibodies raised against purified tobacco PR proteins of the PR-1, PR-2, PR-3, and PR-5 families (Stintzi et al., 1993).
Five days after the infiltration of laminarin or water, in situ evaluation of cell death was performed using the vital dye trypan blue (Koch and Slusarenko, 1990). For comparison, the same procedure was used to evaluate the cell death caused by a necrosis-inducing concentration of 50 nm of β-megaspermin, used as a positive control (Dorey et al., 1999).
Challenge Inoculation with Erwinia carotovora subsp. carotovora and Disease Evaluation
One leaf for each 30-d-old tobacco plant was infiltrated with 50 μL of laminarin (200 μg mL−1) or water (control) with a syringe without a needle. Five days later, a suspension of E. carotovora strain 797 was infiltrated (1 × 106 CFU mL−1) into the previously infiltrated areas. One day after the second inoculation, the extension of soft rot disease symptoms was evaluated. Bacterial multiplication in the plant tissue was measured as follows: Leaves were homogenized in an MgSO4 solution and serial dilutions of the extracts were plated on LB-Agar medium for quantification of bacterial colonies.
ACKNOWLEDGMENTS
We are grateful to D. Expert (INAPG, Paris) for kindly providing the isolate of E. carotovora and to M. Hahn (Complex Carbohydrate Research Center, Athens, GA) for the generous gifts of the Psg glucan fraction and the β-1,6–1,3 heptaglucoside. We also thank S. Kauffmann and P. Saindrenan for helpful discussions and critical reviews of the manuscript.
LITERATURE CITED
- Albersheim P, Darvill AG, McNeil M, Valent BS, Sharp JK, Nothnagel EA, Davis KR, Yamazaki N, Gollin DJ, York WS, Dudman WF, Darvill JE, Dell A. Oligosaccharins: naturally occurring carbohydrates with biological regulatory functions. In: Ciferri O, Dure L III, editors. Structure and Function of Plant Genomes. New York: Plenum Publishing Corporation; 1983. pp. 293–312. [Google Scholar]
- Alvarez ME, Pennell RI, Meijer P, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Ayers AR, Ebel J, Finelli F, Berger N, Albersheim P. Host-pathogen interactions: IX. Quantitative assays of elicitor activity and characterization of the elicitor present in the extracellular medium of cultures of Phytophthora megasperma var. sojae. Plant Physiol. 1976a;57:751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers AR, Ebel J, Valent B, Albersheim P. Host-pathogen interactions: X. Fractionation and biological activity of an elicitor isolated from the mycelial walls of Phytophthora megasperma var. sojae. Plant Physiol. 1976b;57:760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillieul F, Genetet I, Kopp M, Saindrenan P, Fritig B, Kauffmann S. A new elicitor of the hypersensitive response in tobacco: a fungal glycoprotein elicits cell death, expression of defense genes, production of salicylic acid, and induction of systemic acquired resistance. Plant J. 1995;8:551–560. doi: 10.1046/j.1365-313x.1995.8040551.x. [DOI] [PubMed] [Google Scholar]
- Beffa RS, Hofer R-M, Thomas M, Meins F., Jr Decreased susceptibility to viral disease of β-1,3-glucanase-deficient plants generated by antisense transformation. Plant Cell. 1996;8:1001–1011. doi: 10.1105/tpc.8.6.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blée E. Phytooxylipins and plant defense reactions. Prog Lipid Res. 1998;37:33–72. doi: 10.1016/s0163-7827(98)00004-6. [DOI] [PubMed] [Google Scholar]
