Abstract
Objective
The objective was to identify risk factors that were associated with the progression from endometriosis to ovarian cancer based on medical insurance data.
Methods
The study was performed on a dataset obtained from the National Health Insurance Research Database, which covered all the inpatient claim data from 2000 to 2013 in Taiwan. The International Classification of Diseases (ICD) code 617 was used to screen the dataset for the patients who were admitted to hospital due to endometriosis. They were then tracked for subsequent diagnosis of ovarian cancer, and available biological, socioeconomic and clinical information was also collected. Univariate and multivariate analyses were then performed based on the Cox regression model to identify risk factors. C-index was calculated and cross validated.
Results
A total of 229,617 patients who were admitted to hospital due to endometriosis from 2000 to 2013 were included in the study, out of whom 1,473 developed ovarian cancer by the end of 2013. A variety of factors, including age, residence, hospital stratification, premium range, and various comorbidities had significant impact on the progression (p<0.05). Among them, age, urbanization of residence, hospital stratification, premium range, post-endometriosis childbearing, pelvic inflammation, and depression all had independent, significant impact (p<0.05). The validated C-index was 0.69.
Conclusion
For a woman diagnosed with endometriosis, increased age, residing in a highly urbanized area, low or high income, depression, pelvic inflammation, and absence of childbearing post-endometriosis all put her at high-risk to develop ovarian cancer. The findings may be of help to gynecologists to identify high-risk patients.
Keywords: Endometriosis, Ovarian Neoplasms, Risk Factors, Cohort Studies
INTRODUCTION
Endometriosis is a condition in which endometrial glands and stroma grow outside of the endometrial lining and uterine musculature [1,2]. It affects 1% to 10% women of reproductive age and can be associated with substantial morbidity such as pelvic pain and infertility [3,4]. Although a benign gynecologic disorder itself, endometriosis puts one at risk for ovarian cancer [5,6,7].
Ovarian cancer is the second leading cause of death among gynecologic cancers worldwide [8]. Unfortunately, most women are diagnosed at an advanced stage, and the 5-year survival rate ranges from 30% to 90% while the recurrence rate, 20% to 75%, according to the stage of the disease [9]. The global burden of ovarian cancer is immense, not only to patients and their families, but also to healthcare institutes and social economies.
The link between endometriosis and ovarian cancer has been well established although the exact mechanisms underlying the progression are not entirely understood. There is some evidence that atypical endometriosis may represent a transition to carcinoma, while 2 histotypes of ovarian cancer, clear cell and endometrioid carcinomas, are particularly associated with endometriosis [10,11]. On top of pathogenesis, there may also remain other factors that potentially affect the progression from endometriosis to ovarian cancer, and, unfortunately, few studies have established convincing results. This study therefore was aimed to identify risk factors that were associated with the progression from endometriosis to ovarian cancer based on a cohort.
MATERIALS AND METHODS
The study was based on the National Health Insurance Research Database (NHIRD, case number: NHIRD-104-071). The dataset used covered all the inpatient claim data from 2000 to 2013 in Taiwan. The study was approved by the Internal Review Board of Kaohsiung Veterans General Hospital (IRB number: VGHKS15-EM4-01) and was in accordance with the Declaration of Helsinki.
The International Classification of Diseases (ICD) code 617 (617.0 through 617.9) was used to screen the dataset for the patients who were admitted to hospital due to endometriosis. The search resulted in 233,277 patients. They were then tracked for subsequent diagnosis of ovarian cancer (ICD code 183.0–183.9), and available biological, socioeconomic, and clinical information was also collected. Then 144 patients were excluded from the study since they had been diagnosed with ovarian cancer before being hospitalized for endometriosis and 887 were later excluded due to scanty information available. The patient information collected included age, urbanization of residence [12], premium ranges [13], hospital stratification, a wide range of comorbidity such as coronary heart disease, diabetes, depression and dementia, malignancies other than ovarian cancer, and post-endometriosis parity. A list of variables used can be found in Table 1.
