Abstract
Objective
Uterine serous carcinoma (USC) is an aggressive type 2 endometrial cancer. Data on prognostic factors for patients with early-stage USC without adjuvant therapy are limited. This study aims to assess the baseline recurrence risk of early-stage USC patients without adjuvant treatment and to identify prognostic factors and patients who need adjuvant therapy.
Methods
Sixty-eight patients with International Federation of Gynecology and Obstetrics (FIGO) stage I–II USC between 1997 and 2016 were included. All the cases did not undergo adjuvant treatment as institutional practice. Clinicopathological features, recurrence patterns, and survival outcomes were analyzed to determine prognostic factors.
Results
FIGO stages IA, IB, and II were observed in 42, 7, and 19 cases, respectively. Median follow-up time was 60 months. Five-year disease-free survival (DFS) and overall survival (OS) rates for all cases were 73.9% and 78.0%, respectively. On multivariate analysis, cervical stromal involvement and positive pelvic cytology were significant predictors of DFS and OS, and ≥1/2 myometrial invasion was also a significant predictor of OS. Of 68 patients, 38 patients had no cervical stromal invasion or positive pelvic cytology and showed 88.8% 5-year DFS and 93.6% 5-year OS.
Conclusion
Cervical stromal invasion and positive pelvic cytology are prognostic factors for stage I–II USC. Patients with stage IA or IB USC showing negative pelvic cytology may have an extremely favorable prognosis and need not receive any adjuvant therapies.
Keywords: Adenocarcinoma; Endometrial Neoplasms; Prognosis; Cytodiagnosis; Chemotherapy, Adjvant
INTRODUCTION
Uterine serous carcinoma (USC) is a rare aggressive type 2 endometrial carcinoma [1]. It accounts for >39% of deaths attributed to endometrial carcinoma even though it represents <10% of all endometrial cancers [2]. The recurrence rate of USC is high, estimated to be 31%–80% even in its early stages (stage I–II) [3]. Recently, histological and genomic studies suggested that USC possibly shares aggressive behavioral and molecular characteristics with ovarian high-grade serous carcinoma or breast cancer with basal-like phenotype rather than endometrial endometrioid adenocarcinoma [4,5].
Frequent recurrences in USC have led to the induction of adjuvant treatment, including systemic chemotherapy or radiation therapy. However, treatment modalities for patients with early-stage USC who can benefit from adjuvant therapy remain controversial. Several retrospective studies have reported on the prognostic impact of adjuvant treatment [6,7,8]. Fader et al. [9] demonstrated that survival outcomes are significantly improved in patients who underwent platinum/taxane chemotherapy±radiation therapy compared with patients without adjuvant therapy or with radiation therapy alone. Conversely, van der Putten et al. [10] reported that adjuvant chemo-radiotherapy has no significant survival benefit for stage IB USC patients. Appropriate treatment modalities are unclear because there are early-stage USC patients with very favorable prognoses who should not receive adjuvant therapy.
Optimal patient selection for adjuvant treatment requires evaluation of baseline recurrence risk and prognostic factors for early-stage USC. However, identifying prognostic factors unaffected by adjuvant therapies is quite difficult because most USC patients receive adjuvant treatment in recent clinical practice. Reports on prognostic factors in USC patients without adjuvant therapy are limited. Furthermore, patients who did not undergo adjuvant therapies were reported to be older, had poor performance status or organ dysfunction, or refused treatment. Such selection bias presents challenges for detecting the true baseline recurrence risk and prognostic factors for early-stage USC patients. In this study, we retrospectively reviewed consecutive early-stage USC patients who did not undergo any adjuvant therapies. Adjuvant treatment as a standard clinical practice was not performed during the study period because solid evidence was lacking.
This study aims to assess the baseline recurrence risk in early-stage USC patients without adjuvant treatment and to identify prognostic factors in patients who are candidates for adjuvant therapy.