- Bohland C, Balkenhohl T, Loers G, Feussner I, Grambow HJ. Differential induction of lipoxygenase isoforms in wheat upon treatment with rust fungus elicitor, chitin oligosaccharides, chitosan, and methyl jasmonate. Plant Physiol. 1997;114:679–685. doi: 10.1104/pp.114.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouarab K, Potin P, Correa J, Kloareg B. Sulfated oligosaccharides mediate the interaction between a marine red alga and its green algal pathogenic endophyte. Plant Cell. 1999;11:1635–1650. doi: 10.1105/tpc.11.9.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KP, Darvill AG, Albersheim P. Activation of a tobacco glycine-rich protein gene by a fungal glucan preparation. Plant J. 1993;4:517–524. doi: 10.1046/j.1365-313x.1993.04030517.x. [DOI] [PubMed] [Google Scholar]
- Cheong JJ, Birberg W, Fügedi P, Pilotti A, Garegg PJ, Hong N, Ogawa T, Hahn MG. Structure-activity relationships of oligo-β-glucoside elicitors of phytoalexin accumulation in soybean. Plant Cell. 1991;3:127–136. doi: 10.1105/tpc.3.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio EG, Feger M, Miller CJ, Antelo L, Ebel J. High-affinity binding of fungal β-glucan elicitors to cell membranes of species of the plant family Fabaceae. Planta. 1996;200:92–99. [Google Scholar]
- Côté F, Hahn MG. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1379–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Côté F, Ham K-S, Hahn MG, Bergmann CW. Biswas, Das, eds, Subcellular Biochemistry: Plant-Microbe Interactions. Vol. 29. New York: Plenum Press; 1998. Oligosaccharide elicitors in host-pathogen interactions: generation, perception, and signal transduction; pp. 385–432. [DOI] [PubMed] [Google Scholar]
- Côté F, Roberts K, Hahn M. Identification of high-affinity binding sites for the hepta-β-glucoside elicitor in membranes of the model legumes Medicago truncatula and Lotus japonicus. Planta. 2000;211:596–605. doi: 10.1007/s004250000325. ) [DOI] [PubMed] [Google Scholar]
- Dixon RA, Harrison MJ, Lamb CJ. Early events in the activation of plant defense responses. Annu Rev Phytopathol. 1994;32:479–501. [Google Scholar]
- Dorey S, Kopp M, Geoffroy P, Fritig B, Kauffmann S. Hydrogen peroxyde from the oxidative burst is neither necessary nor sufficient for hypersensitive cell death induction, phenylalanine ammonia lyase stimulation, salicylic acid accumulation, or scopoletin consumption in cultured tobacco cells treated with elicitin. Plant Physiol. 1999;121:163–171. doi: 10.1104/pp.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyakova LA, Zvyagintseva TN. A study of the laminarins of some Far-Eastern, brown seaweeds. Carbohydr Res. 1974;34:241–248. doi: 10.1016/s0008-6215(00)82899-2. [DOI] [PubMed] [Google Scholar]
- Felix G, Baureithel K, Boller T. Desensitization of the perception system for chitin fragments in tomato cells. Plant Physiol. 1998;117:643–650. doi: 10.1104/pp.117.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Regenass M, Boller T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinisation, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993;4:307–316. [Google Scholar]
- Fritig B, Heitz T, Legrand M. Antimicrobial proteins in induced plant defense. Curr Opin Immunol. 1998;10:16–22. doi: 10.1016/s0952-7915(98)80025-3. [DOI] [PubMed] [Google Scholar]
- Gilchrist DG. Programmed cell death in plant disease: the purpose and promise of cellular suicide. Annu Rev Phytopathol. 1998;36:393–414. doi: 10.1146/annurev.phyto.36.1.393. [DOI] [PubMed] [Google Scholar]
- Ham K-S, Wu S-C, Darvill AG, Albersheim P. Fungal pathogens secrete an inhibitor protein that distinguishes isoforms of plant pathogenesis-related endo-β-1,3-glucanases. Plant J. 1997;11:169–179. [Google Scholar]
- Inui H, Yamaguchi Y, Hirano S. Elicitor actions of N-acetylchitooligosaccharides and laminarioligosaccharides for chitinase and L-phenylalanine ammonia-lyase induction in rice suspension culture. Biosci Biotech Biochem. 1997;61:975–978. doi: 10.1271/bbb.61.975. [DOI] [PubMed] [Google Scholar]