Table 1. Characteristics of the cohort in study.
| Variables (n=229,617) | Medium (range) | |
|---|---|---|
| Age (yr) | 41.07 (2.24–96.41) | |
| Time to ovarian cancer (day) | 2,438 (0–5,113) | |
| Post-endometriosis ovarian cancer (%) | 0.64 | |
| Hospital stratification (%) | ||
| Medical centers | 49.28 | |
| Regional hospitals | 37.32 | |
| District hospitals | 12.58 | |
| Local hospitals | 0.82 | |
| Urbanization (%) | ||
| Highly urbanized | 35.71 | |
| Moderately urbanized | 32.03 | |
| Newly urbanized | 15.40 | |
| Rural areas | 11.02 | |
| Others* | 5.84 | |
| Premium range (monthly income, NTD) | ||
| >15,840 and ≤25,000 | 15.56 | |
| ≤15,840 | 47.73 | |
| >25,000 | 36.71 | |
| Comorbidity | ||
| Myocardial infarction | 0.14 | |
| Congestive heart failure | 0.42 | |
| Peripheral vascular disease | 0.11 | |
| Cerebrovascular diseases | 1.18 | |
| Dementia | 0.05 | |
| Chronic pulmonary disease | 1.08 | |
| Rheumatologic disease | 0.45 | |
| Peptic ulcer disease | 2.02 | |
| Mild liver disease | 0.40 | |
| Diabetes | 2.76 | |
| Diabetes with chronic complications | 0.38 | |
| Hemiplegia/paraplegia | 0.17 | |
| Renal disease | 0.41 | |
| Moderate/severe liver disease | 0.15 | |
| AIDS | 0.00 | |
| Parkinson's disease | 0.09 | |
| Stroke | 1.18 | |
| Coronary heart disease | 1.21 | |
| Immune rheumatism | 0.45 | |
| Depression | 1.36 | |
| Hypertension | 5.22 | |
| Hyperlipidaemia | 1.53 | |
| Pelvic inflammation | 6.36 | |
| Childbearing post endometriosis | 9.65 | |
AIDS, acquired immune deficiency syndrome; NTD, New Taiwan Dollar.
*Others includes areas with aged population, agrarian towns and cities, and remote areas.
All the statistical analysis was carried out with the statistical computing and graphic drawing language, R (R Core Team, Vienna, Austria; http://www.Rproject.org) [14]. The univariate and multivariate analyses were performed based on the Cox regression model. The nomogram was drawn with an R package, rms. In calculating the concordance statistic (C-index), cross validation was performed for 50 times, and at each time, 4/5 of the patients were randomly chosen as the training set and 1/5, testing set. Details of making the nomogram and calculating the C-index with the validation process are provided in the supplementary files (Supplementary Data 1, 2, and Supplementary Table 1).
RESULTS
A total of 229,617 patients who were admitted to hospital due to endometriosis from 2000 to 2013 in Taiwan were included in the study. Out of these patients, 1,473 eventually developed ovarian cancer by the end of 2013. Demographic information of all the patients, including distribution of age, occurrence rate and time-to-event of ovarian cancer, urbanization of residence, premium range and all the comorbidities, is summarized in Table 1.
Univariate analysis was performed as a preliminary screen for factors that impacted the progression to ovarian cancer. All the available variables were tested and a p-value of 0.05 was used as the threshold to select for significant factors. A variety of factors, including age, residence, hospital stratification, premium range, and various comorbidities were shown in the tests to have significant impact on the progression. A complete list of these factors is summarized in Table 2.
