Abstract
Objective
Our previous study showed that insulin resistance (IR) was related to endometrial hyperplasia as well as endometrial cancer. But the exact impact of IR on fertility-sparing treatment in endometrial hyperplasic disease is unclear. This study investigated how IR affects fertility-sparing treatment in endometrial atypical hyperplasia (EAH) patients.
Methods
The 151 EAH patients received fertility-sparing treatment were retrospectively investigated. All patients received high-dose progestin combined with hysteroscopy. Therapeutic effects were evaluated by hysteroscopy every 3 months during the treatment.
Results
The median age was 33.0 years old (range, 21–54 years old). Sixty-one patients (40.4%) were insulin resistant. Three patients were excluded from the analysis because they chose hysterectomy within 3 months after initiation of progestin treatment. The 141 out of 148 (95.3%) patients achieved complete response (CR). No difference was found in cumulative CR rate between those with or without IR (90.2% vs. 95.6%, p=0.320). IR significantly affected therapeutic duration to achieve CR (8.1±0.5 months with IR vs. 6.1±0.4 months without IR, p=0.004). Overweight (body mass index [BMI]≥25 kg/m2) was associated with higher risk of treatment failure (odds ratio=5.61; 95% confidence interval=1.11–28.35; p=0.040) and longer therapeutic duration to achieve CR (7.6±0.5 months vs. 6.3±0.4 months, p=0.019). EAH patients with both IR and overweight (IR+BMI+) had the longest therapeutic time compared with other patients (8.8±0.6 months vs. 5.6±0.7, 6.3±0.4, and 6.4±0.8 months for IR−BMI+, IR−BMI−, and IR+BMI−, respectively, p=0.006).
Conclusion
IR and overweight were associated with longer therapeutic duration in EAH patients receiving progestin-based fertility-sparing treatment.
Keywords: Endometrial Hyperplasia, Conservative Treatment, Overweight, Insulin Resistance
INTRODUCTION
Insulin resistance (IR) has long been regarded as one of the most important risk factors of endometrial cancer (EC). Our previous study also showed that IR is an early event during the development of endometrial hyperplasia to cancer [1]. About 30% of patients suffering from endometrial hyperplasia without atypia, atypical hyperplasia and cancer were complicated with IR. IR might promote the development of endometrial hyperplasia to cancer through several mechanisms such as increasing local estrogen level or estrogen sensitivity in endometrium through inflammation-induced mechanisms [2,3].
However, it is not clear whether IR has any impact on the therapeutic effects of fertility-sparing treatment in endometrial atypical hyperplasia (EAH) and early-stage, well-differentiated EC. Reports showed that body mass index (BMI) ≥25 kg/m2 and elder age at diagnosis were associated with a lower complete response (CR) rate [4,5,6]. Although higher BMI and elder age are both associated with IR, the evidences found between them and the fertility-sparing treatment results cannot be used to postulate directly that IR is associated with poor therapeutic effects in EAH or EC patients. Furthermore, the synergetic effects of IR and BMI ≥25 kg/m2 in fertility-sparing treatment are also unclear.
Investigating the effects of IR on fertility-sparing treatment might help us better understand the role of IR in EAH patients to further improve the conservative therapeutic effects. Also, we explored some other possible risk factors affecting therapeutic effects and their relationship to IR.
MATERIALS AND METHODS
1. Study population
Patients receiving fertility-sparing treatment in Obstetrics and Gynecology Hospital of Fudan University from September 2011 and June 2016 were retrospectively investigated. Clinical and histopathological data as well as follow-up data were collected. This retrospective study was approved by the Ethics Committees of Obstetrics and Gynecology Hospital of Fudan University (approval No. [2014]11-X-2014-42).
All patients were diagnosed with EAH by endometrial biopsy through dilation and curettage (D&C) with or without hysteroscopy. Pathological diagnosis was confirmed by at least 2 experienced gynecological pathologists according to the World Health Organization (WHO) pathological classification (2014). If their opinions differed, a seminar was held in the pathological department for the final diagnosis. At our institution, patients were considered candidates for fertility-sparing treatment when: 1) younger than 45 years old, 2) they had strong desire to preserve fertility, 3) they had no contraindication for progestin treatment, 4) they were not pregnant, and 5) they had good compliance for treatment. Contraindications included: 1) severe medical diseases, such as liver or renal failure, and 2) other malignant diseases in reproductive system or other progestin-dependent cancers such as breast cancer. All patients had signed informed consent for the treatment.
2. Patient evaluation
The evaluation was done after pathological diagnosis was made yet before any treatment was given. General information was collected, including age, weight, height, history of medical complications (e.g., hypertension, diabetes, or heart attacks), and corresponding therapeutic history. Blood samples were tested for fasting blood glucose (FBG), fasting insulin (FINS) and lipid panel.
