Abstract
Objective
To investigate the expression of androgen receptor (AR) and its correlation with disease status and survival outcome in uterine leiomyosarcoma with other hormone receptors.
Methods
The medical records and paraffin blocks of 42 patients were reviewed. The immunohistochemical expression of AR, estrogen receptor (ER), progesterone receptor (PR), gonadotropin releasing hormone (GnRH), and cytochrome P450, family 19, subfamily A, polypeptide 1 (CYP19A1) were assessed using tissue microarray.
Results
In total, AR expression was observed in 11 patients (26.2%). International Federation of Gynecology and Obstetrics (FIGO) stage and AR were independent factors for disease-free survival (DFS) in multivariate regression analysis (odds ratio [OR]=5.8; 95% confidence interval [CI]=1.2–28.4 and OR=0.2; 95% CI=0.05–0.90; p=0.029 and 0.032, respectively). There were no deaths in the AR expression group, whereas the 5-year overall survival (OS) was 54.8% in the no expression group (p=0.014). Co-expression of ER and/or PR with AR was associated with significantly better 5-year DFS and OS than those with negative AR (72.7% vs. 28.6% and 100% vs. 64.3%; p=0.020 and 0.036, respectively). AR may be an independent prognostic marker regardless of ER/PR.
Conclusion
AR can be a potential prognostic biomarker in uterine leiomyosarcoma.
Keywords: Leiomyosarcoma, Androgen Receptor, Immunohistochemistry
INTRODUCTION
Uterine cancer is one of the most common gynecologic malignancies in the United States [1]. Its incidence has more than doubled recently in Korea [2], and it is estimated to become one of the top 10 cancers by the end of 2016 [3]. Uterine sarcoma is a rare type, comprising 2%–5% of uterine cancers. Among uterine sarcomas, uterine leiomyosarcoma is one of the most common subtypes and its incidence is very low—0.4 per 100,000 women [4]. Although most of the patients undergo adjuvant treatment after surgery, the 5-year overall survival (OS) outcome is unfavorable: 50% and 15% when detected at an early and advanced stage, respectively [5]. Given the current situation, investigation into a new therapeutic modality that can be added to the traditional treatment method is required to improve the poor prognosis of uterine leiomyosarcoma.
Recently, several target agents have been introduced for malignant disease. In metastatic non-adipocytic soft-tissue sarcoma, a phase 3 Pazopanib for metastatic soft-tissue sarcoma (PALETTE) trial incorporating pazopanib (a multitargeted tyrosine kinase inhibitor) showed a 3-month prolongation of progression-free survival [6]. Compared to this, a clinical trial incorporating target agents into uterine leiomyosarcomas had a limited effect and further evaluation is required [7,8]. Knowing the prognosis of certain tumors by disease status or staging system should lead to a more informed decision on the type of adjuvant treatment and in identifying a suitable candidate for a clinical trial. So far, none of the American Joint Committee on Cancer (AJCC)'s new and modified International Federation of Gynecology and Obstetrics (FIGO) staging systems have been effective in classifying patients into statistically significantly different stage groups [9,10]. Thus, finding a prognostic biomarker that can assist or be a substitute for the traditional staging system and the analyses of its relevance to disease status is needed. There have been numerous attempts to predict clinicopathologic characteristics and the prognosis of uterine leiomyosarcoma using the immunohistochemical method. One of the most frequently studied biomarkers is hormone receptors and they have showed moderate frequency of expression in uterine leiomyosarcoma in previous studies [11,12,13]. However, previous studies of hormone receptors in uterine leiomyosarcoma had small sample sizes and their results are conflicting. Furthermore, interpretation of immunohistochemical results vary among studies [11,12,14,15].
Identification of a new biomarker that can function as an adjunct prognosticator and predict treatment response can improve prediction of the clinical course of a uterine leiomyosarcoma and can help plan the disease management, as previous studies have disagreed between hormone treatment response and traditional hormone receptor expression [15,16,17]. In contrast to many previous studies on hormone receptors, few studies have further reached its attention to recognize androgen receptor (AR) as a potential prognostic marker in uterine leiomyosarcoma, although AR shares a common deoxyribonucleic acid (DNA)/ligand-binding domain with molecular structure and is evolutionarily closely related with estrogen receptor (ER)/progesterone receptor (PR) [18,19]. Based on observing an indolent disease character, better differentiated histologic feature, and more favorable survival outcome in endometrial cancer and stromal sarcoma with AR expression [20,21], we hypothesized that it may also be associated with a better disease extent and survival outcome in uterine leiomyosarcoma. Thus, we comprehensively investigated the significance of all of the hormone receptors, including AR, in clinicopathological characters and survival outcomes in uterine leiomyosarcoma.