Table 2. Significant factors in univariate analysis.
| Variables | Hazard ratio | p | 95% confidence interval | |
|---|---|---|---|---|
| Age (yr) | 1.06 | <0.001 | 1.06–1.07 | |
| Hospital stratification | ||||
| Medical centers | Reference | |||
| Regional hospitals | 0.61 | <0.001 | 0.54–0.68 | |
| District hospitals | 0.46 | <0.001 | 0.54–0.56 | |
| Local hospitals | 0.19 | <0.001 | 0.06–0.58 | |
| Urbanization | ||||
| Highly urbanized | Reference | |||
| Moderately urbanized | 0.80 | <0.001 | 0.70–0.90 | |
| Newly urbanized | 0.72 | <0.001 | 0.61–0.85 | |
| Rural areas | 0.72 | <0.001 | 0.60–0.87 | |
| Others* | 0.81 | 0.070 | 0.64–1.02 | |
| Premium range (NTD/month) | ||||
| >15,840 and ≤25,000 | Reference | |||
| ≤15,840 | 1.25 | <0.001 | 1.08–1.44 | |
| >25,000 | 1.23 | <0.001 | 1.10–1.38 | |
| Comorbidity | ||||
| Congestive heart failure | 2.46 | <0.001 | 1.50–4.03 | |
| Cerebrovascular diseases | 1.79 | <0.001 | 1.27–2.52 | |
| Peptic ulcer disease | 1.83 | <0.001 | 1.41–2.38 | |
| Mild liver disease | 1.89 | 0.030 | 1.07–3.34 | |
| Diabetes | 1.99 | <0.001 | 1.60–2.47 | |
| Renal disease | 2.87 | <0.001 | 1.80–4.57 | |
| Stroke | 1.79 | <0.001 | 1.27–2.52 | |
| Depression | 1.86 | <0.001 | 1.35–2.56 | |
| Hypertension | 2.05 | <0.001 | 1.74–2.41 | |
| Pelvic inflammation | 1.85 | <0.001 | 1.58–2.17 | |
| Childbearing | 0.33 | <0.001 | 0.25–0.44 | |
NTD, New Taiwan Dollar.
*Others includes areas with aged population, agricultural towns and cities, and remote areas.
Multivariate analysis was then performed on the above factors to identify independent risk factors in the progression. As shown in Table 3, age, urbanization of residence, hospital stratification, premium range, post-endometriosis childbearing, pelvic inflammation and depression all had independent, significant impact on the progression to ovarian cancer.
Table 3. Independent risk factors in multivariate analysis.
| Variables | Hazard ratio | p | 95% confidence interval | |
|---|---|---|---|---|
| Age (yr) | 1.06 | <0.001 | 1.06–1.07 | |
| Hospital stratification | ||||
| Medical centers | Reference | |||
| Regional hospitals | 0.64 | <0.001 | 0.57–0.72 | |
| District hospitals | 0.50 | <0.001 | 0.41–0.61 | |
| Local hospitals | 0.28 | 0.030 | 0.09–0.86 | |
| Urbanization | ||||
| Highly urbanized | Reference | |||
| Moderately urbanized | 0.85 | 0.010 | 0.75–0.96 | |
| Newly urbanized | 0.77 | <0.001 | 0.66–0.91 | |
| Rural areas | 0.79 | 0.010 | 0.65–0.95 | |
| Others* | 0.87 | 0.240 | 0.69–1.10 | |
| Premium range (NTD/month) | ||||
| >15,840 and ≤25,000 | Reference | |||
| ≤15,840 | 1.27 | <0.001 | 1.10–1.46 | |
| >25,000 | 1.23 | <0.001 | 1.10–1.38 | |
| Comorbidity | ||||
| Depression | 1.67 | <0.001 | 1.21–2.30 | |
| Pelvic inflammation | 2.73 | <0.001 | 2.32–3.22 | |
| Childbearing | 0.69 | 0.010 | 0.52–0.92 | |
NTD, New Taiwan Dollar.
*Others includes areas with aged population, agricultural towns and cities, and remote areas.