MATERIALS AND METHODS
Following approval from the Institutional Review Board of National Cancer Center, patients with histologically confirmed stage I and II (International Federation of Gynecology and Obstetrics [FIGO] 2008) USC from 1997 to 2016 were identified from the tumor registry database of our institution. For mixed cell carcinoma, diagnosis of USC was made when at least 5% serous carcinoma component in the tumor was observed [11]. Clinicopathological data were obtained by retrospective medical chart review.
Clinical follow-up in most cases consisted of vaginal examinations, Pap smear and chest X-rays every 3–6 months, and computed tomography (CT) of the chest/abdomen/pelvis every 6 months for the first 2 years. During the third and fourth follow-up years, patients underwent physical examinations and Pap smear twice and imaging examinations annually. After 5 years, patients still underwent vaginal examinations and Pap smears annually, and those suspected of recurrent USC underwent CT, magnetic resonance imaging, and histological examination.
Follow-up time and time to event was measured from the date of surgery to the last known visit or death. Disease-free survival (DFS) and overall survival (OS) were estimated according to the Kaplan-Meier product limit method and were compared using the log-rank test. Two or more recurrences were calculated from the date of the first relapse. Prognostic factors were obtained by univariate and multivariate analyses. Factors with a p-value <0.05 by univariate analysis were included in the subsequent multivariate analysis. Cox-proportional hazards analysis was used for univariate and multivariate modeling. A 2-sided log-rank test was employed to analyze DFS and OS among the groups divided according to prognostic factors. Statistical analysis was performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). A p-value <0.05 was considered statistically significant in each test.
RESULTS
1. Patients and treatment
Seventy patients with FIGO stage I and II USC without any adjuvant treatment were identified. Two patients were excluded (one died from choriocarcinoma and the other from breast cancer). A total of 68 patients who fulfilled the inclusion criteria were included in the final analysis. The clinicopathological features are summarized in Table 1. The median age was 64 years (range 43–81 years). Sixty-two (91%) patients were post-menopausal, and the median body mass index was 31.1 (range 19.4–44.7).
Table 1. Patients' characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| Age | ||
| <65 | 37 (54) | |
| ≥65 | 31 (46) | |
| Menopause | ||
| Pre-menopause | 6 (9) | |
| Post-menopause | 62 (91) | |
| BMI | ||
| <30 | 15 (22) | |
| ≥30 | 53 (78) | |
| Histology | ||
| Pure | 51 (75) | |
| Mixed | 17 (25) | |
| Depth of invasion | ||
| Endometrium | 16 (24) | |
| Myometrium <50% | 38 (56) | |
| Myometrium ≥50% | 14 (21) | |
| Cervical stromal involvement | ||
| Present | 19 (28) | |
| Absent | 49 (72) | |
| LVSI | ||
| Present | 21 (31) | |
| Absent | 47 (69) | |
| Pelvic cytology | ||
| Positive | 14 (21) | |
| Negative | 53 (78) | |
| Unknown | 1 (1) | |
| Stage (FIGO 2008) | ||
| IA | 42 (62) | |
| IB | 7 (10) | |
| II | 19 (28) | |
BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LVSI, lymphovascular space invasion.
In this study, the surgical staging procedure involved total hysterectomy and bilateral salpingo-oophorectomy. All the patients underwent abdominal hysterectomy and bilateral salpingo-oophorectomy. Pelvic lymph node sampling was performed in 48 (71%) patients, and para-aortic nodes were sampled in 3 patients. The number of removed lymph node ranged from 1 to 46 (median 15). Omentectomy was performed in 8 (12%) patients. Intraoperative peritoneal cytology was performed in 67 (99%) patients, of which 14 (21%) showed adenocarcinoma.
All specimens were reviewed by experienced gynecologic pathologists, and histological diagnoses were made according to the WHO classification criteria [11]. FIGO stages IA, IB, and II were observed in 42 (62%), 7 (10%), and 19 (28%) patients, respectively. Sixteen (24%) patients had diseases confined to the endometrium, and the depth of myometrial invasion was limited to the inner half in 54 (79%) patients. Lymphovascular space invasion (LVSI) was present in 48 (71%) cases.