All blood samples were collected and examined in the laboratory of the Obstetrics and Gynecology Hospital. FBG and blood lipid levels were measured by a Hitachi fully automatic biochemical analyzer (Hitachi, Tokyo, Japan). FINS was measured by an insulin analyzer (MODULEVO E170; Roche Diagnostics, Indianapolis, IN, USA). Tests were repeated when results were beyond the normal range.
BMI was calculated as weight (kg)/height (m2). BMI ≥25 kg/m2 was considered as overweight. The homeostasis model assessment-insulin resistance (HOMA-IR) index was used to evaluate IR status. The HOMA-IR value was calculated as FBG (mmol/L)×FINS (μU/mL)/22.5. According to the distribution of non-diabetic patients' HOMA-IR values in our previous study [1], we chose 2.95, the lower limit of the top quarter, as the cut off value. When HOMA-IR was ≥2.95, we considered the patient to be insulin resistant. The metabolic syndrome (MS) criteria were as follows: 1) elevated waist circumference, which is ≥80 cm for Chinese women, 2) elevated triglycerides (TGs; patients on treatment for elevated TGs was an alternative indicator): TGs ≥150 mg/dL, 3) reduced high density lipoprotein cholesterol (HDL-C; patients on treatment for reduced HDL-C is an alternative indicator): HDL-C <50 mg/dL in female, 4) elevated blood pressure (antihypertensive treatment in a patient was an alternative indicator): systolic pressure ≥130 mmHg and/or diastolic pressure ≥85 mmHg, and 5) elevated FBG (patients on treatment for elevated FBG was an alternative indicator): FBG ≥100 mg/dL. Patients who met any 3 of the 5 criteria above were diagnosed with MS.
3. Fertility-sparing treatment and evaluation
All patients received medication after diagnosis. Progestin therapy was oral megestrol acetate (MA) at a dose of 160 mg/day. Some patients also received metformin 1,500 mg/day according to the doctor's choice.
Complete hysteroscopic evaluation was carried out every 3 months during the medical treatment. If only endometrial biopsy was done or the endometrial lesion was removed incompletely, another hysteroscopy within one month would be given to complete the evaluation after initiation of progestin treatment. In each hysteroscopic evaluation, the whole uterine cavity, especially area around the orifices of fallopian tubes and the lower segment near internal orifice of cervix were carefully evaluated. Suspected lesions were removed completely. Curettage was the most common way to remove the lesion so that endometrial basal layer could be preserved. Electronic resection was used only if myometrial invasion was suspected or the lesion was less than 2 cm in diameter. If there was no lesion suspected, a random endometrial biopsy would be done to evaluate the therapeutic effects.
The response to progestin treatment was assessed histologically using specimens obtained during each hysteroscopic evaluation. CR was defined as the absence of hyperplasia or carcinoma. Partial response (PR) was defined as presence of hyperplasia without atypia. No response (NR) was defined as persistence of atypical hyperplasia. Progression was defined as evidence of potential EC.
Once the patient achieved CR, the same regimen would be administered for another 2–3 months for consolidation. The patient was asked to receive assisted reproductive technology as soon as consolidating treatment was completed.
Definitive hysterectomy was recommended if NR was found after 6 months of treatment or progression was found at any time during treatment. For patients remained NR after 6 months of treatment but refused hysterectomy, alternative treatment would be given to the patient according to doctor's choice. These alternative treatments included 160 mg MA per day combined with 1,500 mg metformin per day (for those who used MA alone), ethinylestradiol cyproterone one pill per day for 21 days out of 28 day-cycle combined with metformin 1,500 mg per day or levonorgestrel intrauterine system (LNG-IUS) insertion.
4. Maintenance and follow-up
Low-dose cyclic progestin, or oral contraceptives, or a LNG-IUS was administered to patients without close pregnancy or after delivery to prevent recurrence. The patient was followed up every 3 to 6 months. Ultrasound and endometrial biopsy by Pipelle were used to evaluate the endometrium.
Adverse effects were recorded during the entire treatment and follow-up period, including weight gain, thrombosis, lactic acidosis, abnormal liver and renal function, and other toxicities or complaints.
All patients were followed up till July 2017.
5. Statistical analysis
Duration to achieve CR was measured from the time point of initiating progestin treatment to the time point diagnosing CR pathologically by hysteroscopy. Recurrence-free survival (RFS) was defined as the time, in months, from the date of achieving CR to the date of relapse or censoring.
All data were presented descriptively as medians, means or proportions. Values between 2 groups were compared using Student's t-test or the Mann-Whitney U test. Frequency distributions were compared using the χ2 test, except if the expected frequency was <5, when Fisher's exact test was employed. A logistic regression model was used for univariate analysis and multivariate analysis of the relationship between covariates and CR in response to treatment. Therapeutic duration and RFS were estimated by the Kaplan-Meier method and compared between groups using log-rank test. The p-values <0.05 in 2-sided tests were regarded as significant. All statistical analyses were performed using SPSS for Mac (version 20.0; IBM, Armonk, NY, USA).