MATERIALS AND METHODS
1. Patients
Seventy-one patients were selected who were diagnosed with uterine leiomyosarcoma, and were treated, and had regular follow-ups at the Asan Medical Center, Seoul, Korea, between January 2000 and December 2014. Overall, 42 paraffin blocks of the patients who were diagnosed with uterine leiomyosarcoma were preserved. Clinicopathological data were reviewed and the manufacture of tissue microarray (TMA) was performed under the approval of the Institutional Review Board of Asan Medical Center (approval number: 2009-0450). It thus meets the standards of the Declaration of Helsinki in its revised version of 1975 and its amendments of 1983, 1989, and 1996 [22]. FIGO 2009 uterine sarcoma classification was adopted to stage uterine leiomyosarcoma [23].
2. TMA
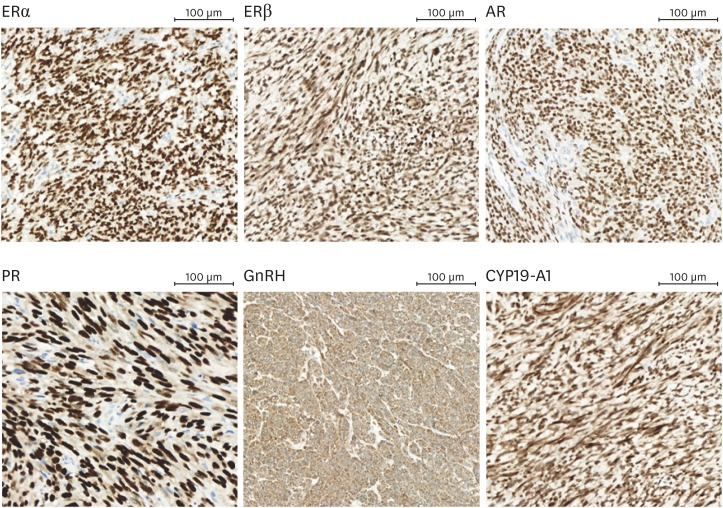
Tumor tissues obtained during surgery for routine diagnostic pathologic examinations were used for immunohistochemistry (IHC) studies. The most apparent and dense part of the tumors in paraffin blocks were obtained using a 2-mm diameter puncture needle, and were sent to Bio-Resource Center (BRC) in the hospital to make TMA. Three punctures at different parts of tumor were performed in each case to minimize selection bias and control quality of results by confirming tumor heterogeneity. Formalin-fixed, paraffin-embedded tissue sections were stained by immunohistochemical method for antibodies using a BenchMark ULTRA automatic immunostaining device (Ventana Medical Systems, Tucson, AZ, USA) with an OptiView 3,3′-diaminobenzidine (DAB) IHC Detection Kit (Ventana Medical Systems) according to the manufacturer's manual in each case. Four-micrometer-thick sections which were obtained with a microtome, were placed onto silanized charged slides and allowed to dry for 10 minutes at room temperature, followed by 20 minutes in an incubator at 65°C. Sections were performed by the heat-induced epitope retrieval (HIER) method using a Cell Conditioning 1 (CC1) buffer for 32 minutes and incubated for 16 minutes with rabbit anti-AR (1:100; AC-0071; Epitomics, Inc., Burlingame, CA, USA), rabbit anti-cytochrome P450, family 19, subfamily A, polypeptide 1 (CYP19A1) (1:50; PAB2971; Abnova Corporation, Taipei, Taiwan), mouse anti-ERα (1:100; NCL-L-ER-6F11, Novocastra™; Leica Biosystems, Newcastle, UK), rabbit anti-ERβ (1:100; SC-8974; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-gonadotropin releasing hormone (GnRH) receptor (1:25; NLS209; Novus Biologicals, San Diego, CA, USA), mouse anti-PR (1:200; NCL-L-PGR-312; Zymed, Carlsbad, CA, USA). Antigen-antibody reactions were visualized using Ventana OptiView DAB IHC Detection Kit (Optiview HQ Linker 8 minutes, Optiview horseradish peroxidase [HRP] Multimer 8 minutes, Optiview hydrogen peroxidase [H2O2]/DAB 8 minutes, Optiview Copper 4 minutes). Counterstaining was performed by using Ventana Hematoxylin II and bluing reagent for 32 and 4 minutes, respectively. All slides were removed from the stainer, dehydrated, and coverslipped for interpretation. For positive controls for each antibody, adequate immune-reactive prostate tissue samples were used. By omission of the primary antibodies, negative controls were produced. Immune-reaction of AR, ERα, ERβ, and PR were evaluated by observing expression pattern of nucleus. Immune-reaction of GnRH and CYP19A1 were assessed by analyzing both nucleus/cytoplasm and membrane, respectively (Fig. 1).
Fig. 1.
Immunohistochemical strong expression (3+) of each biomarker in uterine leiomyosarcoma (images were provided by Department of Pathology, Asan Medical Center, magnification ×200).
AR, androgen receptor; CYP19A1, cytochrome P450, family 19, subfamily A, polypeptide 1; ER, estrogen receptor; GnRH, gonadotropin releasing hormone; PR, progesterone receptor.