2. Patients' outcomes
The median follow-up time was 60 months (range 8–214 months). Recurrences were observed in 18 (26%) cases, the median time to recurrence was 13.5 months (range 4–67 months). Clinicopathological features, treatment, and prognosis are summarized in Table 2. Of the 18 patients, only 1 showed metachronous recurrences in the vagina and lung. Six patients had recurrences at multiple sites. Recurrences in the vagina, para-aortic nodes, and other distant sites were observed in 2, 2, and 7 cases, respectively.
Table 2. Clinicopathological characteristics, treatment, and outcomes of patients with recurrence of uterine serous carcinoma.
| Case | Age | Stage | Depth of invasion | Cervical stromal involvement | LVSI | Pelvic cytology | Tumor size (mm) | Site of recurrence | Treatment for recurrence | DFS (mo) | Status at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | IA | <50% | Present | Present | Positive | 38 | Peritoneum+PLN | Chemotherapy | 11 | DOD |
| 2 | 61 | II | <50% | Absent | Absent | Positive | 55 | Peritoneum | Chemotherapy | 15 | DOD |
| 3 | 65 | II | ≥50% | Absent | Absent | Negative | 25 | Peritoneum | Chemotherapy | 19 | DOD |
| 4 | 72 | II | ≥50% | Absent | Absent | Positive | 33 | Peritoneum | Chemotherapy | 5 | DOD |
| 5 | 72 | IA | Endometrium | Present | Absent | Positive | 25 | Peritoneum | Chemotherapy | 10 | LOF |
| 6 | 76 | IA | Endometrium | Present | Absent | Negative | 25 | Lung+pericardium+cervical lymph node | Chemotherapy | 36 | DOD |
| 7 | 58 | IA | <50% | Present | Absent | Positive | 37 | Lung, abdominal mass | Chemotherapy | 5 | DOD |
| 8 | 77 | II | ≥50% | Absent | Present | Negative | 42 | Lung | Chemotherapy | 22 | DOD |
| 9 | 66 | II | <50% | Absent | Absent | Negative | 25 | Brain+lung | RT | 67 | DOD |
| 10 | 64 | IA | <50% | Present | Absent | Negative | 29 | Vagina (12 mo), lung (27 mo) | IVRT+surgery | 12 | NED |
| 11 | 78 | II | ≥50% | Absent | Present | Negative | 90 | Vagina | IVRT | 4 | DOD |
| 12 | 77 | II | ≥50% | Absent | Present | Negative | 60 | Vagina | IVRT | 7 | DOD |
| 13 | 73 | IA | Endometrium | Present | Absent | Positive | 20 | Liver+pelvic mass | Chemotherapy | 41 | DOD |
| 14 | 58 | IB | ≥50% | Present | Present | Positive | 40 | Pleura | Chemotherapy | 26 | DOD |
| 15 | 63 | IA | <50% | Present | Present | Positive | 15 | Mesentery | Surgery+RT | 21 | NED |
| 16 | 61 | IB | ≥50% | Present | Present | Negative | 35 | PAN+Virchow's node+subcutaneous tumor | Chemotherapy+RT | 17 | DOD |
| 17 | 62 | IA | <50% | Present | Absent | Negative | 25 | PAN | Surgery+RT | 10 | NED |
| 18 | 67 | II | <50% | Absent | Absent | Negative | 100 | PAN | Chemotherapy | 8 | NED |
DFS, disease-free survival; DOD, death on disease; IVRT, intravaginal radiotherapy; LOF, loss of follow-up; LVSI, lymphovascular space invasion; NED, no evidence of disease; PAN, para-aortic lymph node; PLN, pelvic lymph node; RT, radiation therapy.