RESULTS
1. Patient characteristics
A total of 151 EAH patients receiving fertility-sparing treatment were evaluated. The characteristics of patients are shown in Table 1. The median age at diagnosis was 33 years old with a range from 21 to 54 years old. Three patients with age ≥45 years old (47, 48, 54 years old, respectively) who insisted on uterus preservation were also included after being fully informed.
Table 1. General characteristics of the study population (n=151).
| Variables | Total | IR | Non-IR | p-value* | |
|---|---|---|---|---|---|
| No. of patients | 151 | 61 | 90 | - | |
| Age at diagnosis (yr) | 33.0 (21–54) | 33.0 (21–44) | 33.5 (21–54) | 0.130 | |
| BMI (kg/m2) | 24.23 (17.07–37.95) | 27.41 (18.75–37.95) | 22.17 (17.07–34.93) | <0.001 | |
| HOMA-IR | 2.49 (0.44–16.50) | 4.54 (3.03–16.50) | 1.64 (0.44–2.86) | <0.001 | |
| MS | 60 (39.7) | 40 (65.6) | 20 (22.2) | <0.001 | |
| Hypertension | 9 (6.0) | 6 (9.8) | 3 (3.3) | 0.100 | |
| Diabetes mellitus | 5 (3.3) | 5 (8.2) | 0 (0.0) | 0.010 | |
| Nulliparous | 107 (70.2) | 43 (70.5) | 64 (71.1) | 1.000 | |
| Progestin therapy | 0.170 | ||||
| MA | 82 (54.3) | 29 (47.5) | 53 (58.9) | - | |
| MA+metformin | 69 (45.7) | 32 (52.5) | 37 (41.1) | - | |
Values are presented as median (range) or number (%).
BMI, body mass index; HOMA-IR, homeostasis model assessment-insulin resistance; MA, megestrol acetate; MS, metabolic syndrome; IR, insulin resistance.
*p-value: comparison between IR and non-IR group.
Of the 151 patients, 82 (54.3%) received daily oral MA only. The 69 patients (45.7%) received daily oral MA plus metformin.
The 61 out of 151 (40.4%) patients were classified as insulin resistant. No significant difference was found in age, parity and metformin use between IR and non-IR patients. The distribution of BMI, MS, and diabetes mellitus, which are associated with IR, were significantly different between groups with and without IR. IR patients had higher BMI level (p<0.001), less MS (p<0.001), and less diabetes mellitus (p=0.010) than non-IR patients.
The median follow-up time from patients achieving CR to our final follow-up time point was 12 months (range, 1–55 months).
2. Outcome of fertility-sparing treatment
Out of the 151 patients, 3 patients chose hysterectomy only with progestin treatment for 3 months. They were all diagnosed as EAH with the pathological reports post-hysterectomy and refused for further fertility-sparing treatment. We excluded these 3 patients for calculation of cumulative CR rate because 3 months of treatment was too short for progestin treatment to achieve response for most of patients. Among the remaining 148 patients, 141 (95.3%) achieved CR after progestin treatment. The mean duration of progestin treatment for achieving CR was 6.9±0.3 months (range, 1–15 months).
Seven patients did not achieve CR in our program. Three of them quit the progestin treatment in the middle with hysterectomy, who were one patient diagnosed as endometrioid cancer by hysteroscopy 3 months after progestin treatment (the pathological report showed grade 1 endometrioid cancer with superficial myometrium invasion for the patient with cancer); one EAH patient with 6 months of treatment and one EAH patient with 12 months of treatment. The other 4 were still on the progestin treatment. One patient was partially recovered after 9 months of treatment and turned to oral cyclic medroxyprogesterone acetate treatment. Three patients remained NR after 6 to 9 months of MA treatment then turned to LNG-IUS, and they all received a total of 15 months of treatment by the end point of our follow-up and refused definitive surgery regardless of our recommendation.
No severe adverse effect was noted in all patients studied.
Among the 141 patients achieved CR, 56 patients prepared to get pregnant recently. The 25 of them achieved at least one pregnancy (median follow-up duration, 7 months, with the range of 1–25 months), for whom, 22 received assisted reproduction treatment while the other 3 got pregnant spontaneously.