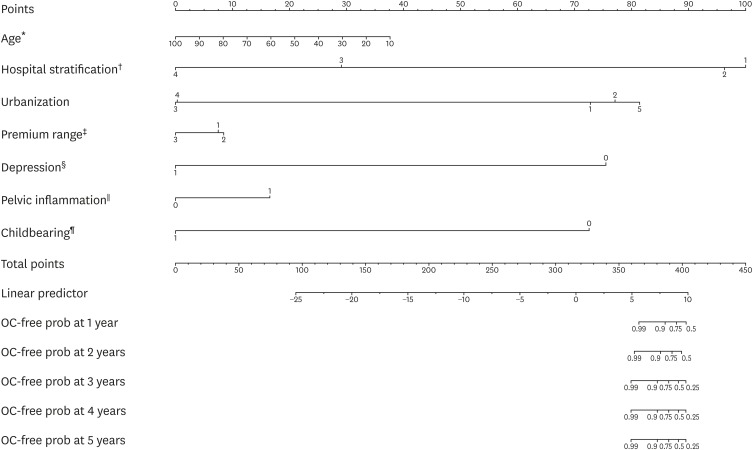
The above independent factors were combined together and a nomogram was calculated in Fig. 1 in order to show the predicted risk of a patient with endometriosis to progress to ovarian cancer at various time points. In the nomogram, the first line of points gives the weights of each risk factor while the line of total points, obtained by adding up the weights of all the risk factors, is used to locate the survival probability with ovarian cancer as the end event at a given time point. Therefore, for a woman aged at 50 (with 50 points), diagnosed with endometriosis at a medical center (hospital stratification 1, corresponding to 21 points), living in a highly urbanized area (urbanization 1, corresponding to 4 points), paying at the highest premium range (with 3 points), with a history of depression (with 9 points) and pelvic inflammation (with 17 points), and without a history of giving birth (with 6 points), she has a total points of 110, and that puts her probability of surviving 5 years post-endometriosis diagnosis without developing ovarian cancer at 0.95. The reliability of such a probability may be tested by calculating the C-index, which tests the risk factors for their predictivity of ovarian cancer occurrence following diagnosis of endometriosis. We performed the calculation with 5-fold cross validation and the validated C-index was 0.69.
Fig. 1.
Nomogram of risk factors in the progression from endometriosis to ovarian cancer.
OC-free prob, ovarian cancer-free probability; NTD, New Taiwan Dollar.
The scales of the risk factors are as follows: *Age: continuous; †Hospital stratification: 1, medical center; 2, regional hospitals; 3, district hospitals; 4, local hospitals; ‡Premium range in NTD: 1, >15,840 and ≤25,000; 2, ≤15,840; 3, >25,000; §Depression: 0, none; 1, history of depression; ∥Pelvic inflammation: 0, none, 1, history of pelvic inflammation; ¶Childbearing: 0, none after endometriosis; 1, history of birth-giving after endometriosis.
DISCUSSION
Many studies have established that endometriosis is a precursor of ovarian cancer, and many more are directed to explore the factors that trigger the former to take a turn for malignant transition. The present study, although not touching on molecular or pathologic mechanisms of the transition, identified factors associated with the progression from endometriosis to ovarian cancer, and was thus helpful with identification of high-risk patients.
The study started with a cohort of patients who were hospitalized due to endometriosis. We did not resort to patient surveys on medical history as patients' own recollection may not be entirely accurate. After all, aside from errors in memories, some affected by endometriosis may not even be aware of it as many may have none or little morbidity. Nor did we resort to out-patient records to screen for endometriosis patients because, unfortunately, there was usually substantial overlap of symptoms between endometriosis and other causes, and out-patient records would not be enough to ascertain the diagnosis of endometriosis [15]. By starting with the in-patient cohort, we could be assured that the subsequent analysis was firmly based on clear diagnosis of endometriosis.