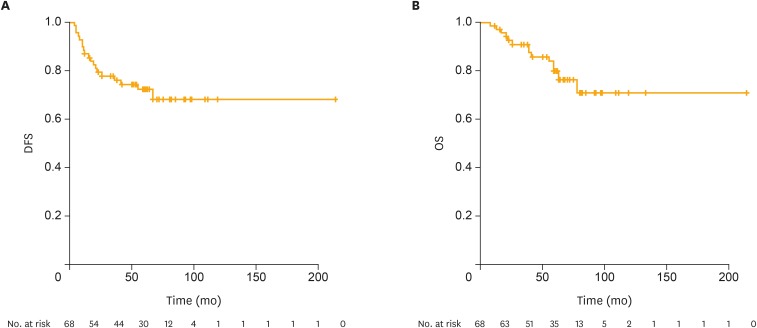
Of the 2 isolated cases of para-aortic node relapses, one patient was salvaged through surgery and the other received systemic chemotherapy. Both patients survived without evidence of disease at 68 and 133 months from the primary surgery. The patient with metachronous recurrences in the vagina and lung underwent surgical resections for each lesion and showed no evidence of disease at 34 months from the last surgery. Two of the patients with vaginal recurrences were treated with radiation therapy, but expired due to the disease. All other patients with recurrences died of the disease after chemotherapy. The 5-year DFS and 5-year OS rates for all patients were 73.9% and 78.0%, respectively (Fig. 1). The median DFS and OS were 59.0 (range 4–214 months) and 60.0 (range 8–214 months), respectively.
Fig. 1.
DFS and OS of patients with stage I and II USC.
DFS, disease-free survival; OS, overall survival; USC, uterine serous carcinoma.
3. Prognostic factors
Prognostic factors in 68 early-stage USC patients without adjuvant therapy reported from previous studies were examined. On univariate analysis, ≥1/2 myometrial invasion (DFS: hazard ratio [HR]=2.71; 95% confidence interval [CI]=1.06–6.88; p=0.037; OS: HR=4.50; 95% CI 1.58–12.90; p=0.005), cervical stromal involvement (DFS: HR=2.64; 95% CI=1.07–6.50; p=0.035; OS: HR=3.80; 95% CI=1.32–11.00; p=0.014), positive pelvic cytology (DFS: HR=3.35; 95% CI=1.34–8.33; p=0.010; OS: HR=3.00; 95% CI=1.04–8.69; p=0.042), and lymph node sampling (DFS: HR=3.30; 95% CI=1.32–8.23; p=0.011; OS: HR=3.88; 95% CI=1.34–11.30; p=0.013) were independently associated with both DFS and OS (Table 3). Age ≥65 years (DFS: HR=0.55; 95% CI=0.22–1.37; p=0.200; OS: HR=0.39; 95% CI=0.13–1.17; p=0.091), presence of myometrial invasion (DFS: HR=1.83; 95% CI=0.53–6.30; p=0.340; OS: HR=1.75; 95% CI=0.39–7.82; p=0.470), and LVSI (DFS: HR=0.73; 95% CI=0.28–1.84; p=0.510; OS: HR=0.55; 95% CI=0.19–1.58; p=0.270) showed no significant effect on DFS and OS.
Table 3. Univariate and multivariate analyses.