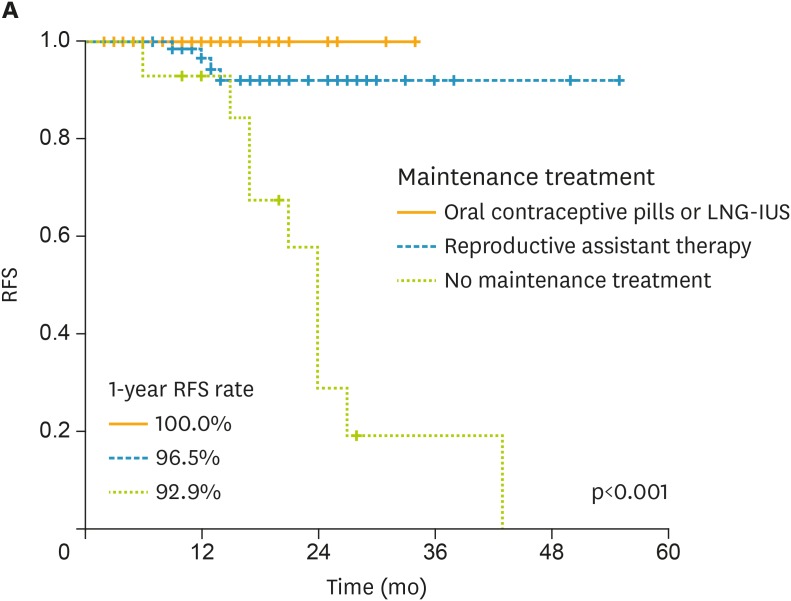
The relapse rate was 10.1% (14/138) and the median time interval to recurrence was 14 months (range, 3–55 months). Ten out of the 14 relapsed as they did not use any preventive regimen after achieving CR. Another 4 relapsed during reproductive assistant therapy. No patient on continuous cyclic oral contraceptive pills or LNG-IUS relapsed (Fig. 1).
Fig. 1.
RFS of 141 EAH patients achieving CR. The RFS of 141 EAH patients achieving CR was calculated according to their different maintenance strategies. No recurrence was found in patients receiving oral contraceptive pills or LNG-IUS.
CR, complete response; EAH, endometrial atypical hyperplasia; LNG-IUS, levonorgestrel intrauterine system; RFS, recurrence-free survival.
3. IR affected fertility-sparing treatment duration in EAH patients
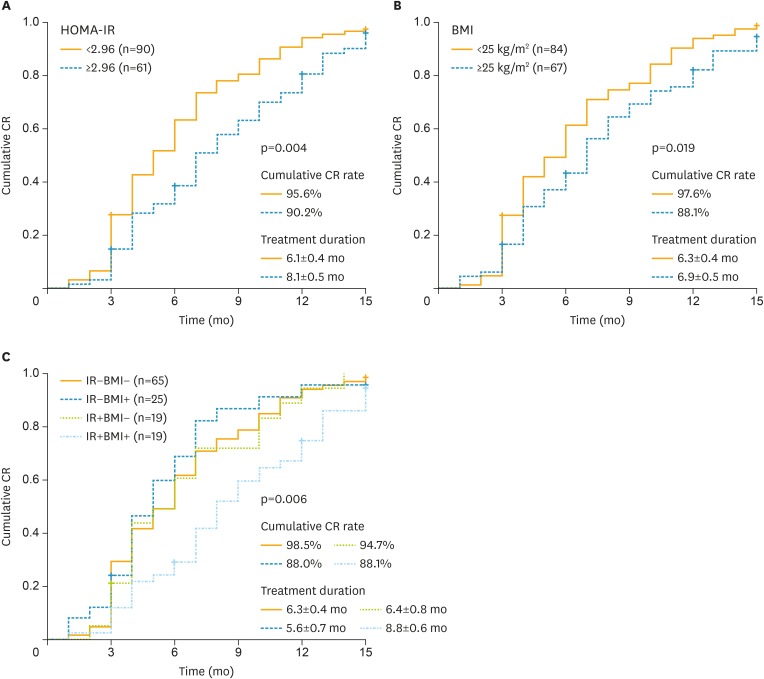
To answer whether IR affected the therapeutic results in EAH patients on fertility-sparing treatment, we first analyzed possible factors affecting CR rate and therapeutic duration to achieve CR. We found that IR and BMI significantly affected fertility-sparing therapeutic effects (Table 2). No difference was found in cumulative CR rate between groups with and without IR (90.2% vs. 95.6%, p=0.320). But patients with IR took longer to achieve CR compared with those without IR (8.1±0.5 months vs. 6.1±0.4 months, p=0.004) (Fig. 2A).