- Jabs T, Tschöpe M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2 from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci USA. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H. Callose biosynthesis as a Ca2+-regulated process and possible relations to the induction of other metabolic changes. J Cell Sci Suppl. 1985;2:89–103. doi: 10.1242/jcs.1985.supplement_2.5. [DOI] [PubMed] [Google Scholar]
- Keen NT, Yoshikawa M. β-1,3 Endoglucanase from soybean releases elicitor active carbohydrates from fungal cell walls. Plant Physiol. 1983;71:460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Tai A, Kanzaki H, Kawazu K. Elicitor-active oligosaccharides from algal laminaran stimulate the production of antifungal compounds in alfalfa. Z Naturforsch. 1993;48c:575–579. [Google Scholar]
- Koch E, Slusarenko A. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell. 1990;2:437–445. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E, Somssich IE. Defense responses of plants to pathogens. Adv Bot Res. 1995;21:1–34. [Google Scholar]
- Kopp M, Rouster J, Fritig B, Darvill A, Albersheim P. Host-pathogen interactions: XXXII. A fungal glucan preparation protects Nicotianae against infection by viruses. Plant Physiol. 1989;90:208–216. doi: 10.1104/pp.90.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuc J. Phytoalexins, stress metabolism, and disease resistance in plants. Annu Rev Phytopathol. 1995;33:275–297. doi: 10.1146/annurev.py.33.090195.001423. [DOI] [PubMed] [Google Scholar]
- Kurosaki F, Tashiro N, Nishi A. Role of chitinase and chitin oligosaccharides in lignification response of cultured carrot cells treated with mycelial walls. Plant Cell Physiol. 1988;29:527–531. [Google Scholar]
- Lapous D, Mathieu Y, Guern J, Laurière C. Increase of defense gene transcripts by cytoplasmic acidification in tobacco cell suspensions. Planta. 1998;205:452–458. [Google Scholar]
- Legrand M, Fritig B, Hirth L. Enzymes of the phenylpropanoid pathway and the necrotic reaction of hypersensitive tobacco to tobacco mosaic virus. Phytochemistry. 1976;15:1353–1359. [Google Scholar]
- Lépagnol-Descamps V, Richard C, Lahaye M, Potin P, Yvin JC, Kloareg B. Purification and determination of the action pattern of Haliotis tuberculata laminarinase. Carbohydr Res. 1998;310:283–289. doi: 10.1016/s0008-6215(98)00181-5. [DOI] [PubMed] [Google Scholar]
- Lyon GD, Forrest RS, Newton AC. British Crop Protection Council, ed, The 1996 Brighton Conference: Pests & Diseases. 8A. Farnham, UK: British Crop Protection Council; 1996. SAR: the potential to immunize plants against infection; pp. 939–946. [Google Scholar]
- Maeda M, Nisizawa K. Laminaran of Ishige okamurai. Carbohydr Res. 1968;7:94–97. [Google Scholar]
- Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury S, Geoffroy P, Legrand M. Tobacco O-methyltransferases involved in phenylpropanoid metabolism: the different CCoAOMT and COMT classes have distinct substrate specificities and expression patterns. Plant Physiol. 1999;121:215–224. doi: 10.1104/pp.121.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milat ML, Ricci P, Bonnet P, Blein JP. Capsidiol and ethylene production by tobacco cells in response to cryptogein, an elicitor from Phytophthora cryptogea. Phytochemistry. 1991;30:2171–2173. [Google Scholar]
- Miller KJ, Hadley JA, Gustine DL. Cyclic β-1,6–1,3-glucans of Bradyrhizobium japonicum USDA 110 elicit isoflavonoid production in the soybean (Glycine max) host. Plant Physiol. 1994;104:917–923. doi: 10.1104/pp.104.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Bhagwat AA, Feger M, Ebel J. Suppression of fungal β-glucan-induced plant defense in soybean (Glycine max. L.) by cyclic 1: 3–1,6-β-glucans from the symbiont Bradyrhizobium japonicum. Planta. 1996;199:270–275. [Google Scholar]