| Characteristic | No. | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5-year DFS | 5-year OS | 5-year DFS | 5-year OS | |||||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |||
| Age | ||||||||||
| ≥65 (vs. <65) | 31 | 0.55 (0.22–1.37) | 0.200 | 0.39 (0.13–1.17) | 0.091 | - | - | - | - | |
| Myometrial invasion | ||||||||||
| Present (vs. absent) | 51 | 1.83 (0.53–6.30) | 0.340 | 1.75 (0.39–7.82) | 0.470 | - | - | - | - | |
| ≥1/2 (vs. <1/2) | 14 | 2.71 (1.06–6.88) | 0.037 | 4.50 (1.58–12.90) | 0.005 | 2.01 (0.78–5.21) | 0.150 | 3.87 (1.31–11.40) | 0.014 | |
| Tumor location | ||||||||||
| LUS (vs. others) | 12 | 1.66 (0.60–4.60) | 0.330 | 1.87 (0.59–5.98) | 0.290 | - | - | - | - | |
| LVSI | ||||||||||
| Present (vs. absent) | 21 | 0.73 (0.28–1.84) | 0.510 | 0.55 (0.19–1.58) | 0.270 | - | - | - | - | |
| Cervical stromal invasion | ||||||||||
| Present (vs. absent) | 19 | 2.64 (1.07–6.50) | 0.035 | 3.80 (1.32–11.0) | 0.014 | 3.67 (1.35–9.95) | 0.011 | 7.09 (1.92–26.22) | 0.003 | |
| Pelvic cytology | ||||||||||
| Positive (vs. negative) | 14 | 3.35 (1.34–8.33) | <0.001 | 3.00 (1.04–8.69) | 0.042 | 4.24 (1.56–11.52) | 0.005 | 4.96 (1.34–18.33) | 0.017 | |
| Lymph node sampling | ||||||||||
| Not done (vs. done) | 20 | 3.30 (1.32–8.23) | 0.011 | 3.88 (1.34–11.26) | 0.013 | 3.47 (1.37–8.79) | 0.009 | 4.98 (1.63–15.25) | 0.005 | |
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; LUS, lower uterine segment; LVSI, lymphovascular space invasion; OS, overall survival.
On multivariate analysis, cervical stromal involvement (DFS: HR=3.67; 95% CI=1.35–9.95; p=0.011; OS: HR=7.09; 95% CI=1.92–26.20; p=0.003), positive pelvic cytology (DFS: HR=4.24; 95% CI=1.56–11.50; p=0.005; OS: HR=4.96; 95% CI=1.34–18.30; p=0.017), and lymph node sampling (DFS: HR=3.47; 95% CI=1.37–8.79; p=0.009; OS: HR=4.98; 95% CI=1.63–15.30; p=0.005) remained as significant prognosticators of both DFS and OS (Table 3). Although ≥1/2 myometrial invasion (OS: HR=3.87; 95% CI=1.31–11.40; p=0.014) was also a prognostic factor for OS, it is not associated with DFS (HR=2.01; 95% CI=0.78–5.21; p=0.150).
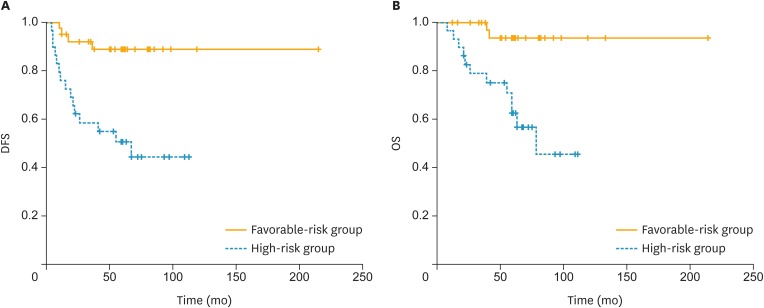
Furthermore, we identified a subgroup of early-stage USC patients with extremely favorable prognosis who did not need adjuvant therapy. Of the 68 patients, 38 (56%) patients had no cervical stromal invasion or positive pelvic cytology. We classified these patients into the favorable-risk group. Twenty-nine (43%) patients had cervical stromal invasion and/or positive pelvic cytology and were classified as the high-risk group. The 5-year DFS rates for the favorable-risk and high-risk groups were 88.8% and 50.6%, respectively (p<0.001) (Fig. 2 and Table 4). The 5-year OS rates for the favorable-risk and high-risk groups were 93.6% and 62.6%, respectively (p<0.001). A statistically significant difference in the 5-year OS and DFS was found between the 2 groups.
Fig. 2.
DFS and OS of patients of favorable-risk group and high-risk group. Favorable-risk group: negative pelvic cytology and absence of cervical stromal invasion; High-risk group: positive pelvic cytology and/or presence of cervical stromal invasion.
DFS, disease-free survival; OS, overall survival.