Table 2. Factors associated with effect of conservative treatment (n=151).
| Characteristics | Total | CR | p-value | Treatment duration (mo) | p-value | |
|---|---|---|---|---|---|---|
| Total | 151 | 141 (93.4) | - | - | - | |
| Age at diagnosis (yr) | <30 | 46 | 44 (95.7) | 0.720 | 7.0±0.6 | 0.620 |
| ≥30 | 105 | 97 (92.4) | - | 6.8±0.4 | - | |
| BMI (kg/m2) | <25.0 | 84 | 82 (97.6) | 0.023 | 6.3±0.4 | 0.019 |
| ≥25.0 | 67 | 59 (88.1) | - | 7.6±0.5 | - | |
| IR | No | 90 | 86 (95.6) | 0.320 | 6.1±0.4 | 0.004 |
| Yes | 61 | 55 (90.2) | - | 8.1±0.5 | - | |
| MS | No | 91 | 85 (93.4) | 0.620 | 6.5±0.4 | 0.220 |
| Yes | 60 | 56 (93.3) | - | 7.4±0.5 | - | |
| Hypertension | No | 142 | 132 (93.0) | 0.530 | 6.9±0.3 | 0.880 |
| Yes | 9 | 9 (100.0) | - | 7.0±1.6 | - | |
| Diabetes mellitus | No | 146 | 136 (93.2) | 1.000 | 6.8±0.3 | 0.430 |
| Yes | 5 | 5 (100.0) | - | 8.8±1.8 | - | |
| Nulliparous | Yes | 107 | 103 (96.3) | 0.064 | 6.9±0.4 | 0.660 |
| No | 44 | 38 (86.4) | - | 6.7±0.5 | - | |
| Progestin therapy | MA alone | 82 | 78 (95.1) | 0.510 | 6.7±0.4 | 0.650 |
| MA+metformin | 69 | 63 (91.3) | - | 7.1±0.5 | - | |
Data are shown as number (%) or mean ± standard deviation.
BMI, body mass index; CR, complete response; MA, megestrol acetate; MS, metabolic syndrome; IR, insulin resistance.
Fig. 2.
Cumulative CR rate in EAH patients. (A) The cumulative CR rate in patients with or without IR. Patients with IR needed longer time to achieve CR. (B) The cumulative CR rate in patients with BMI ≥25 kg/m2 or BMI <25 kg/m2. Patients with BMI ≥25 kg/m2 had poor CR rate and needed longer time to achieve CR compared with those with BMI <25 kg/m2. (C) The cumulative CR rate in patients with different IR status and BMI. Patients with both IR and BMI ≥25 kg/m2 needed the longest time to achieve CR.
BMI, body mass index; CR, complete response; EAH, endometrial atypical hyperplasia; HOMA-IR, homeostasis model assessment-insulin resistance; IR, insulin resistance.
Univariate analysis showed that only BMI ≥25 kg/m2 was significantly associated with poor CR rate (odds ratio [OR]=5.56; 95% confidence interval [CI]=1.14–27.13; p=0.030). The CR rate in patients with BMI ≥25 kg/m2 and those <25 kg/m2 were 88.1% and 97.6%, respectively. After adjusting for patient age, medical co-morbidity, parity and metformin use, BMI ≥25 kg/m2 remained to be significantly correlated with poor CR rate (OR=5.77; 95% CI=1.12–29.73; p=0.040). Also, patients with BMI ≥25.0 kg/m2 needed longer treatment to achieve CR compared with BMI <25.0 kg/m2 (7.6±0.5 months vs. 6.3±0.4 months, p=0.019) (Fig. 2B).
As not all patients with IR are complicated with high BMI, the next question we asked was whether there is any difference in the therapeutic effects between IR patients with or without high BMI. We divided patients into 4 groups according to their IR and BMI status as shown in Table 3 and Fig. 2C. The result showed that IR+BMI+ patients (insulin resistant and BMI ≥25 kg/m2) had the longest therapeutic duration (8.8±0.6 months) to achieve CR compared with other 3 groups (p=0.010). Treatment duration in IR+BMI+ group was significantly longer than all the other 3 groups (IR+BMI+ 8.8±0.6 months vs. IR−BMI+ 5.6±0.7 months, p=0.004; vs. IR−BMI− 6.3±0.4 months, p=0.003; vs. IR+BMI− 6.4±0.8 months, p=0.019).
Table 3. The length of treatment to achieve CR and pregnancy outcome according to BMI and IR (n=151).
| Groups | IR−BMI− (n=65) | IR−BMI+ (n=25) | IR+BMI− (n=19) | IR+BMI+ (n=42) | p-value |
|---|---|---|---|---|---|
| IR | No | No | Yes | Yes | - |
| BMI ≥25.0 kg/m2 | No | Yes | No | Yes | - |
| CR | 64 (98.5) | 22 (88.0) | 18 (94.7) | 37 (88.1) | 0.071 |
| Treatment duration (mo) | 6.3±0.4 | 5.6±0.7 | 6.4±0.8 | 8.8±0.6 | 0.006 |
| Recent pregnant preparation | 27 | 8 | 11 | 11 | - |
| Pregnant | 12 (44.0) | 4 (33.3) | 7 (63.6) | 3 (27.3) | 0.601 |
Data are shown as number (%) or mean ± standard deviation.
BMI, body mass index; CR, complete response; IR, insulin resistance.