- Narváez-Vásquez J. Expression of proteinase inhibitor genes in transgenic plants: effects on insect resistance, levels of expression in four plant species, and cellular compartmentalization. PhD thesis. Pullman: Washington State University; 1991. [Google Scholar]
- Nelson TE, Lewis BA. Separation and characterization of the soluble and insoluble components of insoluble laminaran. Carbohydr Res. 1974;33:63–74. doi: 10.1016/s0008-6215(00)82940-7. [DOI] [PubMed] [Google Scholar]
- O'Donnell V, Tew DG, Jones OTG, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva TK, Holmström K-O, Heino P, Palva ET. Induction of plant defense response by exoenzymes of Erwinia carotovora subsp. carotovora. Mol Plant-Microbe Interact. 1992;6:190–196. [Google Scholar]
- Palva TK, Hurtig M, Saindrenan P, Palva ET. Salicylic acid induced resistance to Erwinia carotovora subsp. carotovora in tobacco. Mol Plant-Microbe Interact. 1994;7:356–363. [Google Scholar]
- Read SM, Currie G, Basic A. Analysis of the structural heterogeneity of laminarin by electrospray-ionization-mass spectrometry. Carbohydr Res. 1996;281:187–201. doi: 10.1016/0008-6215(95)00350-9. [DOI] [PubMed] [Google Scholar]
- Reglinski T, Lyon GD, Newton AC. Induction of resistance mechanisms in barley by yeast-derived elicitors. Ann Appl Biol. 1994;124:509–517. [Google Scholar]
- Rouhier P, Kopp M, Begot V, Bruneteau M, Fritig B. Structural features of fungal β-D-glucans for the efficient inhibition of the initiation of virus infection on Nicotiana tabacum. Phytochemistry. 1995;39:57–62. doi: 10.1016/0031-9422(94)00852-k. [DOI] [PubMed] [Google Scholar]
- Ruiz-Herrera J. Fungal Cell Wall: Structure, Synthesis and Assembly. Boca Raton, FL: CRC Press; 1992. [Google Scholar]
- Sharp JK, McNeil M, Albersheim P. The primary structures of one elicitor-active and seven elicitor-inactive hexa (β-D-glucopyranosyl)-D-glucitols isolated from the mycelial walls of Phytophthora megasperma f.sp. glycinea. J Biol Chem. 1984b;259:11321–11336. [PubMed] [Google Scholar]
- Sharp JK, Valent B, Albersheim P. Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J Biol Chem. 1984a;259:11312–11320. [PubMed] [Google Scholar]
- Siegrist J, Glenewinkel D, Kolle C, Schmidtke M. Chemically induced resistance in green bean against bacterial and fungal pathogens. J Plant Dis Prot. 1997;104:599–610. [Google Scholar]
- Spiro MD, Kates KA, Koller AL, O'Neill MA, Albersheim P, Darvill A. Purification and characterization of biologically active (1–4)-linked α-D-oligogalacturonides after partial digestion of polygalacturonic acid with endopolygalacturonase. Carbohydr Res. 1993;247:9–20. [Google Scholar]
- Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B. Plant “pathogenesis-related” proteins and their role in defense against pathogens. Biochimie. 1993;75:687–706. doi: 10.1016/0300-9084(93)90100-7. [DOI] [PubMed] [Google Scholar]
- Tai A, Kawazu K, Kobayashi A. Species-specificity of an elicitor-active oligosaccharide, LN-3, to leguminous plants. Z Naturforsch. 1996a;51:371–378. [Google Scholar]
- Tai A, Ohsawa E, Kawazu K, Kobayashi A. A minimum essential structure of LN-3 elicitor activity in bean cotyledons. Z Naturforsch. 1996b;51:15–19. doi: 10.1515/znc-1996-1-205. [DOI] [PubMed] [Google Scholar]
- Usui T, Toriyama T, Mizuno T. Structural investigation of laminaran of Eisenia bicyclis. Agric Biol Chem. 1979;43:603–611. [Google Scholar]
- Van Loon LC, Van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol. 1999;55:85–97. [Google Scholar]
- Yu I-C, Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA. 1998;95:7819–7824. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]