Table 4. Comparison of prognosis between the favorable-risk group and high-risk group of early-stage USC patients.
| Group | No. | DFS | OS | ||
|---|---|---|---|---|---|
| 5-years DFS rate (%) | p | 5-years OS rate (%) | p | ||
| Favorable-risk group* | 38 | 88.8 | <0.001 | 93.6 | <0.001 |
| High-risk group† | 29 | 50.6 | 62.6 | ||
DFS, disease-free survival; OS, overall survival; USC, uterine serous carcinoma.
*Negative pelvic cytology and absence of cervical stromal invasion; †Positive pelvic cytology and/or presence of cervical stromal invasion.
Survival analyses of cases in each FIGO stage were also performed (Supplementary Fig. 1). Positive pelvic cytology has significant worse prognostic impact on OS and RFS in stage IA disease (n=42), but not in stage IB (n=7) and II (n=19). However, the number of cases with stage IB and II disease were smaller and follow-up periods were shorter than those of stage IA disease.
DISCUSSION
In this study, we evaluated 68 early-stage USC patients without adjuvant treatment, determined their baseline recurrence risk, and identified the prognostic factors. We discriminated against patients with favorable prognoses who may not need adjuvant therapy.
The 5-year DFS and OS of the entire cohort of our study were similar to those previously reported. Most previous studies on USC were compromised by their relatively small sample sizes, short follow-up period, and selection bias for adjuvant therapy. Nevertheless, prior large-scale studies including USC patients with adjuvant therapy reported survival outcomes similar to those of our study. van der Putten et al. [10] examined 127 patients with stage I USC and reported that the 5-year DFS was 69.8%. Creasman et al. [12] reported that the 5-year OS rates were 72% in 148 stage I USC patients, 66% in patients with surgery alone, and 74% in patients with adjuvant treatment. Hamilton et al. [13] showed that in patients with stage I and II USC, the 5-year OS rate was 74%. Hence, the 5-year DFS and OS of our cohort, despite the lack of adjuvant therapy, were comparable to those previously reported in early-stage USC patients with adjuvant treatment.
Omentectomy had no prognostic impact on early-stage USC in this study. Omentectomy was performed in 12% of patients only, and it was not associated with DFS and OS on multivariate analysis (DFS: HR=0.65; 95% CI=0.085–4.930; p=0.990; OS: HR=0; 95% CI=0).
Our findings suggest that in early-stage USC patients without adjuvant therapy, cervical stromal involvement, positive pelvic cytology, and lymph node sampling are significant predictors of DFS, and ≥1/2 myometrial invasion, cervical stromal involvement, positive pelvic cytology, and lymph node sampling are significantly associated with OS. However, we found considerable variation in the number and site of removed lymph nodes, which ranged from 1 to 46 (median 15). This quantitative and qualitative variation of lymph node sampling/dissection made it difficult to interpret the results of comparisons between 2 simple categorical (lymph node sampling not done vs. done) groups. For USC patients with adjuvant therapy, positive pelvic cytology has been reported to be a prognostic factor. A previous retrospective study on 42 cases with type 2 endometrial cancer showed that FIGO stage III–IV and positive pelvic cytology are prognostic factors of recurrence and positive pelvic cytology is also associated with DFS especially in the early stages of the disease [14]. Moreover, a database study on 14,704 patients with early-stage endometrial cancer, including 1,153 with USC or clear cell carcinoma, showed that age, FIGO stage, and positive pelvic cytology are associated with DFS, and age and positive pelvic cytology are prognostic factors of survival [15]. “FIGO stage” in the study was synonymous with cervical stromal involvement and myometrial invasion in our study. These previously reported prognostic factors for patients with adjuvant therapy were also associated with DFS and OS in our study including only patients with early-stage USC without adjuvant therapy. Additionally, positive pelvic cytology was observed in 21% of patients in our study, which is comparable to previously reported studies. Previous studies on pelvic cytology in type 2 endometrial cancer have reported a positive rate of 17.5%–21.4% [14,15,16].