IR+BMI+ Patients had a pregnant rate of 27.3%, which was lower than other 3 groups but without significant differences (p=0.601) (Table 3). Neither IR nor BMI ≥25.0 kg/m2 was found to affect relapse rate in this study (data not shown).
DISCUSSION
Epidemiological studies have shown that IR is an important risk factor for the development of EC [7]. In our previous study, we found that increased insulin levels and IR were remarkably related to disordered proliferative endometrium, endometrial hyperplasia and EC [1].
Studies have demonstrated that IR plays an important role in the development of EC [8,9]. However, the role of IR in EAH and EC patients on fertility-sparing treatment in is unclear. Our study demonstrated that IR negatively affected progestin-based conservative treatment duration in EAH patients. Patients complicated with IR needed longer time to achieve CR compared with those without IR.
Our findings are consistent with current reports that higher BMI correlates with worse therapeutic effects and higher recurrence in EAH and EC patients [6,10,11]. Our study confirmed that BMI ≥25 kg/m2 was associated with lower CR rate and longer treatment duration to achieve CR in EAH patients. As IR is positively related to BMI [12,13], IR might also play a role in the poor prognosis of overweight EAH and EC patients.
The fact that both IR and BMI ≥25 kg/m2 negatively affected progestin-based fertility-sparing treatment duration in EAH or EC patients indicated that IR and overweight might play a synergistic role in counteracting progestin function and therefore compromised its therapeutic effects. We suppose that IR or overweight induced overproduction of peripheral and local estrogen [14], abnormal function of endometrial stromal cells [15], and the local inflammatory environment induced in the endometrium [3] might be the culprit causing less favorable therapeutic effects in these patients. Study showed that IR and increased body fat promoted estrogen production in both ovary and peripheral adipose tissue [16]. Hyperinsulinemia and higher BMI also indirectly down-regulated sex hormone-binding globulin (SHBG) level, which increased circulating free estradiol level [17,18]. Some literatures reported that IR up-regulated estrogen sensitivity in endometrium through sensitizing the estrogen receptor and G-protein coupled receptor (GPER) [19,20]. Piltonen et al. [15] showed that endometrial stromal fibroblasts from women with polycystic ovary syndrome (PCOS; for whom IR is a main characteristic) exhibited an aberrant decidualization response to progesterone and concomitant changes in pro-inflammatory cytokine, chemokine, and matrix metalloproteinase (MMP) release and immune cell chemoattraction. Inflammatory microenvironment induced by IR might result in progestin resistance in endometrium [21]. Tumor necrosis factor (TNF)-α and interleukin (IL)-1β could directly decrease progesterone receptor isoforms, possibly via epigenetic modifications [22,23]. Pro-inflammatory cytokines may disrupt receptor function through alterations in steroid receptor chaperone proteins, by directly competing for receptor co-regulators or interfering the functional bridges connecting PR and transcription factors, such as FOXO1, which is essential for expression of key PR target genes [24,25]. In general, these possible mechanisms potentially work together leading to the progestin resistance in endometrium for overweight women with or without IR.
Metformin is an insulin sensitizer, which has been widely investigated to treat various malignant diseases adjunctively. Studies have been carried out to explore the effects of metformin on fertility-sparing treatment for EAH and EC patients [26,27]. Whether metformin could improve the therapeutic effects in them especially with IR is unclear yet. Our pilot study showed that metformin combined with MA might shorten therapeutic duration to achieve CR in EAH patients, while no significant improvement in CR rate [28]. A prospective randomized controlled trial (NCT01968317: Megestrol Acetate Plus Metformin to Megestrol Acetate in Patients with Endometrial Atypical Hyperplasia or Early Stage Endometrial Adenocarcinoma) done by our team is recently closed and the results will be provided in the near future which might help answer these questions.
In our study, the CR rate in the 148 EAH patients was 95.3% and the treatment duration to achieve CR was 6.9 months, which is a relatively decent outcome for fertility-sparing treatment [29,30]. A recent study showed that the overall response rate, evaluated by curettage or endometrial biopsy every 3 or 6 months, in 88 patients with EAH or EC was 87.5% with the median treatment time at 8 months [10]. In the study conducted by Pronin et al. [31], treatment of the LNG-IUS insertion showed 92% (35/38 cases) CR rate at 9 to 12 months, evaluated by Pipelle endometrial biopsy or curettage by hysteroscopy. Our treatment efficacy was better compared with these data. We suppose that thorough evaluation of the uterine cavity and complete removal of endometrial lesion by hysteroscopy might help improve the fertility-sparing treatment effects in EAH and EC patients. Hysteroscopy can help remove endometrial lesion under direct visualization without harming other healthy endometrium and basal layer [32,33,34]. Further study of hysteroscopy combined with progestin in fertility-sparing treatment in EAH and EC patients is being carried out in our hospital.