Slides were reviewed by a pathologist subspecializing in gynecologic oncology and a gynecologic oncologist without access to the clinical data of the patients. An assessment of the immunohistochemical results was performed using a semi-quantitative scoring system. This was calculated by multiplying the intensity of stain and the percentage of the total width (0 [0–20 points], 1+ [21–80 points], 2+ [81–180 points], and 3+ [181–300 points]). Patient group was categorized according to the expression of biomarkers by negative (0) versus positive (1+, 2+, and 3+) cases in ERα, PR, and AR. In ERβ and CYP19A1, patients group was categorized by moderate (2+) versus strong (3+) positive cases because all of them showed moderate to strong expression in this study. Patient categorization was not possible in GnRH because all of the patients showed strong (3+) positivity, and this was not included in the analysis.
3. Statistics
For univariate analyses, Mann-Whitney U test and Student's t-test were used to analyze median and mean value of each variable, respectively (Table 1). Chi-square test and Fisher's exact test were used to test for statistical differences among frequencies of each variable (Tables 1 and 2). The Kaplan-Meier method was used to analyze survival outcome, and the difference between each group was analyzed by log-rank test (Fig. 2) and Cox's proportional regression analysis (Table 3). Disease-free survival (DFS) was defined as from the date of operation to the date of recurrence or the last follow-up, and OS was defined as from the date of operation to the date of death or the last follow-up. Statistical significance was defined as p<0.05. SPSS software version 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Table 1. Patient characteristics (n=42).
| Variables | AR | p | ||||
|---|---|---|---|---|---|---|
| Negative | Positive | |||||
| Age (yr) | 47 (33–69) | 49 (33–69) | 45 (39–61) | 0.390 | ||
| Parity | 0.323 | |||||
| 0 | 3 (7.1) | 2 (6.5) | 1 (9.1) | |||
| 1 | 6 (14.3) | 3 (9.7) | 3 (27.3) | |||
| ≥2 | 33 (78.6) | 26 (83.9) | 7 (63.6) | |||
| BMI (kg/m2) | 23.06 (18.04–32.79) | 23.50 (18.65–32.79) | 22.55 (18.04–26.18) | 0.112 | ||
| Menopause | 0.273 | |||||
| Yes | 27 (64.3) | 18 (41.9) | 9 (81.8) | |||
| No | 15 (35.7) | 13 (58.1) | 2 (18.2) | |||
| Size of tumor (cm) | 7.8 (2.5–21.0) | 8.8 (2.5–21.0) | 6.5 (3.5–14.0) | 0.439 | ||
| <10 | 27 (64.3) | 19 (61.3) | 8 (72.7) | 0.717 | ||
| ≥10 | 15 (35.7) | 12 (38.7) | 3 (27.3) | - | ||
| FIGO stage | 0.311 | |||||
| I | 26 (61.9) | 18 (58.1) | 8 (72.7) | |||
| II | 4 (9.5) | 2 (6.5) | 2 (18.2) | |||
| III | 3 (7.1) | 3 (9.7) | 0 (0) | |||
| IV | 9 (21.4) | 8 (25.8) | 1 (9.1) | |||
| Mitotic count (mitoses/10 high-power fields) | 1.000 | |||||
| ≤10 | 13 (31.0) | 9 (37.5) | 4 (40.0) | |||
| >10 | 21 (50.0) | 15 (62.5) | 6 (60.0) | |||
| Unknown | 8 (19.0) | - | - | |||
| Histologic subtype | 0.642 | |||||
| Spindle cell | 30 (71.4) | 23 (74.2) | 7 (71.4) | |||
| Epithelioid | 7 (16.7) | 5 (16.1) | 2 (18.2) | |||
| Myxoid | 4 (9.5) | 2 (6.5) | 2 (18.2) | |||
| Other | 1 (2.4) | 1 (3.2) | 0 (0) | |||
| Recurrence | 23 (54.8) | 20 (64.5) | 3 (27.3) | 0.043 | ||
| Pattern of recurrence | 0.791 | |||||
| Loco-regional | 9 (39.1) | 8 (40.0) | 1 (33.3) | |||
| Distant | 12 (52.2) | 10 (50.0) | 2 (66.7) | |||
| Both | 2 (9.7) | 2 (10.0) | 0 (0) | |||
| Death | 14 (33.3) | 14 (45.2) | 0 (0) | 0.007 | ||
| Hysterectomy | 0.481 | |||||
| With unilateral ovarian preservation | 15 (35.7) | 10 (32.3) | 5 (45.5) | |||
| With BSO | 27 (64.3) | 21 (67.7) | 6 (54.5) | |||
| Lymph node dissection | 19 (45.2) | 14 (45.2) | 5 (45.5) | 1.000 | ||
| LNM | 1.000 | |||||
| Positive | 1 (5.3) | 1 (7.1) | 0 (0) | |||
| Negative | 18 (94.7) | 13 (92.9) | 5 (100.0) | |||
| Adjuvant treatment | 0.761 | |||||
| None | 15 (35.7) | 10 (32.3) | 5 (45.5) | |||
| Chemotherapy | 25 (59.5) | 19 (61.3) | 6 (54.5) | |||
| Radiotherapy | 1 (2.4) | 1 (3.2) | 0 (0) | |||
| Both (chemotherapy+radiotherapy) | 1 (2.4) | 1 (3.2) | 0 (0) | |||
Values are presented as median (range) or number (range or %).