The main aim of this study is to determine candidates and non-candidates for adjuvant therapy. Patients who have neither cervical stromal involvement nor positive pelvic cytology (i.e., those with stage I USC without positive pelvic cytology) have favorable DFS and OS compared with other patients with early-stage USC. Previous studies suggested that recurrence and survival outcomes are improved in stage I USC patients receiving adjuvant therapy. Fader et al. [9] evaluated 142 patients with stage I USC, and the 5-year DFS rates for patients with adjuvant chemotherapy±radiation therapy and those without adjuvant therapy were 81.5% and 64.7%, respectively. However, pelvic cytology was not considered in the report. The 5-year DFS and OS rates of stage I USC without positive pelvic cytology in our study were almost similar to those of patients with adjuvant therapy and those of patients with stage I type 1 endometrial cancer [17,18]. Hence, adjuvant therapy in patients with stage I USC without positive pelvic cytology may not be necessary. Nevertheless, our findings need to be validated in another large-scale cohort study.
Furthermore, our findings also suggest another distinctive subgroup with an extremely favorable prognosis. Six pre-menopausal patients survived without recurrence at ≥58 months. Stage IA, IB, and II were observed in 1, 1, and 4 cases, respectively, which included patients with adverse prognostic factors, such as cervical stromal involvement or positive pelvic cytology. All of their tumors were located in the isthmus of the uterus. In patients who were menopausal for at least the previous 2 years, the tumors were localized in the uterine fundus or uterine body or occupied the entire area of the uterus. This observation suggests that USC located in the lower uterine segments may have a different mechanism of tumorigenesis and clinical behavior, especially in younger patients. Several recent studies showed the unique characteristics of tumors in the lower uterine segment. In the largest comparison study, patients with lower uterine segment tumors were significantly younger than those with uterine corpus tumors, and the median ages of onset were 54.2 and 62.9 years old, respectively [19]. Moreover, a large descriptive study reported that muscular invasion was deeper and the histology grade higher in 16 patients with low uterine segment tumors compared to patients with uterine corpus tumors. Nevertheless, no difference in the 10-year survival rate was noted [20]. Collectively, these observations along with our data suggest that serous carcinoma in the lower uterine segment may have distinct biological and clinicopathological features.
This study has several limitations. The sample size was relatively small and the study was retrospective in nature. However, obtaining large-scale data including detailed clinicopathological information and conducting relevant prospective studies appear quite challenging. Nevertheless, the size of our study cohort was sufficient for analyzing the prognostic factors that were independently associated with DFS and OS. Furthermore, our study is one of the largest studies of stage I and II USC without adjuvant therapy. In most previous reports on prognostic factors for early-stage USC patients, adjuvant treatment almost always influenced patients' prognosis and concealed the natural history or baseline recurrence risk of USC. Our study is free from bias in patient selection for adjuvant therapy and we provided the natural history of early-stage USC.
In conclusion, cervical stromal involvement and positive pelvic cytology are prognostic factors for early-stage USC patients. Patients with stage IA or IB USC showing that patients with negative pelvic cytology may have a favorable prognosis and need not receive any adjuvant therapies.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.jp) for English language editing.
Footnotes
Funding: This work was supported by the National Cancer Center Research and Development Fund.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: T.K., Y.H., I.M.
- Data curation: T.K.
- Formal analysis: T.K., Y.H., I.M.
- Funding acquisition: Y.H.
- Investigation: T.K., Y.H., I.M.
- Methodology: T.K., Y.H., I.M.
- Project administration: H.N., K.T.
- Resources: T.K., Y.H., I.M., U.T., I.S., H.N., K.T.
- Software: T.K.
- Supervision: H.N., K.T.
- Validation: T.K., Y.H., I.M.
- Visualization: T.K.
- Writing - original draft: T.K., Y.H.
- Writing - review & editing: T.K., Y.H., I.M., U.T., I.S., H.N., K.T.
SUPPLEMENTARY MATERIAL
Survival impact of pelvic cytology: analysis within same FIGO stage.