We identified a few independent factors that were associated with the progression of endometriosis to ovarian cancer, and some of them had been previously suggested to be linked to incidence of ovarian cancer. Depression is a common public health problem and has been found to be associated with a variety of health concerns such as obesity and metabolic dysfunction while a few studies also linked it with an increased risk of ovarian cancer [16,17,18]. In addition, pelvic inflammatory disease has also been associated with higher risk of ovarian cancer and there is some evidence that it may be histotype specific [19,20].
Among the other risk factors were those associated with one's biological and socioeconomic status. Higher age, residing in a highly urbanized area and both low and high income all facilitated the progression to ovarian cancer. The socioeconomic status of the patients in this study was embodied by their medical insurance premiums, and the lower cutoff value used here, 15,840 New Taiwan Dollar (NTD), corresponded to the amount of monthly income at the level of the government-stipulated minimum wage for full-time employees in Taiwan, and the higher cutoff, 25,000 NTD, was previously described to indicate high socioeconomic status [13]. Although the classifications here were based on local standards, the findings associated with the classifications may still bring insights for other regions around the world. After all, what the findings pointed to was not any absolute amount of income, but one's socioeconomic status in a society. We argue that higher age is associated with diseases of any kind and the other factors may have something to do with a highly stressed environment while the stress may be caused by urbanization, work pressure that is potentially associated with both high and low income, poor living conditions associated with low income and unhealthy lifestyles associated with high income. The results highlighted the importance to watch one's living condition and life styles in prevention of the malignant transformation.
The last but the not least risk factor was absence of childbearing after endometriosis. It is estimated that 90% of the women with infertility has endometriosis [1]. Childbearing after hospital discharge post-endometriosis suggests remission of the disorder, and it is therefore reasonable to deduct that treatment for endometriosis, especially those focused on infertility, may help prevent the progression to malignancy.
The predictivity of the risk factors was illustrated visually with the nomogram and statistically with C-index. The nomogram demonstrated the probability of any given patient with endometriosis to develop ovarian cancer at a given year when her points of all the factors were added up. Such a risk measurement shall be of help to gynecologists when assessing patient risk as well as to patients themselves seeking for medical consultation. In addition, the C-index calculated in the study was also validated, i.e., the patients were randomly classified into a training set and a testing set, and the procedure was repeated for 50 times. The validated C-index highlighted the predictivity of the risk factors.
There were a few limitations to the study. First, it was a retrospective study and thus had limitations inherent in all such studies. Second, although based on a large sample size (n=229,617), it lacked details on clinical characteristics such as subtypes of endometriosis, diagnostic tools, histological information of ovarian cancer and cancer stage. Furthermore, there have been a growing body of studies on the accuracy of ICD codes [21,22], and unfortunately, the study was based on ICD codes to identify diseases of interest, and was thus subject to the errors and limitations of the ICD coding system. In addition, the overall incidence of ovarian cancer is low and only 0.64% of the cohort eventually developed the disease. The imbalance between those with ovarian cancer and those without might have resulted in bias in the conclusion.
In conclusion, for a woman diagnosed with endometriosis, increased age, residing in a highly urbanized area, low or high income, depression, pelvic inflammation, and absence of childbearing post-endometriosis all put her at high-risk to develop ovarian cancer. The findings may be of help to gynecologists to identify high-risk patients.
ACKNOWLEDGMENTS
We thank Mr. Yi-Jie Chou for his help with data analysis and Statistic Center of Department of Health and Welfare and Research Center of Medical Informatics, Kaohsiung Veterans General Hospital for access of the database.
Footnotes
Funding: This study was supported in part by a grant from the Ministry of Science and Technology of ROC (104-2118-M-110-001) and 3 grants from Kaohsiung Veterans General Hospital (VGHKS103-G02, VGHKS105-104, and VGHNSU103-006).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.A.J., C.C., C.J.
- Data curation: C.C., H.C.H., C.J.
- Formal analysis: H.C.H.
- Funding acquisition: C.A.J., C.C.
- Investigation: C.A.J., C.C., H.C.H., H.W.C., K.Y.Y., C.J.
- Methodology: C.C., H.C.H., C.J.