AR, androgen receptor; BMI, body mass index; BSO, bilateral salpingo-oophorectomy; FIGO, international federation of gynecology and obstetrics; LNM, lymph node metastasis.
Table 2. Expression of hormone receptors in patients with leiomyosarcoma (semi-quantitative scoring system) (n=42).
| Hormone receptors | Negative | Positive | ||
|---|---|---|---|---|
| 1+ | 2+ | 3+ | ||
| ERα | 24 (57.1) | 3 (7.1) | 6 (14.3) | 9 (21.4) |
| ERβ | 0 (0) | 0 (0) | 7 (16.7) | 35 (83.3) |
| PR | 22 (52.4) | 4 (9.5) | 4 (9.5) | 12 (28.6) |
| AR | 31 (73.8) | 4 (9.5) | 5 (11.9) | 2 (4.8) |
| GnRH | 0 (0) | 0 (0) | 0 (0) | 42 (100.0) |
| CYP19A1 | 0 (0) | 0 (0) | 7 (16.7) | 35 (83.3) |
Values are presented as number (%).
AR, androgen receptor; CYP19A1, cytochrome P450, family 19, subfamily A, polypeptide 1; ER, estrogen receptor; GnRH, gonadotropin releasing hormone; PR, progesterone receptor.
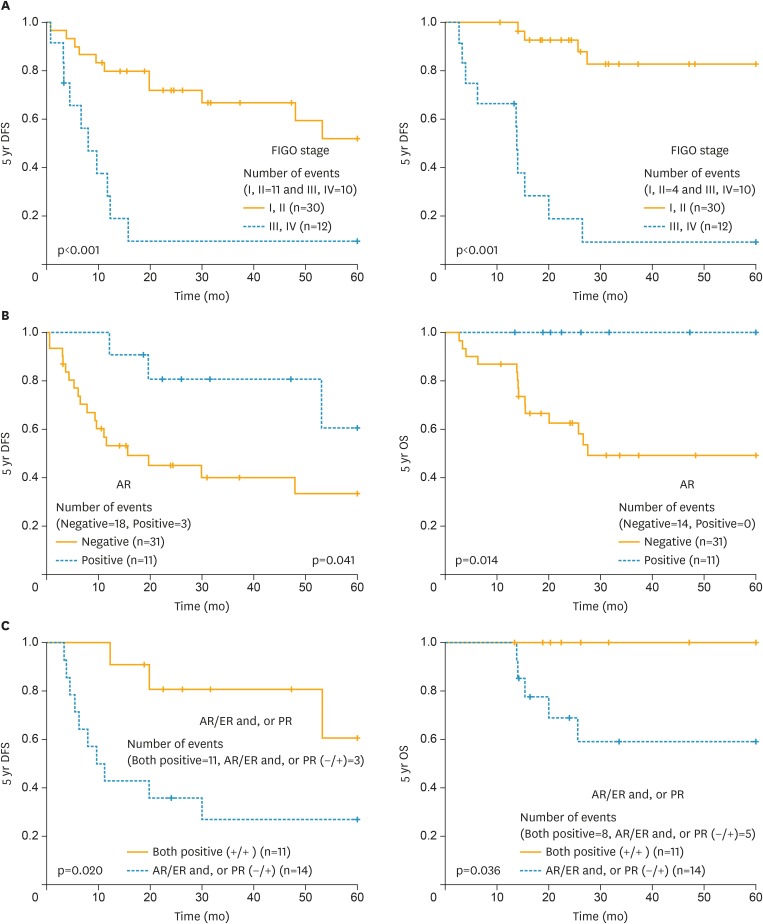
Fig. 2.
DFS and OS difference according to FIGO (A), the immunohistochemical expression of AR (B), and AR with ER and/or PR (C) in uterine leiomyosarcoma.
AR, androgen receptor; DFS, disease-free survival; ER, estrogen receptor; FIGO, International Federation of Gynecology and Obstetrics; OS, overall survival; PR, progesterone receptor.