References
- 1.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 2.Boruta DM, 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115:142–153. doi: 10.1016/j.ygyno.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 3.del Carmen MG, Birrer M, Schorge JO. Uterine papillary serous cancer: a review of the literature. Gynecol Oncol. 2012;127:651–661. doi: 10.1016/j.ygyno.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin Cancer Res. 2002;8:1271–1279. [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huh WK, Powell M, Leath CA, 3rd, Straughn JM, Jr, Cohn DE, Gold MA, et al. Uterine papillary serous carcinoma: comparisons of outcomes in surgical stage I patients with and without adjuvant therapy. Gynecol Oncol. 2003;91:470–475. doi: 10.1016/j.ygyno.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich CS, 3rd, Modesitt SC, DePriest PD, Ueland FR, Wilder J, Reedy MB, et al. The efficacy of adjuvant platinum-based chemotherapy in stage I uterine papillary serous carcinoma (UPSC) Gynecol Oncol. 2005;99:557–563. doi: 10.1016/j.ygyno.2005.07.104. [DOI] [PubMed] [Google Scholar]
- 8.Kelly MG, O’malley DM, Hui P, McAlpine J, Yu H, Rutherford TJ, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol Oncol. 2005;98:353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Fader AN, Drake RD, O’Malley DM, Gibbons HE, Huh WK, Havrilesky LJ, et al. Platinum/taxane-based chemotherapy with or without radiation therapy favorably impacts survival outcomes in stage I uterine papillary serous carcinoma. Cancer. 2009;115:2119–2127. doi: 10.1002/cncr.24247. [DOI] [PubMed] [Google Scholar]
- 10.van der Putten LJ, Hoskins P, Tinker A, Lim P, Aquino-Parsons C, Kwon JS. Population-based treatment and outcomes of stage I uterine serous carcinoma. Gynecol Oncol. 2014;132:61–64. doi: 10.1016/j.ygyno.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Zaino RJ, Carnelli SG. Tumours of the uterine corpus. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO classification of tumours of female reproductive organs. Lyon: IARC Press; 2014. pp. 121–135. [Google Scholar]
- 12.Creasman WT, Kohler MF, Odicino F, Maisonneuve P, Boyle P. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95:593–596. doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han KH, Park NH, Kim HS, Chung HH, Kim JW, Song YS. Peritoneal cytology: a risk factor of recurrence for non-endometrioid endometrial cancer. Gynecol Oncol. 2014;134:293–296. doi: 10.1016/j.ygyno.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Garg G, Gao F, Wright JD, Hagemann AR, Mutch DG, Powell MA. Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol. 2013;128:77–82. doi: 10.1016/j.ygyno.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fader AN, Java J, Tenney M, Ricci S, Gunderson CC, Temkin SM, et al. Impact of histology and surgical approach on survival among women with early-stage, high-grade uterine cancer: an NRG Oncology/Gynecologic Oncology Group ancillary analysis. Gynecol Oncol. 2016;143:460–465. doi: 10.1016/j.ygyno.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Wárlám-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 18.Burke WM, Orr J, Leitao M, Salom E, Gehrig P, Olawaiye AB, et al. Endometrial cancer: a review and current management strategies: part II. Gynecol Oncol. 2014;134:393–402. doi: 10.1016/j.ygyno.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Westin SN, Lacour RA, Urbauer DL, Luthra R, Bodurka DC, Lu KH, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol. 2008;26:5965–5971. doi: 10.1200/JCO.2008.18.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hachisuga T, Fukuda K, Iwasaka T, Hirakawa T, Kawarabayashi T, Tsuneyoshi M. Endometrioid adenocarcinomas of the uterine corpus in women younger than 50 years of age can be divided into two distinct clinical and pathologic entities based on anatomic location. Cancer. 2001;92:2578–2584. doi: 10.1002/1097-0142(20011115)92:10<2578::aid-cncr1610>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survival impact of pelvic cytology: analysis within same FIGO stage.