- Project administration: C.A.J., C.C., C.J.
- Resources: C.A.J., H.W.C., K.Y.Y.
- Software: C.C., H.C.H.
- Supervision: C.A.J., C.C., C.J.
- Validation: H.C.H.
- Visualization: H.C.H., C.J.
- Writing - original draft: C.J.
- Writing - review & editing: C.A.J., C.C., C.J.
SUPPLEMENTARY MATERIALS
Nomogram codes.
C-index calculation and cross validation.
Example of the data format used to plot the nomogram
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Viganò P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004;18:177–200. doi: 10.1016/j.bpobgyn.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Eisenberg VH, et al. Epidemiology of endometriosis: a large population-based database study from a healthcare provider with 2 million members. BJOG. 2018;125:55–62. doi: 10.1111/1471-0528.14711. [DOI] [PubMed] [Google Scholar]
- 5.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 6.Melin A, Sparén P, Persson I, Bergqvist A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum Reprod. 2006;21:1237–1242. doi: 10.1093/humrep/dei462. [DOI] [PubMed] [Google Scholar]
- 7.Vlahos NF, Kalampokas T, Fotiou S. Endometriosis and ovarian cancer: a review. Gynecol Endocrinol. 2010;26:213–219. doi: 10.1080/09513590903184050. [DOI] [PubMed] [Google Scholar]
- 8.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 10.Munksgaard PS, Blaakaer J. The association between endometriosis and ovarian cancer: a review of histological, genetic and molecular alterations. Gynecol Oncol. 2012;124:164–169. doi: 10.1016/j.ygyno.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Worley MJ, Welch WR, Berkowitz RS, Ng SW. Endometriosis-associated ovarian cancer: a review of pathogenesis. Int J Mol Sci. 2013;14:5367–5379. doi: 10.3390/ijms14035367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CY, Hung YT, Chuang YL. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4:1–22. [Google Scholar]
- 13.Wang JY, Wang CY, Juang SY, Huang KY, Chou P, Chen CW, et al. Low socioeconomic status increases short-term mortality of acute myocardial infarction despite universal health coverage. Int J Cardiol. 2014;172:82–87. doi: 10.1016/j.ijcard.2013.12.082. [DOI] [PubMed] [Google Scholar]
- 14.R Development Core Team. R: a language and environment for statistical computing. Vienna: the R Foundation for Statistical Computing; 2011. [Google Scholar]
- 15.Kodaman PH. Current strategies for endometriosis management. Obstet Gynecol Clin North Am. 2015;42:87–101. doi: 10.1016/j.ogc.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, Wallace RB, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888–1893. doi: 10.1093/jnci/90.24.1888. [DOI] [PubMed] [Google Scholar]
- 17.Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control. 2010;21:191–199. doi: 10.1007/s10552-009-9449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang T, Poole EM, Okereke OI, Kubzansky LD, Eliassen AH, Sood AK, et al. Depression and risk of epithelial ovarian cancer: results from two large prospective cohort studies. Gynecol Oncol. 2015;139:481–486. doi: 10.1016/j.ygyno.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HW, Tu YY, Lin SY, Su WJ, Lin WL, Lin WZ, et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. Lancet Oncol. 2011;12:900–904. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen CB, Kjaer SK, Albieri V, Bandera EV, Doherty JA, Høgdall E, et al. Pelvic inflammatory disease and the risk of ovarian cancer and borderline ovarian tumors: a pooled analysis of 13 case-control studies. Am J Epidemiol. 2017;185:8–20. doi: 10.1093/aje/kww161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40:1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burles K, Innes G, Senior K, Lang E, McRae A. Limitations of pulmonary embolism ICD-10 codes in emergency department administrative data: let the buyer beware. BMC Med Res Methodol. 2017;17:89. doi: 10.1186/s12874-017-0361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nomogram codes.
C-index calculation and cross validation.
Example of the data format used to plot the nomogram