Table 3. Analyses of survival outcome by clinicopathologic characteristics, treatment type, and expression of hormone receptors (n=42).
| Variables | Univariate | Multivariate | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|
| DFS (%) | p | OR | p | OS (%) | p | OR | p | ||
| Clinicopathologic characteristics | |||||||||
| Age (<47 vs. ≥47) | 47.6 vs. 42.9 | 0.198 | 76.2 vs. 57.1 | 0.094 | |||||
| Parity (≤2 vs. >2) | 77.8 vs. 36.4 | 0.057 | 88.9 vs. 60.6 | 0.127 | |||||
| Menopause (no vs. yes) | 48.1 vs. 40.0 | 0.050 | 77.8 vs. 46.7 | 0.005 | 1.7 (0.5–5.5) | 0.366 | |||
| BMI (<23 vs. ≥23) | 31.6 vs. 56.5 | 0.253 | 68.4 vs. 65.2 | 0.777 | |||||
| Tumor size (<10 vs. ≥10) | 59.3 vs. 20.0 | 0.013 | 0.6 (0.2–2.4) | 0.471 | 81.5 vs. 40.0 | 0.005 | 0.8 (0.2–3.8) | 0.745 | |
| Mitotic count (≤10 vs. >10) | 69.2 vs. 28.6 | 0.042 | 1.1 (0.3–4.7) | 0.888 | 76.9 vs. 71.4 | 0.815 | |||
| FIGO stage (I, II vs. III, IV) | 60.0 vs. 8.3 | <0.001 | 5.8 (1.2–28.4) | 0.029 | 86.7 vs. 16.7 | <0.001 | 12.0 (2.5–56.6) | 0.002 | |
| Ovarian preservation (no vs. yes) | 44.4 vs. 46.7 | 0.509 | 66.7 vs. 66.7 | 0.794 | |||||
| Lymphadenectomy (no vs. yes) | 52.2 vs. 36.8 | 0.049 | 1.7 (0.6–5.3) | 0.333 | 73.9 vs. 57.9 | 0.143 | |||
| Adjuvant therapy (no vs. yes) | 60.0 vs. 37.0 | 0.349 | 66.7 vs. 66.7 | 0.746 | |||||
| Hormone receptors | |||||||||
| ERα (0 vs. 1+, 2+, 3+) | 50.0 vs. 38.9 | 0.961 | 54.2 vs. 83.3 | 0.054 | |||||
| ERβ (2+ vs. 3+) | 57.1 vs. 42.9 | 0.191 | 71.4 vs. 65.7 | 0.614 | |||||
| PR (0 vs. 1+, 2+, 3+) | 36.4 vs. 55.0 | 0.043 | 1.1 (0.3–3.7) | 0.853 | 54.5 vs. 80.0 | 0.039 | 0.4 (0.1–1.8) | 0.250 | |
| AR (0 vs. 1+, 2+, 3+) | 35.5 vs. 72.7 | 0.023 | 0.2 (0.0–0.9) | 0.032 | 54.8 vs. 100.0 | 0.014 | 0 (0.0–2.9) | 0.968 | |
| GnRH (3+) | 45.2 | - | 66.7 | - | |||||
| CYP19A1 (2+ vs. 3+) | 71.4 vs. 40.0 | 0.035 | 7.5 (0.7–81.9) | 0.098 | 85.7 vs. 62.9 | 0.179 | |||
AR, androgen receptor; BMI, body mass index; CYP19A1, cytochrome P450, family 19, subfamily A, polypeptide 1; DFS, disease-free survival; ER, estrogen receptor; FIGO, International Federation of Gynecology and Obstetrics; GnRH, gonadotropin releasing hormone; OR, odds ratio; OS, overall survival; PR, progesterone receptor.
RESULTS
1. Patient characteristics
Twenty-six patients (61.9%) were FIGO stage I. Recurrence and death occurred in 23 (54.8%) and 14 (33.3%) of 42 patients with uterine leiomyosarcoma, respectively. Distant metastasis with/without loco-regional recurrence was observed in 14 of 23 recurrent patients (61.9%). The lung (n=11) was one of the most common site of distant metastasis (lung=8, abdominal peritoneum=1, lung/bone=1, lung/liver=1, and lung/liver/spleen/pancreas/neck/thigh/bone=1). Two patients showed metastasis at both loco-region and distant site (pelvis/abdominal wall/omentum=1 and pelvis/abdominal peritoneum=1). A hysterectomy was performed in all of the patients. Lymphadenectomy was performed in 19 (45.2%) of all patients. Lymph node metastasis (LNM) was found in 1 case (5.3%). Twenty-seven patients (64.3%) received adjuvant treatment. Adjuvant treatment was given when patients had extra-uterine tumor extension (FIGO II–IV). Omission of adjuvant treatment was decided by consulting the patients when they were diagnosed as FIGO stage I and had a good compliance to make a regular follow-up. Chemotherapy was performed in 25 patients (59.5%) and was the most common type of treatment (Table 1). Among them, the majority of the patients received ifosfamide based regimen (ifosfamide+cisplatin=11, etoposide+ifosfamide+cisplatin=3, and ifosfamide=1). The other regimens were doxorubicin based (paclitaxel+doxorubicin+cisplatin=2, doxorubicin+cisplatin=4, and doxorubicin=3) except one patient who received docetaxel+gemcitabine. AR expression group showed significantly less recurrences with no death events. Expression of AR showed no significant relevance with other clinic-pathological characteristics.