Our study still has some limitations. We only analyzed EAH patients in our study retrospectively. A prospective study in both EAH and EC patients with a standardized evaluation and treatment protocol might provide more information about the effects of IR on fertility-sparing treatment in these patients.
In conclusion, the results of our study indicated that IR and BMI ≥25 kg/m2 negatively affected progestin-based fertility-sparing treatment duration in EAH patients. As this is an initial study, we want to support the future investigations with these findings and contribute to the improvement of the therapeutic effects of fertility-sparing treatment for EAH and EC patients.
ACKNOWLEDGMENTS
We are grateful for the data work of Weiwei Shan, Chengcheng Ning, Bingying Xie, and Bing Li as well as editing assistance received from Yue Shi.
Footnotes
Funding: The authors' work is funded by National Natural Science Foundation of China (Grant No. 81370688, 81671417), Shanghai Science and Technology Development Medical Guide Project (Grant No. 17411961000), and Talent training program from Shanghai Municipal Commission of Health and Family Planning (No. 2017BR035).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.X., L.X.
- Formal analysis: Y.B., X.L., D.Y., Z.H., Z.Q.
- Funding acquisition: C.X.
- Investigation: Y.B., X.L., D.Y., Z.H., Z.Q.
- Methodology: Y.B., X.L.
- Resources: Y.B., X.L.
- Software: D.Y.
- Supervision: L.X.
- Validation: Y.B., X.L.
- Writing - original draft: Y.B.
- Writing - review & editing: C.X., L.X.
References
- 1.Shan W, Ning C, Luo X, Zhou Q, Gu C, Zhang Z, et al. Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: a prospective cross-sectional study. Gynecol Oncol. 2014;132:606–610. doi: 10.1016/j.ygyno.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Mitsuhashi A, Uehara T, Hanawa S, Shozu M. Prospective evaluation of abnormal glucose metabolism and insulin resistance in patients with atypical endometrial hyperplasia and endometrial cancer. Support Care Cancer. 2017;25:1495–1501. doi: 10.1007/s00520-016-3554-y. [DOI] [PubMed] [Google Scholar]
- 3.Ning C, Xie B, Zhang L, Li C, Shan W, Yang B, et al. Infiltrating macrophages induce ERα expression through an IL17A-mediated epigenetic mechanism to sensitize endometrial cancer cells to estrogen. Cancer Res. 2016;76:1354–1366. doi: 10.1158/0008-5472.CAN-15-1260. [DOI] [PubMed] [Google Scholar]
- 4.Eftekhar Z, Izadi-Mood N, Yarandi F, Shojaei H, Rezaei Z, Mohagheghi S. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: our experiences with 21 patients. Int J Gynecol Cancer. 2009;19:249–252. doi: 10.1111/IGC.0b013e31819c5372. [DOI] [PubMed] [Google Scholar]
- 5.Simpson AN, Feigenberg T, Clarke BA, Gien LT, Ismiil N, Laframboise S, et al. Fertility sparing treatment of complex atypical hyperplasia and low grade endometrial cancer using oral progestin. Gynecol Oncol. 2014;133:229–233. doi: 10.1016/j.ygyno.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Park JY, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002) Eur J Cancer. 2013;49:868–874. doi: 10.1016/j.ejca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez AV, Pasupuleti V, Benites-Zapata VA, Thota P, Deshpande A, Perez-Lopez FR. Insulin resistance and endometrial cancer risk: A systematic review and meta-analysis. Eur J Cancer. 2015;51:2747–2758. doi: 10.1016/j.ejca.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Merritt MA, Strickler HD, Einstein MH, Yang HP, Sherman ME, Wentzensen N, et al. Insulin/IGF and sex hormone axes in human endometrium and associations with endometrial cancer risk factors. Cancer Causes Control. 2016;27:737–748. doi: 10.1007/s10552-016-0751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu N, Zhu Y, Wang Y, Zhang H, Xue F. Insulin resistance: a significant risk factor of endometrial cancer. Gynecol Oncol. 2012;125:751–757. doi: 10.1016/j.ygyno.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Yang YF, Liao YY, Liu XL, Su SG, Li LZ, Peng NF. Prognostic factors of regression and relapse of complex atypical hyperplasia and well-differentiated endometrioid carcinoma with conservative treatment. Gynecol Oncol. 2015;139:419–423. doi: 10.1016/j.ygyno.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Jin Y, Li Y, Bi Y, Shan Y, Pan L. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynaecol Obstet. 2016;132:34–38. doi: 10.1016/j.ijgo.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 12.Yazıcı D, Sezer H. Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol. 2017;960:277–304. doi: 10.1007/978-3-319-48382-5_12. [DOI] [PubMed] [Google Scholar]