2. Expression of biomarkers
Of the 42 patients with uterine leiomyosarcoma, overall immune-positivity of ERα, ERβ, PR, GnRH, and CYP19A1 were 18 (42.9%), 42 (100%), 20 (47.6%), 42 (100%), and 42 (100%), respectively. Eleven patients (26.2%) showed immunoreactivity in AR. Among them, 7 patients (16.7%) showed moderate to strong immune-reaction (2+ to 3+). GnRH showed strong immune-reaction (3+) in all cases. ERβ and CYP19A1 showed a high proportion of strong immunoreactivity (3+), 35 cases (83.3%) and 35 cases (83.3%), respectively. ERα and PR showed low rates of strong immuno-reaction (3+) with 9 cases (21.4%) and 12 cases (28.6%), respectively (Fig. 1, Table 2).
3. The impact of expression of biomarkers on survival outcome, pattern of recurrence, LNM, tumor size, and FIGO stage
The mean follow-up period was 42.6±41.2 months (range, 2.6–172.6 months). In terms of tumor size and mitotic count, previous studies showed that tumor size or mitotic count greater than 10 cm or 10 mitoses/10 high-power fields were strongly associated with worse survival outcome [24,25], and we adopted these as cut-off values for more accurate analysis. In univariate analysis, tumor size, mitotic index, FIGO stage, lymphadenectomy, PR, AR, and CYP19A1 were significantly associated with DFS. The multivariate analysis was performed with variables that were statistically significant in univariate analyses, and FIGO stage and AR were an independent prognostic factor for DFS. Menopause, tumor size, FIGO stage, PR, and AR were all statistically significant in the univariate analyses for OS. There were no events in 5-year OS in uterine leiomyosarcoma patients showing AR immune-reaction compared to the no expression group (100% vs. 54.8%; p=0.014), and patients having a co-expression of AR with ER and/or PR showed statistically significant better 5-year DFS and OS compared with ER and/or PR positive patients with negative AR expression. However, FIGO stage was the only independent prognostic factor for OS in the multivariate analyses (Fig. 2, Table 3).
DISCUSSION
AR acts as a ligand-inducible transcription factor and is involved in proliferation, differentiation, and metabolism of various cells, including apoptosis [26]. Recent findings on ovarian dysfunction in female AR-knockout mice suggest that there may be a yet unknown important additional role of AR in female reproduction and tumor biology [27].
The prevalence of AR did not reach in conclusion in previous investigations, and the discrepancy between series varies from 0% to 40% [18,19]. In our study, AR expression was found in 11 patients (26.2%) and was lower than that of ER/PR. Expression of AR was higher in endometrial stromal sarcoma which has indolent clinical course than high grade undifferentiated stromal sarcoma [20]. Overall, AR expression was higher in pre-malignant lesion, more favorable histology, and lower tumor grade [28,29], and these previous findings may be the answer why AR expression is low in uterine leiomyosarcoma.
Also, AR expression was more common in early stage, lower tumor differentiation, and type 1 endometrial cancer [21]. Furthermore, it was significantly related with no lymph-vascular invasion, LNM, higher DFS, and delayed recurrence event in endometrial cancer [21]. As with previous studies in endometrial cancer, the expression of AR was related to the lower rate of recurrence and death events in our study [21]. Leitao et al. [13] reported better DFS in patients with no PR and AR expression [19]. AR positivity in uterine leiomyosarcoma independently correlated with better DFS in this study as it did in Leitao et al. [13]. Moreover, although PR was a predictive factor for a better survival outcome in the univariate analyses, AR was the only independent predictive factor for recurrence in the multivariate analyses in our study. In our series, uterine leiomyosarcoma having co-expression of AR with ER and/or PR showed significantly better DFS and OS than those with ER and/or PR positive patients with negative AR expression. Co-expression also seems to be a favorable prognostic factor in uterine leiomyosarcoma, and AR was an independent useful marker even in the presence of the expression of hormone receptors.
Most of the previous analyses on prevalence and its relationship with survival outcome have focused mainly on ER and PR in uterine sarcoma, and the results are conflicting. Hormone receptors were expressed in uterine leiomyosarcoma from 25% to 100% in previous studies [11,12,13,14,15,18,19,30,31]. In our study, there was 42.9%, 100%, and 47.6% expression for ERα, ERβ and PR, which is consistent with previous studies (30%–50%) [11,13,14,19]. Hormone therapy offers significant clinical response with disease control in recurrent endometrial stromal sarcoma [32], and also should be considered in uterine leiomyosarcoma which shares a same root. But, incorporation of hormone treatment in patients with uterine leiomyosarcoma who showed hormone receptor immune-reaction did not always show response or prognostic improvement in uterine leiomyosarcoma. Also, there are numerous different hormone treatments and we do not know which ones will show a promising clinical response, and further clinical trials are essential to confirm their efficacy. Aromatase inhibitor has a wide range of tolerance with few side effects [15,16,17], and can be administered to hormone receptor positive patients, and also to those who are resistant to progestin [31]. In one prospective phase 2 clinical study, letrozole (an aromatase inhibitor) was administered and there was a prolongation of DFS in patients with a metastatic sarcoma, which showed a strong expression of ER/PR [17]. However, despite all the patients who showed the expression of a hormone receptor, an aromatase inhibitor showed low clinical response with 9%–11% overall in previous studies [15,16,17]. A biomarker that can tell who will more likely to show a response in LMS patients regardless of ER and PR status may help to identify which case will show better response and benefit to design tailored treatment plan. Recent studies report that AR expression is significantly higher in metastatic lesion than in primary tumor [28,29], and Enzalutamide which is a direct AR inhibitor can be a promising targeted agent in certain clinical situation.
In the study by Leitao et al. [13], 6 patients (24%) underwent hormonal therapies. Among them, 3 patients were negative for hormone receptors, 2 had positive ER/PR/AR, and 1 had positive AR but negative ER/PR. Because of the small sample size, further analyses were not performed [19]. In our study, 20 and 3 patients received megestrol acetate and medroxyprogesterone acetate, respectively. Furthermore, all patients used megestrol acetate to counteract anorexia and weight loss during the pre/post-operative treatment period, taking a low dose for a short period, and 1 patient used medroxyprogesterone acetate pre-operatively to inhibit the progress of the disease. It is hard to consider or determine whether these managements are “hormone therapy,” and we were also not able to perform further analyses.
After confirming promising results of AR as a prognosticator, we expect larger and better-designed research into the role of AR in uterine leiomyosarcoma as a predictor of response to hormone treatment, disease status, and survival outcome. Currently, prospective clinical trial “NCT00414076, Letrozole versus observation in patients with newly diagnosed uterine leiomyosarcoma” is ongoing. AR showed expression in selected group of patients and may show more specific picture. Not only, incorporating traditional hormone receptors, but also AR on future research will show a better picture on tailored treatment which will eventually lead us closer to precision medicine.
There are several limitations to our study. We used archived paraffin-embedded tissue instead of fresh frozen tissue, which was not possible because of the retrospective nature of our study. Our study included patients who were diagnosed more than 10 years ago. Changes in diagnosis and management policies during the long study period must be considered as a potential cause of selection bias. Also, it would have been a comprehensive analysis if histologic subtypes of uterine leiomyosarcoma were analyzed separately. Intra-specimen and patient expression variability were not shown due to limited resource sample size although most of the cases showed homogenous expression pattern. Consistency in pathological diagnosis and measurement of expression of each biomarker is one strength of our study. We adopted generally and widely accepted criteria in the field, which is a semi-quantitative scoring system to assess the immunoreactivity of each biomarker, and compared our findings with those of other studies.
We believe that this study will help determine novel target agents for future clinical trials and help understand the characteristics of this rare type of cancer.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: P.J.Y.
- Data curation: S.D.S.
- Formal analysis: K.K.R.
- Funding acquisition: P.J.Y.
- Investigation: B.M.H.
- Methodology: P.Y.
- Project administration: K.Y.T.
- Resources: K.D.Y.
- Software: P.J.Y.
- Supervision: N.J.H.
- Validation: B.M.H.
- Visualization: K.Y.M.
- Writing - original draft: B.M.H.
- Writing - review & editing: K.J.H.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013;24:298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee JK, et al. Prediction of cancer incidence and mortality in Korea, 2016. Cancer Res Treat. 2016;48:451–457. doi: 10.4143/crt.2016.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koivisto-Korander R, Martinsen JI, Weiderpass E, Leminen A, Pukkala E. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: results from NORDCAN and NOCCA databases. Maturitas. 2012;72:56–60. doi: 10.1016/j.maturitas.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 5.D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 6.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 7.Hensley ML, Miller A, O'Malley DM, Mannel RS, Behbakht K, Bakkum-Gamez JN, et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: an NRG Oncology/Gynecologic Oncology Group study. J Clin Oncol. 2015;33:1180–1185. doi: 10.1200/JCO.2014.58.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hensley ML, Sill MW, Scribner DR, Jr, Brown J, Debernardo RL, Hartenbach EM, et al. Sunitinib malate in the treatment of recurrent or persistent uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2009;115:460–465. doi: 10.1016/j.ygyno.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zivanovic O, Leitao MM, Iasonos A, Jacks LM, Zhou Q, Abu-Rustum NR, et al. Stage-specific outcomes of patients with uterine leiomyosarcoma: a comparison of the international Federation of gynecology and obstetrics and american joint committee on cancer staging systems. J Clin Oncol. 2009;27:2066–2072. doi: 10.1200/JCO.2008.19.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim D, Wang WL, Lee CH, Dodge T, Gilks B, Oliva E. Old versus new FIGO staging systems in predicting overall survival in patients with uterine leiomyosarcoma: a study of 86 cases. Gynecol Oncol. 