- 13.Greenfield JR, Campbell LV. Insulin resistance and obesity. Clin Dermatol. 2004;22:289–295. doi: 10.1016/j.clindermatol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Lu J, Wu S, Bi Y, Mu Y, Zhao J, et al. Association of insulin resistance with breast, ovarian, endometrial and cervical cancers in non-diabetic women. Am J Cancer Res. 2016;6:2334–2344. [PMC free article] [PubMed] [Google Scholar]
- 15.Piltonen TT, Chen JC, Khatun M, Kangasniemi M, Liakka A, Spitzer T, et al. Endometrial stromal fibroblasts from women with polycystic ovary syndrome have impaired progesterone-mediated decidualization, aberrant cytokine profiles and promote enhanced immune cell migration in vitro. Hum Reprod. 2015;30:1203–1215. doi: 10.1093/humrep/dev055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 17.Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26:376–383. doi: 10.1016/j.tem.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Simó R, Saez-Lopez C, Lecube A, Hernandez C, Fort JM, Selva DM. Adiponectin upregulates SHBG production: molecular mechanisms and potential implications. Endocrinology. 2014;155:2820–2830. doi: 10.1210/en.2014-1072. [DOI] [PubMed] [Google Scholar]
- 19.Lv QY, Xie BY, Yang BY, Ning CC, Shan WW, Gu C, et al. Increased TET1 expression in inflammatory microenvironment of hyperinsulinemia enhances the response of endometrial cancer to estrogen by epigenetic modulation of GPER. J Cancer. 2017;8:894–902. doi: 10.7150/jca.17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie BY, Lv QY, Ning CC, Yang BY, Shan WW, Cheng YL, et al. TET1-GPER-PI3K/AKT pathway is involved in insulin-driven endometrial cancer cell proliferation. Biochem Biophys Res Commun. 2017;482:857–862. doi: 10.1016/j.bbrc.2016.11.124. [DOI] [PubMed] [Google Scholar]
- 21.Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96:623–632. doi: 10.1111/aogs.13156. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Starzinski-Powitz A, Guo SW. Prolonged stimulation with tumor necrosis factor-alpha induced partial methylation at PR-B promoter in immortalized epithelial-like endometriotic cells. Fertil Steril. 2008;90:234–237. doi: 10.1016/j.fertnstert.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Pierce S, Roberson AE, Hyatt K, Singleton K, Deschamps D, Myers DA. Interaction between progesterone and interleukin-1β in modulating progesterone receptor expression and the inflammatory phenotype in human cervical fibroblasts. Reprod Sci. 2017 doi: 10.1177/1933719117725826. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 24.Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358:208–215. doi: 10.1016/j.mce.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 25.Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update. 2015;21:155–173. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitsuhashi A, Sato Y, Kiyokawa T, Koshizaka M, Hanaoka H, Shozu M. Phase II study of medroxyprogesterone acetate plus metformin as a fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Ann Oncol. 2016;27:262–266. doi: 10.1093/annonc/mdv539. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Guo YR, Lin JF, Feng Y, Billig H, Shao R. Combination of Diane-35 and metformin to treat early endometrial carcinoma in PCOS women with insulin resistance. J Cancer. 2014;5:173–181. doi: 10.7150/jca.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan W, Wang C, Zhang Z, Gu C, Ning C, Luo X, et al. Conservative therapy with metformin plus megestrol acetate for endometrial atypical hyperplasia. J Gynecol Oncol. 2014;25:214–220. doi: 10.3802/jgo.2014.25.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266.e1–266.12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Pronin SM, Novikova OV, Andreeva JY, Novikova EG. Fertility-sparing treatment of early endometrial cancer and complex atypical hyperplasia in young women of childbearing potential. Int J Gynecol Cancer. 2015;25:1010–1014. doi: 10.1097/IGC.0000000000000467. [DOI] [PubMed] [Google Scholar]
- 32.De Marzi P, Bergamini A, Luchini S, Petrone M, Taccagni GL, Mangili G, et al. Hysteroscopic resection in fertility-sparing surgery for atypical hyperplasia and endometrial cancer: safety and efficacy. J Minim Invasive Gynecol. 2015;22:1178–1182. doi: 10.1016/j.jmig.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Arendas K, Aldossary M, Cipolla A, Leader A, Leyland NA. Hysteroscopic resection in the management of early-stage endometrial cancer: report of 2 cases and review of the literature. J Minim Invasive Gynecol. 2015;22:34–39. doi: 10.1016/j.jmig.2014.08.782. [DOI] [PubMed] [Google Scholar]
- 34.Mazzon I, Corrado G, Masciullo V, Morricone D, Ferrandina G, Scambia G. Conservative surgical management of stage IA endometrial carcinoma for fertility preservation. Fertil Steril. 2010;93:1286–1289. doi: 10.1016/j.fertnstert.2008.12.009. [DOI] [PubMed] [Google Scholar]