2013;128:322–326. doi: 10.1016/j.ygyno.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Akhan SE, Yavuz E, Tecer A, Iyibozkurt CA, Topuz S, Tuzlali S, et al. The expression of Ki-67, p53, estrogen and progesterone receptors affecting survival in uterine leiomyosarcomas. A clinicopathologic study. Gynecol Oncol. 2005;99:36–42. doi: 10.1016/j.ygyno.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Ioffe YJ, Li AJ, Walsh CS, Karlan BY, Leuchter R, Forscher C, et al. Hormone receptor expression in uterine sarcomas: prognostic and therapeutic roles. Gynecol Oncol. 2009;115:466–471. doi: 10.1016/j.ygyno.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Leitao MM, Jr, Hensley ML, Barakat RR, Aghajanian C, Gardner GJ, Jewell EL, et al. Immunohistochemical expression of estrogen and progesterone receptors and outcomes in patients with newly diagnosed uterine leiomyosarcoma. Gynecol Oncol. 2012;124:558–562. doi: 10.1016/j.ygyno.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Garcia C, Kubat JS, Fulton RS, Anthony AT, Combs M, Powell CB, et al. Clinical outcomes and prognostic markers in uterine leiomyosarcoma: a population-based cohort. Int J Gynecol Cancer. 2015;25:622–628. doi: 10.1097/IGC.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 15.O'Cearbhaill R, Zhou Q, Iasonos A, Soslow RA, Leitao MM, Aghajanian C, et al. Treatment of advanced uterine leiomyosarcoma with aromatase inhibitors. Gynecol Oncol. 2010;116:424–429. doi: 10.1016/j.ygyno.2009.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman AD, Nelson GS, Chu P, Nation J, Ghatage P. Uterine sarcoma and aromatase inhibitors: Tom Baker Cancer Centre experience and review of the literature. Int J Gynecol Cancer. 2012;22:1006–1012. doi: 10.1097/IGC.0b013e31825b7de8. [DOI] [PubMed] [Google Scholar]
- 17.George S, Feng Y, Manola J, Nucci MR, Butrynski JE, Morgan JA, et al. Phase 2 trial of aromatase inhibition with letrozole in patients with uterine leiomyosarcomas expressing estrogen and/or progesterone receptors. Cancer. 2014;120:738–743. doi: 10.1002/cncr.28476. [DOI] [PubMed] [Google Scholar]
- 18.Koivisto-Korander R, Butzow R, Koivisto AM, Leminen A. Immunohistochemical studies on uterine carcinosarcoma, leiomyosarcoma, and endometrial stromal sarcoma: expression and prognostic importance of ten different markers. Tumour Biol. 2011;32:451–459. doi: 10.1007/s13277-010-0138-1. [DOI] [PubMed] [Google Scholar]
- 19.Leitao MM, Soslow RA, Nonaka D, Olshen AB, Aghajanian C, Sabbatini P, et al. Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer. 2004;101:1455–1462. doi: 10.1002/cncr.20521. [DOI] [PubMed] [Google Scholar]
- 20.Roy M, Kumar S, Bhatla N, Ray MD, Kumar L, Jain D, et al. Androgen receptor expression in endometrial stromal sarcoma: correlation with clinicopathologic features. Int J Gynecol Pathol. 2017;36:420–427. doi: 10.1097/PGP.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 21.Mahdi Z, Abdulfatah E, Pardeshi V, Hassan O, Schultz D, Morris R, et al. The impact of androgen receptor expression on endometrial carcinoma recurrence and survival. Int J Gynecol Pathol. 2017;36:405–411. doi: 10.1097/PGP.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 22.World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 23.Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet. 2009;104:177–178. doi: 10.1016/j.ijgo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 25.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71:1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 26.Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamal AM, Bulmer JN, DeCruze SB, Stringfellow HF, Martin-Hirsch P, Hapangama DK. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br J Cancer. 2016;114:688–696. doi: 10.1038/bjc.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangen IL, Onyango TB, Kopperud R, Berg A, Halle MK, Øyan AM, et al. Androgen receptor as potential therapeutic target in metastatic endometrial cancer. Oncotarget. 2016;7:49289–49298. doi: 10.18632/oncotarget.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lusby K, Savannah KB, Demicco EG, Zhang Y, Ghadimi MP, Young ED, et al. Uterine leiomyosarcoma management, outcome, and associated molecular biomarkers: a single institution's experience. Ann Surg Oncol. 2013;20:2364–2372. doi: 10.1245/s10434-012-2834-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thanopoulou E, Thway K, Khabra K, Judson I. Treatment of hormone positive uterine leiomyosarcoma with aromatase inhibitors. Clin Sarcoma Res. 2014;4:5. doi: 10.1186/2045-3329-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamazaki H, Todo Y, Mitsube K, Hareyama H, Shimada C, Kato H, et al. Long-term survival of patients with recurrent endometrial stromal sarcoma: a multicenter, observational study. J Gynecol Oncol. 2015;26:214–221. doi: 10.3802/jgo.2015.26.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]